Abstract
Surface proteins of Streptococcus pyogenes are important virulence factors. Here we describe a novel collagen-like surface protein, designated SclA (streptococcal collagen-like surface protein). The sclA gene was identified in silico using the Streptococcal Genome Sequencing Project with the recently identified protein GRAB as the probe. SclA has a signal sequence and a cell wall attachment region containing the prototypic LPXTGX motif. The surface-exposed part of SclA contains a unique NH2-terminal domain of 73 amino acids, followed by a collagen-like region. The sclA gene was found to be positively regulated by Mga, a transcriptional activator of several S. pyogenes virulence determinants. A mutant lacking cell wall-associated SclA was constructed and was found to be as effective as wild-type bacteria in platelet aggregation, survival in fresh human blood, and adherence to pharyngeal cells. The sclA gene was found in all 12 S. pyogenes strains that were investigated using PCR. Sequence analysis revealed that the signal sequence and the cell wall attachment region are highly conserved. The collagen-like domain is variable in its NH2-terminal region and has conserved repeated domains in its COOH-terminal part. SclA proteins from most strains have additional proline-rich repeats spacing the collagen-like domain and the cell wall attachment sequence. The unique NH2-terminal region is hypervariable, but computer predictions indicate a common secondary structure, with two alpha helices connected by a loop region. Immune selection may explain the hypervariability in the NH2-terminal region, whereas the preserved secondary structure implies that this region has a common function. These features and the Mga regulation are shared with the M protein of S. pyogenes. Moreover, as with the gene encoding the M protein, phylogenetic analysis indicates that horizontal gene transfer has contributed to the evolution of sclA.
Streptococcus pyogenes is an important pathogen causing the common diseases pharyngitis, erysipelas, and impetigo. Sometimes this organism causes severe invasive diseases, such as a toxic shock-like syndrome and necrotizing fasciitis. Nonsuppurative sequelae following infection with S. pyogenes include acute rheumatic fever and poststreptococcal glomerulonephritis. The cell wall-anchored proteins of S. pyogenes are regarded as major virulence determinants and share common features, including a signal sequence and a cell wall attachment signal containing a so-called LPXTGX motif (39). Among the best-characterized and most important cell wall-attached proteins are the alpha-helical coiled-coil M and M-like proteins (see references 15 and 51). Each S. pyogenes strain carries one to three genes encoding M or M-like proteins, and these genes are colocated on the chromosome with the mga gene encoding their positive regulator, Mga (7, 22, 36, 42, 45). The scpA gene encoding the cell wall-attached C5a-peptidase is also located at this chromosomal site (44, 60). The M and M-like proteins bind several plasma proteins, such as fibrinogen (30), IgA (37), IgG (1, 20), serum albumin (16, 50, 56), plasminogen (4), kininogens (3), and regulatory proteins of the complement system (23, 59). The binding sites for these ligands are found both in the conserved COOH- and in the variable NH2-terminal regions of the protein (39). The binding activities are probably important for the antiphagocytic properties of M proteins. Other cell wall-anchored proteins in S. pyogenes containing the LPXTGX motif include the fibronectin binding protein F (Sfb1) and PFBP (18, 52, 58), the fibronectin binding apolipoproteinase serum opacity factor (47, 55), and the α2-macroglobulin binding protein GRAB (48). In this work we describe a novel and widespread gene, regulated by Mga, encoding a cell wall-anchored protein of S. pyogenes. SclA (streptococcal collagen-like surface protein) has a typical cell wall anchor and signal sequence, whereas the surface-exposed part of the molecule comprises a region with homology to collagen and a hypervariable NH2-terminal part.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
S. pyogenes strains designated AP are from the Institute of Hygiene and Epidemiology, Prague, Czech Republic. The KTL3 and KTL9 strains are blood isolates from the Finnish Institute for Health. The SF370 strain is the ATCC 700294 strain. BMJ71 is an isogenic AP1 mutant containing a Tn916 insertion within mga (33). BMJ71pMGA.1 is a derivate of BMJ71 containing mga on a plasmid (33). For molecular cloning purposes, the BL21 or the DH5α strain of Escherichia coli was used. Streptococci were grown in Todd-Hewitt broth (Difco, Detroit, Mich.) with 0.2% yeast extract (THY) (Difco) in 5% CO2 at 37°C. For growth of mga mutants, appropriate antibiotics were added (33). E. coli strains were grown in Luria Bertani broth (10 g of tryptone [Difco]/liter, 10 g of NaCl/liter, 5 g of yeast extract [Difco]/liter). For growth on Petri dishes, 15 g of bacto agar (Difco)/liter was added. When E. coli contained a plasmid, either 100 μg of ampicillin (Sigma, St. Louis, Mo.)/ml or 50 μg of kanamycin (Sigma)/ml was added to the medium.
PCR, cloning procedures, and sequencing.
Genomic DNA was prepared from S. pyogenes as described previously (43) with modifications as described previously (48). PCR was performed using Taq polymerase (Gibco-BRL, Gaithersburg, Md.) and synthetic oligonucleotides hybridizing to sclA. Primers hybridized to the following nucleotides found in the Streptococcal Genome Sequencing database (the introduced restriction site and the position of the site are given in parentheses): F1, nucleotide (nt) 199208-199236; F2, nt 199372-199400 (BamHI at position 199377-199383); F3, nt 199430-199455 (BamHI at position 199436-199442); R1, nt 200120-200100 (HindIII at position 200114-200109); and R2, 200206-200183 (XhoI at position 200201-200196). Restriction enzymes and ligase were from Gibco-BRL, and standard ligation, transformation, and plasmid isolation methods were used (54). PCR products were generated using primers F1 and R2, and DNA sequences were determined using an ABI-470 prism and Taq-dyed dideoxy terminator kit (Perkin and Elmer, Norwalk, Conn.). For expression cloning, primers F2 and R2 were used in PCR with AP1 DNA, and the restriction enzyme-cleaved product was ligated into the corresponding site of pGEX-5X-3 (Pharmacia Biotech, Uppsala, Sweden). The resulting plasmid was transformed into BL21 bacteria, which upon induction with 0.5 mM isopropyl-β-thiogalactopyranoside (Promega) produced a fusion protein between glutathione S-transferase (GST) and SclA. The fusion protein was purified by affinity chromatography according to the instructions of the manufacturer. Sequencing of the plasmid insert confirmed that the correct sequence had been cloned. To generate a mutant devoid of SclA on its surface, a fragment of sclA, lacking the part encoding the putative cell wall attachment region, was generated by PCR from the AP1 strain using primers F3 and R1. The fragment was treated with appropriate restriction enzyme, ligated into the streptococcal suicide plasmid pFW13 (46), a kind gift from Andreas Podbielski, to generate pFW-sclA, and transformed into DH5α. The plasmid was purified, and 2 μg of the plasmid was electroporated into the AP1 strain (19) for homologous recombination to occur. Transformants were plated on THY plus 15 g of agar/liter with 150 μg of kanamycin/ml.
