Abstract
While heredity is predominantly controlled by what deoxyribonucleic acid (DNA) sequences are passed from parents to their offspring, a small but growing number of traits have been shown to be regulated in part by the non-genetic inheritance of information. Transgenerational epigenetic inheritance is defined as heritable information passed from parents to their offspring without changing the DNA sequence. Work of the past seven decades has transitioned what was previously viewed as rare phenomenology, into well-established paradigms by which numerous traits can be modulated. For the most part, studies in model organisms have correlated transgenerational epigenetic inheritance phenotypes with changes in epigenetic modifications. The next steps for this field will entail transitioning from correlative studies to causal ones. Here, we delineate the major molecules that have been implicated in transgenerational epigenetic inheritance in both mammalian and non-mammalian models, speculate on additional molecules that could be involved, and highlight some of the tools which might help transition this field from correlation to causation.
Keywords: Epigenetic Inheritance, noncoding RNAs, histone modifications, Transgenerational, DNA methylation, lipid methylation, rRNA methylation
Graphical Abstract
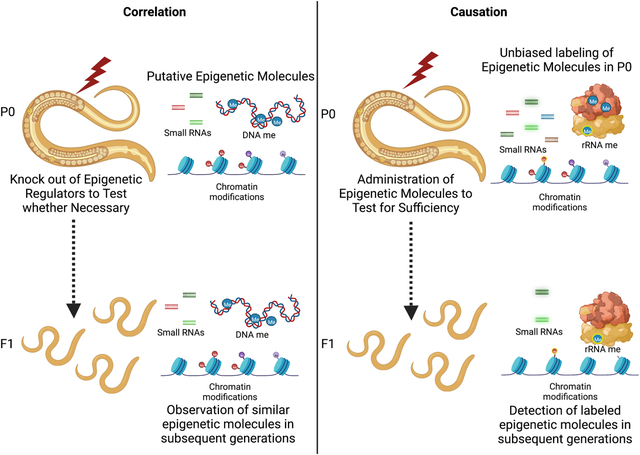
Transgenerational epigenetic inheritance is the non-Mendelian non-genetic transmission of phenotypes, from ancestors to their descendants, without changes in genetic information. Here, we highlight how the field is shifting from correlative observations of how epigenetic inheritance is regulated, towards causal experiments to label and track epigenetic cues, and to determine whether epigenetic molecules are sufficient to induce epigenetic inheritance.
Introduction
Traits such as lifespan, metabolism, and morphology have been shown to be regulated in part by the inheritance of non-genetic information. Non-genetic information being transmitted for a single generation is termed intergenerational epigenetic inheritance while non-genetic information which is transmitted for multiple generations is termed transgenerational epigenetic inheritance [1,2]. In the case of transgenerational epigenetic inheritance none of the genetic material of the descendants was present or exposed to the initiating environmental or genetic signal. To date, much of the examination of these heritable phenotypes has correlated changes in potential epigenetic molecules including histone modifications, DNA methylation, small RNA inheritance, prions or microbiota with transgenerational epigenetic inheritance phenotypes [3,4]. The preponderance of work examining the molecular mechanisms of epigenetic inheritance has been done in yeast, C. elegans and D. melanogaster, however an increasing number of studies are beginning to be performed in mammalian species. Each of the putative epigenetic carriers on non-genetic information do not usually function in isolation and tend to have crosstalk with other pathways in regulating gene expression [5]. Therefore, identification of critical initiating factors is confounded by the understanding that there is a reinforcement by other factors during transmission between parent and progeny. Here, we will briefly summarize a small portion of the work on epigenetic inheritance that has been validated in independent laboratories and different species, the established epigenetic factors, before discussing some of the emerging epigenetic factors that are just beginning to be examined. These “established” epigenetic factors need to be further examined mechanistically to determine whether they are causal for the inheritance of non-genetic phenotypes or if they are passenger epigenetic cues that contribute to amplifications of non-genetic signals.
Histone Modifications
DNA is wrapped around nucleosomes which facilitates compaction and packaging of the entire genome within the nucleus [6,7]. The level of chromatin compaction affects accessibility for the transcription machinery to turn on gene expression. Histones possess extruding tails that can be post translationally modified with specific chemical modifications, affecting the level of DNA compaction and serving as markers for gene regulation [8]. There have been numerous studies supporting histone modifications as pivotal for epigenetic inheritance of a variety of phenotypes. In Caenorhabditis elegans, mutation of a histone H3 lysine 4 (H3K4) trimethylation complex can extend lifespan in a transgenerational manner [9,10]. Mutation of an H3K4me1/me2 demethyltransferase also induces a progressive fertility decline and a transgenerational lifespan extension [11,12]. Histone modifications, particularly H3K27me3, have been demonstrated to serve as an imprinting marker independent of DNA methylation in mammals [13]. Work in C. elegans and D. melanogaster has also suggested H3K27me3 itself is transmitted across generations [14–16]. Histone modification changes can be triggered by environmental conditions that persist in several generations. In a study examining the effect of heat stress in C. elegans, it was revealed that a decrease of H3K9me3 is required for perpetuating the increased levels of a repetitive transgenic array expressing heat-shock response protein, and that this memory can be transmitted through multiple generations [17]. Earlier studies have reported that silencing of repetitive transgenic arrays in C. elegans germline is stable and epigenetically inherited due to the maintenance of the chromatin state [18,19]. Similarly, in D. melanogaster high-sugar and high-fat diets affect heritable obesity and cardiac function correlating with changes in H3K27me3 [20,21]. These examples highlight how changing levels of histone modifications can correlate with transgenerational phenotypes, and how manipulation of the enzymes responsible for regulating histone modifications are necessary for transgenerational phenotypes. However, it remains to be seen whether changes in histone modifications are the transmissible signal and therefore responsible for regulating transgenerational phenotypes, or whether they are simply an integral component of an epigenetic amplification cascade.
Although there have been many studies pointing at histone modifications as a key epigenetic factor, mechanistic insight into how histones bearing specific modifications are passed on to preserve the gene regulatory state at specific genomic regions is still being elucidated. This is in part due to the DNA replication process, where the original nucleosomes have to be split between the parental and new cells [22]. Since modified histones are sometimes retained through cell divisions and the H3K27 methylation complex can be retained on chromatin through DNA replication, this provides a plausible mechanism by which epigenetic information could be transmitted across generations [23–25] (Figure 1A). Future work will be required to identify molecular mechanisms by which histone modifications can respond to environmental changes known to induce epigenetic inheritance, and to determine how prevalent the transmission of these altered histones is for the perpetuation of epigenetic phenotypes. A deeper understanding of how histone modifications are interpreted and reinforced by other epigenetic pathways, will determine whether the communication between histone modifications and other epigenetic factors, or the histone modifications themselves are the essential carriers of non-genetic information.
Figure 1. Types of Epigenetic Factors & Mechanisms.
