Abstract
Unhealthy alcohol consumption is a global health problem. Individual and public health, and socioeconomic consequences are attributable to harmful alcohol use. Epidemiological studies have shown that alcohol use disorder (AUD) and alcohol-associated liver disease (ALD) are the top two pathologies among alcohol-related diseases. Consistent with the major role that the liver plays in alcohol metabolism, uncontrolled drinking may cause significant damage to the liver. This damage is initiated with excessive fat accumulation in the liver, which can further progress to advanced liver disease. The only effective therapeutic strategies currently available for ALD are alcohol abstinence or liver transplantation. Any molecule with dual pronged effects at the central and peripheral organs controlling addictive behaviors and associated metabolic pathways could be a promising therapeutic target to treat AUD and ALD. Ghrelin, a hormone primarily derived from the stomach, has such properties and regulates both behavioral and metabolic functions. In this review, we highlight recent advances in understanding the peripheral and central functions of the ghrelin system and its role in AUD and ALD pathogenesis. We first discuss the correlation between blood ghrelin concentrations and alcohol use or abstinence. Next, we discuss the role of ghrelin in alcohol-seeking behaviors, and finally its role in development of fatty liver by metabolic regulations and organ-crosstalk. We propose that understanding the ghrelin system may open an innovative avenue for improved treatments for AUD and associated medical consequences, including ALD.
Keywords: Ghrelin, Gut-brain axis, Alcohol, Alcohol use disorder, Alcohol-associated liver disease
Introduction:
Excessive alcohol use is a serious health concern in the US and worldwide that inflicts significant social and economic burden on individuals and society at large (Axley et al., 2019). According to the World Health Organization (WHO), 43% of the global population were current alcohol drinkers in 2016, and alcohol use resulted in ~3 million deaths and 132.6 million disability-adjusted life years in 2016 (Axley et al., 2019). In addition to alcohol-related mental illnesses, chronic excessive alcohol use can lead to liver disease, cancers, and increased transmission of infectious diseases, (Rehm et al., 2017, Scott-Sheldon et al., 2016), which highlights some of the harmful effects of excessive alcohol use.
Chronic, heavy alcohol consumption disrupts normal organ function in virtually every tissue of the body, but the liver typically sustains the greatest damage (Axley et al., 2019). This is primarily because the liver is the first organ exposed to alcohol absorbed in the gastrointestinal tract via the portal circulation and secondly, because the liver is the principal site of alcohol metabolism (Zakhari and Li, 2007). Indeed, approximately half of global liver cirrhosis cases are attributed to excessive alcohol use (Mellinger, 2019). Ninety percent of individuals with excessive alcohol use develop fatty liver (steatosis), characterized by accumulation of lipids in hepatocytes (Ohashi et al., 2018). About 20–40% of current drinkers with continued excessive alcohol use develop alcohol-associated steatohepatitis (ASH). ASH is characterized by fatty liver, inflammation, and lobular fibrosis. Repeated episodes of ASH with continued drinking can lead to advanced liver diseases, including fibrosis/cirrhosis and hepatocellular carcinoma (Lieber, 2004, Ohashi et al., 2018, Leggio and Lee, 2017). Moreover, the rapidly growing prevalence of obesity exacerbates the progression of liver damage in individuals with ALD (Alkhouri et al., 2022). The effective therapeutic strategies currently available for ALD are abstinence or liver transplantation (Ohashi et al., 2018). Thus, abstinence is a vital therapeutic goal for patients with ALD, as it can improve outcomes at nearly all stages of the disease and is particularly important pre- and post-liver transplantation (Mathurin and Lucey, 2020, Shawcross and O’Grady, 2010, Wackernah et al., 2014). Despite this, abstinence can be challenging to achieve in patients where chronic excessive alcohol use occurs as a result of alcohol use disorder (AUD).
AUD is a medical condition characterized by loss of control over alcohol consumption despite adverse social, occupational, and/or health consequences (Leggio and Lee, 2017). In addition to liver toxicity, chronic alcohol use and AUD are accompanied by changes in the brain, motivational behavior, and negative emotional states when alcohol is not used (Leggio and Lee, 2017). These changes ultimately reinforce excessive alcohol consumption and make total abstinence from alcohol difficult to achieve for patients with AUD. Given that alcohol abstinence is important for the improvement of ALD outcomes, AUD treatment becomes a necessary component of ALD management to prevent continued liver damage and disease progression. Moreover, AUD treatment is a critical factor in lowering pre- and post- transplantation relapse rates (Arab et al., 2022). Therefore, understanding the common pathophysiological mechanisms related to AUD and ALD is crucial for developing dual treatment approaches for AUD and ALD. Considering that recent epidemiological data suggest an increase in the prevalence of AUD, alcohol-associated cirrhosis, and liver transplantations in many countries, including the USA (Lee et al., 2019), there is critical need for therapeutic strategies that address both AUD and ALD.
Accumulating evidence demonstrates that gut-derived hormones often play a role in communications between peripheral organs and the brain (Czerwinska et al., 2021). There is a growing interest in understanding the role of gut-derived peptides in organ crosstalk as well as in the development of psychological and metabolic diseases, including AUD and ALD (Vadnie et al., 2014). One such hormone of interest is ghrelin, a gut-derived peptide hormone that exerts regulatory effects on both the central nervous system and peripheral organs. Ghrelin is an acylated 28-amino-acid peptide that is mainly secreted from the stomach, but is also expressed in the intestine, pancreas, kidney, adipose tissue and brain (Ariyasu et al., 2001). The ghrelin gene (GHRL) produces a ghrelin-obestatin preproprotein, which is cleaved/converted to proghrelin. Proghrelin undergoes acylation by ghrelin-O-acyltransferase (GOAT) and is then cleaved to its active form, acyl-ghrelin/acylated ghrelin (here referred to as ghrelin) (Muller et al., 2015). Notably, des-acyl ghrelin (DAG; also referred to as unacylated ghrelin) can also be formed. DAG is devoid of action at the ghrelin receptor but may play a distinct role in metabolic regulation (Asakawa et al., 2005, Fernandez et al., 2016). The specific receptor for ghrelin is the growth hormone secretagogue receptor (GHSR1a) a G-protein-coupled receptor (GPCR) expressed both centrally and peripherally that mediates the metabolic and neurobehavioral effects of ghrelin (Muller et al., 2015). Through GHSR1a, ghrelin modulates a wide range of physiological functions, such as glucose homeostasis (Lin et al., 2019, Mani et al., 2019b, Sovetkina et al., 2020), lipid metabolism (Barazzoni et al., 2005, Li et al., 2014), inflammation (Baatar et al., 2011), and apoptotic cell death (Bonfili et al., 2013), which makes it of interest in identifying new ALD therapies. Moreover, ghrelin has been shown to play a role in the rewarding effects of food, alcohol, and other addictive drugs, demonstrating an effect on centrally mediated consummatory behaviors (Al Massadi et al., 2019, Zallar et al., 2017). Significant evidence suggests that targeting the ghrelin system is a viable therapeutic strategy for AUD (Farokhnia et al., 2019). Thus, current knowledge of ghrelin’s central and peripheral actions suggests that the ghrelin system is a promising target for novel therapies that address both ALD and AUD.
In this review, we discuss recent advances in understanding the peripheral and central functions of the ghrelin system in AUD and ALD (Fig.1), obtained from studies conducted in humans and experimental animals.
