Abstract
Study Design:
Retrospective study at a single academic institution
Objective:
The purpose of this study is to utilize machine learning to predict hospital length of stay (LOS) and discharge disposition following adult elective spine surgery, and to compare performance metrics of machine learning models to the ACS NSQIP prediction calculator.
Summary of Background Data:
3,678 adult patients undergoing elective spine surgery between 2014–2019, acquired from the Electronic Health Record (EHR).
Methods:
Patients were divided into 3 stratified cohorts: cervical degenerative, lumbar degenerative, and adult spinal deformity groups (ASD). Predictive variables included demographics, BMI, surgical region, surgical invasiveness, surgical approach, and comorbidities. Regression, classification trees, and Least Absolute Shrinkage and Selection Operator (LASSO) were used to build predictive models. Validation of the models was conducted on 16% of patients (N=587), using area under the receiver operator curve (AUROC), sensitivity, specificity, and correlation. Patient data were manually entered into the ACS NSQIP online risk calculator to compare performance. Outcome variables were discharge disposition (home vs rehabilitation) and LOS (days).
Results:
Of 3,678 patients analyzed, 51.4% were male (n=1,890) and 48.6% were female (n=1,788). The average LOS was 3.66 days. 78% were discharged home and 22% discharged to rehabilitation. Compared to NSQIP (Pearson R2=0.16), the predictions of poisson regression (R2=0.29) and LASSO (R2=0.29) models were significantly more correlated with observed LOS (p=0.025 and p=0.004, respectively). Of the models generated to predict discharge location, logistic regression yielded an AUROC of 0.79, which was statistically equivalent to the AUROC of 0.75 for NSQIP (p= 0.135).
Conclusion:
The predictive models developed in this study can enable accurate preoperative estimation of LOS and risk of rehabilitation discharge for adult patients undergoing elective spine surgery. The demonstrated models exhibited better performance than NSQIP for prediction of LOS and equivalent performance to NSQIP for prediction of discharge location.
MINI ABSTRACT
Accurate preoperative identification of patients at risk for extended length of stay (LOS) and discharge to rehabilitation can provide substantial benefit for patients undergoing elective spine surgery. We built machine learning models that predicted both outcomes and compared performance metrics to that of the ACS NSQIP prediction calculator.
Introduction
Complications and delayed recovery following elective spine surgery can greatly impact patient quality of life and perception of improvement [1]. Given perioperative medical complication rates between 25–52% for highly invasive spine surgeries, such as long-segment fusions for adult spinal deformity, patients may require extended hospital length of stay (LOS) and rehabilitation services postoperatively [2,3]. LOS is notable as a composite measurement of the postoperative course, as patients with systemic illnesses, elderly age, and those with hospital-acquired infections stay longer in the hospital [4,5]. Discharge to rehabilitation may be an avenue for patients requiring need for extended care and aid in return to function [6,7]. From a cost perspective, extended LOS has been identified as a reliable predictor for catastrophic costs over $100,000 following spine surgery, while usage of rehabilitation services can account for 30% of the cost of care [8–10]. Even in cases where rehabilitation discharge is not due to a complication, an alternative disposition to home incurs a substantial cost in a shared risk payment model and represents an important outcome. Hence, both extended LOS and discharge to rehabilitation care may reflect a combination of worsening patient morbidity, cost, and postoperative outcome.
Accurate preoperative identification of patients at risk for extended LOS and discharge to rehabilitation can provide substantial benefit, including more transparent communication on expected benefits and risks of surgery, postoperative planning, cost savings, preemptive administrative action, and optimization of modifiable patient risk factors [11–13]. Many studies have determined significant risk factors in spine surgery, but most have been unable to create robust predictive models due to small cohort sizes and limited granularity of patient data [14,15]. Of the reported predictive models, few capture the breadth of elective spinal cases, normally focusing on single types of procedures, diagnosis, or patient groups [16]. A notable tool used for all types of surgeries is the American College of Surgeon’s National Surgical Quality Improvement Program’s (ACS NSQIP) online calculator, which utilizes 21 inputted preoperative factors to predict both LOS and discharge status [17]. While met with moderate accuracy for general surgical procedures, numerous studies have shown that it does not provide accurate predictions for patients undergoing spine surgery [18–21].
The purpose of this study is to estimate LOS and likelihood of discharge to rehabilitation following adult elective spine surgery while creating a machine learning prediction tool to assess patient-specific risk, which cannot be accomplished using traditional statistical association or regression techniques. Comorbidities, demographic, and operative risk factors will be used to inform model training. Finally, we compare performance metrics of the predictive models for both LOS and discharge outcome to those of the ACS NSQIP prediction calculator.
Methods
Data Sources
Data were gathered retrospectively on patients undergoing spine surgery from the Electronic Health Record (EHR) at a single academic tertiary care institution from 2014–2019. Patient demographical information (age, gender, ethnicity), body mass index (BMI), and written procedure description were collected for all patients through automated data acquisition from the EHR. Patient diagnostic category and select medical comorbidities were determined by utilization of International Classification of Diseases, Volume 10 codes (ICD10) [22]. No identifying patient information was acquired. Information from the dataset was utilized to determine predictive factors for LOS and discharge to rehabilitation for adult patients undergoing elective spine surgery.
Study Sample and Selection Criteria
The study population consisted of adult patients undergoing elective spine fusions and/or decompressions of the cervical, thoracic, and lumbar spine. Patients with diagnosis that indicated a non-elective procedure were excluded. The criteria queued for exclusion included: age<18, malignancy, spinal infection, extradural and subdural abscess, spinal fractures/trauma, collapsed vertebra, spinal dislocation, preexisting neuromuscular disorders, and revision procedures [23]. Cases of missing BMI, demographical information, LOS, and unknown discharge location were removed. Patients were selected using the criteria provided in Supplementary Table 1 and Supplementary Table 2 and underwent further manual review of the procedure description notes.
Predictors and Outcomes
The study outcomes included LOS and discharge to rehabilitation or skilled nursing facility (SNF) following elective spine surgery. LOS was measured in days, while discharge disposition to rehab/SNF was captured as a binary variable (yes/no). Demographics, comorbidities, and operative variables were used as predictors of LOS and discharge disposition.
Demographic variables included age (18–39, 40–49, 50–59, 60–69, 70–79, ≥80), gender, and race/ethnicity (Asian, Black, White, Hispanic/Latino, Other). BMI was classified as normal (BMI<25), overweight(25≤BMI<30), obese(30≤BMI<35), and morbidly obese (BMI≥35).
Operative variables included surgical approach (anterior alone, posterior alone, combined approach – same day, and combined staged approach), surgical invasiveness index (1–2, 3–6, 7–12, 13–18, 19–24, ≥ 25), and diagnosis group (cervical degenerative, lumbar degenerative, and adult spinal deformity). Diagnoses were derived from ICD10 codes, while surgical invasiveness index and surgical approach were manually assessed based on textual information contained in the written procedure description. Of note, combined staged approach indicated when anterior and posterior components of the surgery were conducted during the same episode of healthcare but completed sequentially on different days. Surgical invasiveness is a robust indicator developed by Mirza et al. that is obtained by adding the number of vertebral levels receiving decompression, fusion, and/or instrumentation in the anterior and posterior approaches, with a scoring range between 0–48 [24]. The index was manually calculated for each individual patient in this study and has successfully predicted estimated blood loss, operative time, and surgical site infections in the reported literature [25].
Preoperative risk factors included cardiovascular, respiratory, smoking status, renal, metabolic, and other comorbidities as indicated by ICD10 codes (Supplementary Table 3). Cardiovascular risk factors included history of hypertension (HTN), heart failure, peripheral arterial disease (PAD), heart block, past stroke, cardiomyopathy, arrythmia, and myocarditis. Respiratory risk factors consisted of chronic obstructive pulmonary disease (COPD), dyspnea at presentation, asthma, and ventilator dependence. Smoking status was classified as current smoker, former smoker, and never smoker. Renal risk factors included acute renal failure, renal dialysis, and chronic kidney disease (CKD). Metabolic risk factors were diabetes and acute liver damage. Other comorbidities were cancer history, osteoporosis, inflammatory disease, insomnia, sleep apnea, depression, anxiety, gastroesophageal reflux disease (GERD), and opioid addiction disorder.
