Abstract
Innate B cells are a heterogeneous group of cells that function in maintaining homeostatic levels of circulating natural antibodies and as the first line of defense against infections. Innate B-1 cells and marginal zone (MZ) B cells may relocate to lymphoid follicles, and differentiate into cytokine and antibody-secreting cells in T-independent and T-dependent manners. While MZ B cells are widely described in humans, the presence of B-1 cells is more controversial. Here, we review the basic features of the innate B cell subsets identified in mice and their equivalent in humans, as well as their potential roles in transplantation. We summarize the findings of Cascalho and colleagues on the unexpected protective role of TNF receptor superfamily member 13B (TNFRSF13B) in regulating circulating levels of protective natural IgM, and the studies by Zorn and colleagues on the potential pathogenic role for polyreactive innate B cells infiltrating allograft explants. Finally, we discuss our studies that took a transcriptomic approach to identify innate B cells infiltrating kidney allografts with antibody-mediated rejection (AMR) and to demonstrate that local antigens within the allograft together with inflammation may induce a loss of B cell tolerance.
INTRODUCTION
The mammalian immune system comprises the innate and adaptive arms; innate immunity senses pathogens through germline coded receptors to generate protective responses whereas the adaptive immune system uses clonal antigen receptors that are generated by gene rearrangements, to sense antigens. Overall, the two systems seem to be well compartmentalized in terms of cell subsets, with innate immunity driving adaptive immunity mediated by classical T and B cells, and innate T and B cells playing an important role that precedes, or is complementary to, adaptive immune responses1–3. Three major subsets of peripheral mature B cells have been identified, based primarily on data from mice: B2 follicular, marginal zone (MZ) B cells, and B-1 B cells (CD5+ B-1a and CD5- B-1b cells)4(Table 1). Innate B1 and MZB produce germline encoded natural antibodies that spontaneous arise without known antigen exposure, and typically bind with low affinity to non-protein antigens such as phospholipids or carbohydrates expressed by pathogens and self-antigens5,6. In contrast, B2 cells are responsible for T-dependent class-switched and affinity-matured antibody responses, and they may also be costimulated by innate immune receptors7.
Table 1.
Features of B cell subsets.
While the most recognized role of B cells is the secretion of antibodies, it is not their only function. Activated B cells upregulate MHC Class II antigens and function as professional antigen-presenting cells (APCs) following their efficient capture and uptake of low concentrations of antigen with their B cell receptors (BCRs)8. B cells can also produce anti- and pro-inflammatory cytokines depending on their activation states9. Regulatory B cells secrete anti-inflammatory cytokines IL-10 or TGFβ−1, while effector B cell populations produce cytokines such as IL-2, IL-4, TNFα, IFNγ, and IL-12, and their roles in shaping T cell effector function and transplant rejection has been suggested9,10. This review will discuss the characteristics and functions of innate B cells in mice and humans and on their potential roles in allograft rejection.
Innate B cells
Two major innate B cell subsets have been identified in mice, namely the MZ and B-1 B cells. MZ B cells have classically been considered as exclusively residing in the marginal sinus of the spleen, but more recently have been identified in the subcapsular sinuses of mouse lymph nodes11. B-1 B cells are divided into CD5+ B-1a, and CD5− B-1b, and they primarily reside in the peritoneal and pleural cavity, and are present in low numbers in the lymph nodes and spleen12. Both MZ B cells and B1 B cells typically produce low-affinity, broadly cross-reactive antibodies that provide early protection to particulate bacterial antigens13. MZ B cells are well-described in the human spleen, while the existence of human B-1 cells remains controversial14,15.
MZ B cells.
MZ B cells (IgMhiIgDlowCD21hiCD23−CD1dhi) are fully mature innate B cells and account for 5% of splenic B cells in mice16. Like follicular B cells, murine MZ B cells continuously develop from transitional T2 B cells emerging from the bone marrow, and upon reaching the spleen MZ and specifically receiving Notch signals, the T2 B cells complete their differentiation into MZ B cells17. Compared to follicular B cells, surface IgM levels are higher and IgD levels are lower in MZ B cells. In addition, MZ B cells express high levels of CD21, CD1, and CD9, but low levels of CD23, CD5, and CD11b, consistent with their pre-activated state that requires lower activation thresholds, and their propensity to produce IgG and IgA upon class switch recombination18–23. Finally, human MZ B cells can undergo somatic hypermutation in the absence of immunization or infection at very early developmental phases, although the process by which this is achieved is not well understood24.