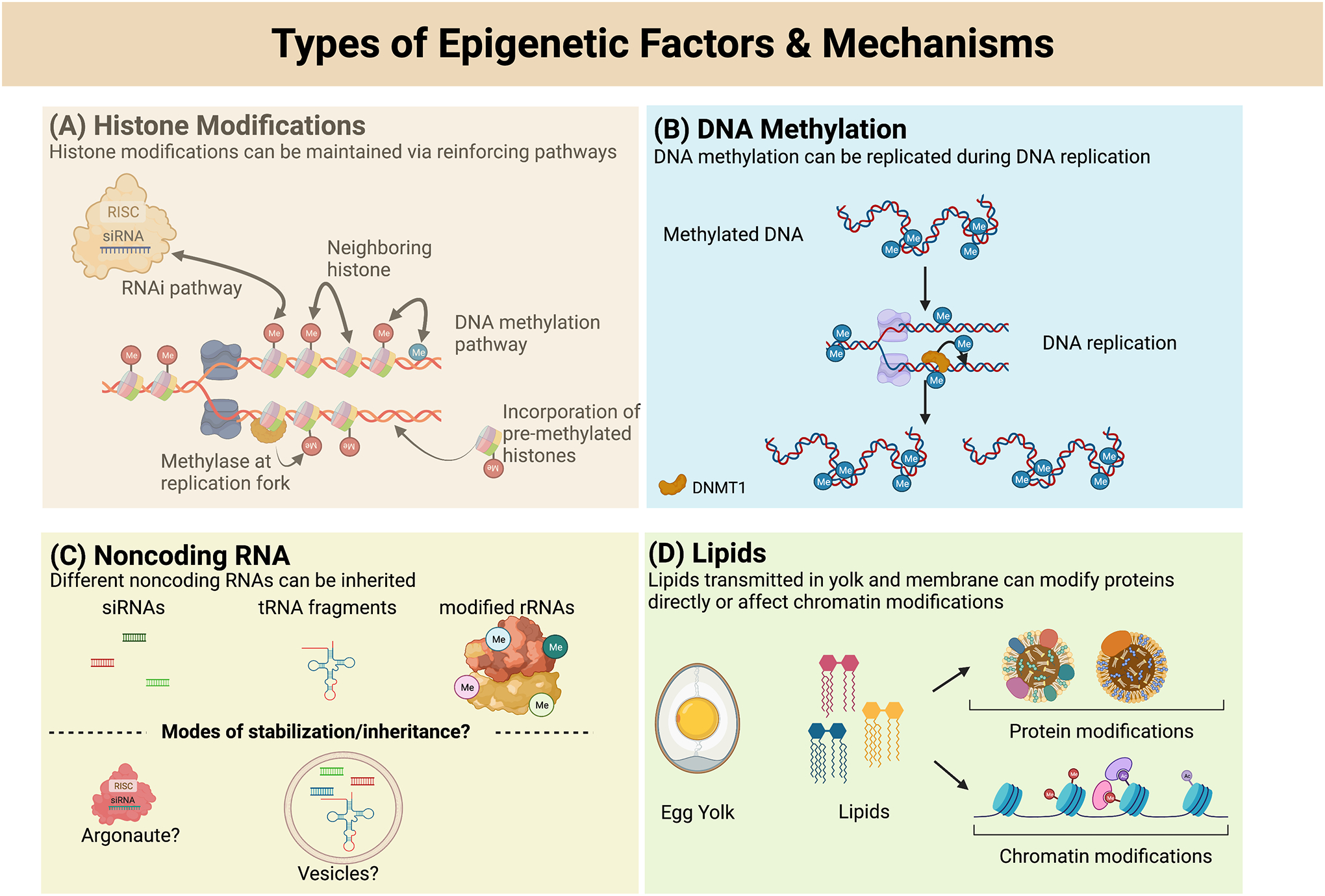
A) Histone modifications could be transmitted through cell division and generations by multiple methods. Non-coding RNAs can direct methylation to specific genomic locations, complete methylated histones could segregate alternatively to each daughter strand and a neighboring modified histone could inform the reacquisition of modifications on the newly incorporated histone, DNA methylation can direct methylation of histones (and vice versa), histone methyltransferases can exist at the replication fork to immediately methylate newly incorporated histones, or pre-modified histones could be incorporated during replication. None of these methods are mutually exclusive. B) DNA methylation has been shown to be retained through DNA replication through the actions of the maintenance DNA methyltransferase, DNMT1. C) Various non-coding RNAs have been suggested or demonstrated to be inherited across generations, however, how these non-coding RNAs are stabilized sufficiently to persist across generations is still unknown. RNAs could be stabilized through incorporation into protein complexes or vesicles to protect them from degradation. D) Lipids are putative carriers of non-genetic information that could be transmitted through the yolk, newly synthesized membranes, or signaling lipids such as eicosanoids. Transmitted lipids could be used to directly modify protein function or to confer altered chromatin modifications.
DNA methylation
Chemical modification of nucleic acids, such as DNA methylation [26], present another option for transmitting epigenetic information across generations. In eukaryotic systems, DNA methylation is present primarily on the C5 position of cytosines (5mC) and can occur in the CG, CHG and CHH sequence context (H = A/T/G) [27]. DNA methylation can affect the chromatin state by acting as a marker recruiting gene silencing machineries, that deposits other repressive modifications such as H3K9me2, leading to the entire chromatin region becoming highly compact and inaccessible for transcription [28]. As an epigenetic factor, DNA methylation has been one of the most well studied transmissible marks. This is due to the fact that DNA maintenance pathways, where DNA methyltransferases such as DNMT1/MET1, immediately deposit DNA methylation on the nascent daughter strands during DNA replication, preserving epigenetic memory without discontinuity [29(p. 1),30(p. 1)] (Figure 1B). This is evident in plant systems where DNA methylation remains present in the egg cell during the fertilization process and embryonic development [31]. Hence, this allows the formation of stable epialleles which can persist through many generations in plants. Amongst the epialleles, the most well-known is the paramutation affecting maize pigmentation, where the mutant epiallele B’, is able to convert wildtype maize upon crossing into the mutant phenotype, persisting through future generations [32,33]. DNA methylation represents one of the most parsimonious methods of how epigenetic information can be directly transmissible, as DNA itself needs to be perpetuated and DNA methylation maintenance pathways function during DNA replication.