Development of Predictive Models
Regression, Decision Learning, and Least Absolute Shrinkage and Selection Operator (LASSO) were used to build predictive models. Of note, prediction models were optimized to output a numerical estimation of LOS and a percentage probability for discharge to rehabilitation. For discharge prediction, the models utilized included decision learning, logistic regression, and LASSO given a binary outcome. For LOS prediction, generalized linear regression (GLM): Poisson distribution was chosen given that LOS is a non-parametric outcome, consists of independently associated count data, and is bounded at zero. Cohorts were split into 84% training and 16% validation cohorts. To assess diagnostic performance for numerical LOS predictions, correlation, root mean squared error (RMSE), and mean absolute error (MAE) were calculated. To assess diagnostic performance for discharge prediction, area under the receiver operator curve (AUROC), sensitivity, and specificity were determined.
Comparison to ACS NSQIP Calculator and Statistical Tests
Patient data from the 16% validation cohort were manually entered into the online ACS NSQIP risk calculator, and NSQIP predictions for LOS and probability of discharge to rehabilitation were retrieved. Diagnostic performance, such as AUROC, sensitivity, specificity, correlation, RMSE, and MAE were calculated for the NSQIP predictions. Statistical tests were conducted to compare performance of the predictive models and NSQIP calculator. Correlation coefficients were compared via Fisher’s z-transformation, and model AUROCs were compared through DeLong’s test [26]. P-values less than 0.05 were considered significant. Analysis was conducted in MATLAB version 2020b [27].
Results
Exploratory data analysis
Among 7,554 patients in the spine dataset, 3,678 patients met the inclusion criteria (Supplemental Digital Content Figure 1)). Of these, 22% were discharged to rehabilitation, and 15.5% had an extended LOS beyond seven days (Table 1 and Table 2). The average LOS was 3.7 days. Mean age was 60.1 years, with a nearly even male to female ratio (51.4 % vs 48.6%). 71.1% of patients fell into BMI categories of overweight, obese, or morbidly obese. The most common surgical approach was posterior approach (61.5%), followed by anterior approach (15.3%). Surgical procedures conducted on the selected cohort are described in Table 3. The most common diagnosis group was lumbar degenerative, consisting of 50.1% of total procedures. Highly prevalent comorbidities in the cohort included hypertension (45.9%) and former smoking history (39.3%).
Figure 1:
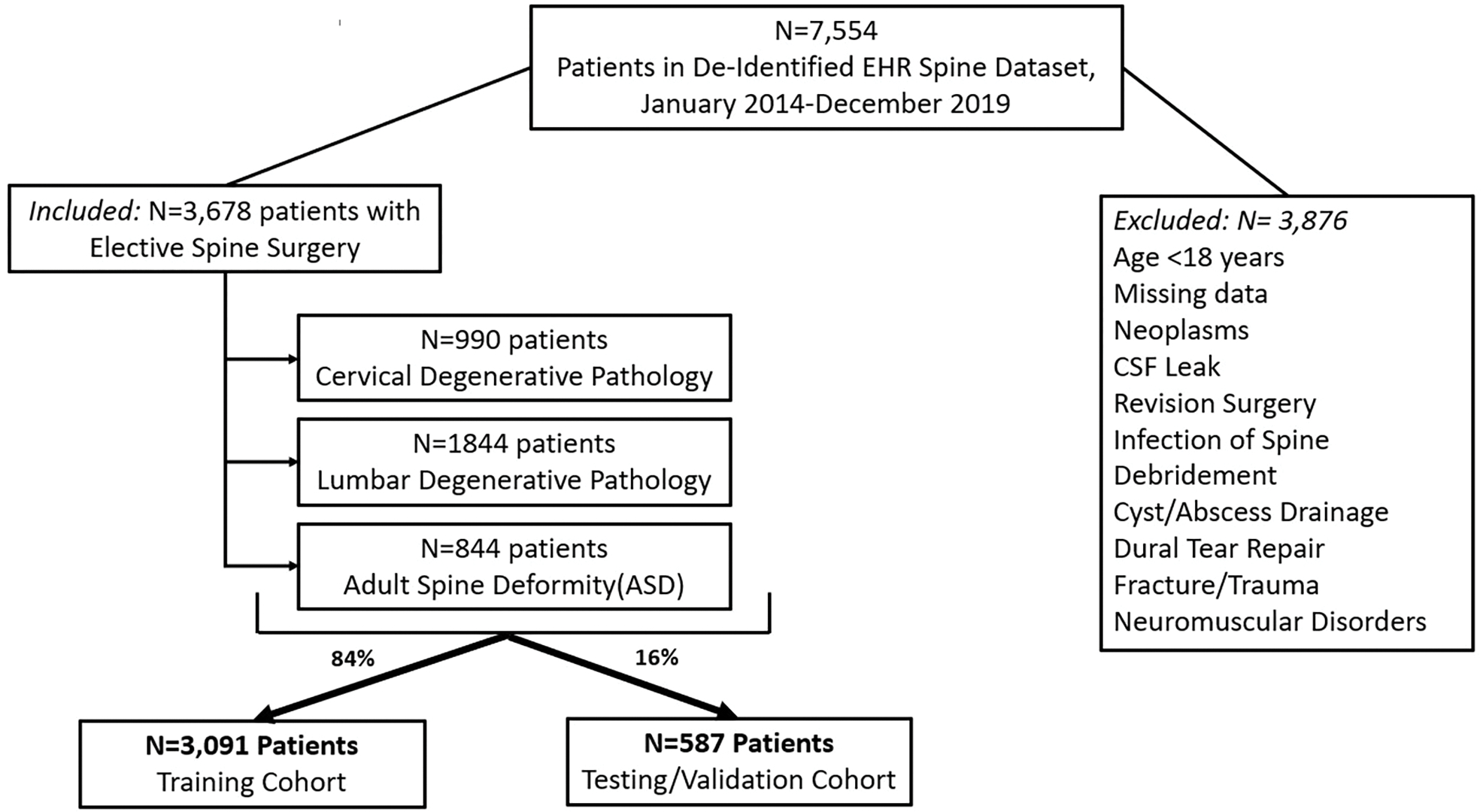
Patient Selection Map
Table 1:
Cohort demographics, interventions, comorbidities, stratified by discharge disposition
| VARIABLE | N (% of Cohort) | Discharge to Home (%) | Discharge to Rehab/SNF (%) |
|---|---|---|---|
|
| |||
| Population | 3,678 (100.0%) | 2,869 (78.0%) | 809 (22.0%) |
|
| |||
| Age (Mean, SD) | 60.1 ± 13.8 | 57.9 ± 13.9 | 67.7 ± 10.3 |
| 18–39 | 349 (9.5%) | 338 (96.8%) | 11 (3.2%) |
| 40–49 | 424 (11.5%) | 393 (92.7%) | 31 (7.3%) |
| 50–59 | 804 (21.9%) | 688 (85.6%) | 116 (14.4%) |
| 60–69 | 1123 (30.5%) | 849 (75.6%) | 274 (24.4%) |
| 70–79 | 807 (21.9%) | 513 (63.6%) | 294 (36.4%) |
| ≥80 | 171 (4.6%) | 88 (51.5%) | 83 (48.5%) |
|
| |||
| Gender | |||
| Male | 1890 (51.4%) | 1576 (83.4%) | 314 (16.6%) |
| Female | 1788 (48.6%) | 1293 (72.3%) | 495 (27.7%) |
|
| |||
| Ethnicity | |||
| Asian | 231 (6.3%) | 178 (77.1%) | 53 (22.9%) |
| Black | 171 (4.6%) | 115 (67.3%) | 56 (32.7%) |
| White | 2842 (77.3%) | 2240 (78.8%) | 602 (21.2%) |
| Hispanic/Latino | 310 (8.4%) | 246 (79.4%) | 64 (20.6%) |
| Other | 124 (3.4%) | 90 (72.6%) | 34 (27.4%) |
|
| |||
| BMI | |||
| Normal BMI(BMI<25) | 1062 (28.9%) | 840 (79.1%) | 222 (20.9%) |
| Overweight(25≤ BMI<30) | 1397 (38.0%) | 1116 (79.9%) | 281 (20.1%) |
| Obese(30≤BMI<35) | 787 (21.4%) | 617 (78.4%) | 170 (21.6%) |
| Morbidly Obese (BMI≥35) | 432 (11.7%) | 296 (68.5%) | 136 (31.5%) |
|
| |||
| Diagnosis | |||
| Cervical Degenerative | 990 (26.9%) | 855 (86.4%) | 135 (13.6%) |
| Lumbar Degenerative | 1844 (50.1%) | 1592 (86.3%) | 252 (13.7%) |
| Spine Deformity | 844 (22.9%) | 422 (50.0%) | 422 (50.0%) |
|
| |||
| Surgical Invasiveness | |||
| 1–2 | 978 (26.6%) | 934 (95.5%) | 44 (4.5%) |
| 3–6 | 622 (16.9%) | 516 (83.0%) | 106 (17.0%) |
| 7–12 | 922 (25.1%) | 760 (82.4%) | 162 (17.6%) |
| 13–18 | 616 (16.7%) | 427 (69.3%) | 189 (30.7%) |