Figure 1:
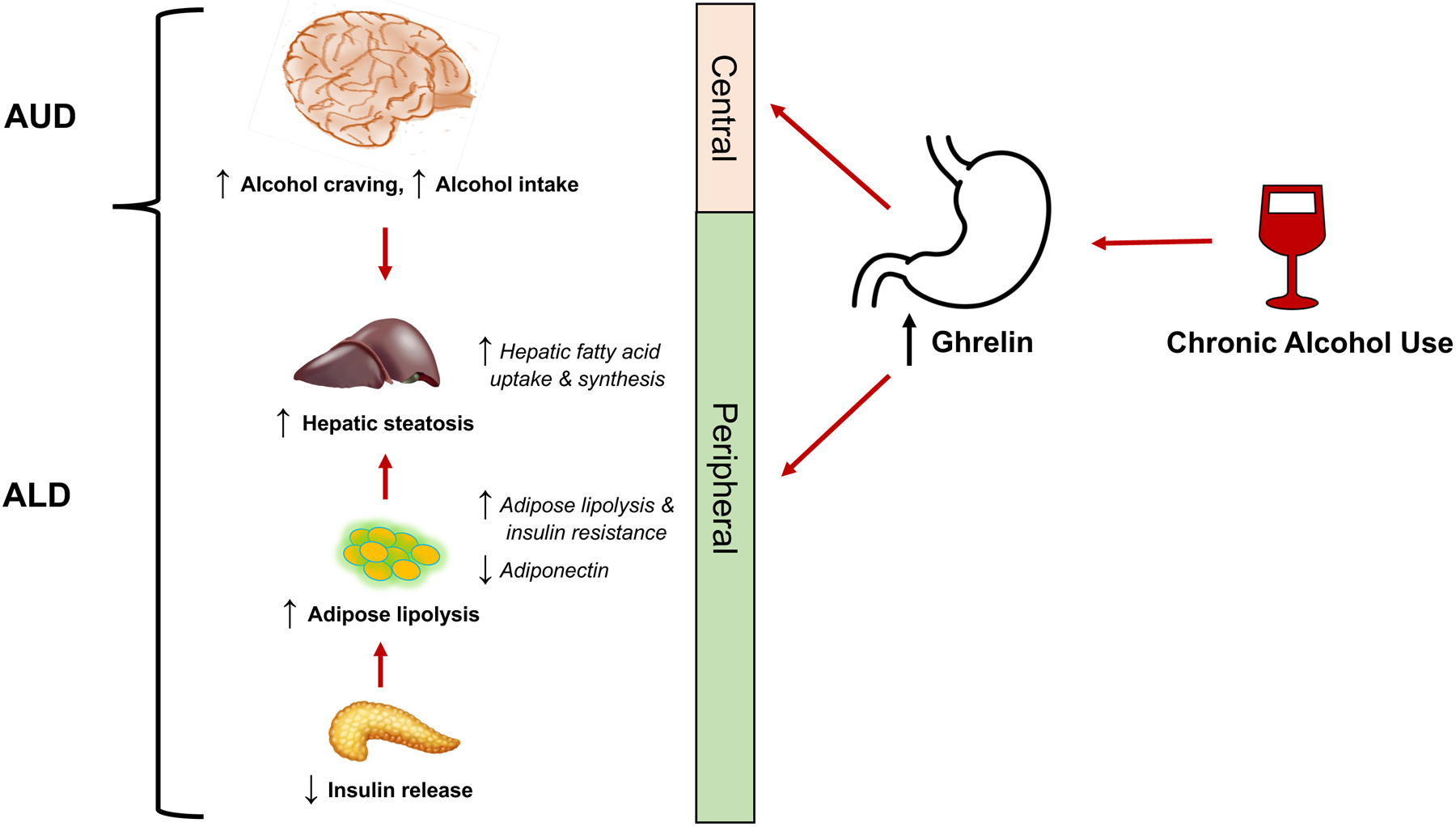
Schematic of the potential role of the ghrelin system in alcohol use disorder (AUD) and alcohol-associated liver disease (ALD).
a). Alcohol consumption and blood ghrelin concentration
There are only a few experimental studies which explore the relationship between alcohol intake and serum/plasma ghrelin concentrations in humans and rodents. Studies conducted in male Wistar rats fed the Lieber-DeCarli ethanol-containing liquid diet (6.7% v/v or 36% calories) for 6 weeks showed a significant increase in serum ghrelin concentrations (acylated ghrelin), as well as ghrelin and GOAT gene expression in stomach, compared to their isocaloric control liquid diet-fed rats (Rasineni et al., 2019a, Rasineni et al., 2019b). Further, mice administered ethanol (20% ethanol in water) for 10 days showed an increase of both serum acylated and des-acylated ghrelin (Godlewski et al., 2019).
In agreement with these rodent data, large population-based studies found higher serum total ghrelin (acylated and des-acylated ghrelin) among alcohol drinkers than non-drinkers (Farokhnia et al., 2021), and that alcohol consumption was positively associated with serum total ghrelin in alcohol drinkers (Wittekind et al., 2018). In addition, patients with alcohol-associated cirrhosis and chronic alcohol dependence showed higher plasma ghrelin than healthy volunteers (Goodyear et al., 2010, Kim et al., 2005, Kraus et al., 2005). Interestingly, studies conducted in patients with AUD showed that abstainers have higher blood ghrelin compared to current drinkers (Kim et al., 2005, Kim et al., 2013, Koopmann et al., 2012, Kraus et al., 2005, Leggio et al., 2012). Further, acute alcohol administration to both healthy volunteers and rodents reduces plasma ghrelin (Calissendorff et al., 2005, Calissendorff et al., 2006, Leggio et al., 2013, Ralevski et al., 2017, Zimmermann et al., 2007). In contrast to former hypotheses, recent data suggests that this suppressive effect of acute alcohol on ghrelin does not occur through direct action of alcohol on ghrelin-secreting gastric mucosal cells or in proportion to the caloric value of alcohol administered (Deschaine et al., 2021). These data demonstrate that alcohol has an acute suppressive effect on ghrelin secretion, and that this effect likely occurs through an indirect mechanism separate from the effects of alcohol metabolism on energy homeostasis.
Overall, the reports on the effects of alcohol on blood ghrelin concentrations are mixed, mainly because of differences between acute vs chronic exposure to alcohol, but also because of differences in, for example, the dose, duration, mode of alcohol administration, and the time of ghrelin measurement post-alcohol intake. When different effects of acute vs chronic alcohol consumption on ghrelin concentration are considered, side-by side, the following hypothesis may explain their relationship. With prolonged, harmful alcohol consumption, the acute alcohol-induced suppression of ghrelin secretion may lead to compensatory physiological changes that promote a rebound increase in circulating ghrelin concentrations. This hypothesized mechanism may explain why increased ghrelin concentrations are observed in chronic alcohol drinkers compared to non-drinkers and during alcohol abstinence vs chronic alcohol drinkers. However, additional controlled animal and human studies are needed to confirm this hypothesis and obtain more conclusive information on changes in peripheral ghrelin concentrations following acute vs chronic and continuous vs intermittent alcohol consumption in fed vs fasted conditions. Nevertheless, consistent observations of increased ghrelin concentrations following prolonged alcohol consumption, while reduction in ghrelin concentrations following acute alcohol intake, are reported. Moreover, serum ghrelin is increased in patients with alcohol-associated cirrhosis and in abstinent patients with AUD, suggesting that upregulation of ghrelin secretion may be a pathophysiological component or consequence of chronic alcohol consumption that can be therapeutically leveraged.
b). Ghrelin and alcohol-mediated behavior: Clinical and experimental studies
Ghrelin is traditionally known to stimulate hunger and meal initiation through a GHSR1a-stimulated complex network of neuronal circuits (Muller et al., 2015), although its effects are complex and not fully understood (Deschaine and Leggio, 2022). GHSR1a is expressed in reward-related brain areas, including the ventral tegmental area (VTA) and nucleus accumbens (NAc) (Zigman et al., 2006, Shevchouk et al., 2021, Suchankova et al., 2016a), suggesting that ghrelin may regulate reward processing. Growing evidence from preclinical experiments and human studies shows that ghrelin is also involved in alcohol craving and alcohol consumption (Deschaine and Leggio, 2022, Farokhnia et al., 2019, Shevchouk et al., 2021). These findings indicate that increased circulating ghrelin following chronic alcohol consumption may in turn increase alcohol craving and consumption and lead to more severe AUD and/or ALD. Below we review the literature describing the relationship between ghrelin and alcohol craving and consumption.
Ghrelin and alcohol craving:
Systemic or central administration of ghrelin increases alcohol consumption in rodents (Jerlhag et al., 2009). Genetic knock-out or pharmacological blockage of the ghrelin system reduces alcohol consumption (Jerlhag et al., 2009, Suchankova et al., 2016b, Zallar et al., 2019, Godlewski et al., 2019). Moreover, suppression of either GHSR1a (genetic or pharmacological) or ghrelin production (genetic) suppresses conditioned place preference (CPP) for alcohol (Bahi et al., 2013, Jerlhag et al., 2009, Jerlhag et al., 2011). Indeed, acute treatment with a GHSR1a antagonist reduces alcohol intake in rodents (Bahi et al., 2013, Gomez et al., 2015, Gomez and Ryabinin, 2014, Jerlhag et al., 2009, Kaur and Ryabinin, 2010, Landgren et al., 2012, Stevenson et al., 2015). Besides regular alcohol intake, GHSR1a antagonism decreases operant self-administration of alcohol (Gomez et al., 2015, Landgren et al., 2012). Following abstinence from alcohol, pharmacological suppression of GHSR1a prevents relapse in male rats (Suchankova et al., 2013b, Jerlhag et al., 2009).