Unlike follicular B cells which express monoreactive BCRs, MZ B cells express polyreactive BCRs that bind to multiple antigenic patterns, and high levels of Toll-like receptors (TLRs)25. The engagement of BCR and TLR with conserved pathogen-associated molecular patterns such as lipopolysaccharide (LPS) or peptidoglycan stimulates MZ B cells to initiate low-affinity antibody responses earlier than the high-affinity antibody production by follicular B cells. Belperron et al. reported that MZ B cells started secreting Borrelia hermsii specific IgM as early as 24h post-infection26, as such, MZ B cells function as an important bridge between innate and adaptive immune responses. More recently, it has become appreciated that gut commensals can induce innate-like IgM memory B cells in both mice and humans, and that MZ B cells can enter germinal centers where they may acquire somatic mutations and emerge as IgM memory B cells27–29.
In addition to their role as early antibody-producers, Attanavich and Kearney showed that MZ B cells are good antigen-presenting cells, and are able to induce the clonal expansion of antigen-specific T cells in vivo and in vitro30. When MZ B cells are depleted, the infection burden of Borrelia burgdorferi increases drastically due to decreased levels of Borrelia burgdorferi specific IgM and IgG. In addition, MZ B cells were responsible for B. burgdorferi specific CD4+ T cell priming and early CD4+-dependent IgG response 31. Additionally, MZ B cells act as sensors for TLR ligands, and the in vivo stimulation of MZ B cells with TLR agonists leads to MZ B cell activation and accelerated antigen-specific IgM responses32.
Human MZ B cells are phenotypically characterized as IgM+IgD+CD21+CD23−CD1c+CD27+ and comprise around 15–20% of splenic B cells and around 15% of B cells in peripheral blood. In addition, MZ B cells also inhabit the inner wall of the subcapsular sinus of lymph nodes, the epithelium of tonsillar crypts, and the subepithelial dome of intestinal Peyer’s patches24. Their expression of CD21 and CD1c is indicative of MZ B cells responding to the complement fragment C3b and interacting with NK-T cells33. Furthermore, the expression of costimulatory and memory B cell marker; CD27, provides insight into the signals driving their differentiation into plasma cells 34,35. The majority of human MZ B cells are somatically mutated even in infants suggesting that they may undergo pre-immune hypermutation19,36. Upon encounter with antigen, MZ B cells can undergo both T-independent pathways to generate antibodies specific for microbial polysaccharides, as well as T-dependent antibodies. Compared to follicular B2 cells, MZ B cells have a distinct mechanism of IgV gene repertoire diversification during ontogeny, a different pattern of IgV gene usage, fewer IgV gene mutations, a slower rate of accumulation of IgV gene mutations, and a lesser dependence on germinal centers and CD40L-expressing CD4+ T cells24. Notably, a subpopulation of human cells with a transitional-to-MZ B cell phenotype is enriched for IL-10 production, a feature of regulatory, IL-10–producing B regulatory (Breg) cells37.
B1 B cells
B-1 cells comprise a very small portion of total B cells in mice. They have high surface IgM, CD19 expression, low to no surface IgD, CD23, and B220, and can be either CD5+ (B-1a) or CD5− (B-1b)38,39. B-1 cells are localized in the peritoneal cavity and pleural cavity as CD11b+ B-1a cells, in the spleen and bone marrow as CD11b− B-1a, but they are barely detectable in the blood and lymph nodes39–41. Montecino-Rodriguez et al. reported that B-1a cells are derived from a unique CD19+, B220− and Lin− progenitor lineage found in fetal liver and fetal bone marrow42, and their development largely depends on IL-7Rα and Flt-3 ligand43. B-1a B cells maintain their number by self-renewal in adulthood44, and while B-1 progenitor cells can be found in the adult bone marrow, their contribution to the maintenance of B-1a cell numbers in the periphery is still an enigma42,45.