| 19–24 | 247 (6.7%) | 140 (56.7%) | 107 (43.3%) |
| ≥25 | 293 (8.0%) | 92 (31.4%) | 201 (68.6%) |
|
| |||
| Surgical Approach | |||
| Anterior | 561 (15.3%) | 512 (91.3%) | 49 (8.7%) |
| Posterior | 2261 (61.5%) | 1838 (81.3%) | 423 (18.7%) |
| Combined- Same Day | 498 (13.5%) | 374 (75.1%) | 124 (24.9%) |
| Combined - Staged Different Day | 358 (9.7%) | 145 (40.5%) | 213 (59.5%) |
|
| |||
| Cardiovascular Risk Factors | |||
| Hypertension | 1690 (45.9%) | 1201 (71.1%) | 489 (28.9%) |
| Heart Failure | 110 (3.0%) | 68 (61.8%) | 42 (38.2%) |
| Peripheral Arterial Disease | 92 (2.5%) | 60 (65.2%) | 32 (34.8%) |
| Past Myocardial Infarction | 46 (1.3%) | 34 (73.9%) | 12 (26.1%) |
| Heart Block | 40 (1.1%) | 24 (60.0%) | 16 (40.0%) |
| Past Stroke | 51 (1.4%) | 27 (52.9%) | 24 (47.1%) |
| Cardiomyopathy | 39 (1.1%) | 29 (74.4%) | 10 (25.6%) |
| Arrhythmia | 526 (14.3%) | 335 (63.7%) | 191 (36.3%) |
| Myocarditis | 65 (1.8%) | 45 (69.2%) | 20 (30.8%) |
|
| |||
| Respiratory Risk Factors | |||
| COPD | 203 (5.5%) | 109 (53.7%) | 94 (46.3%) |
| Dyspnea | 32 (0.9%) | 22 (68.8%) | 10 (31.3%) |
| Asthma History | 453 (12.3%) | 329 (72.6%) | 124 (27.4%) |
| Ventilator Dependent | 20 (0.5%) | 7 (35.0%) | 13 (65.0%) |
|
| |||
| Smoking Status | |||
| Current Smoker | 242 (6.6%) | 197 (81.4%) | 45 (18.6%) |
| Former Smoker | 1445 (39.3%) | 1066 (73.8%) | 379 (26.2%) |
| Never Smoker | 1991 (54.1%) | 1606 (80.7%) | 385 (19.3%) |
|
| |||
| Renal Risk Factors | |||
| Acute Renal Failure | 94 (2.6%) | 44 (46.8%) | 50 (53.2%) |
| Renal Dialysis | 12 (0.3%) | 5 (41.7%) | 7 (58.3%) |
| Chronic Kidney Disease | 329 (8.9%) | 192 (58.4%) | 137 (41.6%) |
|
| |||
| Metabolic Risk Factors | |||
| Diabetes | 564 (15.3%) | 381 (67.6%) | 183 (32.4%) |
| Acute Liver Damage | 53 (1.4%) | 37 (69.8%) | 16 (30.2%) |
|
| |||
| Other Risk Factors | |||
| Cancer History | 581 (15.8%) | 444 (76.4%) | 137 (23.6%) |
| Osteoporosis | 297 (8.1%) | 162 (54.5%) | 135 (45.5%) |
| Inflammatory Disease | 114 (3.1%) | 78 (68.4%) | 36 (31.6%) |
| Insomnia | 60 (1.6%) | 34 (56.7%) | 26 (43.3%) |
| Sleep Apnea | 516 (14.0%) | 356 (69.0%) | 160 (31.0%) |
| Depression | 511 (13.9%) | 342 (66.9%) | 169 (33.1%) |
| Anxiety | 397 (10.8%) | 280 (70.5%) | 117 (29.5%) |
| GERD | 841 (22.9%) | 575 (68.4%) | 266 (31.6%) |
| Opioid Addiction Disorder | 40 (1.1%) | 20 (50.0%) | 20 (50.0%) |
Table 2:
Cohort demographics, interventions, comorbidities, stratified by Length of Stay (LOS)
| VARIABLE | N (% of Cohort) | LOS<7 Days | LOS≥7 Days |
|---|---|---|---|
|
| |||
| Population | 3,678 (100.0%) | 3,107 (84.5%) | 571 (15.5%) |
|
| |||
| Age (Mean, SD) | 60.1 ± 13.8 | 59.1 ± 14.2 | 65.2 ± 10.2 |
| 18–39 | 349 (9.5%) | 340 (97.4%) | 9 (2.6%) |
| 40–49 | 424 (11.5%) | 390 (92.0%) | 34 (8.0%) |
| 50–59 | 804 (21.9%) | 701 (87.2%) | 103 (12.8%) |
| 60–69 | 1123 (30.5%) | 905 (80.6%) | 218 (19.4%) |
| 70–79 | 807 (21.9%) | 632 (78.3%) | 175 (21.7%) |
| ≥80 | 171 (4.6%) | 139 (81.3%) | 32 (18.7%) |
|
| |||
| Gender | |||
| Male | 1890 (51.4%) | 1667 (88.2%) | 223 (11.8%) |
| Female | 1788 (48.6%) | 1440 (80.5%) | 348 (19.5%) |
|
| |||
| Ethnicity | |||
| Asian | 231 (6.3%) | 204 (88.3%) | 27 (11.7%) |
| Black | 171 (4.6%) | 142 (83.0%) | 29 (17.0%) |
| White | 2842 (77.3%) | 2393 (84.2%) | 449 (15.8%) |
| Hispanic/Latino | 310 (8.4%) | 260 (83.9%) | 50 (16.1%) |
| Other | 124 (3.4%) | 108 (87.1%) | 16 (12.9%) |
|
| |||
| BMI | |||
| Normal BMI(BMI<25) | 1062 (28.9%) | 901 (84.8%) | 161 (15.2%) |
| Overweight(25≤BMI<30) | 1397 (38.0%) | 1204 (86.2%) | 193 (13.8%) |
| Obese(30≤BMI<35) | 787 (21.4%) | 652 (82.8%) | 135 (17.2%) |
| Morbidly Obese (BMI≥35) | 432 (11.7%) | 350 (81.0%) | 82 (19.0%) |
|
| |||
| Diagnosis | |||
| Cervical Degenerative | 990 (26.9%) | 935 (94.4%) | 55 (5.6%) |
| Lumbar Degenerative | 1844 (50.1%) | 1722 (93.4%) | 122 (6.6%) |
| Spine Deformity | 844 (22.9%) | 450 (53.3%) | 394 (46.7%) |
|
| |||
| Surgical Invasiveness | |||
| 1–2 | 978 (26.6%) | 966 (98.8%) | 12 (1.2%) |
| 3–6 | 622 (16.9%) | 589 (94.7%) | 33 (5.3%) |
| 7–12 | 922 (25.1%) | 834 (90.5%) | 88 (9.5%) |
| 13–18 | 616 (16.7%) | 504 (81.8%) | 112 (18.2%) |
| 19–24 | 247 (6.7%) | 138 (55.9%) | 109 (44.1%) |
| ≥25 | 293 (8.0%) | 76 (25.9%) | 217 (74.1%) |
|
| |||
| Surgical Approach | |||
| Anterior | 561 (15.3%) | 534 (95.2%) | 27 (4.8%) |
| Posterior | 2261 (61.5%) | 2046 (90.5%) | 215 (9.5%) |
| Combined- Same Day | 498 (13.5%) | 428 (85.9%) | 70 (14.1%) |
| Combined - Staged Different Day | 358 (9.7%) | 99 (27.7%) | 259 (72.3%) |