In clinical studies, peripheral endogenous ghrelin is positively correlated with alcohol craving, subjective response to alcohol, and brain activity in response to alcohol (Bach et al., 2019, Koopmann et al., 2019, Leggio et al., 2012). In a randomized, between-subject, double-blind, placebo-controlled, human laboratory study, intravenous (IV) ghrelin administration significantly increased cue-induced craving for alcohol in a bar-like laboratory, with no significant effect on craving for juice (used as a non-alcoholic appetitive control) (Leggio et al., 2014). In a subsequent study, the potential role of ghrelin administration in influencing motivation to self-administer alcohol and/or brain functional activity during reward anticipation was investigated in a randomized, crossover, double-blind, placebo-controlled human laboratory study with IV ghrelin. This study revealed that IV ghrelin infusion motivates participants to self-administer more alcohol and increases amygdala activity during alcohol reward anticipation (Farokhnia et al., 2018).
Sites and mode of ghrelin action:
While the impact of ghrelin on alcohol-related behaviors has been widely explored, the brain circuits responsible for this interaction remain to be fully mapped. Some of the brain regions expressing GHSR1a have been identified as potentials sites of action for ghrelin to modulate alcohol-related behaviors (Cruz et al., 2013, Landgren et al., 2011, Zigman et al., 2006). One of these is the VTA, an important site for reinforcement, as its dopaminergic neurons project to the NAc shell. Indeed, local infusion of ghrelin into the VTA increases alcohol intake in male mice (Jerlhag et al., 2009), and GHSR1a antagonism reduces the ability of alcohol to cause somatodendritic dopamine release in this area (Edvardsson et al., 2021). Another implicated brain region is the amygdala, as ghrelin administration to alcohol-dependent male rats enhances the GABA levels in this area (Cruz et al., 2013, Yoshimoto et al., 2017). Additionally, findings that alcohol elevates both c-fos in the Edinger-Westphal nucleus (Kaur and Ryabinin, 2010) and both acyl- and des-acyl ghrelin within the lateral hypothalamus (Yoshimoto et al., 2017), combined with findings that local ghrelin infusion into the laterodorsal tegmental area (LDT) increases alcohol intake (Jerlhag et al., 2009), indicate that areas outside of the mesolimbic dopamine system are of importance for the ghrelin-alcohol link.
The influence of ghrelin signaling on brain regions involved in reward processing and memory have been examined in a few human functional magnetic resonance imaging (fMRI) studies by assessing the effects of acute exogenous ghrelin administration on the blood oxygen level dependent (BOLD) signal. In the fed state (when endogenous plasma ghrelin is reduced), in healthy adults without obesity, IV or subcutaneous (SC) ghrelin administration increased BOLD signal during viewing of food pictures in areas implicated in reward processing, such as the orbitofrontal cortex (OFC) and hippocampus (mimicking the effects of fasting where there is endogenous hyperghrelinemia), and amygdala, anterior insula and striatum, and increased appeal rating of high-energy food pictures (Goldstone et al., 2014, Malik et al., 2008). Note that, SC ghrelin administration had no effect on BOLD signal in primary auditory, motor, or visual cortices during control auditory, motor or visual tasks, respectively, suggesting a lack of non-specific effects of ghrelin on neurovascular coupling (Goldstone et al., 2014).
In fed, adults without obesity, but with alcohol dependence, IV ghrelin administration decreased BOLD signal during anticipation of delayed food reward in the medial OFC and increased BOLD signal in the NAc; increased BOLD signal in the amygdala during anticipation of an IV alcohol administration was also observed (Farokhnia et al., 2018). Additionally, IV ghrelin administration decreased the latency to initiate self-administration of IV alcohol (Farokhnia et al., 2018). In detoxified patients with alcohol dependence (21 days of controlled alcohol abstinence), an fMRI study found that alcohol cue-induced brain activity in a network of brain clusters, including the bilateral ventral striatum, showed a significant positive association with plasma acylated ghrelin (Koopmann et al., 2019).
Furthermore, the association between plasma acylated ghrelin and alcohol craving was mediated by a cue-induced brain response in the ventral striatum and mesolimbic pathway (Koopmann et al., 2019)
Des-acyl ghrelin:
Although acyl-ghrelin modulates alcohol-related behaviors in rodents, the effects of DAG are not yet clear. DAG is the major circulating form of ghrelin and was initially thought to be inactive because it does not bind to GHSR1a at physiologically relevant concentrations. However, new findings reveal various physiological properties of DAG. Some studies showed that DAG inhibits ghrelin-enhanced food intake (Fernandez et al., 2016) and decreases feeding (Asakawa et al., 2005) in rodents. In addition, DAG decreases gastric emptying (Asakawa et al., 2005) and regulates body temperature, glucose homeostasis, and lipid metabolism (Heppner et al., 2014) in rodents. Nevertheless, recent human studies reported that only plasma acylated ghrelin is associated with alcohol craving (Koopmann et al., 2012, Koopmann et al., 2019, Sha et al., 2021). This was also confirmed by fMRI studies which documented that only acylated ghrelin, and not DAG, positively correlate with BOLD signal in the brain to alcohol cues (Bach et al., 2019, Koopmann et al., 2019).
Preliminary human work on ghrelin receptor blockade in AUD:
Given the role of ghrelin in increasing alcohol craving and intake, and that GHSR1a antagonists reduce alcohol intake and alcohol preference in preclinical studies (Jerlhag et al., 2009, Kaur and Ryabinin, 2010, Stevenson et al., 2016, Landgren et al., 2012), blocking the ghrelin system is under clinical investigation as a potential pharmacotherapeutic approach for AUD.
PF-5190457 is a ghrelin receptor inverse agonist, which can inhibit GHSR1a constitutive activity, as well as ghrelin mediated activity. In rodents and humans (heavy drinking individuals), PF-5190457, combined with alcohol, was shown to be safe and no alcohol-drug interactions were detected (Lee et al., 2020b). Furthermore, in a preliminary alcohol/food cue-reactivity session, PF-5190457, compared to placebo, reduced cue-induced alcohol craving and attention to alcohol cues, as well as cue-induced food craving, in a bar-like laboratory (Lee et al., 2020b). In addition, despite reducing ghrelin mediated alcohol craving, PF-5190457 did not significantly affect the serum concentrations of appetitive/metabolic and stress-related hormones or inflammatory markers, confirming the safety profile of this compound, especially when co-administered with alcohol (Farokhnia et al., 2020, Lee et al., 2020b, Lee et al., 2020a).
In summary, a significant body of literature demonstrates that stimulating the ghrelin system increases, while blocking the ghrelin system decreases, alcohol craving and consumption. Although the mechanism(s) and potential brain regions involved in the effect of ghrelin on alcohol craving and consumption, as well as the role of DAG in this relationship, have yet to be fully elucidated, the evidence thus far suggests that inhibiting the ghrelin system can be a potential therapeutic strategy for AUD. Considered together, observations of (i) an association between chronic alcohol consumption and increased circulating ghrelin, and (ii) a positive association between ghrelin and alcohol craving and consumption, suggest the presence of a pathological positive feedback loop, where chronic alcohol increases ghrelin secretion which, in turn, further promotes alcohol consumption and together lead to increased AUD and/or ALD severity. Inhibiting the ghrelin system may therefore halt this harmful cycle.
c). Ghrelin in development of ALD
Liver and adipose tissue play a prominent role in glucose and fatty acid metabolism. Liver-adipose crosstalk and energy homeostasis is regulated by peptide hormones, such as ghrelin, insulin, and adiponectin secreted by the gut, pancreas, and adipose tissue, respectively. From clinical and experimental studies, it is well known that ghrelin reduces insulin secretion by suppressing intracellular Ca2+ levels in pancreatic β cells (Broglio et al., 2001, Dezaki et al., 2004, Dezaki et al., 2008). An inverse relationship between plasma ghrelin and insulin concentrations were reported in alcohol-fed rats (Rasineni et al., 2019b). Further studies revealed that an alcohol-induced increase in serum ghrelin impaired insulin secretion from pancreatic β-cells (Rasineni et al., 2019b, Rasineni et al., 2019a). Since insulin promotes export of lipoproteins from the liver and facilitates fat storage in adipose tissue (Saltiel and Kahn, 2001), suppression of circulating insulin by ethanol-induced ghrelin increases results in increased adipose lipolysis. The consequent rise in circulating fatty acids level and their enhanced hepatic uptake and esterification, leads to the development of alcohol-associated steatosis (Kang et al., 2007, Wei et al., 2013). Treatment of chronic alcohol-fed rats with the GHSR1a antagonist, [D-Lys-3] GHRP-6, increased serum insulin and by reduced circulating free fatty acids attenuated hepatic steatosis (Rasineni et al., 2019a). These results highlight the important role of ghrelin in modulating the pancreas-adipose-liver axis to promote the development of ALD. Since gene knockout of either GHRL or GHSR or inhibition of GOAT reduces the incidence of hepatic steatosis of diverse etiology (Li et al., 2014, Wortley et al., 2005, Zhang et al., 2018), underscores the importance of ghrelin in regulating hepatic lipid metabolism.