Blotting techniques.
Twenty micrograms of chromosomal DNA, purified as described above, was cleaved by HindIII, separated by agarose gel electrophoresis, and blotted onto Hybond-N filters (Amersham, Amersham, United Kingdom) using standard protocols (54). Total RNA from S. pyogenes was purified using a Fastprep cell disrupter (Savant, Holbrok, N.Y.) as previously described (9). Streptococci were cultured in THY medium to an optical density at 620 nm (OD620) of 0.3 (early logarithmic phase), an OD620 of 0.6 (late logarithmic phase), or an OD620 of 0.8 (early stationary phase) before harvest by centrifugation at 3,800 × g for 10 min at 4°C. Pellets were resuspended in water, followed by disruption for 20 s (2 times) at setting 6.0 using a FastRNA kit with glass beads (BIO 101, Vista, Calif.) according to the instructions from the manufacturers. For Northern blot experiments, 5 μg of total RNA was separated on 1% agarose in 1× HEPES buffer (0.2 M Na-HEPES [pH 7.0], 50 mM NaAc, 10 mM EDTA) containing 2.3 M formaldehyde and blotted onto Hybond-N. RNA or DNA was cross-linked to the filter using a Spectrolinker XL-1000 UV Crosslinker, prehybridized for 2 h, and hybridized overnight at 50°C with a probe generated by PCR from AP1 using primers F2 and R2 and labeled as described (48). This probe hybridizes with the part of sclA encoding the A, CLR, and W regions (see Fig. 1). Filters were also hybridized with a probe constructed from a 16S ribosomal RNA sequence of S. pyogenes (48). After hybridization, the membranes were washed in 6 −0.1× SSC–0.1% sodium dodecyl sulfate (SDS) (1× SSC is 0.15 M NaCl plus 0.115 M sodium citrate), followed by exposure of a BAS-III imaging plate and scanning with a Bio-Imaging Analyzer BAS-2000 (Fuji Photo Films Co. Ltd.). Intensities of bands were calculated using the Image Gauche program.
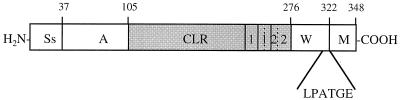
FIG. 1.
Schematic representation of the SclA protein from strain SF370. A putative signal sequence (Ss) is followed by an A domain and a CLR in gray. The CLR contains two partially overlapping repeats, denoted by 1 and 2. In the COOH-terminal part is a putative cell wall-spanning domain (W), including the typical LPXTGX motif and a hydrophobic membrane-spanning domain (M).
Antiserum, protein separation, and Western blotting.
Rabbit antiserum against SclA was generated by injecting 50 μg of purified GST-SclA protein with an equal volume of Freunds adjuvant (1/3 complete and 2/3 incomplete) subcutaneously into rabbits. This was repeated after 4 weeks, blood was drawn 6 weeks after the first immunization, and serum was prepared. Proteins were separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE) (40) and transferred to a Protane nitrocellulose filter (Schleicher & Shuell, Dassel, Germany) using a Trans-blot semidry transfer cell (Bio-Rad, Hercules, Calif.). Filters were blocked using phosphate-buffered saline with 0.05% Tween 20 and 5% dry milk powder (blocking buffer), followed by incubation of the filter with antiserum (1:5,000) in 5 ml of the same buffer for 30 min at 37°C. Membranes were washed 3 times for 10 min using phosphate-buffered saline with 0.05% Tween 20 and 0.5 M NaCl, followed by incubation of the filter with a peroxidase-conjugated antibody against rabbit immunoglobulin (1:3,000) in blocking buffer. Filters were washed as described above, and detection of bound antibodies was performed with the chemiluminescence method.
Other methods.
The tBLASTn service (www.genome.ou.edu/strep.html) was used to search the database of the Streptococcal Genome Sequencing Project. Other homology searches were made using the BLAST 2.0 program (www.ncbi.nlm.nih.gov). Signal sequence predictions were made using the SignalP V1.1 program (www.cbs.dtu.dk) (41). Phylogenetic analyses of the NH2-terminal region of the M protein and the A domain of SclA were performed (http: //bioweb.pasteur.fr) using parsimony analysis. Secondary structure and pI predictions were performed using MacVector (Oxford Molecular Ltd., version 6.5). Platelet aggregonometry was performed as described previously (35). Adherence of streptococci to the human pharynx carcinoma cell line Detroit 562 (ATCC CCL-138) was tested as described previously (5). Proteins were precipitated by incubation with 6% trichloroacetic acid (TCA) for 30 min on ice, followed by centrifugation at 15,000 × g (4°C for 20 min).
Nucleotide sequence accession numbers.
The sclA and SclA sequences have been deposited in GenBank under accession no. AF296329 to AF296339.
RESULTS
Identification of a novel collagen-like surface protein (SclA) in S. pyogenes.
Cell wall-anchored proteins of gram-positive bacteria share common structural features, including an LPXTGX motif, a cell membrane-spanning hydrophobic region, and a short charged intracellular tail (for a review see reference 39). To identify open reading frames encoding putative cell wall-anchored proteins, a tBLASTn search against the Streptococcal Genome Sequencing Project was performed using the W and M regions of the recently discovered protein GRAB (48) as the query sequence. Besides grab, this search revealed three open reading frames encoding proteins with an LPXTGX motif, followed by a hydrophobic sequence and a short charged sequence. One of the predicted proteins lacked a putative signal sequence, while the second was similar to an alkaline amylopullolanase from Bacillus spp. The third open reading frame (positions 199241 to 200284 in the Streptococcal Genome Sequencing Project database) encodes a protein with similarities to collagen, and the present work is focused on this streptococcal collagen-like surface protein (SclA).