|
| |||
| Cardiovascular Risk Factors | |||
| Hypertension | 1690 (45.9%) | 1354 (80.1%) | 336 (19.9%) |
| Heart Failure | 110 (3.0%) | 82 (74.5%) | 28 (25.5%) |
| Peripheral Arterial Disease | 92 (2.5%) | 65 (70.7%) | 27 (29.3%) |
| Past Myocardial Infarction | 46 (1.3%) | 37 (80.4%) | 9 (19.6%) |
| Heart Block | 40 (1.1%) | 32 (80.0%) | 8 (20.0%) |
| Past Stroke | 51 (1.4%) | 40 (78.4%) | 11 (21.6%) |
| Cardiomyopathy | 39 (1.1%) | 34 (87.2%) | 5 (12.8%) |
| Arrhythmia | 526 (14.3%) | 394 (74.9%) | 132 (25.1%) |
| Myocarditis | 65 (1.8%) | 55 (84.6%) | 10 (15.4%) |
|
| |||
| Respiratory Risk Factors | |||
| COPD | 203 (5.5%) | 143 (70.4%) | 60 (29.6%) |
| Dyspnea | 32 (0.9%) | 25 (78.1%) | 7 (21.9%) |
| Asthma History | 453 (12.3%) | 368 (81.2%) | 85 (18.8%) |
| Ventilator Dependent | 20 (0.5%) | 9 (45.0%) | 11 (55.0%) |
|
| |||
| Smoking Status | |||
| Current Smoker | 242 (6.6%) | 216 (89.3%) | 26 (10.7%) |
| Former Smoker | 1445 (39.3%) | 1163 (80.5%) | 282 (19.5%) |
| Never Smoker | 1991 (54.1%) | 1728 (86.8%) | 263 (13.2%) |
|
| |||
| Renal Risk Factors | |||
| Acute Renal Failure | 94 (2.6%) | 51 (54.3%) | 43 (45.7%) |
| Renal Dialysis | 12 (0.3%) | 6 (50.0%) | 6 (50.0%) |
| Chronic Kidney Disease | 329 (8.9%) | 237 (72.0%) | 92 (28.0%) |
|
| |||
| Metabolic Risk Factors | |||
| Diabetes | 564 (15.3%) | 439 (77.8%) | 125 (22.2%) |
| Acute Liver Damage | 53 (1.4%) | 38 (71.7%) | 15 (28.3%) |
|
| |||
| Other Risk Factors | |||
| Cancer History | 581 (15.8%) | 503 (86.6%) | 78 (13.4%) |
| Osteoporosis | 297 (8.1%) | 198 (66.7%) | 99 (33.3%) |
| Inflammatory Disease | 114 (3.1%) | 95 (83.3%) | 19 (16.7%) |
| Insomnia | 60 (1.6%) | 40 (66.7%) | 20 (33.3%) |
| Sleep Apnea | 516 (14.0%) | 404 (78.3%) | 112 (21.7%) |
| Depression | 511 (13.9%) | 398 (77.9%) | 113 (22.1%) |
| Anxiety | 397 (10.8%) | 312 (78.6%) | 85 (21.4%) |
| GERD | 841 (22.9%) | 642 (76.3%) | 199 (23.7%) |
| Opioid Addiction Disorder | 40 (1.1%) | 12 (30.0%) | 28 (70.0%) |
Table 3:
Operative Procedures Conducted on Selected Cohort, Stratified by Spinal Region
| VARIABLE | N (% of Cohort) |
|---|---|
|
| |
| Population | 3,768 (100.0%) |
|
| |
| Cervical | |
| ACDF, 1 level | 85 (3.2%) |
| ACDF, 2–3 level | 441 (11.7%) |
| ACDF, 4 level | 35 (0.9%) |
| Laminoforaminotomy | 102 (2.7%) |
| Laminectomy/Laminoplasty | 257 (6.8%) |
| Cervical fusion (posterior or anterior/posterior), 1–3 Level | 237 (6.3%) |
| Cervical fusion (posterior or anterior/posterior), 4–6 Level | 206 (5.5%) |
|
| |
| Lumbar | |
| Decompression, 1–2 level | 885 (23.5%) |
| Decompression, 3–4 level | 73 (1.9%) |
| Fusion (posterior or anterior/posterior), 1–4 level | 883 (23.4%) |
|
| |
| Thoracolumbar | |
| Fusion (posterior or anterior/posterior), 4–6 level | 133 (3.5%) |
| Fusion (posterior or anterior/posterior), 7–12 level | 220 (5.8%) |
| Fusion (posterior or anterior/posterior), >12 level | 121 (3.2%) |
ACDF = Anterior Cervical Discectomy and Fusion
Predictive Models: Discharge Disposition
Three predictive models were created for prediction of discharge to rehabilitation. These included logistic regression (AUROC=0.79), LASSO (AUROC=0.79), and decision tree learning (AUROC=0.69). 84% of the cohort (N=3,091) were used to generate each model with validation on the remaining 16% (N=587). The NSQIP calculator produced an AUROC= 0.75 (Supplemental Digital Content Figure 2). Beta coefficients, odds ratios, and p-values for the logistic model, which had the highest AUROC, are displayed in Table 4. Significant variables that increased likelihood of discharge to rehabilitation included age, black race, BMI, surgical invasiveness index, arrhythmia, COPD, current smoker, diabetes, and depression. Variables that decreased likelihood of discharge to rehabilitation included male gender, Asian race, anterior approach, and combined same-day staged approach. Of the models trained to predict discharge location, the AUROC of 0.79 for both logistic and LASSO regression were statistically equivalent to the AUROC of 0.75 for NSQIP (p=0.135). At a predictive probability threshold of 0.16, the logistic regression model produced a sensitivity of 0.80 and a specificity of 0.64.
Figure 2:
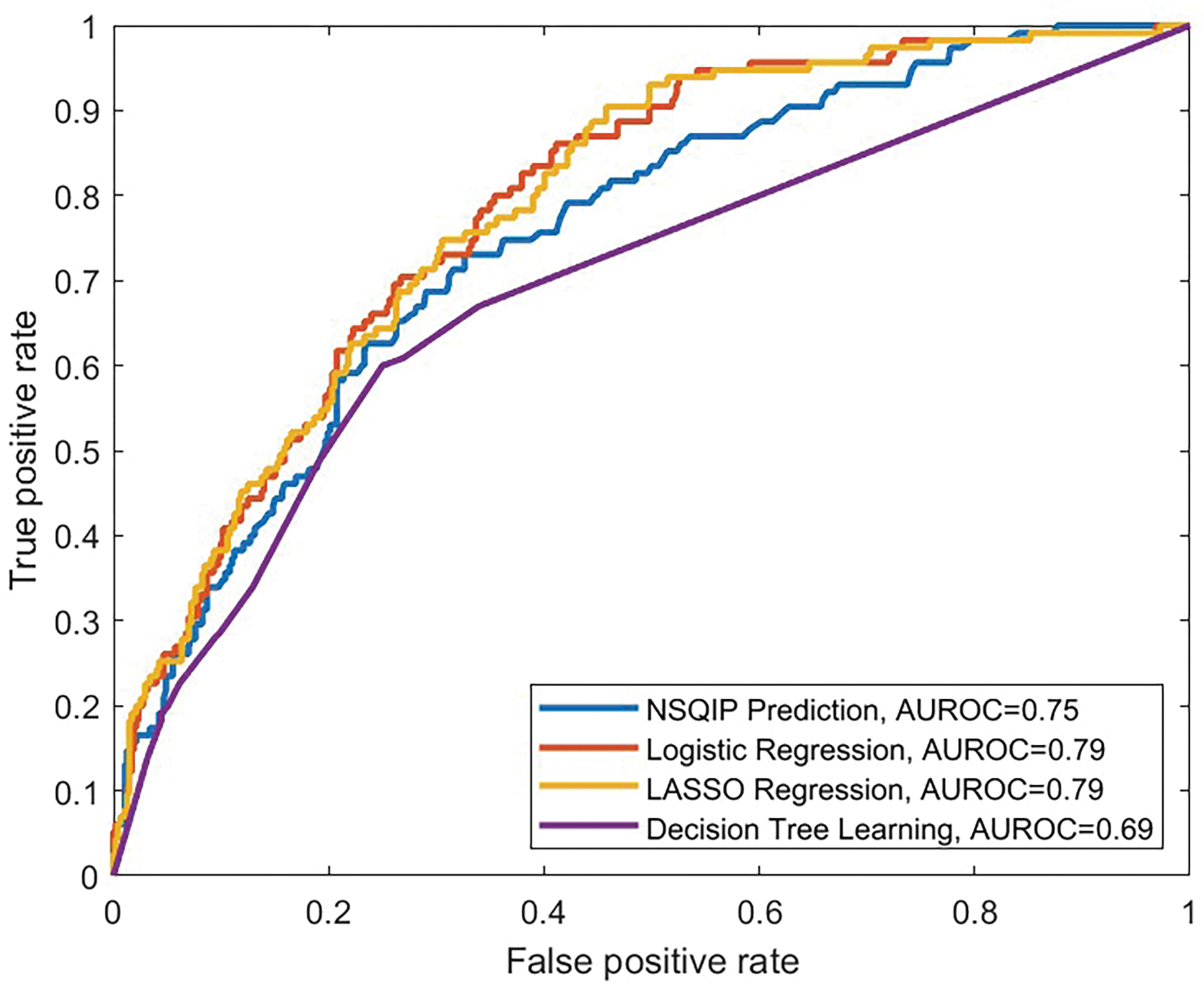
Receiver Operating Curve (ROC) for ACS NSQIP calculator and trained predictive models, which included: logistic regression, LASSO, and decision tree learning for discharge to rehabilitation. The AUROCs were 0.75, 0.79, 0.79, and 0.69, respectively.