Ghrelin also directly promotes fat accumulation in hepatocytes by increasing fatty acid transport as well as de novo fatty acid synthesis and esterification (Barazzoni et al., 2005, Li et al., 2014, Rasineni et al., 2019a). In vitro studies conducted on isolated hepatocytes revealed that ghrelin promotes lipid accumulation via a similar mechanism as alcohol (Rasineni et al., 2019b). In support of these results, ghrelin directly increases lipogenesis in hepatocytes by activating the mammalian target of rapamycin (mTOR) and peroxisome proliferator-activated receptor gamma (PPARγ) signaling pathway (Li et al., 2014). Additionally, several other in vivo studies reported that ghrelin infusion increased hepatic lipid accumulation by elevating levels of fatty acid synthase enzymes (acetyl coenzyme A carboxylase, stearoyl-CoA desaturase-1) and decreasing fatty acid oxidation enzymes (carnitine palmitoyl transferase and PPARγ) (Dallak, 2018, Sangiao-Alvarellos et al., 2009).
In addition to its indirectly affecting adipose tissue via decreasing pancreatic insulin, ghrelin can directly alter adipose tissue metabolism. Ghrelin inhibits adipocyte differentiation/maturation, as well as increases lipolysis and release of free fatty acid from differentiated adipocytes in in vitro (Rasineni et al., 2020, Salmeron et al., 2015, Zhang et al., 2004, Miao et al., 2019). These studies were corroborated by in vivo studies, showing that ghrelin infusion reduces peripheral insulin sensitivity and increases lipolysis in adipose and muscle tissue (Vestergaard et al., 2008a, Vestergaard et al., 2008b, Theander-Carrillo et al., 2006). In addition, ghrelin decreases secretion of adiponectin (Rasineni et al., 2020, Ott et al., 2002), a hormone that protects liver from fat accumulation by increasing hepatic lipid oxidation (You et al., 2005). Indeed, clinical and experimental studies showed that chronic ethanol treatment/intake is associated with low serum adiponectin (Chen et al., 2007, Jung et al., 2013, Nishise et al., 2010). That this decrease with alcohol administration is likely a consequence of ghrelin increase was shown by the improved serum adiponectin in chronic alcohol-fed rats treated with the ghrelin receptor antagonist [D-Lys-3] GHRP-6 (Rasineni et al., 2020). In contrast to these studies, brain ghrelin infusion (for 8 days) increased visceral adipose tissue deposition in rats (Sangiao-Alvarellos et al., 2009), which may likely be due to increased food intake.
d). Future perspectives
Gene polymorphisms in ghrelin system
Several single nucleotide polymorphisms (SNPs) have been reported for GHRL and GHSR (Mora et al., 2015, Pabalan et al., 2014, Suchankova et al., 2013a). Several publications document an association of these SNPS with increased risk of alcohol and/or other substance abuse in humans (Suchankova et al., 2017, Landgren et al., 2010, Landgren et al., 2008, Suchankova et al., 2016b, Suchankova et al., 2013a). There is also clinical evidence showing that SNPs in the coding region of the GHRL are associated with differences in body mass index, fat mass, susceptibility to type 2 diabetes mellitus and development of chronic hepatitis B related liver cirrhosis (Mora et al., 2015, Ukkola et al., 2002, Choi et al., 2006, Bing et al., 2005, Zhang et al., 2015). However, future studies are required to examine whether there is a link between polymorphism in the ghrelin system and ALD pathogenesis.
Ghrelin receptor dimerization
Another relevant aspect is that GHSR1a can interact with other GPCRs, such as melanocortin-3 receptor, dopamine D1 and D2 receptors, serotonin 2C receptor, oxytocin receptor and prostanoid receptors (Price et al., 2021, Ringuet et al., 2021). The co-interaction may lead to decrease or increase in GHSR1a mediated signal transduction (Price et al., 2021, Xiao et al., 2020). Further investigation is warranted on whether such receptor-receptor interactions also exist in peripheral organs and tissues and the possible role of such interactions in modulating ALD pathogenesis.
Endogenous antagonist of ghrelin receptor
Liver-expressed antimicrobial peptide-2 (LEAP-2) is a recently discovered antimicrobial peptide predominantly expressed in the liver and gut. In addition to its antimicrobial role (Li et al., 2015, Townes et al., 2009), LEAP-2 is an inverse agonist at GHSR1a, attenuating the effects of ghrelin on food intake and stimulation of growth hormone (Al-Massadi et al., 2018, Ge et al., 2018, Hagemann et al., 2022, Mani et al., 2019a). Interestingly, a recent study revealed that IV ghrelin administration in mice reduces plasma LEAP-2 and treatment of hepatocytes with ghrelin decreased LEAP-2 synthesis and secretion (Islam et al., 2020). Since ghrelin reduces the levels of LEAP-2 which has an important role in innate immunity via its antibacterial activity, it is likely that the increased ghrelin seen with alcohol consumption may (i) play a role in the development of hepatic steatosis (an early event in ALD pathogenesis) and (ii) promote ALD progression by increasing intestinal hyperpermeability and gut dysbiosis - crucial modulators of hepatic inflammation and fibrosis (Bajaj, 2019, Fairfield and Schnabl, 2021). Besides, the possibility that LEAP2 modulate addiction processes should also be evaluated in man and rodents.
Conclusions:
Emerging evidence documents a close, yet complex, interaction between the ghrelin system and alcohol-related outcomes (Fig.1). Consistent observations indicate that chronic alcohol consumption is associated with an increased in blood ghrelin concentrations in rodents, and clinical studies of patients with alcohol-associated cirrhosis report increased ghrelin levels, compared with healthy individuals. This increase in circulating ghrelin may drive an increase in alcohol consumption as preclinical and clinical experiments demonstrates that ghrelin stimulates alcohol-related behaviors, such as alcohol craving and consumption. These findings lead us to hypothesize a potentially unique therapeutic strategy for AUD where inhibiting the ghrelin system may reduce the effects of increasing circulating ghrelin on alcohol consumption. Indeed, preliminary studies in humans demonstrate that a ghrelin receptor inverse agonist/antagonist reduces cue-induced alcohol attention and craving, suggesting promising effects of ghrelin receptor antagonism on alcohol craving. The identified bi-directional relationship between alcohol consumption and circulating ghrelin concentrations is also of interest in the treatment of ALD. In addition to increasing alcohol consumption, ghrelin also increases ALD severity by (i) reducing insulin secretion from pancreatic β-cells and thereby stimulating adipose-derived fatty acid mobilization to ultimately contribute to hepatic steatosis; (ii) directly promoting fat accumulation in hepatocytes by increasing fatty acid transport, as well as de novo fatty acid synthesis and esterification; and (iii) reducing insulin sensitivity and impairing adipocyte differentiation and maturation consistent with an inflammatory state. Ghrelin system inhibition could therefore serve as a possible dual therapeutic strategy to decrease alcohol craving and consumption as well the effects of ghrelin on ALD pathogenesis.
Future studies are required to (i) conclusively catalog the relationship between serum/plasma ghrelin and varying degrees and types of alcohol consumption (i.e., acute vs chronic and intermittent vs continuous) with more carefully controlled studies; (ii) parse out the central and peripheral mechanisms by which ghrelin stimulates alcohol craving and consumption, (iii) understand the role of other components of the ghrelin system in AUD and ALD pathophysiology, such as DAG, LEAP-2, and GHSR1a heterodimers, and (iv) investigate the relationship between genetic variations in the ghrelin system and AUD and ALD pathogenesis. Altogether, the known interplay between alcohol, ghrelin, and ghrelin-mediated organ crosstalk suggests that future research on the ghrelin system represents a viable target for dual treatment of AUD and ALD.