In mammals however, global reprogramming and erasure of DNA methylation at the primordial and early embryo stages has made it more difficult to rationalize the role of DNA methylation as a transgenerational epigenetic mark in animals [34]. Interestingly, mechanisms such as genomic imprinting via DNA methylation is commonly utilized to carry paternal or maternal epigenetic memory in mammals [35]. This has been studied in the agouti mice model, where the Avy alleles become methylated by maternal imprinting which causes the progeny to express light yellow fur instead of dark brown [2]. When progeny is generated from yellow furred mice, the litter will contain a range of fur colors even though they are isogenic. There are also other regions in the genome in mammals which escape the global reprogramming demethylation step, making it comparable to epialleles present in plants [36]. It is still not understood how some of these regions can escape reprogramming but DNA methylation seems to be a robust transgenerational epigenetic factor in systems that possess it. Beyond 5mC there are other DNA methylation events, such as N6-methyladenosine [37] and N4-methylcytosine [38] and other modifications to DNA, including N4-acetylcytosine [39] and 5-hydoxymethyluracil [40], which could each play a role in transmitting non-genetic information across generations. Having a deeper mechanistic understanding of these additional modifications could help reveal whether these rarer DNA modifications could play a role specifically in the inheritance of non-genetic information. Future studies are required to differentiate what makes some DNA modifications impervious to the epigenetic erasure that occurs upon fertilization, while others are readily removed. Comprehensive understanding of the underlying mechanisms behind this selectivity would allow potential therapies or treatments for a wide variety of imprinting disorders.
Noncoding RNAs
Small RNAs (sRNA) are comprised of noncoding RNA which are 18–30 nucleotides (nt) and are involved in transcriptional and post-transcriptional gene silencing pathways [41]. In plants and mammals, transcriptional gene silencing pathways utilize short interfering RNA (siRNA) and long noncoding RNA (lncRNA) to target genomic regions with DNA methylation for silencing transposable elements (TEs) [42,43]. This then feeds into DNA methylation pathways which will maintain silencing in a transgenerational manner. In fungi and worms, noncoding RNA-induced silencing is reinforced by the repressive chromatin modification H3K9me3 [44]. In animals, there is a germline-specific RNA-based system known as the PIWI-interacting small RNAs (piRNAs) system that also functions to silence target regions complementary to the piRNA sequences [45,46]. In the piRNA system, this can occur through chromatin changes induced by sRNA or heritable silencing by RNAi through secondary siRNAs. It is thought that RNA-induced epigenetic silencing function more towards responding to immediate environmental stress or changes, which allows faster reversal of epigenetic states once conditions improve or return to normal [44]. For example, C. elegans that possess a repetitive transgenic array carrying part of the Flock House virus (FHV) genome, can generate siRNA which leads to heritable silencing of viral genomes present in the animal for several generations [47]. It is worth noting that a study using only natural C. elegans virus did not show vertical transmission of small RNAs [48]. Starvation can also induce an increase in longevity, lower fertility and an increased resistance to stress, which sets the worms into an epigenetic state, preparing them and future generations for increased survivability in low food conditions through heritable sRNAs [1]. Small RNAs can be transmitted from the paternal or maternal side to induce heritable silencing [49]. These examples highlight how small RNAs can play a crucial role in regulating gene expression and are easily transmissible from parent to child due to their trans-acting characteristics.
Although there have been many examples of sRNA-mediated epigenetic inheritance in C. elegans, other organisms which do not have RNA-dependent RNA polymerases (RdRP), do not seem to exhibit a similar repertoire for inheriting various stress induced responses. Mammals who lack RdRPs, for instance, seem to depend more on chromatin modifications for creating stable epialleles and even then, global reprogramming in early embryonic development resets most of the epigenetic marks [34]. However, mammalian systems do have other small RNAs which have been found to be present in reproductive tissues, such as tRNA derived small RNA fragments [50,51]. Recent studies which utilized new techniques such as Ordered Two-Template Relay (OTTR) and panoramic RNA display by overcoming RNA modification aborted sequencing (PANDORA-seq), have uncovered a large number of novel small RNA fragments which appear to show tissue-specific expression as well as cell-specific expression in mammalian cells [52,53]. Long non-coding RNAs have also been proposed as carriers of epigenetic information. Studies examining the role of long noncoding RNA (lncRNA) in sperm-dependent epigenetic phenotypes, through either exposure to maternal stress or injection of toxic chemicals such as vinclozolin in the parental generation, showed strong correlation between the changes in lncRNA transcripts with chromatin modifications in the following generations[54,55]. As the mechanisms of non-coding RNA inheritance are further elucidated it will be important to decipher if non-coding RNAs are themselves specifically transmitted via vacuoles or if the epigenetic signal is transmitted via subsequent secondary epigenetic cues (such as chromatin modifications) which then allow for a reestablishment and perpetuation of the non-coding RNA signal. Further studies focused on the role of noncoding RNAs in transgenerational epigenetic inheritance remains important due to its prevalent presence in most animals and plants, which will likely reveal mechanistic insights that are either fully or partially conserved between different organisms. This in turn, could potentially open further avenues for RNA-based therapies or agricultural innovations, which could function in a transgenerational manner. Because of the inherently unstable nature of RNA, future studies will need to determine how non-coding RNAs can be protected to transmit non-genetic information across generations (Figure 1C). It will also be interesting to determine whether specific small RNAs are sufficient to elicit transgenerational phenotypes.
Emerging Epigenetic Factors
While chromatin modifications and small RNAs are some of the most extensively studied putative carriers of non-genetic information, almost any molecule present in the zygote, which is not the DNA itself, could be a carrier of epigenetic information across generations. Here, we catalogue some of the evidence for less well studied epigenetic factors, which have promise as putative carriers of nongenetic information across generations.
Ribosome modifications
The ribosome is composed of four ribosomal RNAs (rRNA), the 28S, 18S, 5.8S and 5S, and over 80 ribosomal proteins that form a complex essential for protein synthesis [56]. Recent studies have demonstrated that changes in which ribosomal proteins [57–59] or chemical modifications to the rRNAs [60–62] are integrated into the ribosome, helps to specify which transcripts are translated to regulate a variety of processes such as environmental stress, lifespan and development [60,61,63]. During fertilization, the bulk of ribosomes and proteins are transmitted from the maternal lineage, while the paternal side mostly contributes DNA and tRNA cargo [51,64]. It is likely that if rRNA modifications are involved in epigenetic inheritance, that it is passed on through the maternal side. These initial ribosomes are required for early translation events before the new organism generates its own ribosomes [65]. rRNAs have a half-life of 1–7 days under basal conditions and this could potentially be altered by localizing to specialized compartments or through modifications, raising the possibility for prolonged persistence of these non-genetic signals. Interestingly, the composition of the ribosome can change in response to environmental stress, for example, yeast increase monomethylation at the N6 position (m6A) and decrease N6-dimethylation (m6,2A) of adenosines 1781 and 1782 in the 18S rRNA, in response to sulfur starvation [62]. These changes in rRNA modifications lead to differential translation, likely due to alterations in ribosome conformation, which causes preferential binding to specific mRNA transcripts [62]. This is one example, amongst several where rRNA modifications have been shown to play an important role in regulating translational activity through ribosome heterogeneity.