Table 4:
Logistic regression coefficients for prediction of discharge to rehabilitation
| Variable | Beta Coefficient | Odds Ratio | 95% CI | P-Value | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Intercept | −5.25 | -- | -- | -- | <0.001 | |
|
| ||||||
| Age(categorical)* | 0.67 | 1.96 | 1.75 | - | 2.19 | <0.001 |
|
| ||||||
| Gender | ||||||
| Female | Ref | -- | -- | -- | -- | |
| Male | −0.62 | 0.54 | 0.43 | - | 0.68 | <0.001 |
|
| ||||||
| Race | ||||||
| White | Ref | -- | -- | -- | -- | |
| Asian | 0.47 | 1.60 | 1.03 | - | 2.47 | 0.035 |
| Black | 1.04 | 2.83 | 1.77 | - | 4.54 | <0.001 |
| Hispanic/Latino | 0.19 | 1.21 | 0.80 | - | 1.82 | 0.371 |
|
| ||||||
| BMI** | 0.23 | 1.25 | 1.11 | - | 1.41 | <0.001 |
|
| ||||||
| Diagnosis | ||||||
| Lumbar Degenerative | Ref | -- | -- | -- | -- | |
| Cervical Degenerative | 0.30 | 1.35 | 0.95 | - | 1.90 | 0.091 |
| Spine Deformity | 0.00 | 1.00 | 0.67 | - | 1.50 | 0.981 |
|
| ||||||
| Surgical Invasiveness Index*** | 0.69 | 2.00 | 1.72 | - | 2.32 | <0.001 |
|
| ||||||
| Surgical Approach | ||||||
| Posterior | Ref | -- | -- | -- | -- | |
| Anterior | −1.31 | 0.27 | 0.17 | - | 0.42 | <0.001 |
| Combined - Staged Same Day | −0.54 | 0.59 | 0.40 | - | 0.86 | 0.007 |
| Combined - Staged Different Day | 0.07 | 1.07 | 0.73 | - | 1.57 | 0.741 |
|
| ||||||
| Cardiovascular Risk Factors | ||||||
| Hypertension | −0.02 | 0.98 | 0.78 | - | 1.23 | 0.867 |
| Heart Failure | −0.18 | 0.83 | 0.45 | - | 1.54 | 0.562 |
| Peripheral Arterial Disease | 0.27 | 1.31 | 0.68 | - | 2.53 | 0.422 |
| Past Myocardial Infarction | −0.40 | 0.67 | 0.23 | - | 1.91 | 0.452 |
| Heart Block | 0.29 | 1.34 | 0.55 | - | 3.29 | 0.521 |
| Past Stroke | 0.69 | 1.99 | 0.90 | - | 4.40 | 0.091 |
| Cardiomyopathy | −0.15 | 0.86 | 0.31 | - | 2.39 | 0.779 |
| Arrhythmia | 0.53 | 1.71 | 1.27 | - | 2.30 | <0.001 |
| Myocarditis | −0.02 | 0.98 | 0.40 | - | 2.40 | 0.962 |
|
| ||||||
| Respiratory Risk Factors | ||||||
| COPD | 0.67 | 1.96 | 1.31 | - | 2.94 | 0.001 |
| Dyspnea | −0.59 | 0.55 | 0.16 | - | 1.87 | 0.341 |
| Asthma History | 0.08 | 1.09 | 0.78 | - | 1.51 | 0.621 |
| Ventilator Dependent | 0.84 | 2.31 | 0.69 | - | 7.79 | 0.177 |
|
| ||||||
| Smoking Status | ||||||
| Never Smoker | Ref | -- | -- | -- | -- | |
| Current Smoker | 0.54 | 1.72 | 1.07 | - | 2.76 | 0.024 |
| Former Smoker | 0.16 | 1.17 | 0.94 | - | 1.47 | 0.165 |
|
| ||||||
| Renal Risk Factors | ||||||
| Acute Renal Failure | 0.65 | 1.91 | 0.96 | - | 3.81 | 0.065 |
| Renal Dialysis | 0.43 | 1.54 | 0.30 | - | 7.83 | 0.605 |
| Chronic Kidney Disease(CKD) | 0.10 | 1.10 | 0.72 | - | 1.68 | 0.661 |
|
| ||||||
| Metabolic Risk Factors | ||||||
| Diabetes | 0.35 | 1.42 | 1.06 | - | 1.90 | 0.019 |
| Acute Liver Damage | 0.16 | 1.17 | 0.55 | - | 2.48 | 0.682 |
|
| ||||||
| Other Risk Factors | ||||||
| Cancer History | 0.19 | 1.21 | 0.89 | - | 1.63 | 0.224 |
| Osteoporosis | 0.31 | 1.36 | 0.97 | - | 1.92 | 0.074 |
| Inflammatory Disease | −0.25 | 0.78 | 0.47 | - | 1.30 | 0.341 |
| Insomnia | 0.82 | 2.27 | 1.00 | - | 5.15 | 0.050 |
| Sleep Apnea | 0.05 | 1.05 | 0.78 | - | 1.43 | 0.733 |
| Depression | 0.58 | 1.79 | 1.29 | - | 2.48 | <0.001 |
| Anxiety | 0.19 | 1.21 | 0.84 | - | 1.74 | 0.316 |
| GERD | 0.12 | 1.13 | 0.89 | - | 1.45 | 0.317 |
| Opioid Addiction Disorder | 0.24 | 1.27 | 0.54 | - | 3.01 | 0.582 |
Per 1 point category increase. 18–39=0, 40–49=1, 50–59=2, 60–69=3, 70–79=4, ≥80=5
Per 1 point category increase. BMI<25 = 0, 25≤BMI<30 =1, 30≤BMI<35 =2, BMI≥35=3
Per 1 point category increase. Index 1–2 = 0, 3–6=1, 7–12=2, 13–18=3, 19–24=4, ≥25=5
Predictive Models: Length of Stay (LOS)
Two predictive models were created for numerical prediction of LOS. These included GLM: Poisson Distribution (R2=0.29, RMSE=5.94 days, MEA=1.66 days) and LASSO regression (R2=0.29, RMSE=7.28 days, MEA=1.72 days). 84% of the cohort (n=3,091) were used to train each model with validation on the remaining 16% (n=587). The NSQIP calculator produced a R2=0.16, RMSE=17.81 days, and MAE=1.84 days. Plots of predicted LOS vs observed LOS for each model and NSQIP predictions of the n=587 validation patients are displayed in Supplemental Digital Content Figure 3.
Figure 3:
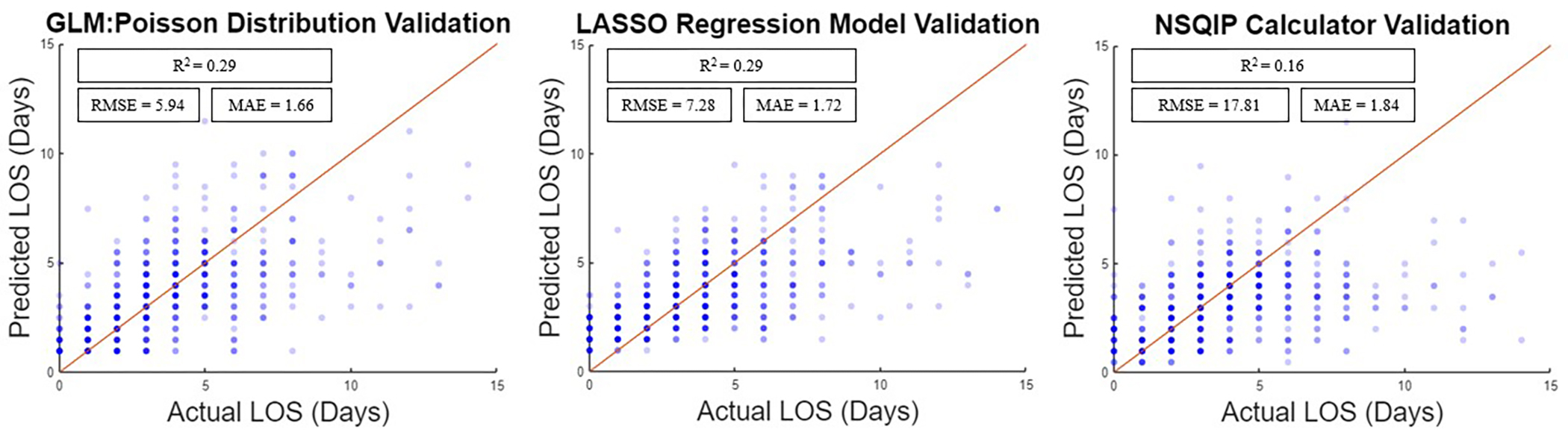
Predicted vs observed Length of Stay (LOS) for ACS NSQIP Calculator and Predictive models.