Acknowledgements:
This work was supported by the National Institutes of Health/NIAAA funding R01AA028504 (Rasineni); National Institutes of Health/NIAAA funding R01AA026723 (Kharbanda) and United States Department of Veterans Affairs Biomedical Laboratory Research and Development Merit Review grants, BX004053 (Kharbanda); National Institutes of Health, intramural funding ZIA-DA000635 (Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section), jointly supported by the NIDA Intramural Research Program and the NIAAA Division of Intramural Clinical and Biological Research (Farokhnia, Deschaine, Leggio); Swedish Research Council (2015-03219;2019-01676), The Swedish Brain Foundation, and LUA/ALF (723941) from the Sahlgrenska University Hospital (Jerlhag); UK Medical Research Council MR/T017279/1 (Goldstone). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or other funding organizations.
Footnotes
Conflicts of Interest: Authors Kharbanda, Farokhnia, Deschaine, Bhargava, Flores, Casey, Holm, Leggio, and Rasineni have no conflict of interest. Goldstone: Medical Advisory Board, Millendo Therapeutics; Consultant, Helsinn Healthcare S.A.; Consultant, Rhythm Pharmaceuticals.
References:
- Global status report on alcohol and health 2018, in Series Global status report on alcohol and health 2018, Geneva: World Health Organization; 2018. Licence: CC BY-NC-SA 3.0 IGO. [Google Scholar]
- Al-Massadi O, Muller T, Tschop M, Dieguez C, Nogueiras R (2018) Ghrelin and LEAP-2: Rivals in Energy Metabolism. Trends Pharmacol Sci 39:685–694. [DOI] [PubMed] [Google Scholar]
- Al Massadi O, Nogueiras R, Dieguez C, Girault JA (2019) Ghrelin and food reward. Neuropharmacology 148:131–138. [DOI] [PubMed] [Google Scholar]
- Alkhouri N, Almomani A, Le P, Payne JY, Asaad I, Sakkal C, Vos M, Noureddin M, Kumar P (2022) The prevalence of alcoholic and nonalcoholic fatty liver disease in adolescents and young adults in the United States: analysis of the NHANES database. BMC Gastroenterol 22:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arab JP, Izzy M, Leggio L, Bataller R, Shah VH (2022) Management of alcohol use disorder in patients with cirrhosis in the setting of liver transplantation. Nat Rev Gastroenterol Hepatol 19:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, Shirakami G, Usui T, Shimatsu A, Doi K, Hosoda H, Kojima M, Kangawa K, Nakao K (2001) Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab 86:4753–4758. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Inui A, Fujimiya M, Sakamaki R, Shinfuku N, Ueta Y, Meguid MM, Kasuga M (2005) Stomach regulates energy balance via acylated ghrelin and desacyl ghrelin. Gut 54:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axley PD, Richardson CT, Singal AK (2019) Epidemiology of Alcohol Consumption and Societal Burden of Alcoholism and Alcoholic Liver Disease. Clin Liver Dis 23:39–50. [DOI] [PubMed] [Google Scholar]
- Baatar D, Patel K, Taub DD (2011) The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol 340:44–58. [DOI] [PubMed] [Google Scholar]
- Bach P, Bumb JM, Schuster R, Vollstadt-Klein S, Reinhard I, Rietschel M, Witt SH, Wiedemann K, Kiefer F, Koopmann A (2019) Effects of leptin and ghrelin on neural cue-reactivity in alcohol addiction: Two streams merge to one river? Psychoneuroendocrinology 100:1–9. [DOI] [PubMed] [Google Scholar]
- Bahi A, Tolle V, Fehrentz JA, Brunel L, Martinez J, Tomasetto CL, Karam SM (2013) Ghrelin knockout mice show decreased voluntary alcohol consumption and reduced ethanol-induced conditioned place preference. Peptides 43:48–55. [DOI] [PubMed] [Google Scholar]
- Bajaj JS (2019) Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol 16:235–246. [DOI] [PubMed] [Google Scholar]
- Barazzoni R, Bosutti A, Stebel M, Cattin MR, Roder E, Visintin L, Cattin L, Biolo G, Zanetti M, Guarnieri G (2005) Ghrelin regulates mitochondrial-lipid metabolism gene expression and tissue fat distribution in liver and skeletal muscle. Am J Physiol Endocrinol Metab 288:E228–235. [DOI] [PubMed] [Google Scholar]
- Bing C, Ambye L, Fenger M, Jorgensen T, Borch-Johnsen K, Madsbad S, Urhammer SA (2005) Large-scale studies of the Leu72Met polymorphism of the ghrelin gene in relation to the metabolic syndrome and associated quantitative traits. Diabet Med 22:1157–1160. [DOI] [PubMed] [Google Scholar]
- Bonfili L, Cuccioloni M, Cecarini V, Mozzicafreddo M, Palermo FA, Cocci P, Angeletti M, Eleuteri AM (2013) Ghrelin induces apoptosis in colon adenocarcinoma cells via proteasome inhibition and autophagy induction. Apoptosis 18:1188–1200. [DOI] [PubMed] [Google Scholar]
- Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, van der Lely AJ, Deghenghi R, Ghigo E (2001) Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 86:5083–5086. [DOI] [PubMed] [Google Scholar]
- Calissendorff J, Danielsson O, Brismar K, Rojdmark S (2005) Inhibitory effect of alcohol on ghrelin secretion in normal man. Eur J Endocrinol 152:743–747. [DOI] [PubMed] [Google Scholar]
- Calissendorff J, Danielsson O, Brismar K, Rojdmark S (2006) Alcohol ingestion does not affect serum levels of peptide YY but decreases both total and octanoylated ghrelin levels in healthy subjects. Metabolism 55:1625–1629. [DOI] [PubMed] [Google Scholar]
- Chen X, Sebastian BM, Nagy LE (2007) Chronic ethanol feeding to rats decreases adiponectin secretion by subcutaneous adipocytes. Am J Physiol Endocrinol Metab 292:E621–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi HJ, Cho YM, Moon MK, Choi HH, Shin HD, Jang HC, Kim SY, Lee HK, Park KS (2006) Polymorphisms in the ghrelin gene are associated with serum high-density lipoprotein cholesterol level and not with type 2 diabetes mellitus in Koreans. J Clin Endocrinol Metab 91:4657–4663. [DOI] [PubMed] [Google Scholar]
- Cruz MT, Herman MA, Cote DM, Ryabinin AE, Roberto M (2013) Ghrelin increases GABAergic transmission and interacts with ethanol actions in the rat central nucleus of the amygdala. Neuropsychopharmacology 38:364–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerwinska M, Czarzasta K, Cudnoch-Jedrzejewska A (2021) New Peptides as Potential Players in the Crosstalk Between the Brain and Obesity, Metabolic and Cardiovascular Diseases. Front Physiol 12:692642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallak MA (2018) Acylated ghrelin induces but deacylated ghrelin prevents hepatic steatosis and insulin resistance in lean rats: Effects on DAG/ PKC/JNK pathway. Biomed Pharmacother 105:299–311. [DOI] [PubMed] [Google Scholar]
- Deschaine SL, Farokhnia M, Gregory-Flores A, Zallar LJ, You ZB, Sun H, Harvey DM, Marchette RCN, Tunstall BJ, Mani BK, Moose JE, Lee MR, Gardner E, Akhlaghi F, Roberto M, Hougland JL, Zigman JM, Koob GF, Vendruscolo LF, Leggio L (2021) A closer look at alcohol-induced changes in the ghrelin system: novel insights from preclinical and clinical data. Addict Biol:e13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschaine SL, Leggio L (2022) From “Hunger Hormone” to “It’s Complicated”: Ghrelin Beyond Feeding Control. Physiology (Bethesda) 37:5–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezaki K, Hosoda H, Kakei M, Hashiguchi S, Watanabe M, Kangawa K, Yada T (2004) Endogenous ghrelin in pancreatic islets restricts insulin release by attenuating Ca2+ signaling in beta-cells: implication in the glycemic control in rodents. Diabetes 53:3142–3151. [DOI] [PubMed] [Google Scholar]
- Dezaki K, Sone H, Yada T (2008) Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol Ther 118:239–249. [DOI] [PubMed] [Google Scholar]
- Edvardsson CE, Vestlund J, Jerlhag E (2021) A ghrelin receptor antagonist reduces the ability of ghrelin, alcohol or amphetamine to induce a dopamine release in the ventral tegmental area and in nucleus accumbens shell in rats. Eur J Pharmacol 899:174039. [DOI] [PubMed] [Google Scholar]