A schematic representation of the SclA protein is shown in Fig. 1. In the NH2-terminal region is a putative 37-amino-acid (aa)-long signal sequence as predicted from the signalP program. This region is followed by a 73-aa A domain with no significant similarity to any other protein. The collagen-like region (CLR) consists of 171 amino acid residues with a glycine in every third position and is rich in proline residues (10%). In the COOH-terminal part of the CLR are two partially overlapping repeated motifs. The type 1 repeats are 15 aa in length and are composed entirely of collagen-like GXY units, while the type 2 repeat is 16 aa and consists predominantly of GXY units. Each pair of repeats contains a single mismatch. The CLR is similar to several types of collagen from different species, including human collagens. The CLR is followed by a putative cell wall-spanning domain (W) which includes the LPATGE sequence. The membrane-spanning domain (M) consists of 19 hydrophobic residues followed by 6 charged residues in the extreme COOH-terminal region. The W and M regions are similar to the corresponding domains of several M proteins. The mature SclA protein has a calculated molecular mass of 29 kDa and an estimated pI of 5.26.
The sclA gene is transcriptionally controlled by Mga.
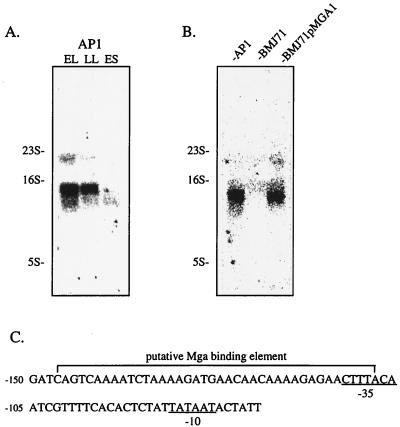
The expression of sclA was investigated using Northern blotting, in which total RNA from the AP1 strain was prepared at different stages of growth. Maximal expression of sclA was seen in early logarithmic growth phase, with lower amounts of transcript in late logarithmic growth phase (Fig. 2A). In early stationary phase, almost no sclA transcript was detected. The same filter was hybridized with a 16S probe, showing that the same amount of RNA had been applied to each well (data not shown). Several surface proteins implicated in the virulence of S. pyogenes are transcriptionally activated by a protein termed Mga (7, 42, 45). An isogenic mutant of AP1, with a transposon insertion within mga, was therefore tested for sclA expression, and it was found that this mutant (BMJ71) was devoid of the sclA transcript in three independent experiments (Fig. 2B). The expression of sclA was almost completely restored in the BMJ71pMGA.1 strain (77 and 92% of the AP1 level in two separate experiments), which harbors mga on a plasmid (Fig. 2B). Again, the filter shown in Fig. 2B was hybridized with a 16S probe, confirming that the same amount of RNA had been applied to each well (data not shown). The Mga protein exerts its effects by binding to a so-called Mga-binding element, and the consensus sequence for this element (38) was compared to the DNA sequence upstream from sclA. Indeed, a DNA sequence was identified for which 34 out of 40 nt were identical to the consensus sequence. This sequence was found 108 to 147 bp upstream from the start codon of sclA (Fig. 2C). Moreover, putative −35 and −10 boxes, similar to those described for the emm and scpA genes (38), were located within and downstream from this element, respectively (Fig. 2C).
FIG. 2.
(A) Total RNA was obtained from the AP1 strain at early logarithmic growth phase (EL), late logarithmic growth phase (LL), and early stationary phase (ES). RNA was subjected to Northern blotting using a probe hybridizing with sclA. A part of the gel was stained with ethidium bromide to visualize rRNA bands, used as molecular weight markers. (B) Total RNA from AP1, BMJ71, and BMJ71pMGA.1 was prepared from bacteria in early logarithmic growth phase and subjected to Northern blotting using the same probe as for panel A. (C) Representation of the putative Mga-binding element located at −107 to −147 bp from the start codon of sclA. Putative −35 and −10 boxes are indicated.
Generation and characterization of a mutant lacking surface-associated SclA.
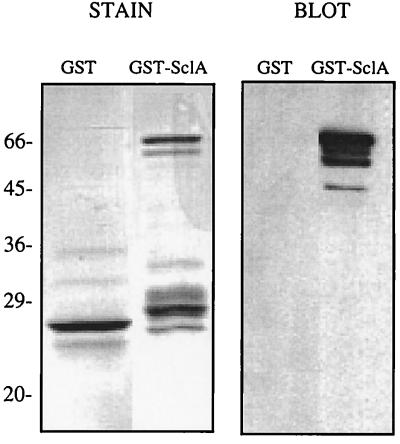
The entire mature SclA (aa 37 to 322 in Fig. 1) was expressed as a fusion protein with GST. The protein could be purified in small quantities and had a tendency to precipitate. The GST-SclA protein migrated with an apparent size of approximately 65 kDa, which is somewhat larger than expected (Fig. 3). However, it is common for the sizes of cell wall proteins from gram-positive bacteria to be overestimated by SDS-PAGE (31, 48). The fusion protein was used to immunize rabbits, and the polyclonal antiserum reacted specifically with the GST-SclA protein fusion (Fig. 3, right panel).
FIG. 3.
The SclA protein was expressed as a GST fusion. GST and GST-SclA samples were separated by SDS-PAGE (12% acrylamide; reducing conditions), and two identical gels were run. One gel was stained with Coomassie brilliant blue (STAIN), and the other was subjected to Western blotting using an antiserum to GST-SclA (BLOT).