Model coefficients for GLM: Poisson distribution, which produced the lowest RMSE and MEA, are displayed in Table 5. Significant variables that increased LOS included age, black race, diagnosis of spine deformity, surgical invasiveness index, combined staged approach on a different day, PAD, arrythmia, former smoking history, COPD history, acute renal failure, diabetes, anxiety, GERD, and opioid addiction disorder. Significant variables that decreased LOS included male gender, diagnosis of cervical degenerative pathology, anterior approach, combined same-day staged approach, and inflammatory disease. Compared to NSQIP (R2=0.16), the predictions of GLM: Poisson distribution (R2=0.29) and LASSO (R2=0.29) models were significantly more correlated with observed LOS (p=0.025 and p=0.004, respectively).
Table 5:
Generalized Linear Model: Poisson Distribution coefficients for numerical prediction of Length of Stay(LOS)
| Variable | Coefficient | Standard Error (SE) | t-statistic | P-Value |
|---|---|---|---|---|
|
| ||||
| Intercept | −0.15 | 0.05 | −2.69 | 0.007 |
|
| ||||
| Age | ||||
| 18–39 | Ref | -- | -- | -- |
| 40–49 | 0.05 | 0.06 | 0.95 | 0.343 |
| 50–59 | 0.17 | 0.05 | 3.37 | 0.001 |
| 60–69 | 0.24 | 0.05 | 4.82 | <0.001 |
| 70–79 | 0.27 | 0.05 | 5.34 | <0.001 |
| ≥80 | 0.42 | 0.06 | 6.80 | <0.001 |
|
| ||||
| Gender | ||||
| Female | Ref | -- | -- | -- |
| Male | −0.08 | 0.02 | −3.74 | <0.001 |
|
| ||||
| Race | ||||
| White | Ref | -- | -- | -- |
| Asian | 0.02 | 0.04 | 0.38 | 0.705 |
| Black | 0.23 | 0.04 | 5.19 | <0.001 |
| Hispanic/Latino | 0.07 | 0.03 | 1.95 | 0.051 |
|
| ||||
| BMI | ||||
| Normal BMI(BMI<25) | Ref | -- | -- | -- |
| Overweight(25≤BMI<30) | 0.01 | 0.02 | 0.46 | 0.646 |
| Obese(30≤BMI<35) | 0.04 | 0.03 | 1.38 | 0.167 |
| Morbidly Obese (BMI≥35) | 0.05 | 0.03 | 1.44 | 0.149 |
|
| ||||
| Diagnosis | ||||
| Lumbar Degenerative | Ref | -- | -- | -- |
| Cervical Degenerative | −0.17 | 0.04 | −4.66 | <0.001 |
| Deformity | 0.14 | 0.04 | 3.43 | 0.001 |
|
| ||||
| Surgical Invasiveness Index | ||||
| 1–2 | Ref | -- | -- | -- |
| 3–6 | 1.06 | 0.04 | 24.88 | <0.001 |
| 7–12 | 1.25 | 0.05 | 27.64 | <0.001 |
| 13–18 | 1.25 | 0.05 | 24.57 | <0.001 |
| 19–24 | 1.43 | 0.06 | 22.05 | <0.001 |
| ≥25 | 1.50 | 0.07 | 21.63 | <0.001 |
|
| ||||
| Surgical Approach | ||||
| Posterior | Ref | -- | -- | -- |
| Anterior | −0.38 | 0.04 | −8.48 | <0.001 |
| Combined - Staged Same Day | −0.06 | 0.04 | −1.72 | 0.085 |
| Combined - Staged Different Day | 0.31 | 0.03 | 9.27 | <0.001 |
|
| ||||
| Cardiovascular Risk Factors | ||||
| Hypertension | 0.02 | 0.02 | 0.88 | 0.379 |
| Heart Failure | −0.06 | 0.06 | −1.07 | 0.284 |
| Peripheral Arterial Disease | 0.18 | 0.06 | 3.21 | 0.001 |
| Past Myocardial Infarction | 0.01 | 0.10 | 0.10 | 0.918 |
| Heart Block | 0.00 | 0.08 | −0.03 | 0.980 |
| Past Stroke | −0.02 | 0.08 | −0.27 | 0.786 |
| Cardiomyopathy | −0.13 | 0.11 | −1.20 | 0.230 |
| Arrhythmia | 0.20 | 0.03 | 7.09 | <0.001 |
| Myocarditis | 0.05 | 0.08 | 0.64 | 0.521 |
|
| ||||
| Respiratory Risk Factors | ||||
| COPD | 0.07 | 0.04 | 1.96 | 0.050 |
| Dyspnea | −0.19 | 0.11 | −1.78 | 0.075 |
| Asthma History | 0.02 | 0.03 | 0.74 | 0.459 |
| Ventilator Dependent | 0.14 | 0.09 | 1.49 | 0.135 |
|
| ||||
| Smoking Status | ||||
| Never Smoker | Ref | -- | -- | -- |
| Current Smoker | 0.08 | 0.04 | 1.94 | 0.053 |
| Former Smoker | 0.06 | 0.02 | 2.98 | 0.003 |
|
| ||||
| Renal Risk Factors | ||||
| Acute Renal Failure | 0.30 | 0.06 | 5.38 | <0.001 |
| Renal Dialysis | 0.23 | 0.14 | 1.65 | 0.099 |
| Chronic Kidney Disease (CKD) | 0.02 | 0.04 | 0.50 | 0.618 |
|
| ||||
| Metabolic Risk Factors | ||||
| Diabetes | 0.13 | 0.03 | 4.85 | <0.001 |
| Acute Liver Damage | 0.03 | 0.07 | 0.36 | 0.722 |
|
| ||||
| Other Risk Factors | ||||
| Cancer History | −0.01 | 0.03 | −0.21 | 0.832 |
| Osteoporosis | 0.04 | 0.03 | 1.32 | 0.186 |
| Inflammatory Disease | −0.10 | 0.05 | −2.07 | 0.038 |
| Insomnia | −0.02 | 0.07 | −0.27 | 0.790 |
| Sleep Apnea | 0.03 | 0.03 | 1.24 | 0.214 |
| Depression | 0.04 | 0.03 | 1.28 | 0.200 |
| Anxiety | 0.10 | 0.03 | 3.15 | 0.002 |
| GERD | 0.07 | 0.02 | 3.34 | 0.001 |
| Opioid Addiction Disorder | 0.19 | 0.06 | 3.07 | 0.002 |
Discussion
The goal of this study was to develop a machine learning model that could predict LOS and discharge location following adult elective spine surgery with a higher accuracy than the ACS NSQIP risk calculator. With a statistically significant R2=0.29 for predicting LOS and a statistically equivalent AUROC=0.79 for predicting discharge to rehabilitation, the predictive models are valuable tools that can be utilized preoperatively to assess patient risk. The logistic and GLM: Poisson regression models, with provided coefficients, can be easily translated for preoperative implementation.
The associations found within this study are concordant with those reported in the literature. The association between increasing age and BMI with perioperative complications and non-home discharges is well documented and demonstrates a more profound effect at higher ends of the spectrum [28,29]. Worse outcomes in women may be explained by the fact that women undergo surgery at more advanced diseased states compared to men [30]. Patients with diagnosis of adult spinal deformity represent a particularly disabled group with more functional limitations than those with other chronic medical conditions and have been associated with worse postoperative outcomes [31]. The relation between increasing surgical invasiveness index with increased LOS and likelihood of rehab discharge makes intuitive sense, as higher scores have been associated with multiple medical complications [32]. Risk factors such as arrythmias, COPD, and diabetes have been thoroughly reported to worsen perioperative morbidity [7,33]. As expected, there was overlap between risk factors that were significantly associated with discharge to rehabilitation and those associated with longer LOS.