- Fairfield B, Schnabl B (2021) Gut dysbiosis as a driver in alcohol-induced liver injury. JHEP Rep 3:100220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Faulkner ML, Piacentino D, Lee MR, Leggio L (2019) Ghrelin: From a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav 204:49–57. [DOI] [PubMed] [Google Scholar]
- Farokhnia M, Grodin EN, Lee MR, Oot EN, Blackburn AN, Stangl BL, Schwandt ML, Farinelli LA, Momenan R, Ramchandani VA, Leggio L (2018) Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry 23:2029–2038. [DOI] [PubMed] [Google Scholar]
- Farokhnia M, Murphy G, Weinstein SJ, Shah NN, Parisi D, Albanes D, Leggio L (2021) A population-based investigation of the association between alcohol intake and serum total ghrelin concentrations among cigarette-smoking, non-alcohol-dependent male individuals. Drug Alcohol Depend 226:108835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Portelli J, Lee MR, McDiarmid GR, Munjal V, Abshire KM, Battista JT, Browning BD, Deschaine SL, Akhlaghi F, Leggio L (2020) Effects of exogenous ghrelin administration and ghrelin receptor blockade, in combination with alcohol, on peripheral inflammatory markers in heavy-drinking individuals: Results from two human laboratory studies. Brain Res 1740:146851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez G, Cabral A, Cornejo MP, De Francesco PN, Garcia-Romero G, Reynaldo M, Perello M (2016) Des-Acyl Ghrelin Directly Targets the Arcuate Nucleus in a Ghrelin-Receptor Independent Manner and Impairs the Orexigenic Effect of Ghrelin. J Neuroendocrinol 28:12349. [DOI] [PubMed] [Google Scholar]
- Ge X, Yang H, Bednarek MA, Galon-Tilleman H, Chen P, Chen M, Lichtman JS, Wang Y, Dalmas O, Yin Y, Tian H, Jermutus L, Grimsby J, Rondinone CM, Konkar A, Kaplan DD (2018) LEAP2 Is an Endogenous Antagonist of the Ghrelin Receptor. Cell Metab 27:461–469 e466. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Cinar R, Coffey NJ, Liu J, Jourdan T, Mukhopadhyay B, Chedester L, Liu Z, Osei-Hyiaman D, Iyer MR, Park JK, Smith RG, Iwakura H, Kunos G (2019) Targeting Peripheral CB1 Receptors Reduces Ethanol Intake via a Gut-Brain Axis. Cell Metab 29:1320–1333 e1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstone AP, Prechtl CG, Scholtz S, Miras AD, Chhina N, Durighel G, Deliran SS, Beckmann C, Ghatei MA, Ashby DR, Waldman AD, Gaylinn BD, Thorner MO, Frost GS, Bloom SR, Bell JD (2014) Ghrelin mimics fasting to enhance human hedonic, orbitofrontal cortex, and hippocampal responses to food. Am J Clin Nutr 99:1319–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Cunningham CL, Finn DA, Young EA, Helpenstell LK, Schuette LM, Fidler TL, Kosten TA, Ryabinin AE (2015) Differential effects of ghrelin antagonists on alcohol drinking and reinforcement in mouse and rat models of alcohol dependence. Neuropharmacology 97:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Ryabinin AE (2014) The effects of ghrelin antagonists [D-Lys(3)]-GHRP-6 or JMV2959 on ethanol, water, and food intake in C57BL/6J mice. Alcohol Clin Exp Res 38:2436–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodyear SJ, Mottershead M, Sung EZ, Wong LS, McTernan PG, Kumar S, Nwokolo CU (2010) Dysregulation of plasma ghrelin in alcoholic cirrhosis. Clin Endocrinol (Oxf) 73:323–329. [DOI] [PubMed] [Google Scholar]
- Hagemann CA, Jensen MS, Holm S, Gasbjerg LS, Byberg S, Skov-Jeppesen K, Hartmann B, Holst JJ, Dela F, Vilsboll T, Christensen MB, Holst B, Knop FK (2022) LEAP2 reduces postprandial glucose excursions and ad libitum food intake in healthy men. Cell Rep Med 3:100582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppner KM, Piechowski CL, Muller A, Ottaway N, Sisley S, Smiley DL, Habegger KM, Pfluger PT, Dimarchi R, Biebermann H, Tschop MH, Sandoval DA, Perez-Tilve D (2014) Both acyl and des-acyl ghrelin regulate adiposity and glucose metabolism via central nervous system ghrelin receptors. Diabetes 63:122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam MN, Mita Y, Maruyama K, Tanida R, Zhang W, Sakoda H, Nakazato M (2020) Liver-expressed antimicrobial peptide 2 antagonizes the effect of ghrelin in rodents. J Endocrinol 244:13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salome N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A 106:11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E, Landgren S, Egecioglu E, Dickson SL, Engel JA (2011) The alcohol-induced locomotor stimulation and accumbal dopamine release is suppressed in ghrelin knockout mice. Alcohol 45:341–347. [DOI] [PubMed] [Google Scholar]
- Jung SK, Kim MK, Shin J, Choi BY (2013) A cross-sectional analysis of the relationship between daily alcohol consumption and serum adiponectin levels among adults aged 40 years or more in a rural area of Korea. Eur J Clin Nutr 67:841–847. [DOI] [PubMed] [Google Scholar]
- Kang L, Chen X, Sebastian BM, Pratt BT, Bederman IR, Alexander JC, Previs SF, Nagy LE (2007) Chronic ethanol and triglyceride turnover in white adipose tissue in rats: inhibition of the anti-lipolytic action of insulin after chronic ethanol contributes to increased triglyceride degradation. J Biol Chem 282:28465–28473. [DOI] [PubMed] [Google Scholar]
- Kaur S, Ryabinin AE (2010) Ghrelin receptor antagonism decreases alcohol consumption and activation of perioculomotor urocortin-containing neurons. Alcohol Clin Exp Res 34:1525–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DJ, Yoon SJ, Choi B, Kim TS, Woo YS, Kim W, Myrick H, Peterson BS, Choi YB, Kim YK, Jeong J (2005) Increased fasting plasma ghrelin levels during alcohol abstinence. Alcohol Alcohol 40:76–79. [DOI] [PubMed] [Google Scholar]
- Kim JH, Kim SJ, Lee WY, Cheon YH, Lee SS, Ju A, K M, Kim DJ (2013) The effects of alcohol abstinence on BDNF, ghrelin, and leptin secretions in alcohol-dependent patients with glucose intolerance. Alcohol Clin Exp Res 37 Suppl 1:E52–58. [DOI] [PubMed] [Google Scholar]
- Koopmann A, Bach P, Schuster R, Bumb JM, Vollstadt-Klein S, Reinhard I, Rietschel M, Witt SH, Wiedemann K, Kiefer F (2019) Ghrelin modulates mesolimbic reactivity to alcohol cues in alcohol-addicted subjects: a functional imaging study. Addict Biol 24:1066–1076. [DOI] [PubMed] [Google Scholar]
- Koopmann A, von der Goltz C, Grosshans M, Dinter C, Vitale M, Wiedemann K, Kiefer F (2012) The association of the appetitive peptide acetylated ghrelin with alcohol craving in early abstinent alcohol dependent individuals. Psychoneuroendocrinology 37:980–986. [DOI] [PubMed] [Google Scholar]
- Kraus T, Schanze A, Groschl M, Bayerlein K, Hillemacher T, Reulbach U, Kornhuber J, Bleich S (2005) Ghrelin levels are increased in alcoholism. Alcohol Clin Exp Res 29:2154–2157. [DOI] [PubMed] [Google Scholar]
- Landgren S, Engel JA, Hyytia P, Zetterberg H, Blennow K, Jerlhag E (2011) Expression of the gene encoding the ghrelin receptor in rats selected for differential alcohol preference. Behav Brain Res 221:182–188. [DOI] [PubMed] [Google Scholar]
- Landgren S, Jerlhag E, Hallman J, Oreland L, Lissner L, Strandhagen E, Thelle DS, Zetterberg H, Blennow K, Engel JA (2010) Genetic variation of the ghrelin signaling system in females with severe alcohol dependence. Alcohol Clin Exp Res 34:1519–1524. [DOI] [PubMed] [Google Scholar]
- Landgren S, Jerlhag E, Zetterberg H, Gonzalez-Quintela A, Campos J, Olofsson U, Nilsson S, Blennow K, Engel JA (2008) Association of pro-ghrelin and GHS-R1A gene polymorphisms and haplotypes with heavy alcohol use and body mass. Alcohol Clin Exp Res 32:2054–2061. [DOI] [PubMed] [Google Scholar]
- Landgren S, Simms JA, Hyytia P, Engel JA, Bartlett SE, Jerlhag E (2012) Ghrelin receptor (GHS-R1A) antagonism suppresses both operant alcohol self-administration and high alcohol consumption in rats. Addict Biol 17:86–94. [DOI] [PubMed] [Google Scholar]
