The Food and Drug Administration (FDA) recently published their opinion on the inadequate characterization of doses and schedules of oncology drugs, based on their experience with sotorasib, and their post-marketing requirement to evaluate lower doses1. The sotorasib case is illustrative for the speed of development of novel, active anticancer drugs, resulting in incomplete pharmacological characterization and consequent erroneous dose recommendations. Yet, it is unique in that the cause was as basic as pharmacokinetic (PK) futility due to saturated absorption. While PK should have been included as one of the phase 1 primary endpoints for this oral agent, it was insufficiently reported2. In general, oncology dose recommendations are suboptimal due to default selection of the maximum tolerated dose (MTD), based on a small, selected patient sample and single cycle toxicity data. In response, the FDA is drafting guidance as part of their Project Optimus, which will highlight the need for a more thorough evaluation of the optimal risk-benefit ratio prior to registration trials, e.g. through randomized dose-ranging trials.
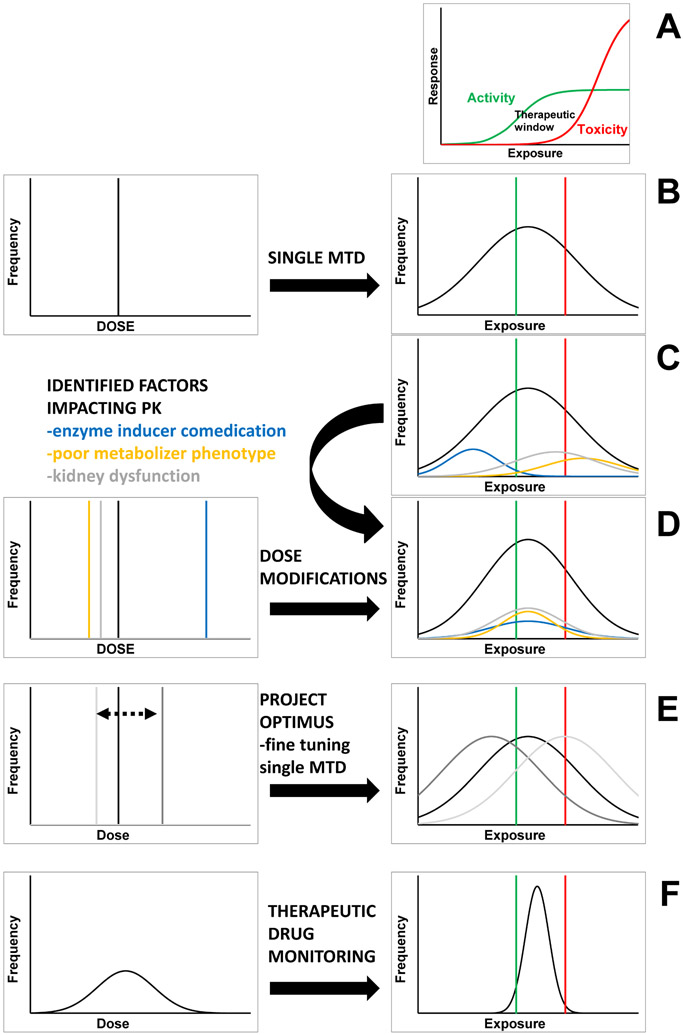
The recommended dose of anticancer agents is often determined in relatively small numbers of patients through rule-based approaches, such as the 3+3 design (Fig.1A-B). The oncology community has made great strides in identifying extrinsic (modifiable, e.g. diet, smoking) and intrinsic (individual, e.g. sex, age, organ function, comorbidity) factors that determine PK variability between individuals, resulting in recommendations for defined subgroups of patients (Fig.1C-D). However, this is usually limited to a “typical” set of factors, and a recent study reported 40% of drugs surveyed had no intrinsic factors associated with PK3. Therefore, beyond cautionary advice or dose recommendations for factors such as enzyme inhibitors/inducers or organ dysfunction, the vast majority of variability in anticancer drug PK is not directly addressable with our current standard approaches. Moreover, this issue of PK variability will not be directly addressed by Project Optimus and the proposed new guidance, which will merely shift the “one-size-fits-all” dose to a dose that is better for the average patient. (Fig.1E).
Figure 1.
A) Many anticancer drugs have a narrow therapeutic window; B) A single maximum tolerable dose for all results in overdosing and underdosing some; C) Extrinsic and intrinsic factors partly explain exposure variability, and associated dose recommendations (D) reduce some, but not the majority, of this variability; E) While FDA Project Optimus shifts the dose recommendation for all patients based on the overall safety profile, the number of patients within the therapeutic window may not increase; F) TDM generates individual patient dose recommendations, increases the number of patients within the therapeutic window, and increases the likelihood of achieving optimal patient benefit.