The main function of B-1a cells in the innate immune system is the spontaneous secretion of natural IgM, thereby maintaining resting IgM levels in the body46. These natural IgM form the first line of humoral defense against infection, and it is estimated that 80 % of serum IgM is derived from B-1a B cells47. Antibodies secreted by B-1a B cells contain little or no somatic hypermutation and minimal N-region addition48, and their repertoire is skewed towards low affinity and polyreactive, and target bacterial antigens, apoptotic cells, and oxidized lipids 47. B-1a B cells have also been shown to be autoreactive, so they can participate in the clearance of cell debris and thus preventing uncontrolled immune activation and further tissue damage49. In addition, Zimecki et al. reported that B-1a B cells can present antigens to CD4+ T cells50, while Zhong et al. showed that B-1a B cells induced T cells to express IL-17 and IFN-γ more effectively than follicular B cells51. Finally, B-1a B cells can produce IL-10, GM-CSF, and IL-3 and when stimulated with LPS and may also have an immunoregulatory role52.
B-1b cells, on the other hand, have been studied far less than B1a B cells. B1b cells are thought to derive from adult bone marrow precursors, in contrast to CD5+ B-1a cells that are largely fetal and neonatal derived53. Two non-mutually exclusive models may explain the presence of B1a and B1b B cells: a “division of labor” model where each subset preferentially responds to different infection, or degree of self-antigen-mediated stimulation of the BCR and/or additional costimulatory signals that determines the responding B1 subset. Finally, despite early reports, the existence, significance, and phenotypic identifiers of human B1 cells, especially in the blood or secondary lymphoid organs, remain in dispute14,54. Nevertheless, Rodriguez-Zhurbenko et al. reported that approximately 2% of circulating CD19+ B cells are CD19+CD20+CD27+CD38low/intCD43+ B-1 B cells55. Another a recent study by Cordero et al. reported on the presence of B cells in the thymus of human neonates (<7 days after birth) that display a unique innate-like B cell gene signature56. Furthermore, some of these B cells differentiate into CD138+ plasma cells that secreted antibodies with a reactivity profile consistent with natural antibodies, prompting the authors to speculate that these intrathymic B cells and plasma cells develop without exposure to a foreign antigen, and are responsible for generating the repertoire of protective natural antibodies in newborn humans. Whether these innate-like B cells derived from the B1 lineage or are MZ B cells remains to be clarified.
Innate B cells in transplant rejection
We will review three main lines of investigations whereby the role of innate B cells and the antibodies they produced might play a role in clinical transplant rejection: the findings of Cascalho and colleagues on the protective role of TNF receptor superfamily member 13B (TNFRSF13B), the studies by Zorn and colleagues on polyreactive innate B cells infiltrating allograft explants, and our studies that took a transcriptomic approach to demonstrate innate B cells infiltrating kidney allografts with antibody-mediated rejection (AMR).
TNFRSF13B polymorphisms control T-independent antibody responses and graft outcomes.
TNF receptor superfamily member 13B (TNFRSF13B) encodes the transmembrane activator and CAML interactor (TACI) expressed by B cells, and it binds to three ligands: a proliferation induced ligand (APRIL), B cell activation factor (BAFF), and calcium modulating ligand (CAML). In addition, TACI binds heparan sulfate chains associated with syndecan-2 and−4 cores and potentiates signaling by Toll-Like receptors (TLRs)57. Initial insights into the role of TACI in regulating humoral immunity came from the observations that TACI-deficient mice have fewer plasma cells in secondary lymphoid organs and the bone marrow, and lower concentrations of IgM, IgA, and IgG in serum58. Furthermore, mutations in TNFRSF13B are associated with common variable immunodeficiency in humans59,60. It is well characterized that TACI signaling drives the expression of the transcription factor, BLIMP-1, which is essential for the development of plasma cells. Paradoxically, TACI knockout mice mount proficient antibody responses and antibody-mediated defenses against pathogenic bacteria, which we now understand is due to the necessity of TACI inducing BLIMP-1 only for T-independent B cell responses, whereas in T-dependent antibody responses, T cell help generates CD40 and IL21/STAT3 signals that bypass the need for TACI to induce BLIMP-1 expression57.