- Lee BP, Vittinghoff E, Dodge JL, Cullaro G, Terrault NA (2019) National Trends and Long-term Outcomes of Liver Transplant for Alcohol-Associated Liver Disease in the United States. JAMA Intern Med 179:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Farokhnia M, Cobbina E, Saravanakumar A, Li X, Battista JT, Farinelli LA, Akhlaghi F, Leggio L (2020a) Endocrine effects of the novel ghrelin receptor inverse agonist PF-5190457: Results from a placebo-controlled human laboratory alcohol co-administration study in heavy drinkers. Neuropharmacology 170:107788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Tapocik JD, Ghareeb M, Schwandt ML, Dias AA, Le AN, Cobbina E, Farinelli LA, Bouhlal S, Farokhnia M, Heilig M, Akhlaghi F, Leggio L (2020b) The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry 25:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, Nesci A, Miceli A, Malandrino N, Capristo E, Canestrelli B, Monteleone P, Kenna GA, Swift RM, Addolorato G (2012) Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol 17:452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Lee MR (2017) Treatment of Alcohol Use Disorder in Patients with Alcoholic Liver Disease. Am J Med 130:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Schwandt ML, Oot EN, Dias AA, Ramchandani VA (2013) Fasting-induced increase in plasma ghrelin is blunted by intravenous alcohol administration: a within-subject placebo-controlled study. Psychoneuroendocrinology 38:3085–3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA (2014) Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry 76:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HX, Lu XJ, Li CH, Chen J (2015) Molecular characterization of the liver-expressed antimicrobial peptide 2 (LEAP-2) in a teleost fish, Plecoglossus altivelis: antimicrobial activity and molecular mechanism. Mol Immunol 65:406–415. [DOI] [PubMed] [Google Scholar]
- Li Z, Xu G, Qin Y, Zhang C, Tang H, Yin Y, Xiang X, Li Y, Zhao J, Mulholland M, Zhang W (2014) Ghrelin promotes hepatic lipogenesis by activation of mTOR-PPARgamma signaling pathway. Proc Natl Acad Sci U S A 111:13163–13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber CS (2004) Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol 34:9–19. [DOI] [PubMed] [Google Scholar]
- Lin Y, Liang Z, He L, Yang M, Liu D, Gu HF, Liu H, Zhu Z, Zheng H, Li L, Yang G (2019) Gut ghrelin regulates hepatic glucose production and insulin signaling via a gut-brain-liver pathway. Cell Commun Signal 17:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, McGlone F, Bedrossian D, Dagher A (2008) Ghrelin modulates brain activity in areas that control appetitive behavior. Cell Metab 7:400–409. [DOI] [PubMed] [Google Scholar]
- Mani BK, Puzziferri N, He Z, Rodriguez JA, Osborne-Lawrence S, Metzger NP, Chhina N, Gaylinn B, Thorner MO, Thomas EL, Bell JD, Williams KW, Goldstone AP, Zigman JM (2019a) LEAP2 changes with body mass and food intake in humans and mice. J Clin Invest 129:3909–3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Shankar K, Zigman JM (2019b) Ghrelin’s Relationship to Blood Glucose. Endocrinology 160:1247–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathurin P, Lucey MR (2020) Liver transplantation in patients with alcohol-related liver disease: current status and future directions. Lancet Gastroenterol Hepatol 5:507–514. [DOI] [PubMed] [Google Scholar]
- Mellinger JL (2019) Epidemiology of Alcohol Use and Alcoholic Liver Disease. Clin Liver Dis (Hoboken) 13:136–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao H, Pan H, Wang L, Yang H, Zhu H, Gong F (2019) Ghrelin Promotes Proliferation and Inhibits Differentiation of 3T3-L1 and Human Primary Preadipocytes. Front Physiol 10:1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora M, Adam V, Palomera E, Blesa S, Diaz G, Buquet X, Serra-Prat M, Martin-Escudero JC, Palanca A, Chaves JF, Puig-Domingo M, Mataro Aging Study G (2015) Ghrelin Gene Variants Influence on Metabolic Syndrome Components in Aged Spanish Population. PLoS One 10:e0136931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller TD, Nogueiras R, Andermann ML, Andrews ZB, Anker SD, Argente J, Batterham RL, Benoit SC, Bowers CY, Broglio F, Casanueva FF, D’Alessio D, Depoortere I, Geliebter A, Ghigo E, Cole PA, Cowley M, Cummings DE, Dagher A, Diano S, Dickson SL, Dieguez C, Granata R, Grill HJ, Grove K, Habegger KM, Heppner K, Heiman ML, Holsen L, Holst B, Inui A, Jansson JO, Kirchner H, Korbonits M, Laferrere B, LeRoux CW, Lopez M, Morin S, Nakazato M, Nass R, Perez-Tilve D, Pfluger PT, Schwartz TW, Seeley RJ, Sleeman M, Sun Y, Sussel L, Tong J, Thorner MO, van der Lely AJ, van der Ploeg LH, Zigman JM, Kojima M, Kangawa K, Smith RG, Horvath T, Tschop MH (2015) Ghrelin. Mol Metab 4:437–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishise Y, Saito T, Makino N, Okumoto K, Ito JI, Watanabe H, Saito K, Togashi H, Ikeda C, Kubota I, Daimon M, Kato T, Fukao A, Kawata S (2010) Relationship between alcohol consumption and serum adiponectin levels: the Takahata study--a cross-sectional study of a healthy Japanese population. J Clin Endocrinol Metab 95:3828–3835. [DOI] [PubMed] [Google Scholar]
- Ohashi K, Pimienta M, Seki E (2018) Alcoholic liver disease: A current molecular and clinical perspective. Liver Res 2:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott V, Fasshauer M, Dalski A, Meier B, Perwitz N, Klein HH, Tschop M, Klein J (2002) Direct peripheral effects of ghrelin include suppression of adiponectin expression. Horm Metab Res 34:640–645. [DOI] [PubMed] [Google Scholar]
- Pabalan NA, Seim I, Jarjanazi H, Chopin LK (2014) Associations between ghrelin and ghrelin receptor polymorphisms and cancer in Caucasian populations: a meta-analysis. BMC Genet 15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price ML, Ley CD, Gorvin CM (2021) The emerging role of heterodimerisation and interacting proteins in ghrelin receptor function. J Endocrinol 252:R23–R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevski E, Horvath TL, Shanabrough M, Hayden R, Newcomb J, Petrakis I (2017) Ghrelin is Supressed by Intravenous Alcohol and is Related to Stimulant and Sedative Effects of Alcohol. Alcohol Alcohol 52:431–438. [DOI] [PubMed] [Google Scholar]
- Rasineni K, Kubik JL, Casey CA, Kharbanda KK (2019a) Inhibition of Ghrelin Activity by Receptor Antagonist [d-Lys-3] GHRP-6 Attenuates Alcohol-Induced Hepatic Steatosis by Regulating Hepatic Lipid Metabolism. Biomolecules 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasineni K, Kubik JL, Knight KL, Hall L, Casey CA, Kharbanda KK (2020) Ghrelin regulates adipose tissue metabolism: Role in hepatic steatosis. Chem Biol Interact 322:109059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasineni K, Thomes PG, Kubik JL, Harris EN, Kharbanda KK, Casey CA (2019b) Chronic alcohol exposure alters circulating insulin and ghrelin levels: role of ghrelin in hepatic steatosis. Am J Physiol Gastrointest Liver Physiol 316:G453–G461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehm J, Probst C, Shield KD, Shuper PA (2017) Does alcohol use have a causal effect on HIV incidence and disease progression? A review of the literature and a modeling strategy for quantifying the effect. Popul Health Metr 15:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringuet MT, Furness JB, Furness SGB (2021) G protein-coupled receptor interactions and modification of signalling involving the ghrelin receptor, GHSR1a. J Neuroendocrinol:e13077. [DOI] [PubMed] [Google Scholar]
- Salmeron C, Johansson M, Asaad M, Angotzi AR, Ronnestad I, Stefansson SO, Jonsson E, Bjornsson BT, Gutierrez J, Navarro I, Capilla E (2015) Roles of leptin and ghrelin in adipogenesis and lipid metabolism of rainbow trout adipocytes in vitro. Comp Biochem Physiol A Mol Integr Physiol 188:40–48. [DOI] [PubMed] [Google Scholar]
- Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806. [DOI] [PubMed] [Google Scholar]
