We describe the case of a 13-year-old male with a history of truncus arteriosus (Van Praagh type 1A), moderate truncal valve insufficiency, and right aortic arch who underwent neonatal truncus arteriosus repair with ventricular septal defect closure, truncal valve annuloplasty, and placement of a right ventricle-to-pulmonary artery (RV-PA) conduit. Subsequent intervention included conduit upsizing and stenting, implantation of a transcatheter valve within the stented proximal conduit, and device occlusion of a resulting right ventricular outflow tract pseudoaneurysm.
The patient remained physically active without symptoms attributable to the cardiovascular system. Serial transthoracic echocardiograms demonstrated truncal insufficiency that advanced from mild-moderate to moderate-severe and surveillance cardiac magnetic resonance imaging demonstrating moderate-severe truncal insufficiency (52–55%) with progression of left ventricular dilation. He was referred for surgical intervention with anticipated truncal valve replacement with a prosthetic valve and root-sparing ascending aorta graft.
Following review by the Pediatric Valve Center, comprised of a team of cardiologists, cardiology fellows, cardiothoracic surgeons, sonographers, and research assistants, a transesophageal echocardiogram and cardiac computer tomographic angiography (CTA) were performed to delineate the truncal valve morphology and the mechanism of valve insufficiency (Video 1). The retrospectively gated CTA demonstrated a quadricuspid truncal valve with incomplete apposition of the valve leaflets during diastole (Figure 1). The SlicerHeart extension for 3D Slicer (www.slicer.org) was used to perform post-processing morphologic analysis, including volume rendering and focused segmentation of the truncal valve (Figure 2, Videos 2–4).1 Measurements of truncal valve dimensions were performed in a manner analogous to that described by Berrebi et al. for the aortic valve.2 Specifically, the geometric height (gH) and effective height (eH) of each leaflet were determined in the context of the 3D models and volume renderings. Visualization demonstrated a normal commissural post height of the right anterior leaflet, which would serve as the reference leaflet, and three separate symmetrically deficient leaflets with partial fusion of the commissure between the right posterior and left posterior leaflets. Measurements of gH and eH demonstrated relative deficiency of the left anterior leaflet and reduced, but equal effective heights of the left anterior, left posterior, and right posterior leaflets. Modeling demonstrated relative prolapse of the deficient valve leaflets below the level of the annular plane, resulting in regurgitation through a central coaptation defect. The left anterior leaflet was additionally retracted, highly irregular, and had a fenestration adjacent to the left anterior-posterior commissure through which regurgitation occurred. In preoperative planning, the most ‘normal’ appearing leaflet (right anterior) was designated as the “reference leaflet” to which the remaining leaflets were virtually revised to approximate in geometric and effective height. Although the remaining leaflets were not symmetric in size, it was determined that combining those leaflets to create a bicuspid valve would create a structure where the reference leaflet and combined leaflets were more alike in height and leaflet excursion, optimizing coaptation and competence.
Figure 1: Preoperative Imaging of Truncal Valve.

A. 3D Echocardiographic (3DE) image of truncal valve in diastole; B. 3DE image in truncal valve in systole; C. Short-Axis 2D Color Doppler in diastole showing primarily central regurgitation; D. 3D Color Doppler image in diastole demonstrating primarily central regurgitation; E. Volume rendering of CT scan in diastole demonstrating central coaptation defect from a surgical view; F. Volume rendering of CT scan in systole showing partial leaflet fusion. See Video 1 for expanded description and multiphase video. A=Anterior, P=Posterior, L=Left, R=Right.
Figure 2: 3D Image-Derived Modeling to Inform Truncal Valve Repair.

A. Measurement of the geometric height (gH) in a 2D plane bisecting the anterior and rightward truncal leaflet in diastole; B. Same measurement in volume rendered CT scan; C. Measurement of the geometric height (gH) in a 2D plane bisecting the anterior and rightward truncal leaflet in 3D echocardiogram (3DE) in diastole; D. Same measurement in volume rendering of 3DE; E. Segmented model of truncal valve in diastole; F. Segmented model of truncal valve in diastole with upper and lower annular rings demonstrating asymmetry of leaflet heights; F. Segmented model of truncal valve with upper and lower annular rings demonstrating asymmetry of leaflet heights. See Videos 2–4 for expanded description. Blue leaflet = Left anterior, Green leaflet = Right anterior, Yellow leaflet = Left posterior, Red leaflet = Right posterior.