Our group has found, through metabolic methyl labeling experiments, that in response to starvation there is a heritable increase in m6,2A on the 18S rRNA in C. elegans [66] (Figure 1C). Starvation induces a hormesis response with increased heat resistance and a subtle extension in lifespan associated with a decrease in reproduction, in not only the generation that is starved, but also in their naïve well fed children and grandchildren [66]. We identified the enzymes responsible for N6-dimethylating adenosines on the 18S rRNA in C. elegans, and found that deletion of these enzymes eliminated the transmission of hormesis phenotypes in response to parental starvation [66]. Based on these findings, it suggests that rRNA become modified in response to external stresses, to presumably alter which proteins are translated to increase survival in stressful conditions. These modified rRNAs are then transmitted to the next generation to alert subsequent generations that conditions might not be optimal, and the children should also alter which proteins are translated to prepare for that stressed environment [66]. By performing an unbiased metabolic methyl labeling experiment, we were able to track epigenetic material transmitted from parents to their children, and identify critical components necessary for maintaining an epigenetic memory. Our approach has revealed an unprecedented role for specific rRNA modifications in transmitting transgenerational epigenetic traits in response to parental starvation, which raises the question as to whether other rRNA modifications could be involved in transmitting non-genetic information in different paradigms. We feel that this labeling and tracking of non-genetic information provides a critical tool in assessing, in an unbiased manner, what non-genetic information is transmitted directly from parents to children through rRNA modifications or other molecules.
Lipids
In many animals, eggs can form yolk which is a high source of lipids and lipoproteins, and is a source of energy for the developing embryo [67,68]. This can also potentially provide a source for epigenetic signals to be passed on through specifically modified lipids (Figure 1D). Lipids are essential components of all membranes and vesicles [69,70] and are also utilized as signaling molecules within and between cells [71]. The ubiquitous presence of lipids make lipids attractive candidates for transmitting epigenetic information between parents and their offspring [72]. For instance, membrane fusion that occurs during mammalian fertilization could be one way that epigenetic information could be passed on either paternally or maternally [73]. In a recent study, it was shown that lipid metabolism is required for the transgenerational epigenetic inheritance of C. elegans behavioral response to exposure to a pathogenic bacteria Pseudomonas aeruginosa [74]. The investigators showed that worms which have been trained to avoid Pseudomonas aeruginosa through exposure to the pathogen, have increased levels of H3K27me3 and decreases in H3K27 acetylation (H3K27ac) in comparison to naïve worms. These changes in chromatin marks were shown to be dependent on the activity of pod-2, which is an acetyl-coA carboxylase, and on vitellogenins, which are a family of yolk proteins. The combination of pod-2 and vitellogenins help to establish H3K27me3 in specific genomic regions in the parental generation, and these modified histone patterns are subsequently passed on to their progeny [74]. Lipids represent an exciting new potential carrier of epigenetic information, as they can play an important role in regulating histone modifications to control gene expression for epigenetic traits.
In addition, lipids, similarly to nucleic acids and proteins, can be modified by the addition of chemical groups, which presents an additional layer of regulation that can potentially retain epigenetic memory [75,76]. Lipids can also modify proteins to regulate protein function [77]. For example, myristoylation of ciliopathy protein nephrocystin-3 (NPHP3) is required for targeting ciliary proteins to the primary cilium in C. elegans [78]. N-palmitoylation, is another lipid modification present on the Hedgehog protein, which is a morphogen active during embryonic development [79]. These properties of lipids to modulate biological activity of proteins, especially in early life, presents a potential opportunity for transmitting transgenerational inheritance to future generations in an epigenetic manner. As lipids are omnipresent in our food and pharmaceutical products, further studies are needed to determine if lipids play a larger role in facilitating transgenerational epigenetic inheritance, which could potentially have unforeseen effects in individuals as well as their future progenies.
Conclusions
Transgenerational epigenetic inheritance has been a phenomenon shrouded in mystery from its inception and there is still a lot that we do not fully understand. Even though epigenetic phenotypes can often be robustly observed through several generations, the epigenetic markers and factors tend to be obscure. This problem is confounded by the cross-talk and reinforcing nature of how epigenetic cues can induce other epigenetic modifications or molecules. Unlike a DNA sequence where discrepancies are immediately identified and linked to a phenotype, there are usually multiple layers or series of interacting components, which ultimately allow the passage of epigenetic memory between parent and offspring (Figure 1). Hence, a major focus for the field is to develop new techniques and approaches for identifying and tracking molecules, which are heritable and required for the epigenetic phenotype. Recently, a study investigating how C. elegans learns and acquires the ability to avoid pathogenic P. aeruginosa, discovered that the worms achieved this through exposure to the pathogen’s sRNA [80]. The authors demonstrated that specific sRNAs were necessary and sufficient to induce an epigenetic memory of the pathogenic bacteria [80]. Other groups have also utilized histone labeling methods to track the transmission of specific histone modifications from parent to offspring [81–83]. Through a series of genetic tricks, histone modifications present in sperm were shown to be passed on to the next generation, affecting the transcriptomic profile and development of the progeny [81,82]. Our own work performing a metabolic methyl labeling technique across generations allowed us to identify, in an unbiased manner, which methylation events were transmitted from parents to their children, in response to parental starvation [66] (Figure 2). These types of approaches shift the focus in the field from correlation to causation, where we can directly track and monitor the passage of epigenetic information from parent to child. By pursuing unbiased labeling and tracking experiments, the field can identify previously unappreciated epigenetic molecules and can reveal previously undiscovered mechanisms due to discontinuous correlative observations between generations. Coupling unbiased labeling methods with directed manipulations to determine whether epigenetic cues are both necessary and sufficient, will help expand the mechanistic understanding of transgenerational epigenetic inheritance. Presently, there are still large gaps in our mechanistic understanding of existing epigenetic factors as well as those that still remain to be identified in the future, leaving the field at an exciting stage of discovery.
Figure 2. Tracking Heritable Methylation Across Generations.