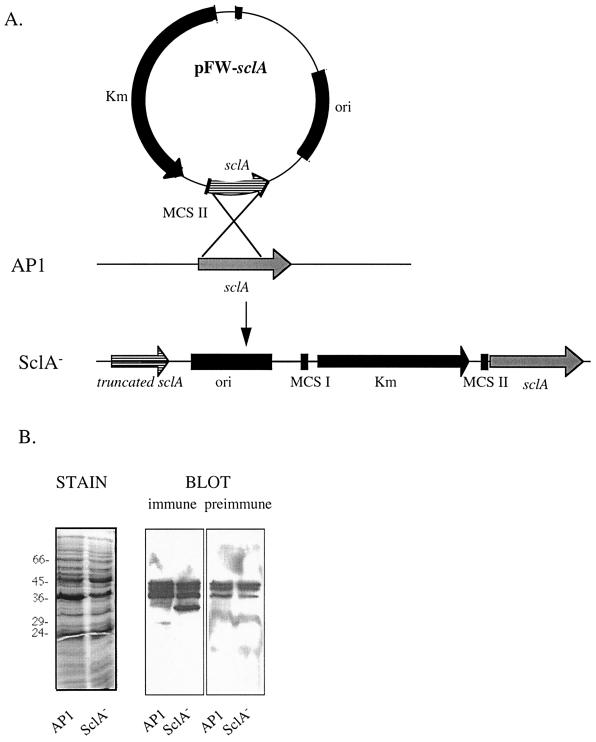
To inactivate sclA, a PCR-generated 692-bp internal fragment (corresponding to aa 74 to 294 in Fig. 1) of sclA lacking the part encoding the cell wall anchoring region was cloned into the pFW13 suicide vector (46) to generate pFW-sclA (Fig. 4A). pFW-sclA was electroporated into AP1 bacteria for homologous recombination (Fig. 4A), and one kanamycine-resistant clone, designated SclA−, was selected for further analysis. Southern blot analysis confirmed that a single crossover event had taken place at the sclA locus (data not shown). With this mutagenesis strategy the mutant should be devoid of surface bound SclA and instead secrete a truncated form (aa 38 to 294 in Fig. 1). Accordingly, when the supernatants from overnight cultures of AP1 and SclA− were precipitated with TCA and subjected to Western blotting using the SclA antiserum, the SclA− strain was found to secrete a protein with an apparent mass of approximately 32 kDa which was not identified in the AP1 medium (Fig. 4B). The other proteins recognized by this antiserum were also recognized by the preimmune serum (Fig. 4B). The predicted size of the SclA protein secreted by SclA− is 27 kDa, but as with the GST-SclA fusion it migrated more slowly in SDS-PAGE. SclA− and AP1 bacteria had similar growth characteristics in THY medium and showed identical binding of fibrinogen and immunoglobulin G (data not shown). Binding of glycoproteins, such as thyroglobulin, to S. pyogenes is mediated by an Mga-regulated protein distinct from the M protein (26). Binding of radiolabeled thyroglobulin to the SclA− mutant was found to be identical to that of MC25 (an M-negative mutant of AP1, generated using pFW13) (11), while the level of binding to BMJ71 was much lower (data not shown). SclA− as well as AP1 survived in fresh human blood and showed identical adherence to a pharyngeal cell line (data not shown). Since several reports have shown that a collagen-like surface protein of Streptococcus sanguis induces aggregation of human platelets (12, 13), the SclA− strain was compared with AP1 in a platelet aggregonometric assay. As was previously reported for other S. pyogenes strains (35), AP1 bacteria were able to induce a complete aggregation of platelets in platelet-rich plasma within less than 1 min. SclA− bacteria were equally effective in inducing aggregation. Moreover, the GST-SclA protein added in concentrations of up to 0.1 mg/ml to platelet-rich plasma was able neither to induce aggregation of platelets nor to influence the aggregation of platelets mediated by AP1 or SclA− bacteria (data not shown).
FIG. 4.
(A) Insertion-duplication mutagenesis was used to delete the part of sclA encoding the membrane-spanning M domain and the COOH-terminal part of the W domain in strain AP1 to generate the SclA− strain. (B) Growth media from AP1 and SclA− were TCA precipitated, and proteins were subjected to SDS-PAGE (12% acrylamide gels; reducing conditions). One gel was stained with Coomassie brilliant blue (STAIN), and two replicas were subjected to Western blotting (BLOT) using SclA antiserum or preimmune serum (dilution, 1:1,000), respectively.
Distribution, sequencing, and genetic analysis of the SclA protein.
The presence of sclA in S. pyogenes strains was investigated by Southern blotting, and all strains (12 of 12) had a single chromosomal fragment reacting with the sclA probe (data not shown). Primers F1 and R2 (see Materials and Methods) were used in PCR to amplify a large fragment of sclA. Depending on the template DNA used, a fragment of 0.9 to 1.5 kb was obtained. These fragments were subjected to sequencing.
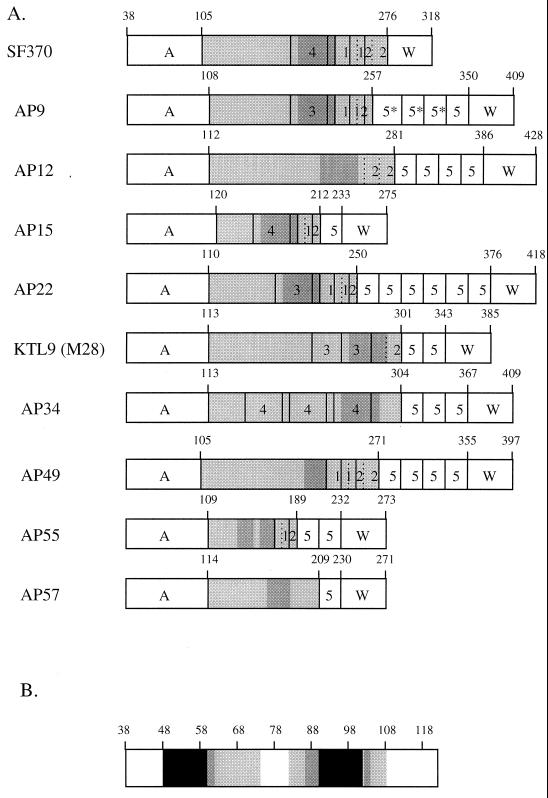
A schematic comparison between the predicted mature SclA protein from the database sequence and the predicted mature SclA proteins from the strains sequenced in this work is shown in Fig. 5A. The SclA proteins from the three M1 strains are identical, while the SclA proteins from different serotypes are divergent. The signal sequences are >95% conserved among the 12 strains (not shown). The A domains, however, differ in sequence, and the identity between the A domains varies between 22 and 70% in a pairwise ClustalW alignment. The A domain of AP15 shows the lowest degree of identity to the other A domains, while the A domains of AP12 and AP49 show the highest degree of identity. The A domains have some features in common, the first of which is a prediction for two alpha helices using two different computer algorithms (10, 17). Figure 5B summarizes the alpha helix predictions for the A domains of SclA from the 10 different serotypes. A region was regarded as helical only when both algorithms predicted it to be so. The predictions indicate that the majority of A domains have one alpha helix from aa 48 to aa 62 and a second alpha helix between aa 86 and 104. The loop region between the two helices is 19 ± 4.5 aa (mean ± the standard deviation), excluding the A domains of M1 and AP15. The A domain of the SclA protein from M1 bacteria is predicted to have a short helix within the loop region, while the A domain of SclA from AP15 has three predicted helices. The second striking feature of the A domain is the high content of aromatic amino acids in the loop region spacing the two helices: 28% ± 7% as compared to 11% ± 3% for the entire A domain (mean ± the standard deviation). None of the A domains showed any significant similarity to sequences deposited in databases.