The notion of personalizing dose arguably started with the administration of body surface area (BSA) normalized doses, a concept derived from animal-to-human dose translation of cytotoxic chemotherapy drugs, which does little to reduce variability within humans. The realization that BSA poorly predicts drug clearance, gained mostly from studying IV administered cytotoxics, resulted in the more recent molecularly targeted agents being administered with a flat dose. In special populations, e.g. patients with kidney or liver impairment, pediatric patients, and to better manage drug-drug and drug-food interactions, dose recommendations are based on targeting the PK exposure of the respective special population to that of the reference population administered the approved dose4. This approach of dose adjustment based on a target exposure-matching is now generally accepted and based on dedicated PK studies without efficacy endpoints or prospective validation studies. An important lesson drawn from cytotoxics and molecularly targeted agents is that the exposure at any single dose remains quite variable, and only individual therapeutic drug monitoring (TDM) can reduce variability in exposure between patients, resulting in optimal outcomes for the individual patient4-6 (Fig.1F). If group-level PK-based dose adjustment is already routine, why has oncology failed to embrace TDM as the logical final step of individual-level PK-based dose adjustment when many indication areas outside of oncology routinely do so? The most straightforward approach for truly personalized therapy would be to adjust exposure of the drug, for each patient, on an individualized basis4-6. The real advantage of TDM is that it can account for all intrinsic and extrinsic factors of PK variability, known and unknown. By identifying and subsequently targeting a specific optimal exposure range, the risk-benefit ratio would be optimized down to the individual patient level4,7. Currently, clinical progression may be due to intrinsic resistance or subtherapeutic exposure, where the latter leads to the emergence of resistant subclones. TDM maximizes the clinical benefit of each treatment line, and disease progression unambiguously signals the need to switch therapy.
One of the objections of oncology to TDM is the absence of phase III randomized trial data, even though existing exposure-response relationships underpin dose recommendations based on kidney or liver function status, or co-medication with enzyme inhibitors or inducers, which are accepted without further question. Another argument is that practical and efficient bioanalytical tools are lacking. This barrier is clearly surmountable as evidenced by the routine use of TDM in non-oncology areas such as transplantation, antiepileptic and infectious disease therapeutics, where inexpensive tools are available and utilized in standard clinical care.
It may remain difficult to change how existing oncology drugs are being dosed. The unfortunate reality is that even the lesson of non-BSA dosing has not been retroactively applied to the cytotoxics themselves. With these agents off-patent, there seems to be no group willing and able to overcome the significant institutional resistance to any change in established standard of care. The rare examples in oncology where drug levels inform standard of care treatment decisions are methotrexate and busulfan, and their package inserts have guidance on the appropriate exposure. For personalized exposure optimization to be realized in oncology, the FDA must provide clear guidance, and stipulate the listing of exposure parameters defining the “therapeutic window” in the product labeling. Sponsors will not do this on their own, and unfortunately, the oncology community has neither the focus nor the perspective to make this happen. Importantly, compared with traditional cytotoxic agents, targeted therapies display an even greater inter-individual variability in exposure, increasing both unnecessary toxicity and unrealized clinical efficacy3. Exposure-response relationships should not merely be evaluated in the NDA/BLA, but should be detailed with an exposure reference range in the package insert. This may prompt national organizations involved in developing guidelines to recommend using TDM in routine clinical practice. Over the past 70 years, cancer treatment has made significant progress, yet there is much knowledge that has not been translated into routine clinical practice. To truly personalize care, TDM needs to be part of the treatment paradigm, and regulatory authorities and national agencies must create the conditions to make it happen.
Support:
U24CA247643, UM1CA186690, P30CA047904, P30CA013330
Footnotes
This viewpoint discusses therapeutic drug monitoring as the logical endpoint of recent discussions by the FDA on how to optimize dose in oncology.
REFERENCES
- 1.Shah M, Rahman A, Theoret MR, Pazdur R. The Drug-Dosing Conundrum in Oncology - When Less Is More. N Engl J Med. Oct 14 2021;385(16):1445–1447. doi: 10.1056/NEJMp2109826 [DOI] [PubMed] [Google Scholar]
- 2.Hong DS, Fakih MG, Strickler JH, et al. KRAS(G12C) Inhibition with Sotorasib in Advanced Solid Tumors. N Engl J Med. Sep 24 2020;383(13):1207–1217. doi: 10.1056/NEJMoa1917239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reyner E, Lum B, Jing J, Kagedal M, Ware JA, Dickmann LJ. Intrinsic and Extrinsic Pharmacokinetic Variability of Small Molecule Targeted Cancer Therapy. Clin Transl Sci. Mar 2020;13(2):410–418. doi: 10.1111/cts.12726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Groenland SL, Verheijen RB, Joerger M, et al. Precision Dosing of Targeted Therapies Is Ready for Prime Time. Clin Cancer Res. Sep 21 2021:Online ahead of print. doi: 10.1158/1078-0432.CCR-20-4555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beumer JH, Chu E, Allegra C, et al. Therapeutic Drug Monitoring in Oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology Recommendations for 5-Fluorouracil Therapy. Clin Pharmacol Ther. Mar 2019;105(3):598–613. doi: 10.1002/cpt.1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke WA, Chatelut E, Fotoohi AK, et al. Therapeutic drug monitoring in oncology: International Association of Therapeutic Drug Monitoring and Clinical Toxicology consensus guidelines for imatinib therapy. Eur J Cancer. Nov 2021;157:428–440. doi: 10.1016/j.ejca.2021.08.033 [DOI] [PubMed] [Google Scholar]
- 7.Beumer JH. Without Therapeutic Drug Monitoring, There Is No Personalized Cancer Care. Research Support, N.I.H., Extramural. Clin Pharmacol Ther. Mar 2013;93(3):228–30. doi: 10.1038/clpt.2012.243 [DOI] [PubMed] [Google Scholar]