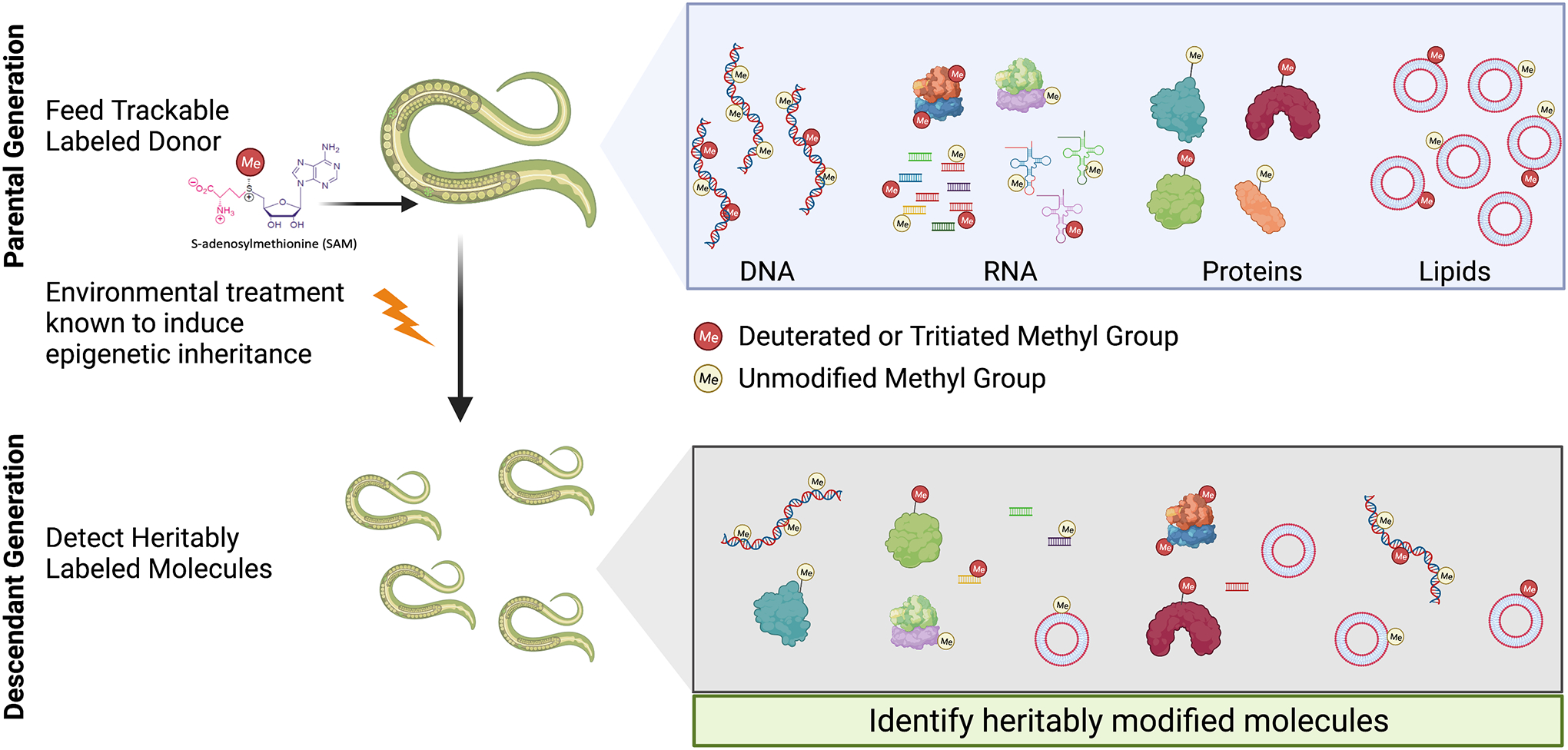
Methods which label and track non-genetic material across generations will be useful for identifying in an unbiased manner what epigenetic cues are physically transmitted and could be causal for epigenetic inheritance. As an example, we illustrate how metabolic methyl labeling is performed by feeding a modified methyl donor (Red methyl group represents deuterated or tritiated methyl) in the parental generation which will label any methylated substrate (shown in top box on right), specifically heritably methylated cues (shown in bottom box on right) will be detected in the subsequent generation through detection of the modified methyl group. To determine whether heritable methylated molecules change in response to an environmental treatment that is known to induce epigenetic inheritance, environmental manipulations can be made in the parental generation.
Acknowledgments
This work was supported by an NIH grant DP2AG055947 to E.L.G. Figures were created using BioRender.com.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created in this study
References
- 1.Rechavi O, Houri-Ze’evi L, Anava S, Goh WSS, Kerk SY, Hannon GJ, & Hobert O (2014). Starvation-Induced Transgenerational Inheritance of Small RNAs in C. elegans. Cell, 158(2), 277–287. 10.1016/j.cell.2014.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morgan HD, Sutherland HGE, Martin DIK, & Whitelaw E (1999). Epigenetic inheritance at the agouti locus in the mouse. Nature Genetics, 23(3), Article 3. 10.1038/15490 [DOI] [PubMed] [Google Scholar]
- 3.Bošković A, & Rando OJ (2018). Transgenerational Epigenetic Inheritance. Annual Review of Genetics, 52(1), 21–41. 10.1146/annurev-genet-120417-031404 [DOI] [PubMed] [Google Scholar]
- 4.Liberman N, Wang SY, & Greer EL (2019). Transgenerational Epigenetic Inheritance: From Phenomena to Molecular Mechanisms. Current Opinion in Neurobiology, 59, 189–206. 10.1016/j.conb.2019.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du J, Johnson LM, Jacobsen SE, & Patel DJ (2015). DNA methylation pathways and their crosstalk with histone methylation. Nature Reviews Molecular Cell Biology, 16(9), 519. 10.1038/nrm4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luger K, Mäder AW, Richmond RK, Sargent DF, & Richmond TJ (1997). Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature, 389(6648), 251–260. 10.1038/38444 [DOI] [PubMed] [Google Scholar]
- 7.Luger K, Dechassa ML, & Tremethick DJ (2012). New insights into nucleosome and chromatin structure: An ordered state or a disordered affair? Nature Reviews Molecular Cell Biology, 13(7), 436–447. 10.1038/nrm3382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannister AJ, & Kouzarides T (2011). Regulation of chromatin by histone modifications. Cell Research, 21(3), Article 3. 10.1038/cr.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greer EL, Maures TJ, Ucar D, Hauswirth AG, Mancini E, Lim JP, Benayoun BA, Shi Y, & Brunet A (2011). Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature, 479(7373), Article 7373. 10.1038/nature10572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee TW, David HS, Engstrom AK, Carpenter BS, & Katz DJ (2019). Repressive H3K9me2 protects lifespan against the transgenerational burden of COMPASS activity in C. elegans. ELife, 8, e48498. 10.7554/eLife.48498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz DJ, Edwards TM, Reinke V, & Kelly WG (2009). A C. elegans LSD1 Demethylase Contributes to Germline Immortality by Reprogramming Epigenetic Memory. Cell, 137(2), 308–320. 10.1016/j.cell.2009.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer EL, Becker B, Latza C, Antebi A, & Shi Y (2016). Mutation of C. elegans demethylase spr-5 extends transgenerational longevity. Cell Research, 26(2), Article 2. 10.1038/cr.2015.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue A, Jiang L, Lu F, Suzuki T, & Zhang Y (2017). Maternal H3K27me3 controls DNA methylation-independent imprinting. Nature, 547(7664), Article 7664. 10.1038/nature23262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaydos LJ, Wang W, & Strome S (2014). H3K27me and PRC2 transmit a memory of repression across generations and during development. Science, 345(6203), 1515–1518. 10.1126/science.1255023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zenk F, Loeser E, Schiavo R, Kilpert F, Bogdanović O, & Iovino N (2017). Germ line–inherited H3K27me3 restricts enhancer function during maternal-to-zygotic transition. Science, 357(6347), 212–216. 10.1126/science.aam5339 [DOI] [PubMed] [Google Scholar]
- 16.Kaneshiro KR, Egelhofer TA, Rechtsteiner A, Cockrum C, & Strome S (2022). Sperm-inherited H3K27me3 epialleles are transmitted transgenerationally in cis. Proceedings of the National Academy of Sciences of the United States of America, 119(40), e2209471119. 10.1073/pnas.2209471119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klosin A, Casas E, Hidalgo-Carcedo C, Vavouri T, & Lehner B (2017). Transgenerational transmission of environmental information in C. elegans. Science, 356(6335), 320–323. 10.1126/science.aah6412 [DOI] [PubMed] [Google Scholar]
- 18.Kelly WG, Xu S, Montgomery MK, & Fire A (1997). Distinct Requirements for Somatic and Germline Expression of a Generally Expressed Caernorhabditis Elegans Gene. Genetics, 146(1), 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strome S, Powers J, Dunn M, Reese K, Malone CJ, White J, Seydoux G, & Saxton W (2001). Spindle Dynamics and the Role of γ-Tubulin in EarlyCaenorhabditis elegans Embryos. Molecular Biology of the Cell, 12(6), 1751–1764. 10.1091/mbc.12.6.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Öst A, Lempradl A, Casas E, Weigert M, Tiko T, Deniz M, Pantano L, Boenisch U, Itskov PM, Stoeckius M, Ruf M, Rajewsky N, Reuter G, Iovino N, Ribeiro C, Alenius M, Heyne S, Vavouri T, & Pospisilik JA (2014). Paternal Diet Defines Offspring Chromatin State and Intergenerational Obesity. Cell, 159(6), 1352–1364. 10.1016/j.cell.2014.11.005 [DOI] [PubMed] [Google Scholar]