FIG. 5.
(A) Schematic comparison of predicted mature SclA proteins from 10 S. pyogenes strains. Amino acid positions are indicated. The unique A domain is followed by the CLR, depicted in light gray. Four types of repeats, denoted by 1, 2, 3, and 4, are present in the CLRs. A region of >95% identity is represented by the dark gray shade. The type 5 repeat is a proline-rich repeat of 21 aa, while the 5* repeats are variants of type 5 with 2 to 5 extra amino acids. In the COOH-terminal part is the conserved wall-spanning region (W) containing the LPATGE motif. (B) Schematic depiction of the two alpha helices in the A domain from the SclA protein of 10 different M serotypes. Amino acid numbers are given on top of the box. The black color indicates that 9 or 10 of the A domains had a helical prediction in the area, the dark gray that 6 to 8 A domains were predicted to be helical, the light gray that 3 to 5 were predicted to be helical, and the white that 0 to 2 were predicted to be helical. Only regions where both predictions indicated alpha helices were regarded as helical.
The CLR varies in length, and most CLRs contain type 1 and type 2 repeats. The CLRs of the SclA protein from AP34 and AP57 have type 1 and type 2 repeats with three additional GXY units. Two additional 30-aa collagen-like repeats can be identified in the CLR. The KTL9 strain harbors two copies of a type 3 repeat, and the AP34 strain has three copies of a type 4 repeat within the CLR. In the COOH-terminal part of the CLR, a stretch of 33 aa is more than 95% conserved between most SclA proteins. This region is represented by a dark gray shading in Fig. 5. Overall, the homology between the SclA proteins is high in the COOH-terminal part of the CLR and lower in the NH2-terminal part. Carboxy-terminal to the CLR, all SclA proteins, except for those from M1 strains, contain one to six copies of a 21-aa-long proline-rich repeat (denoted by 5 in Fig. 5). This repeat does not resemble collagen and shows similarity to several other surface proteins from gram-positive bacteria containing proline-rich repeats, like PspC (6) from Streptococcus pneumoniae and the β antigen (27) from Streptococcus agalactiae. The SclA protein of AP9 has three divergent proline-rich repeats containing two or five additional amino acid residues and one copy of the consensus repeat. The W domain is >95% conserved among all the proteins and contains the LPATGE motif. The calculated molecular mass of the mature SclA protein varies from 25 kDa for AP15 to 40 kDa for AP12. Despite the variation in size and sequence, the proteins all have a predicted acidic pI ranging from 4.7 to 5.2.
The level of similarity between the A domains of different SclA proteins is too low to allow a stringent phylogenetic analysis. However, a few possible phylogenetic dendrograms were obtained and compared with similar dendrograms obtained for the hypervariable NH2-terminal part of different M proteins (61). No correlation was found between any of the dendrograms generated for the SclA and the M proteins (data not shown).
DISCUSSION
In this work we describe a novel surface protein of S. pyogenes containing a collagen-like region. Collagen is a triple-helical, elongated protein structure which is the dominating structural component of the extracellular matrix of all multicellular organisms. Moreover, collagens interact specifically with several macromolecules (see reference 53). Collagen-like sequences are found in other proteins, such as C1q of the complement system (49) and the scavenger receptor on macrophages (34), where these sequences are believed to play both structural and functional roles (53, 57). The CLR of SclA could by analogy serve both as a ligand-binding site and as a stalk on which the hypervariable NH2-terminal A domain is exposed. Collagen-like sequences are not limited to proteins of multicellular organisms but can also be found in bacterial proteins. For example, a collagen-like sequence stabilizes homotrimers of a pullulanase in Klebsiella pneumoniae (8). In S. pyogenes, two extracellular hyaluronidases have been reported to contain short stretches of collagen-like sequences (24, 25). Moreover, a collagen-like surface antigen of S. sanguis has been shown to induce aggregation of platelets (12, 13). A collagen-like heptapeptide was found to be responsible for this effect (14), but the sequence of the intact protein is unknown. The CLR of SclA is, however, the longest collagen-like sequence of a bacterial protein so far described. Moreover, SclA is not involved in platelet aggregation and is thus distinct from the collagen-like immunodeterminant at the surface of S. sanguis.
The SclA protein has several characteristics of a surface protein of gram-positive bacteria, including a signal sequence and a cell wall anchor containing the LPXTGX motif. SclA contains proline-rich repeats in the COOH-terminal part of the protein, which is also common for surface proteins in gram-positive bacteria. The number of proline-rich repeats varies between different streptococcal strains, much like the repeats in the protein GRAB (48). The type 1 to type 4 repeats are all collagen-like and are located in the conserved COOH-terminal part of the CLR. These repeats are less well defined and are sometimes overlapping.