A unique feature of this study is the implementation of a large cohort (n=3,678) with granular patient data. Ability to stratify patients by diagnosis category and include novel variables pertaining to type of surgical intervention (surgical invasiveness index and staged surgery) may be credited for our ability to achieve higher predictive performance. The study alternate hypothesis was that diagnostic category would be an important independent predictor of LOS and discharge, and hence diagnostic category was modelled as a predictor variable. The results demonstrated that patients with adult spinal deformity were indeed significantly associated with extended LOS. Additionally, no studies have developed predictive models utilizing knowledge about type of surgical intervention, even though staged anterior and posterior procedures are routinely conducted and represent an increased chance for hospital acquired complications [34].
Existing predictive models have shown mixed ability to predict outcomes following elective spine surgery. Models such as NSQIP and the Risk Assessment Tool (RAT) use a combination of demographics, procedure codes, operative variables, and comorbidities to determine postoperative outcome following surgery [17,35]. Previous studies have validated NSQIP and RAT tools as having moderate utility in predicting complications and discharge location, with comparable AUROCs between 0.64 and 0.70 [36,37]. NSQIP predictions for LOS have shown to be highly inaccurate, and no studies have conducted validation within spine patients [38]. In this study, the predictive models outperformed NSQIP in predicting LOS and were equivalent to NSQIP in predicting discharge location. Compared to other studies that have developed predictive models for outcomes in spine surgery, this is the first to conduct a high level of stratification among patients, utilize a substantial sample size greater than N=300, and compare performance to a gold-standard [16].
The importance of this study is to develop more accuracy in our ability to predict LOS and discharge disposition to enable medical centers in calculating expected costs of care. Ideally, the predictive models can be used to generate predictions in the preoperative encounter. Especially for health systems utilizing bundled payment models, accurate predictive analytics on LOS and discharge outcomes can facilitate critical financial savings. Currently under bundled payment plans, patients requiring utilization of more-than-expected hospital services incur financial losses that are placed directly on the healthcare team and hospital system [39]. Resulting catastrophic costs and the inability for hospitals to afford high volumes of patients with poor outcomes necessitate usage of predictive analytics to maintain financial viability [10]. Preemptive identification of patients at risk for complications through the predictive models provided could ensure better cost management and treatment planning to reduce unnecessary suffering. Finally, accurate preoperative analytics can empower patient informed choice by increasing transparency on the expected benefits and risks of surgery.
A key limitation of this study is utilization of data from a singular institution. Although the predictive models developed may be less generalizable to other settings, the tradeoff is that single-institution data provides better granularity than that of multi-center datasets. Our goal was to build a predictive model that could incorporate granular variables such as surgical invasiveness index, diagnosis group, staged surgery, and numerous medical comorbidities – information used by few other predictive models. We intend our study to be foundational for development of granular models derived from multiple data sources. Moreover, variables such as socioeconomic status, education, and income level have shown potential in improving predictive accuracy [40]. Measuring social support through the Risk Assessment and Prediction Tool (RAPT) presents a valuable future avenue [41]. Lastly, while this retrospective study utilized ICD10 codes to acquire components of patient data, some uncertainty on accuracy of ICD10 codes has been reported that could have resulted in an underestimation of risk factors [42].
Conclusion
This study utilizing n=3,678 patients facilitated development of predictive models that can preoperatively assess likelihood of discharge to rehabilitation and LOS following adult elective spine surgery. The models utilized highly granular data consisting of patient demographics, BMI, diagnostic category, surgical invasiveness index, surgical region, surgical staging, and patient comorbidities. Performance metrics from the predictive models were tested in comparison to the ACS NSQIP calculator. For prediction of LOS, the GLM: Poisson and LASSO models had a better accuracy than the ACS NSQIP calculator. For prediction of discharge location, the logistic regression model had a statistically equivalent accuracy when compared to the ACS NSQIP calculator.
Supplementary Material
Key Points.
We utilized machine learning to develop predictive models that generated an estimation of LOS and likelihood of discharge to rehabilitation following adult elective spine surgery.
The models utilized highly granular data consisting of patient demographics, BMI, surgical invasiveness index, surgical region, surgical staging, and patient comorbidities.
While accuracy in prediction of discharge was same to that of the ACS NSQIP calculator, the models built outperformed NSQIP in predicting LOS.
Compared to other studies that have developed predictive models for outcomes in spine surgery, this is the first to conduct a high level of stratification among patients, utilize a substantial sample size greater than N=300, and compare performance to a gold-standard.
Acknowledgements
This work was supported by the UCSF Bakar Computational Health Sciences Institute National Center for Advancing Translational Sciences of the National Institutes of Health (NIH) UL1 TR001872.
Footnotes
Conflicts of Interest and Source of Funding:
No funds or financial support were used in support of this study. The authors report no study-specific conflict of interest-associated biases in relation to this manuscript.
IRB Approval/Research Ethics Committee
No IRB was utilized for the purposes of this study.
Device Status/Drug Statement
The manuscript submitted does not contain information about medical device(s)/drug(s).
References
- 1.Hart RA, Cabalo A, Bess S, et al. Comparison of patient and surgeon perceptions of adverse events after adult spinal deformity surgery. Spine (Phila Pa 1976). Apr 20 2013;38(9):732–6. doi: 10.1097/BRS.0b013e31827ae242 [DOI] [PubMed] [Google Scholar]
- 2.Campbell PG, Yadla S, Nasser R, Malone J, Maltenfort MG, Ratliff JK. Patient comorbidity score predicting the incidence of perioperative complications: assessing the impact of comorbidities on complications in spine surgery. J Neurosurg Spine. Jan 2012;16(1):37–43. doi: 10.3171/2011.9.SPINE11283 [DOI] [PubMed] [Google Scholar]
- 3.Reis RC, de Oliveira MF, Rotta JM, Botelho RV. Risk of complications in spine surgery: a prospective study. Open Orthop J. 2015;9:20–5. doi: 10.2174/1874325001509010020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gruskay JA, Fu M, Bohl DD, Webb ML, Grauer JN. Factors affecting length of stay after elective posterior lumbar spine surgery: a multivariate analysis. Spine J Jun 1 2015;15(6):1188–95. doi: 10.1016/j.spinee.2013.10.022 [DOI] [PubMed] [Google Scholar]
- 5.Klineberg EO, Passias PG, Jalai CM, et al. Predicting Extended Length of Hospital Stay in an Adult Spinal Deformity Surgical Population. Spine (Phila Pa 1976). Jul 1 2016;41(13):E798–E805. doi: 10.1097/BRS.0000000000001391 [DOI] [PubMed] [Google Scholar]
- 6.Ames CP, Scheer JK, Lafage V, et al. Adult Spinal Deformity: Epidemiology, Health Impact, Evaluation, and Management. Spine Deform. Jul 2016;4(4):310–322. doi: 10.1016/j.jspd.2015.12.009 [DOI] [PubMed] [Google Scholar]
- 7.Soroceanu A, Burton DC, Oren JH, et al. Medical Complications After Adult Spinal Deformity Surgery: Incidence, Risk Factors, and Clinical Impact. Spine (Phila Pa 1976). Nov 15 2016;41(22):1718–1723. doi: 10.1097/BRS.0000000000001636 [DOI] [PubMed] [Google Scholar]
- 8.Ames CP, Smith JS, Gum JL, et al. Utilization of Predictive Modeling to Determine Episode of Care Costs and to Accurately Identify Catastrophic Cost Nonwarranty Outlier Patients in Adult Spinal Deformity Surgery: A Step Toward Bundled Payments and Risk Sharing. Spine (Phila Pa 1976). Mar 1 2020;45(5):E252–E265. doi: 10.1097/BRS.0000000000003242 [DOI] [PubMed] [Google Scholar]