- 21.Guida MC, Birse RT, Dall’Agnese A, Toto PC, Diop SB, Mai A, Adams PD, Puri PL, & Bodmer R (2019). Intergenerational inheritance of high fat diet-induced cardiac lipotoxicity in Drosophila. Nature Communications, 10(1), Article 1. 10.1038/s41467-018-08128-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramachandran S, & Henikoff S (2015). Replicating nucleosomes. Science Advances, 1(7), e1500587. 10.1126/sciadv.1500587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu M, Long C, Chen X, Huang C, Chen S, & Zhu B (2010). Partitioning of Histone H3-H4 Tetramers During DNA Replication–Dependent Chromatin Assembly. Science, 328(5974), 94–98. 10.1126/science.1178994 [DOI] [PubMed] [Google Scholar]
- 24.Francis NJ, Follmer NE, Simon MD, Aghia G, & Butler JD (2009). Polycomb Proteins Remain Bound to Chromatin and DNA during DNA Replication In Vitro. Cell, 137(1), 110–122. 10.1016/j.cell.2009.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petruk S, Sedkov Y, Johnston DM, Hodgson JW, Black KL, Kovermann SK, Beck S, Canaani E, Brock HW, & Mazo A (2012). TrxG and PcG Proteins but Not Methylated Histones Remain Associated with DNA through Replication. Cell, 150(5), 922–933. 10.1016/j.cell.2012.06.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotchkiss RD (1948). THE QUANTITATIVE SEPARATION OF PURINES, PYRIMIDINES, AND NUCLEOSIDES BY PAPER CHROMATOGRAPHY. Journal of Biological Chemistry, 175(1), 315–332. 10.1016/S0021-9258(18)57261-6 [DOI] [PubMed] [Google Scholar]
- 27.Law JA, & Jacobsen SE (2010). Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nature Reviews Genetics, 11(3), 204. 10.1038/nrg2719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoddard CI, Feng S, Campbell MG, Liu W, Wang H, Zhong X, Bernatavichute Y, Cheng Y, Jacobsen SE, & Narlikar GJ (2019). A Nucleosome Bridging Mechanism for Activation of a Maintenance DNA Methyltransferase. Molecular Cell, 73(1), 73–83.e6. 10.1016/j.molcel.2018.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woo HR, Pontes O, Pikaard CS, & Richards EJ (2007). VIM1, a methylcytosine-binding protein required for centromeric heterochromatinization. Genes & Development, 21(3), 267–277. 10.1101/gad.1512007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shook MS, & Richards EJ (2014). VIM proteins regulate transcription exclusively through the MET1 cytosine methylation pathway. Epigenetics, 9(7), 980–986. 10.4161/epi.28906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slotkin RK, Vaughn M, Borges F, Tanurdžić M, Becker JD, Feijó JA, & Martienssen RA (2009). Epigenetic Reprogramming and Small RNA Silencing of Transposable Elements in Pollen. Cell, 136(3), 461–472. 10.1016/j.cell.2008.12.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brink RA (1956). A GENETIC CHANGE ASSOCIATED WITH THE R LOCUS IN MAIZE WHICH IS DIRECTED AND POTENTIALLY REVERSIBLE. Genetics, 41(6), 872–889. 10.1093/genetics/41.6.872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arteaga-Vazquez MA, & Chandler VL (2010). Paramutation in maize: RNA mediated trans-generational gene silencing. Current Opinion in Genetics & Development, 20(2), 156–163. 10.1016/j.gde.2010.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hackett JA, Sengupta R, Zylicz JJ, Murakami K, Lee C, Down TA, & Surani MA (2013). Germline DNA Demethylation Dynamics and Imprint Erasure Through 5-Hydroxymethylcytosine. Science, 339(6118), 448–452. 10.1126/science.1229277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tang WWC, Kobayashi T, Irie N, Dietmann S, & Surani MA (2016). Specification and epigenetic programming of the human germ line. Nature Reviews Genetics, 17(10), Article 10. 10.1038/nrg.2016.88 [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Liu Y-J, Nakashima H, Umehara H, Inoue K, Matoba S, Tachibana M, Ogura A, Shinkai Y, & Nakano T (2012). PGC7 binds histone H3K9me2 to protect against conversion of 5mC to 5hmC in early embryos. Nature, 486(7403), Article 7403. 10.1038/nature11093 [DOI] [PubMed] [Google Scholar]
- 37.Boulias K, & Greer EL (2022). Means, mechanisms and consequences of adenine methylation in DNA. Nature Reviews Genetics, 1–18. 10.1038/s41576-022-00456-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez F, Yushenova IA, DiCorpo D, & Arkhipova IR (2022). Bacterial N4-methylcytosine as an epigenetic mark in eukaryotic DNA. Nature Communications, 13(1), Article 1. 10.1038/s41467-022-28471-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang S, Xie H, Mao F, Wang H, Wang S, Chen Z, Zhang Y, Xu Z, Xing J, Cui Z, Gao X, Jin H, Hua J, Xiong B, & Wu Y (2022). N4-acetyldeoxycytosine DNA modification marks euchromatin regions in Arabidopsis thaliana. Genome Biology, 23(1), 5. 10.1186/s13059-021-02578-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pfaffeneder T, Spada F, Wagner M, Brandmayr C, Laube SK, Eisen D, Truss M, Steinbacher J, Hackner B, Kotljarova O, Schuermann D, Michalakis S, Kosmatchev O, Schiesser S, Steigenberger B, Raddaoui N, Kashiwazaki G, Müller U, Spruijt CG, … Carell T (2014). Tet oxidizes thymine to 5-hydroxymethyluracil in mouse embryonic stem cell DNA. Nature Chemical Biology, 10(7), Article 7. 10.1038/nchembio.1532 [DOI] [PubMed] [Google Scholar]