The patient underwent truncal valve repair with RV-PA conduit replacement and right pulmonary arterioplasty. Intraoperative inspection confirmed the decision to proceed with bicuspidization of the truncal valve, with preservation of the normal right anterior leaflet and closure of the residual commissures between the posterior leaflets and the right anterior and right posterior leaflets. The raphe between the two posterior leaflets was taken down from the aortic wall and redundant leaflet tissue was resected. The central area of deficiency between these leaflets was plicated with two interrupted sutures. A patch of pericardium was used to repair the fenestration within the left anterior leaflet. The remaining aspects of the coaptation between the left anterior and left posterior leaflets were closed, yielding a functionally bicuspid valve with adequate coaptation height and excursion. Geometric analysis and surgical inspection suggested that sufficient coaptation was achievable by addressing leaflet distortion, prolapse, and fenestration. As such, aortic annular and root interventions were not performed.
Postoperative transesophageal echocardiography demonstrated a normal coaptation height of 8 mm with trivial truncal insufficiency and mild truncal stenosis with a peak gradient of 27 mmHg (Figure 3, Video 5). The patient was managed in the Cardiac Intensive Care Unit postoperatively on milrinone and nicardipine for afterload reduction and was ultimately transitioned to amlodipine and lisinopril. He was discharged on the fifth postoperative day with a discharge echocardiogram demonstrating mild truncal insufficiency and stenosis with a peak gradient of 25 mmHg, while the systolic blood pressure was 121 mmHg.
Figure 3: Comparison of Preoperative Imaging to Postoperative Imaging of Truncal Valve Repair.
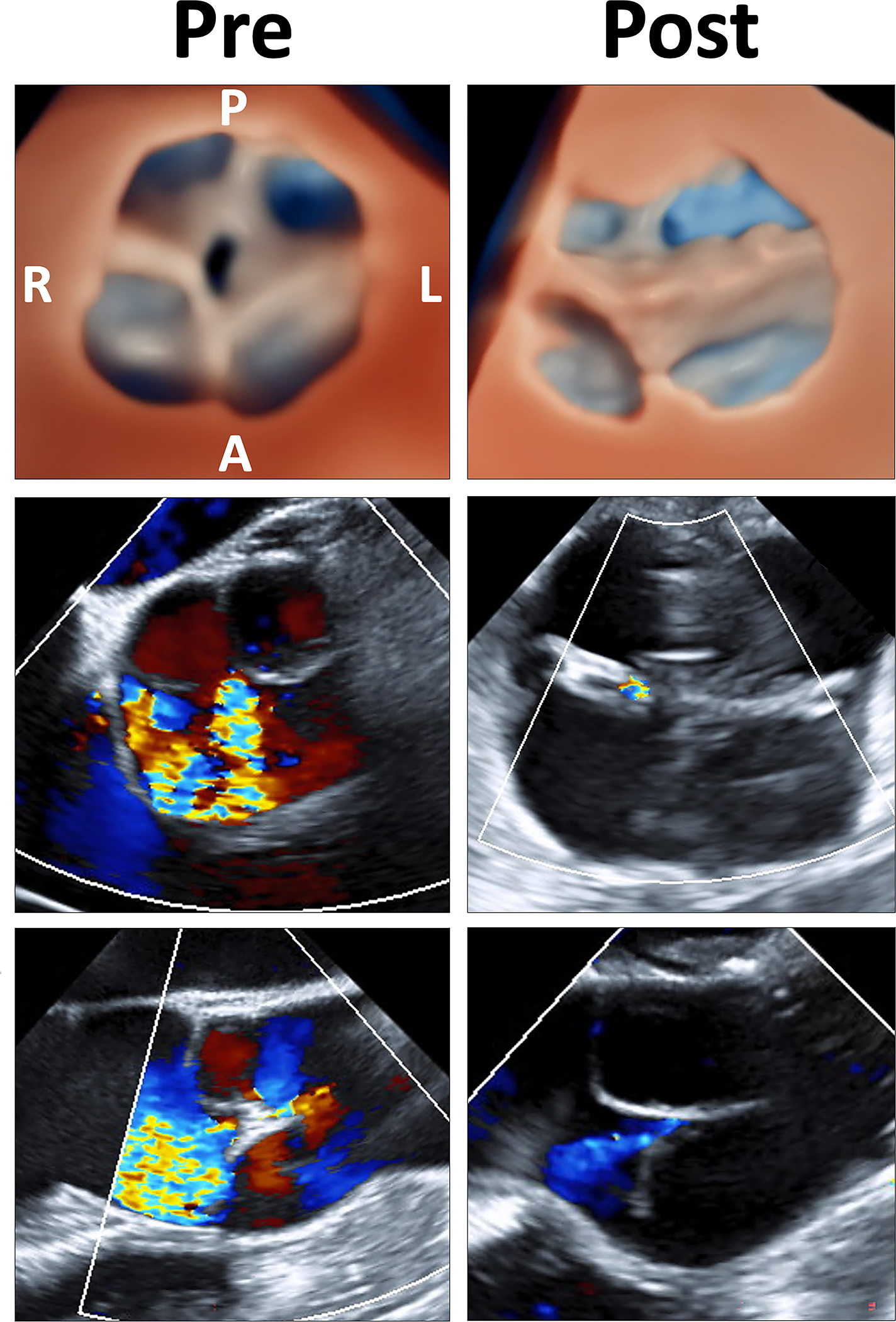
Top. Comparison of 3D echocardiographic images of truncal valve before and after repair in diastole; Middle. Comparison of 2D-color doppler in short axis before and after repair showing significantly reduced regurgitation; Bottom: Comparison of 2D-color doppler in long axis before and after repair demonstrating the same. See Video 5 for expanded description. 3D = three-dimensional; 2D = two-dimensional; L = left; R = right; A = anterior; P= posterior; Pre = pre-repair; Post = post-repair
Persistent truncus arteriosus is an uncommon congenital cardiovascular malformation in which a single arterial vessel arises from the base of the heart and gives rise to the aorta, at least one coronary artery, and at least one pulmonary artery. Truncal valve morphology can be complex and truncal valves are frequently regurgitant. However, truncal valve repair can be difficult in the context of heterogeneous morphology including thickened and dysplastic cusps, cusp size inequality, cusp prolapse, commissural abnormalities, and annular dilation.3