The SclA protein has several features in common with streptococcal M proteins. M proteins are rod-like alpha-helical coiled-coil proteins which protrude from the streptococcal cell surface. In SclA it is likely that the CLR forms such an elongated and rod-like structure, which could serve to present the hypervariable A domain at the bacterial surface. Furthermore, both the M protein and SclA have conserved COOH-terminal parts, while both proteins exhibit hypervariable NH2-terminal domains. The hypervariable regions of SclA proteins, however, share a common secondary structure as judged from computerized predictions. All A domains have a strong prediction to form two alpha helices connected by a loop region rich in aromatic amino acids. Despite the lack of sequence conservation, the hypervariable NH2-terminal regions of several M proteins have recently been shown to bind C4BP, a regulatory protein of the complement system (29). Only one M protein tested failed to bind C4BP, and this M protein had a secondary structure prediction in the hypervariable region different from that for the others (29). Also, the hypervariable NH2-terminal regions of M5 and M6 have an affinity for FHL1, another regulatory protein of the complement system (28). It therefore appears plausible that the hypervariable NH2-terminal regions of SclA showing a conserved secondary structure interact with a common ligand and have common function(s). However, among a large number of tested human proteins, we have failed to identify any that interacts with SclA.
Within a given serotype, the M protein is conserved. Antibodies to the hypervariable part of M proteins are opsonizing, and therefore the differences between M proteins of different serotypes can be explained as a means by which S. pyogenes avoids cross-serotype immunity. It seems likely that the hypervariability of SclA is also due to a selective pressure to avoid cross-serotype-reacting antibodies. Interestingly, there seems to be a correlation between the M serotype and the sequence of SclA. Thus, SclA sequences of the three M1 strains studied here are completely conserved, as is the case for 95% of M1 proteins (21). Therefore, a given M serotype seems to have a distinct SclA type.
Genes encoding M proteins are subjected to horizontal gene transfer (32), and it is possible that at least one case of horizontal gene transfer of the sclA gene has occurred among the S. pyogenes strains studied in this work. There is no special structural similarity between the M12 and M49 proteins and no overall chromosomal relationship of the M12 and M49 strains (61). Nevertheless, the SclA proteins of these two strains are very similar compared to any other pair of SclA proteins. Another striking similarity between the M proteins and SclA is related to their gene regulation. Both genes are under the transcriptional control of the Mga protein. The coregulation of these two surface proteins will result in their simultaneous exposure at the bacterial surface, suggesting that they play a role during the same phase of an infection. It is noteworthy that Mga regulates the expression of additional genes implicated in the virulence of S. pyogenes, including the scpA gene, encoding the C5a peptidase (36, 45, 60) and the sic gene, encoding the complement regulator protein SIC (2, 33). This suggests also that SclA could contribute to the virulence of S. pyogenes, a possibility which will be investigated in future studies.
ACKNOWLEDGMENTS
This work was supported by grants from the Swedish Medical Research Council (project 7480); the Medical Faculty, Lund University; the Foundations of Kock, Lundberg, and Österlund; the Göran Gustavsson Foundation for Research in Natural Sciences and Medicine; and Active Biotech Ltd.
We acknowledge the Streptococcal Genome Sequencing Project, funded by USPHS/NIH grant AI38406, and B. A. Roe, S. P. Linn, L. Song, X. Yuan, S. Clifton, M. McShan, and J. Ferretti. We thank Andreas Podbielski for providing the pFW13 plasmid and Axel Janke for important advice.
ADDENDUM
The gene described herein was originally designated coss, but after the submission of the manuscript, the same sequence from strain SF370 (sequenced in the Streptococcal Genome Sequence Project) was made public in GenBank under accession no. AF252861. The submission was done by S. Lukomski et al. based on unpublished results, and the gene was designated scl. We thus chose to denote the gene described herein sclA since after submission of the manuscript we found that several S. pyogenes strains express an additional Scl-like protein.
REFERENCES
- 1.Åkesson P, Cooney J, Kishimoto F, Björck L. Protein H—a novel IgG binding bacterial protein. Mol Immunol. 1990;6:523–531. doi: 10.1016/0161-5890(90)90071-7. [DOI] [PubMed] [Google Scholar]
- 2.Åkesson P, Sjöholm A G, Björck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–1088. doi: 10.1074/jbc.271.2.1081. [DOI] [PubMed] [Google Scholar]
- 3.Ben Nasr A, Herwald H, Müller-Esterl W, Björck L. Human kininogens interact with M protein, a bacterial surface protein and virulence determinant. Biochem J. 1995;305:173–180. doi: 10.1042/bj3050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berge A, Sjöbring U. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J Biol Chem. 1993;268:25417–25424. [PubMed] [Google Scholar]
- 5.Berry A M, Paton J C. Sequence heterogeneity of PsaA, a 37-kilodalton putative adhesin essential for virulence of Streptococcus pneumoniae. Infect Immun. 1996;64:5255–5262. doi: 10.1128/iai.64.12.5255-5262.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brooks-Walter A, Briles D E, Hollingshead S K. The pspC gene of Streptococcus pneumoniae encodes a polymorphic protein, PspC, which elicits cross-reactive antibodies to PspA and provides immunity to pneumococcal bacteremia. Infect Immun. 1999;67:6533–6542. doi: 10.1128/iai.67.12.6533-6542.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Caparon M G, Scott J R. Identification of a gene that regulates expression of M protein, the major virulence determinant of group A streptococci. Proc Natl Acad Sci USA. 1987;84:8677–8681. doi: 10.1073/pnas.84.23.8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charalambous B M, Keen J N, McPherson M J. Collagen-like sequences stabilize homotrimers of a bacterial hydrolase. EMBO J. 1988;7:2903–2909. doi: 10.1002/j.1460-2075.1988.tb03148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung A L, Eberhardt K I, Fischetti V A. A method to isolate RNA from Gram-positive bacteria and mycobacteria. Anal Biochem. 1994;222:511–514. doi: 10.1006/abio.1994.1528. [DOI] [PubMed] [Google Scholar]