- 41.Carthew RW, & Sontheimer EJ (2009). Origins and Mechanisms of miRNAs and siRNAs. Cell, 136(4), 642–655. 10.1016/j.cell.2009.01.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matzke MA, & Mosher RA (2014). RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nature Reviews Genetics, 15(6), 394. 10.1038/nrg3683 [DOI] [PubMed] [Google Scholar]
- 43.Matzke MA, Kanno T, & Matzke AJM (2015). RNA-Directed DNA Methylation: The Evolution of a Complex Epigenetic Pathway in Flowering Plants. Annual Review of Plant Biology, 66(1), 243–267. 10.1146/annurev-arplant-043014-114633 [DOI] [PubMed] [Google Scholar]
- 44.Duempelmann L, Skribbe M, & Bühler M (2020). Small RNAs in the Transgenerational Inheritance of Epigenetic Information. Trends in Genetics, 36(3), 203–214. 10.1016/j.tig.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 45.Aravin AA, Hannon GJ, & Brennecke J (2007). The Piwi-piRNA Pathway Provides an Adaptive Defense in the Transposon Arms Race. Science, 318(5851), 761–764. 10.1126/science.1146484 [DOI] [PubMed] [Google Scholar]
- 46.Aravin AA, Sachidanandam R, Bourc’his D, Schaefer C, Pezic D, Toth KF, Bestor T, & Hannon GJ (2008). A piRNA Pathway Primed by Individual Transposons Is Linked to De Novo DNA Methylation in Mice. Molecular Cell, 31(6), 785–799. 10.1016/j.molcel.2008.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rechavi O, Minevich G, & Hobert O (2011). Transgenerational Inheritance of an Acquired Small RNA-Based Antiviral Response in C. elegans. Cell, 147(6), 1248–1256. 10.1016/j.cell.2011.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ashe A, Sarkies P, Le Pen J, Tanguy M, & Miska EA (2015). Antiviral RNA Interference against Orsay Virus Is neither Systemic nor Transgenerational in Caenorhabditis elegans. Journal of Virology, 89(23), 12035–12046. 10.1128/JVI.03664-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alcazar RM, Lin R, & Fire AZ (2008). Transmission Dynamics of Heritable Silencing Induced by Double-Stranded RNA in Caenorhabditis elegans. Genetics, 180(3), 1275–1288. 10.1534/genetics.108.089433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma U, Conine CC, Shea JM, Boskovic A, Derr AG, Bing XY, Belleannee C, Kucukural A, Serra RW, Sun F, Song L, Carone BR, Ricci EP, Li XZ, Fauquier L, Moore MJ, Sullivan R, Mello CC, Garber M, & Rando OJ (2016). Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science, 351(6271), 391–396. 10.1126/science.aad6780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng H, Shi J, Zhang Y, Zhang H, Liao S, Li W, Lei L, Han C, Ning L, Cao Y, Zhou Q, Chen Q, & Duan E (2012). A novel class of tRNA-derived small RNAs extremely enriched in mature mouse sperm. Cell Research, 22(11), Article 11. 10.1038/cr.2012.141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi J, Zhang Y, Tan D, Zhang X, Yan M, Zhang Y, Franklin R, Shahbazi M, Mackinlay K, Liu S, Kuhle B, James ER, Zhang L, Qu Y, Zhai Q, Zhao W, Zhao L, Zhou C, Gu W, … Chen Q (2021). PANDORA-seq expands the repertoire of regulatory small RNAs by overcoming RNA modifications. Nature Cell Biology, 23(4), Article 4. 10.1038/s41556-021-00652-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gustafsson HT, Galan C, Yu T, Upton HE, Ferguson L, Kaymak E, Weng Z, Collins K, & Rando OJ (2022). Deep sequencing of yeast and mouse tRNAs and tRNA fragments using OTTR (p. 2022.02.04.479139). bioRxiv. 10.1101/2022.02.04.479139 [DOI]
- 54.Gapp K, van Steenwyk G, Germain PL, Matsushima W, Rudolph KLM, Manuella F, Roszkowski M, Vernaz G, Ghosh T, Pelczar P, Mansuy IM, & Miska EA (2020). Alterations in sperm long RNA contribute to the epigenetic inheritance of the effects of postnatal trauma. Molecular Psychiatry, 25(9), Article 9. 10.1038/s41380-018-0271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ben Maamar M, Sadler-Riggleman I, Beck D, McBirney M, Nilsson E, Klukovich R, Xie Y, Tang C, Yan W, & Skinner MK (2018). Alterations in sperm DNA methylation, non-coding RNA expression, and histone retention mediate vinclozolin-induced epigenetic transgenerational inheritance of disease. Environmental Epigenetics, 4(2), dvy010. 10.1093/eep/dvy010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sergiev PV, Aleksashin NA, Chugunova AA, Polikanov YS, & Dontsova OA (2018). Structural and evolutionary insights into ribosomal RNA methylation. Nature Chemical Biology, 14(3), 226–235. 10.1038/nchembio.2569 [DOI] [PubMed] [Google Scholar]
- 57.Genuth NR, & Barna M (2018). The Discovery of Ribosome Heterogeneity and Its Implications for Gene Regulation and Organismal Life. Molecular Cell, 71(3), 364–374. 10.1016/j.molcel.2018.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ramagopal S, & Ennis HL (1981). Regulation of synthesis of cell-specific ribosomal proteins during differentiation of Dictyostelium discoideum*. Proceedings of the National Academy of Sciences, 78(5), 3083–3087. 10.1073/pnas.78.5.3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parks MM, Kurylo CM, Dass RA, Bojmar L, Lyden D, Vincent CT, & Blanchard SC (2018). Variant ribosomal RNA alleles are conserved and exhibit tissue-specific expression. Science Advances, 4(2), eaao0665. 10.1126/sciadv.aao0665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heissenberger C, Rollins JA, Krammer TL, Nagelreiter F, Stocker I, Wacheul L, Shpylovyi A, Tav K, Snow S, Grillari J, Rogers AN, Lafontaine DLJ, & Schosserer M (2020). The ribosomal RNA m5C methyltransferase NSUN-1 modulates healthspan and oogenesis in Caenorhabditis elegans. ELife, 9, e56205. 10.7554/eLife.56205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liberman N, O’Brown ZK, Earl AS, Boulias K, Gerashchenko MV, Wang SY, Fritsche C, Fady P-E, Dong A, Gladyshev VN, & Greer EL (2020). N6-adenosine methylation of ribosomal RNA affects lipid oxidation and stress resistance. Science Advances, 6(17), eaaz4370. 