We demonstrate how 3D image derived pre-surgical planning may inform the repair of a complex valve (Videos 2–4).3,4 We utilized a custom workflow involving 3D image volume rendering and segmentation to derive quantitative metrics, analogous to those used in aortic valve surgical planning. The integration of visualization enhanced by interactive quantification leveraging a template-based framework subjectively improved understanding of the valve geometry and mechanism of dysfunction relative to multiplanar reconstruction alone. This in turn, informed the decisions leading to successful short-term result.
In the future, we hope to incorporate these tools into a flexible 3D image-derived workflow to allow measurements of the current valve, editing of the valve leaflets, annulus and root to perform “virtual surgery,” and generation of a template for the planned result (Video 4). Such image-derived models may also inform patient-specific application of emerging engineering techniques. We hope that these advances may improve communication between imagers and surgeons, facilitate surgical planning, and inform the complex decision to repair or replace a complex valve. However, further work is needed to demonstrate a benefit to patient outcomes.
Supplementary Material
Sources of Funding:
This work was supported by NIH 1R01HL153166, The Children’s Hospital of Philadelphia (CHOP) Pediatric Valve Center, and the Cora Topolewski Fund at CHOP Pediatric Valve Center
Footnotes
Disclosures: Csaba Pinter is contracted open-source software developer at Pixel Medical. The other authors have no disclosures.
References:
- 1.Scanlan AB, Nguyen AV, Ilina A, Lasso A, Cripe L, Jegatheeswaran A, Silvestro E, McGowan FX, Mascio CE, Fuller S, et al. Comparison of 3D Echocardiogram-Derived 3D Printed Valve Models to Molded Models for Simulated Repair of Pediatric Atrioventricular Valves. Pediatr Cardiol. 2018;39:538–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berrebi A, Monin JL, Lansac E. Systematic echocardiographic assessment of aortic regurgitation-what should the surgeon know for aortic valve repair? Ann Cardiothorac Surg. 2019;8:331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naimo PS, Buratto E, Konstantinov IE. Truncal valve repair in children. J Thorac Cardiovasc Surg. 2021;162:1337–1342. [DOI] [PubMed] [Google Scholar]
- 4.Tretter JT, Izawa Y, Spicer DE, Okada K, Anderson RH, Quintessenza JA, Mori S. Understanding the Aortic Root Using Computed Tomographic Assessment: A Potential Pathway to Improved Customized Surgical Repair. Circ Cardiovasc Imaging. 2021;14:e013134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