- Sangiao-Alvarellos S, Vazquez MJ, Varela L, Nogueiras R, Saha AK, Cordido F, Lopez M, Dieguez C (2009) Central ghrelin regulates peripheral lipid metabolism in a growth hormone-independent fashion. Endocrinology 150:4562–4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott-Sheldon LA, Carey KB, Cunningham K, Johnson BT, Carey MP, Team MR (2016) Alcohol Use Predicts Sexual Decision-Making: A Systematic Review and Meta-Analysis of the Experimental Literature. AIDS Behav 20 Suppl 1:S19–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha L, Dey P, Khess CR, Khitiz KK (2021) The association of plasma acyl ghrelin level with alcohol craving in early abstinent alcohol dependent patients. J Postgrad Med 67:12–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shawcross DL, O’Grady JG (2010) The 6-month abstinence rule in liver transplantation. Lancet 376:216–217. [DOI] [PubMed] [Google Scholar]
- Shevchouk OT, Tufvesson-Alm M, Jerlhag E (2021) An Overview of Appetite-Regulatory Peptides in Addiction Processes; From Bench to Bed Side. Front Neurosci 15:774050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sovetkina A, Nadir R, Fung JNM, Nadjarpour A, Beddoe B (2020) The Physiological Role of Ghrelin in the Regulation of Energy and Glucose Homeostasis. Cureus 12:e7941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson JR, Buirkle JM, Buckley LE, Young KA, Albertini KM, Bohidar AE (2015) GHS-R1A antagonism reduces alcohol but not sucrose preference in prairie voles. Physiol Behav 147:23–29. [DOI] [PubMed] [Google Scholar]
- Stevenson JR, Francomacaro LM, Bohidar AE, Young KA, Pesarchick BF, Buirkle JM, McMahon EK, O’Bryan CM (2016) Ghrelin receptor (GHS-R1A) antagonism alters preference for ethanol and sucrose in a concentration-dependent manner in prairie voles. Physiol Behav 155:231–236. [DOI] [PubMed] [Google Scholar]
- Suchankova P, Engel JA, Jerlhag E (2016a) Sub-chronic Ghrelin Receptor Blockade Attenuates Alcohol- and Amphetamine-Induced Locomotor Stimulation in Mice. Alcohol Alcohol 51:121–127. [DOI] [PubMed] [Google Scholar]
- Suchankova P, Jerlhag E, Jayaram-Lindstrom N, Nilsson S, Toren K, Rosengren A, Engel JA, Franck J (2013a) Genetic variation of the ghrelin signalling system in individuals with amphetamine dependence. PLoS One 8:e61242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Nilsson S, von der Pahlen B, Santtila P, Sandnabba K, Johansson A, Jern P, Engel JA, Jerlhag E (2016b) Genetic variation of the growth hormone secretagogue receptor gene is associated with alcohol use disorders identification test scores and smoking. Addict Biol 21:481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Steensland P, Fredriksson I, Engel JA, Jerlhag E (2013b) Ghrelin receptor (GHS-R1A) antagonism suppresses both alcohol consumption and the alcohol deprivation effect in rats following long-term voluntary alcohol consumption. PLoS One 8:e71284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchankova P, Yan J, Schwandt ML, Stangl BL, Jerlhag E, Engel JA, Hodgkinson CA, Ramchandani VA, Leggio L (2017) The Leu72Met Polymorphism of the Prepro-ghrelin Gene is Associated With Alcohol Consumption and Subjective Responses to Alcohol: Preliminary Findings. Alcohol Alcohol 52:425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theander-Carrillo C, Wiedmer P, Cettour-Rose P, Nogueiras R, Perez-Tilve D, Pfluger P, Castaneda TR, Muzzin P, Schurmann A, Szanto I, Tschop MH, Rohner-Jeanrenaud F (2006) Ghrelin action in the brain controls adipocyte metabolism. J Clin Invest 116:1983–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townes CL, Michailidis G, Hall J (2009) The interaction of the antimicrobial peptide cLEAP-2 and the bacterial membrane. Biochem Biophys Res Commun 387:500–503. [DOI] [PubMed] [Google Scholar]
- Ukkola O, Ravussin E, Jacobson P, Perusse L, Rankinen T, Tschop M, Heiman ML, Leon AS, Rao DC, Skinner JS, Wilmore JH, Sjostrom L, Bouchard C (2002) Role of ghrelin polymorphisms in obesity based on three different studies. Obes Res 10:782–791. [DOI] [PubMed] [Google Scholar]
- Vadnie CA, Park JH, Abdel Gawad N, Ho AM, Hinton DJ, Choi DS (2014) Gut-brain peptides in corticostriatal-limbic circuitry and alcohol use disorders. Front Neurosci 8:288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard ET, Djurhuus CB, Gjedsted J, Nielsen S, Moller N, Holst JJ, Jorgensen JO, Schmitz O (2008a) Acute effects of ghrelin administration on glucose and lipid metabolism. J Clin Endocrinol Metab 93:438–444. [DOI] [PubMed] [Google Scholar]
- Vestergaard ET, Gormsen LC, Jessen N, Lund S, Hansen TK, Moller N, Jorgensen JO (2008b) Ghrelin infusion in humans induces acute insulin resistance and lipolysis independent of growth hormone signaling. Diabetes 57:3205–3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackernah RC, Minnick MJ, Clapp P (2014) Alcohol use disorder: pathophysiology, effects, and pharmacologic options for treatment. Subst Abuse Rehabil 5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Shi X, Zhong W, Zhao Y, Tang Y, Sun W, Yin X, Bogdanov B, Kim S, McClain C, Zhou Z, Zhang X (2013) Chronic alcohol exposure disturbs lipid homeostasis at the adipose tissue-liver axis in mice: analysis of triacylglycerols using high-resolution mass spectrometry in combination with in vivo metabolite deuterium labeling. PLoS One 8:e55382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittekind DA, Kratzsch J, Mergl R, Enzenbach C, Witte AV, Villringer A, Kluge M (2018) Alcohol consumption is positively associated with fasting serum ghrelin in non-dependent adults: Results from the population-based LIFE-Adult-Study. Psychoneuroendocrinology 97:143–148. [DOI] [PubMed] [Google Scholar]
- Wortley KE, del Rincon JP, Murray JD, Garcia K, Iida K, Thorner MO, Sleeman MW (2005) Absence of ghrelin protects against early-onset obesity. J Clin Invest 115:3573–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X, Bi M, Jiao Q, Chen X, Du X, Jiang H (2020) A new understanding of GHSR1a--independent of ghrelin activation. Ageing Res Rev 64:101187. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, Nagao M, Watanabe Y, Yamaguchi T, Ueda S, Kitamura Y, Nishimura K, Inden M, Marunaka Y, Hattori H, Murakami K, Tokaji M, Ochi K (2017) Enhanced alcohol-drinking behavior associated with active ghrelinergic and serotoninergic neurons in the lateral hypothalamus and amygdala. Pharmacol Biochem Behav 153:1–11. [DOI] [PubMed] [Google Scholar]
- You M, Considine RV, Leone TC, Kelly DP, Crabb DW (2005) Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42:568–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakhari S, Li TK (2007) Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology 46:2032–2039. [DOI] [PubMed] [Google Scholar]
- Zallar LJ, Beurmann S, Tunstall BJ, Fraser CM, Koob GF, Vendruscolo LF, Leggio L (2019) Ghrelin receptor deletion reduces binge-like alcohol drinking in rats. J Neuroendocrinol 31:e12663. [DOI] [PubMed] [Google Scholar]
- Zallar LJ, Farokhnia M, Tunstall BJ, Vendruscolo LF, Leggio L (2017) The Role of the Ghrelin System in Drug Addiction. Int Rev Neurobiol 136:89–119. [DOI] [PubMed] [Google Scholar]
- Zhang S, Mao Y, Fan X (2018) Inhibition of ghrelin o-acyltransferase attenuated lipotoxicity by inducing autophagy via AMPK-mTOR pathway. Drug Des Devel Ther 12:873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Zhao L, Lin TR, Chai B, Fan Y, Gantz I, Mulholland MW (2004) Inhibition of adipogenesis by ghrelin. Mol Biol Cell 15:2484–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhai L, Rong C, Qin X, Li S (2015) Association of Ghrelin Gene Polymorphisms and Serum Ghrelin Levels with the Risk of Hepatitis B Virus-Related Liver Diseases in a Chinese Population. PLoS One 10:e0143069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK (2006) Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol 494:528–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann US, Buchmann A, Steffin B, Dieterle C, Uhr M (2007) Alcohol administration acutely inhibits ghrelin secretion in an experiment involving psychosocial stress. Addict Biol 12:17–21. [DOI] [PubMed] [Google Scholar]