10.1126/sciadv.aaz4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu K, Santos DA, Hussmann JA, Wang Y, Sutter BM, Weissman JS, & Tu BP (2021). Regulation of translation by methylation multiplicity of 18S rRNA. Cell Reports, 34(10). 10.1016/j.celrep.2021.108825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tiku V, Jain C, Raz Y, Nakamura S, Heestand B, Liu W, Späth M, Suchiman HED, Müller R-U, Slagboom PE, Partridge L, & Antebi A (2017). Small nucleoli are a cellular hallmark of longevity. Nature Communications, 8(1), Article 1. 10.1038/ncomms16083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang C, Wang M, Li Y, & Zhang Y (2022). Profiling and functional characterization of maternal mRNA translation during mouse maternal-to-zygotic transition. Science Advances, 8(5), eabj3967. 10.1126/sciadv.abj3967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cenik ES, Meng X, Tang NH, Hall RN, Arribere JA, Cenik C, Jin Y, & Fire A (2019). Maternal Ribosomes Are Sufficient for Tissue Diversification during Embryonic Development in C. elegans. Developmental Cell, 48(6), 811–826.e6. 10.1016/j.devcel.2019.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liberman N, Gerashchenko MV, Boulias K, MacWhinnie FG, Ying AK, Taylor AF, Haddad JA, Shibuya H, Roach L, Dong A, Gladyshev VN, & Greer EL (2021). Intergenerational hormesis is regulated by heritable 18S rRNA methylation (p. 2021.09.27.461965). 10.1101/2021.09.27.461965 [DOI]
- 67.Kuksis A (1992). Yolk lipids. Biochimica et Biophysica Acta (BBA) - Lipids and Lipid Metabolism, 1124(3), 205–222. 10.1016/0005-2760(92)90132-F [DOI] [PubMed] [Google Scholar]
- 68.Wiegand MD (1996). Composition, accumulation and utilization of yolk lipids in teleost fish. Reviews in Fish Biology and Fisheries, 6(3), 259–286. 10.1007/BF00122583 [DOI] [Google Scholar]
- 69.Muro E, Atilla-Gokcumen GE, & Eggert US (2014). Lipids in cell biology: How can we understand them better? Molecular Biology of the Cell, 25(12), 1819–1823. 10.1091/mbc.e13-09-0516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Edidin M (2003). Lipids on the frontier: A century of cell-membrane bilayers. Nature Reviews Molecular Cell Biology, 4(5), Article 5. 10.1038/nrm1102 [DOI] [PubMed] [Google Scholar]
- 71.Dennis EA, & Norris PC (2015). Eicosanoid storm in infection and inflammation. Nature Reviews Immunology, 15(8), Article 8. 10.1038/nri3859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Storck EM, Özbalci C, & Eggert US (2018). Lipid Cell Biology: A Focus on Lipids in Cell Division. Annual Review of Biochemistry, 87(1), 839–869. 10.1146/annurev-biochem-062917-012448 [DOI] [PubMed] [Google Scholar]
- 73.Primakoff P, & Myles DG (2007). Cell–cell membrane fusion during mammalian fertilization. FEBS Letters, 581(11), 2174–2180. 10.1016/j.febslet.2007.02.021 [DOI] [PubMed] [Google Scholar]
- 74.Peng D, Wang C, Li K-L, Gan Z-X, Li Y-H, Wang H-W, Li Q-Y, Liu X-W, Sun H-Y, Jing Y-Y, Fang Q, Zhao Q, Zhang L, Chen H-H, Wei H-M, Sun J, Tang H-Y, Yang X-M, Chang J-F, … Sun F-L (2020). The Establishment of Transgenerational Epigenetic Inheritance in the C. elegans Germline is Mediated by Lipid Metabolism (p. 2020.11.04.367854). bioRxiv. 10.1101/2020.11.04.367854 [DOI]
- 75.Zatz M, Dudley PA, Kloog Y, & Markey SP (1981). Nonpolar lipid methylation. Biosynthesis of fatty acid methyl esters by rat lung membranes using S-adenosylmethionine. Journal of Biological Chemistry, 256(19), 10028–10032. 10.1016/S0021-9258(19)68735-1 [DOI] [PubMed] [Google Scholar]
- 76.Kloog Y, Zatz M, Rivnay B, Dudley PA, & Markey SP (1982). Nonpolar lipid methylation—Identification of nonpolar methylated products synthesized by rat basophilic leukemia cells, retina and parotid. Biochemical Pharmacology, 31(5), 753–759. 10.1016/0006-2952(82)90459-2 [DOI] [PubMed] [Google Scholar]
- 77.Resh MD (2013). Covalent lipid modifications of proteins. Current Biology, 23(10), R431–R435. 10.1016/j.cub.2013.04.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, Slusarski DC, & Jackson PK (2011). An ARL3–UNC119–RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes & Development, 25(22), 2347–2360. 10.1101/gad.173443.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petrova E, Rios-Esteves J, Ouerfelli O, Glickman JF, & Resh MD (2013). Inhibitors of Hedgehog acyltransferase block Sonic Hedgehog signaling. Nature Chemical Biology, 9(4), Article 4. 10.1038/nchembio.1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaletsky R, Moore RS, Vrla GD, Parsons LR, Gitai Z, & Murphy CT (2020). C. elegans interprets bacterial non-coding RNAs to learn pathogenic avoidance. Nature, 586(7829), Article 7829. 10.1038/s41586-020-2699-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tabuchi TM, Rechtsteiner A, Jeffers TE, Egelhofer TA, Murphy CT, & Strome S (2018). Caenorhabditis elegans sperm carry a histone-based epigenetic memory of both spermatogenesis and oogenesis. Nature Communications, 9(1), Article 1. 10.1038/s41467-018-06236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaneshiro KR, Rechtsteiner A, & Strome S (2019). Sperm-inherited H3K27me3 impacts offspring transcription and development in C. elegans. Nature Communications, 10(1), Article 1. 10.1038/s41467-019-09141-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma B, Trieu T-J, Cheng J, Zhou S, Tang Q, Xie J, Liu J-L, Zhao K, Habib SJ, & Chen X (2020). Differential Histone Distribution Patterns in Induced Asymmetrically Dividing Mouse Embryonic Stem Cells. Cell Reports, 32(6). 10.1016/j.celrep.2020.108003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created in this study