- 9.Stephens BF 2nd, Khan I, Chotai S, Sivaganesan A, Devin CJ. Drivers of Cost in Adult Thoracolumbar Spine Deformity Surgery. World Neurosurg. Oct 2018;118:e206–e211. doi: 10.1016/j.wneu.2018.06.155 [DOI] [PubMed] [Google Scholar]
- 10.Theologis AA, Lau D, Dalle-Ore C, Tsu A, Deviren V, Ames CP. Costs and utility of post-discharge acute inpatient rehabilitation following adult spinal deformity surgery. Spine Deform May 2021;9(3):817–822. doi: 10.1007/s43390-020-00251-w [DOI] [PubMed] [Google Scholar]
- 11.Maitra S, Mikhail C, Cho SK, Daubs MD. Preoperative Maximization to Reduce Complications in Spinal Surgery. Global Spine J Jan 2020;10(1 Suppl):45S–52S. doi: 10.1177/2192568219882349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menendez JY, Omar NB, Chagoya G, et al. Patient Satisfaction in Spine Surgery: A Systematic Review of the Literature. Asian Spine J Dec 2019;13(6):1047–1057. doi: 10.31616/asj.2019.0032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zambouri A Preoperative evaluation and preparation for anesthesia and surgery. Hippokratia. Jan 2007;11(1):13–21. [PMC free article] [PubMed] [Google Scholar]
- 14.Amin RM, Raad M, Jain A, et al. Risk factors for nonroutine discharge in adult spinal deformity surgery. Spine J Feb 2019;19(2):357–363. doi: 10.1016/j.spinee.2018.06.366 [DOI] [PubMed] [Google Scholar]
- 15.Lovecchio F, Steinhaus M, Elysee JC, et al. Factors Associated With Short Length of Stay After Long Fusions for Adult Spinal Deformity: Initial Steps Toward Developing an Enhanced Recovery Pathway. Global Spine J. Jul 2021;11(6):866–873. doi: 10.1177/2192568220941448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lubelski D, Ehresman J, Feghali J, et al. Prediction calculator for nonroutine discharge and length of stay after spine surgery. Spine J Jul 2020;20(7):1154–1158. doi: 10.1016/j.spinee.2020.02.022 [DOI] [PubMed] [Google Scholar]
- 17.Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg Nov 2013;217(5):833–42 e1–3. doi: 10.1016/j.jamcollsurg.2013.07.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alzahrani SM, Ko CS, Yoo MW. Validation of the ACS NSQIP Surgical Risk Calculator for Patients with Early Gastric Cancer Treated with Laparoscopic Gastrectomy. J Gastric Cancer. Sep 2020;20(3):267–276. doi: 10.5230/jgc.2020.20.e27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson C, Campwala I, Gupta S. Examining the validity of the ACS-NSQIP Risk Calculator in plastic surgery: lack of input specificity, outcome variability and imprecise risk calculations. J Investig Med. Mar 2017;65(3):722–725. doi: 10.1136/jim-2016-000224 [DOI] [PubMed] [Google Scholar]
- 20.McCarthy MH, Singh P, Nayak R, et al. Can the American College of Surgeons Risk Calculator Predict 30-day Complications After Spine Surgery? Spine (Phila Pa 1976). May 1 2020;45(9):621–628. doi: 10.1097/BRS.0000000000003340 [DOI] [PubMed] [Google Scholar]
- 21.Narain AS, Kitto AZ, Braun B, et al. Does the ACS NSQIP Surgical Risk Calculator Accurately Predict Complications Rates After Anterior Lumbar Interbody Fusion Procedures? Spine (Phila Pa 1976). Jun 15 2021;46(12):E655–E662. doi: 10.1097/BRS.0000000000003893 [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. (2004). ICD-10: international statistical classification of diseases and related health problems : tenth revision neWHO. [PubMed]
- 23.Diebo BG, Passias PG, Marascalchi BJ, et al. Primary Versus Revision Surgery in the Setting of Adult Spinal Deformity: A Nationwide Study on 10,912 Patients. Spine (Phila Pa 1976). Nov 2015;40(21):1674–80. doi: 10.1097/BRS.0000000000001114 [DOI] [PubMed] [Google Scholar]
- 24.Mirza SK, Deyo RA, Heagerty PJ, Turner JA, Lee LA, Goodkin R. Towards standardized measurement of adverse events in spine surgery: conceptual model and pilot evaluation. BMC Musculoskelet Disord Jun 20 2006;7:53. doi: 10.1186/1471-2474-7-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cizik AM, Lee MJ, Martin BI, et al. Using the spine surgical invasiveness index to identify risk of surgical site infection: a multivariate analysis. J Bone Joint Surg Am Feb 15 2012;94(4):335–42. doi: 10.2106/JBJS.J.01084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. Sep 1988;44(3):837–45. [PubMed] [Google Scholar]
- 27.Matlab [Computer Software]. Version 2020b. Natick MM. [Google Scholar]
- 28.Bono OJ, Poorman GW, Foster N, et al. Body mass index predicts risk of complications in lumbar spine surgery based on surgical invasiveness. Spine J Jul 2018;18(7):1204–1210. doi: 10.1016/j.spinee.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 29.Passias PG, Poorman GW, Bortz CA, et al. Predictors of adverse discharge disposition in adult spinal deformity and associated costs. Spine J. Oct 2018;18(10):1845–1852. doi: 10.1016/j.spinee.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 30.Siccoli A, Staartjes VE, de Wispelaere MP, Schroder ML. Gender differences in degenerative spine surgery: Do female patients really fare worse? Eur Spine J. Oct 2018;27(10):2427–2435. doi: 10.1007/s00586-018-5737-3 [DOI] [PubMed] [Google Scholar]
- 31.Pellise F, Vila-Casademunt A, Ferrer M, et al. Impact on health related quality of life of adult spinal deformity (ASD) compared with other chronic conditions. Eur Spine J. Jan 2015;24(1):3–11. doi: 10.1007/s00586-014-3542-1 [DOI] [PubMed] [Google Scholar]
- 32.Lee MJ, Konodi MA, Cizik AM, Bransford RJ, Bellabarba C, Chapman JR. Risk factors for medical complication after spine surgery: a multivariate analysis of 1,591 patients. Spine J. Mar 2012;12(3):197–206. doi: 10.1016/j.spinee.2011.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeWald CJ, Stanley T. Instrumentation-related complications of multilevel fusions for adult spinal deformity patients over age 65: surgical considerations and treatment options in patients with poor bone quality. Spine (Phila Pa 1976). Sep 1 2006;31(19 Suppl):S144–51. doi: 10.1097/01.brs.0000236893.65878.39 [DOI] [PubMed] [Google Scholar]
- 34.Warner JL, Zhang P, Liu J, Alterovitz G. Classification of hospital acquired complications using temporal clinical information from a large electronic health record. J Biomed Inform. Feb 2016;59:209–17. doi: 10.1016/j.jbi.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratliff JK, Balise R, Veeravagu A, et al. Predicting Occurrence of Spine Surgery Complications Using “Big Data” Modeling of an Administrative Claims Database. J Bone Joint Surg Am. May 18 2016;98(10):824–34. doi: 10.2106/JBJS.15.00301 [DOI] [PubMed] [Google Scholar]
- 36.Merrill RK, Ibrahim JM, Machi AS, Raphael JS. Analysis and Review of Automated Risk Calculators Used to Predict Postoperative Complications After Orthopedic Surgery. Curr Rev Musculoskelet Med Jun 2020;13(3):298–308. doi: 10.1007/s12178-020-09632-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veeravagu A, Li A, Swinney C, et al. Predicting complication risk in spine surgery: a prospective analysis of a novel risk assessment tool. J Neurosurg Spine. Jul 2017;27(1):81–91. doi: 10.3171/2016.12.SPINE16969 [DOI] [PubMed] [Google Scholar]
- 38.Riley CA, Barton BM, Lawlor CM, et al. NSQIP as a Predictor of Length of Stay in Patients Undergoing Free Flap Reconstruction. OTO Open. Jan-Mar 2017;1(1):2473974X16685692. doi: 10.1177/2473974X16685692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietz N, Sharma M, Alhourani A, et al. Bundled Payment Models in Spine Surgery: Current Challenges and Opportunities, a Systematic Review. World Neurosurg. Mar 2019;123:177–183. doi: 10.1016/j.wneu.2018.12.001 [DOI] [PubMed] [Google Scholar]
- 40.Barrie U, Montgomery EY, Ogwumike E, et al. Household Income as a Predictor for Surgical Outcomes and Opioid Use After Spine Surgery in the United States. Global Spine J Jan 10 2022:21925682211070823. doi: 10.1177/21925682211070823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sconza C, Respizzi S, Grappiolo G, Monticone M. The Risk Assessment and Prediction Tool (RAPT) after Hip and Knee Replacement: A Systematic Review. Joints. Jun 2019;7(2):41–45. doi: 10.1055/s-0039-1693459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roof MA, Lygrisse K, Keitel L, et al. How Accurate Is ICD-10 Coding for Revision Total Knee Arthroplasty? J Arthroplasty. Dec 2021;36(12):3950–3958. doi: 10.1016/j.arth.2021.08.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