- 10.Chou P Y, Fasman G D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1974;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- 11.Collin M, Olsén A. Generation of a mature streptococcal cysteine proteinase is dependent on cell wall-anchored M1 protein. Mol Microbiol. 2000;36:1306–1318. doi: 10.1046/j.1365-2958.2000.01942.x. [DOI] [PubMed] [Google Scholar]
- 12.Erickson P R, Herzberg M C. A collagen-like immunodeterminant on the surface of Streptococcus sanguis induces platelet aggregation. J Immunol. 1987;138:3360–3366. [PubMed] [Google Scholar]
- 13.Erickson P R, Herzberg M C. Purification and partial characterization of a 65-kDa platelet aggregation-associated protein antigen from the surface of Streptococcus sanguis. J Biol Chem. 1990;265:14080–14087. [PubMed] [Google Scholar]
- 14.Erickson P R, Herzberg M C. The Streptococcus sanguis platelet aggregation-associated protein, identification and characterization of the minimal platelet-interacting domain. J Biol Chem. 1993;268:1646–1649. [PubMed] [Google Scholar]
- 15.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frick I-M, Åkesson P, Cooney J, Sjöbring U, Schmidt K-H, Gomi H, Hattori S, Tagawa C, Kishimoto F, Björck L. Protein H—a surface protein of Streptococcus pyogenes with separate binding sites for IgG and albumin. Mol Microbiol. 1994;12:143–151. doi: 10.1111/j.1365-2958.1994.tb01003.x. [DOI] [PubMed] [Google Scholar]
- 17.Garnier J, Osguthorpe D J, Robson B. Analysis of the accuracy and implications of simple methods for predicting the secondary structure of globular proteins. J Mol Biol. 1978;120:97–120. doi: 10.1016/0022-2836(78)90297-8. [DOI] [PubMed] [Google Scholar]
- 18.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanski E, Fogg G, Tovi A, Okada N, Burstein I, Caparon M. Molecular analysis of Streptococcus pyogenes adherence. Methods Enzymol. 1995;253:269–305. doi: 10.1016/s0076-6879(95)53025-8. [DOI] [PubMed] [Google Scholar]
- 20.Heath D G, Cleary P P. Cloning and expression of the gene for an immunoglobulin G Fc receptor protein from a group A streptococcus. Infect Immun. 1987;55:1233–1238. doi: 10.1128/iai.55.5.1233-1238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoe N P, Nakashima K, Lukomski S, Grigsby D, Liu M, Kordari P, Dou S J, Pan X, Vuopio-Varkila J, Salmelinna S, McGeer A, Low D E, Schwartz B, Schuchat A, Naidich S, De-Lorenzo D, Fu Y X, Musser J M. Rapid selection of complement-inhibiting protein variants in group A Streptococcus epidemic waves. Nat Med. 1999;5:924–929. doi: 10.1038/11369. [DOI] [PubMed] [Google Scholar]
- 22.Hollingshead S K, Readdy T L, Yung D L, Bessen D E. Structural heterogeneity of the emm gene cluster in group A streptococci. Mol Microbiol. 1993;8:707–717. doi: 10.1111/j.1365-2958.1993.tb01614.x. [DOI] [PubMed] [Google Scholar]
- 23.Horstmann R D, Sievertsen H J, Knobloch J, Fischetti V A. Antiphagocytic activity of streptococcal M protein: selective binding of complement control protein factor H. Proc Natl Acad Sci USA. 1988;85:1657–1661. doi: 10.1073/pnas.85.5.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hynes W L, Ferretti J J. Sequence analysis and expression in Escherichia coli of the hyaluronidase gene of Streptococcus pyogenes bacteriophage H4489A. Infect Immun. 1989;57:533–539. doi: 10.1128/iai.57.2.533-539.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hynes W L, Hancock L, Feretti J J. Analysis of a second bacteriophage hyaluronidase gene from Streptococcus pyogenes: evidence for a third hyaluronidase involved in extracellular enzymatic activity. Infect Immun. 1995;63:3015–3020. doi: 10.1128/iai.63.8.3015-3020.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hytonen J, Haataja S, Isomäki P, Finne J. Identification of a novel glycoprotein-binding activity in Streptococcus pyogenes regulated by the mga gene. Microbiology. 2000;146:31–39. doi: 10.1099/00221287-146-1-31. [DOI] [PubMed] [Google Scholar]
- 27.Jerlestrom P G, Chhatwal G S, Timmis K N. The IgA-binding beta antigen of the c protein complex of group B streptococci: sequence determination of its gene and detection of two binding regions. Mol Microbiol. 1991;5:843–849. doi: 10.1111/j.1365-2958.1991.tb00757.x. [DOI] [PubMed] [Google Scholar]
- 28.Johnsson E, Berggård K, Kotarsky H, Hellwage J, Zipfel P F, Sjöbring U, Lindahl G. Role of the hypervariable region in streptococcal M proteins: binding of a human complement inhibitor. J Immunol. 1998;161:4894–4901. [PubMed] [Google Scholar]
- 29.Johnsson E, Thern A, Dahlbäck B, Hedén L-O, Wikström M, Lindahl G. A highly variable region in members of the streptococcal M protein family binds the human complement regulator C4BP. J Immunol. 1996;157:3021–3029. [PubMed] [Google Scholar]
- 30.Kantor F S. Fibrinogen precipitation by streptococcal M protein. J Exp Med. 1965;121:849–859. doi: 10.1084/jem.121.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kastern W, Sjöbring U, Björck L. Structure of peptostreptococcal protein L and identification of a repeated immunoglobulin light chain-binding domain. J Biol Chem. 1992;267:12820–12825. [PubMed] [Google Scholar]
- 32.Kehoe M A, Kapur V, Whatmore A M, Musser J M. Horizontal gene transfer among group A streptococci: implications for pathogenesis and epidemiology. Trends Microbiol. 1996;4:436–443. doi: 10.1016/0966-842x(96)10058-5. [DOI] [PubMed] [Google Scholar]
- 33.Kihlberg B-M, Cooney J, Caparon M G, Olsén A, Björck L. Biological properties of a Streptococcus pyogenes mutant generated by Tn916 insertion in mga. Microb Pathog. 1995;19:299–315. doi: 10.1016/s0882-4010(96)80003-9. [DOI] [PubMed] [Google Scholar]
- 34.Kodama T, Freeman M, Rohrer L, Zabrecky J, Matsudaira P, Krieger M. Type I macrophage scavenger receptor contains alpha-helical and collagen-like coiled coils. Nature. 1990;343:531–535. doi: 10.1038/343531a0. [DOI] [PubMed] [Google Scholar]
- 35.Kurpiewski G E, Forrester L J, Campbell B J, Barret J T. Platelet aggregation of Streptococcus pyogenes. Infect Immun. 1983;39:704–708. doi: 10.1128/iai.39.2.704-708.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.La Penta D, Zhang X P, Cleary P P. Streptococcus pyogenes type IIa IgG Fc receptor expression is co-ordinately regulated with M protein and streptococcal C5a peptidase. Mol Microbiol. 1994;12:873–879. doi: 10.1111/j.1365-2958.1994.tb01075.x. [DOI] [PubMed] [Google Scholar]
- 37.Lindahl G, Åkerström B. Receptor for IgA in group A streptococci: cloning of the gene and characterization of the protein expressed in Escherichia coli. Mol Microbiol. 1989;3:239–247. doi: 10.1111/j.1365-2958.1989.tb01813.x. [DOI] [PubMed] [Google Scholar]
- 38.McIver K S, Heath A S, Green B D, Scott J R. Specific binding of the Mga to promoter sequences of the emm and scpA genes in the group A streptococcus. J Bacteriol. 1995;177:6619–6624. doi: 10.1128/jb.177.22.6619-6624.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Navarre W W, Schneewind O. Surface proteins of Gram-positive bacteria and mechanisms of their targeting to the cell wall envelope. Microbiol Mol Biol Rev. 1999;63:174–229. doi: 10.1128/mmbr.63.1.174-229.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neville D M. Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971;246:6328–6334. [PubMed] [Google Scholar]
- 41.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Casal J, Caparon M G, Scott J R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pitcher D G, Saunders N A, Owen R J. Rapid extraction of bacterial genomic DNA with guanidium thiocyanate. Lett Appl Microbiol. 1989;8:151–156. [Google Scholar]
- 44.Podbielski A. Three different types of organization of the vir regulon in group A streptococci. Mol Gen Genet. 1993;237:287–300. doi: 10.1007/BF00282810. [DOI] [PubMed] [Google Scholar]
- 45.Podbielski A, Flosdorff A, Weber-Heynemann J. The group A streptococcal virR49 gene controls expression of four structural vir regulon genes. Infect Immun. 1995;63:9–20. doi: 10.1128/iai.63.1.9-20.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Podbielski A, Spellerberg B, Woischnik M, Pohl B, Lüttiken R. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS) Gene. 1996;177:137–147. doi: 10.1016/0378-1119(96)84178-3. [DOI] [PubMed] [Google Scholar]
- 47.Rakonjac J V, Robbins J C, Fischetti V A. DNA sequence of the serum opacity factor of group A streptococci: identification of a fibronectin-binding domain. Infect Immun. 1995;63:622–631. doi: 10.1128/iai.63.2.622-631.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasmussen M, Müller H-P, Björck L. Protein GRAB of Streptococcus pyogenes regulates proteolysis at the bacterial cell surface by binding α2-macroglobulin. J Biol Chem. 1999;274:15336–15344. doi: 10.1074/jbc.274.22.15336. [DOI] [PubMed] [Google Scholar]
- 49.Reid K B. Complete amino acid sequences of the three collagen-like regions present in subcomponent C1q of the first component of human complement. Biochem J. 1979;179:367–371. doi: 10.1042/bj1790367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Retnoningrum D S, Cleary P P. M12 protein from Streptococcus pyogenes is a receptor for immunoglobulin G3 and human albumin. Infect Immun. 1994;62:2387–2394. doi: 10.1128/iai.62.6.2387-2394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Robinson J H, Kehoe M A. Group A streptococcal M proteins: virulence factors and protective antigens. Immunol Today. 1992;13:362–367. doi: 10.1016/0167-5699(92)90173-5. [DOI] [PubMed] [Google Scholar]
- 52.Rocha C L, Fischetti V A. Identification and characterization of a novel fibronectin-binding protein on the surface of group A streptococci. Infect Immun. 1999;67:2720–2728. doi: 10.1128/iai.67.6.2720-2728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rohrer L, Freeman M, Kodama T, Penman M, Krieger M. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343:570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 55.Saravani G A, Martin D R. Opacity factor from group A streptococci is an apoproteinase. FEMS Microbiol Lett. 1990;56:35–40. doi: 10.1111/j.1574-6968.1990.tb04118.x. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt K-H, Wadström T. A secreted receptor related to M1 protein of Streptococcus pyogenes binds to fibrinogen, IgG, and albumin. Zentbl Bakteriol. 1990;273:216–228. doi: 10.1016/s0934-8840(11)80252-5. [DOI] [PubMed] [Google Scholar]
- 57.Sim R B, Reid K B M. C1: molecular interactions with activating systems. Immunol Today. 1991;12:307–311. doi: 10.1016/0167-5699(91)90004-D. [DOI] [PubMed] [Google Scholar]
- 58.Talay S R, Valentin-Weigand P, Jerlström P G, Timmis K N, Chhatwal G S. Fibronectin-binding protein of Streptococcus pyogenes: sequence of the binding domain involved in adherence of streptococci to epithelial cells. Infect Immun. 1992;60:3837–3844. doi: 10.1128/iai.60.9.3837-3844.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thern A, Stenberg L, Dahlbäck B, Lindahl G. Ig-binding surface proteins of Streptococcus pyogenes also bind human C4b-binding protein (C4BP), a regulatory component of the complement system. J Immunol. 1995;154:375–386. [PubMed] [Google Scholar]
- 60.Wexler D E, Chenoweth D E, Cleary P P. Mechanism of action of the group A streptococcal C5a inactivator. Proc Natl Acad Sci USA. 1985;82:8144–8148. doi: 10.1073/pnas.82.23.8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Whatmore A M, Kapur V, Sullivan D J, Musser J M, Kehoe M A. Non-congruent relationships between variation in emm gene sequences and the population genetic structure of group A streptococci. Mol Microbiol. 1994;14:619–631. doi: 10.1111/j.1365-2958.1994.tb01301.x. [DOI] [PubMed] [Google Scholar]