TNFRSF13B is one of the most polymorphic genes in humans, leading to Cascalho and colleagues to test whether TNFRSF13B alleles might determine the magnitude of innate B cell responses and graft outcome61. Their study showed that human kidney transplant recipients with missense mutations in TNFRSF13B comprised 33% of those with antibody-mediated rejection (AMR) but < 6% of those with stable graft function had TNFRSF13B missense mutations. These observations raised the possibility that WT levels of TACI were protective, and conversely, reduced TACI resulted in more aggressive alloreactivity. To define the mechanisms for these unexpected observations, de Mattos Barbosa et al. used a mouse cardiac transplant model to show that allografts in Tnfrsf13b-mutant recipients underwent early and severe AMR61. The increased propensity for developing AMR in Tnfrsf13b-deficient mice was not caused by increased alloantibodies but rather, was associated with decreased “natural” IgM production.
Natural IgM was postulated by de Mattos Barbaso et al. to be protective because of its polyreactivity results in their binding to circulating C3b, thereby preventing C3b from become activated/fixed on the membrane of eukaryotic cellular targets61. In Tnfrsf13b-deficient recipients, compromised complement regulation as a result of low levels of circulating “natural” IgM resulted in increased complement deposition in heart allografts as well as in the recipient’s kidneys. Thus, WT TACI regulated innate B cell functions by limiting complement-associated inflammation, contrary to some common variants of Tnfrsf13b genes that intensified inflammatory responses. From an evolutionary perspective, these variants may be maintained as low levels of “natural” antibodies help clear microbial infections, but allow inadvertent tissue injury to ensue as in the case of transplant rejection. Indeed, de Mattos Barbosa et al. showed that transplant recipients with TNFRSF13B missense mutations had significantly lower concentrations of IgM natural antibodies and C3 in serum compared to transplant recipients with WT alleles, and an increased risk of AMR61. These elegant studies point to a novel and unexpectedly protective role for natural IgM produced by T-independent B cells in transplantation, and the control that TACI exerts on the levels of circulating protective natural IgM.
Polyreactive B cells and Antibodies promote transplant rejection.
Natural antibodies have been classically defined as antibodies encoded by germline immunoglobulin genes and produced in individuals without overt antigen sensitization6. Recognized examples of natural antibodies are those that are highly specific to ABO blood group antigens or carbohydrate xenoantigens, such as α-(1,3)-galactose (α-Gal) and N-glycolylneuraminic acid (Neu5Gc). These antibodies are driven, at least in part, by gut microbiota and are responsible for precipitating hyperacute rejection in the context of ABO incompatibility or xenotransplantation, respectively62–64. Another class of natural antibodies is characterized by polyreactivity, which is defined as the ability of a single antibody to bind to multiple and apparently unrelated antigenic structures65. In the laboratory, polyreactive antibodies are defined by their ability to bind to a panel of antigens that typically include bacterial antigens such as phosphorylcholine and lipopolysaccharide (LPS), viral proteins such as hemagglutinin, proteins that are targets of autoimmune diseases such as insulin and double or single-stranded DNA, and products generated by oxidative stress, such as malondialdehyde (MDA)66. It should be noted that polyreactivity can only be definitively demonstrated using monoclonal antibodies (mAbs); in contrast, serum with broad reactivity may be explained by the presence of an extended repertoire of antigen-specific antibodies or a limited repertoire of polyreactive antibodies.
Seminal observations by Zorn and colleagues raised the hypothesis of a potential role of polyreactive B cells, and the antibodies they produce, in human transplant rejection 67. Because of the lack of definitive markers for innate human B cells, the approach they took was to isolate B cells infiltrating rejected allografts and interrogate the specificity of those B cells. In their earliest studies, Porcheray et al. investigated B cells isolated from a kidney explanted because of suspected pyelonephritis, and diagnosed with acute cellular rejection superimposed on chronic rejection68. Graft infiltrating B cells were immortalized by EBV transformation, and 102 clones were examined for HLA-reactivity as well as for polyreactivity to double-stranded DNA (dsDNA), whole protein extract from human embryonic kidney cell line (HEK-293), and insulin. One clone was found to be reactive to multiple HLA Class I alleles, and 7 clones were polyreactive, including the clone that was reactive to HLA. Thus, ~7% of the B cells examined were polyreactive, a frequency that is not significantly enriched over 16.7%−26.3% of circulating polyreactive B cells in healthy individuals69. Indeed, Porcheray et al. went on to show that one of the clones with polyreactivity was highly expanded in the blood, and likely contributed to the polyreactive antibodies detected in the serum68. Thus, despite numerous caveats, this study established proof of principle for the expansion of polyreactive B cells in a rejected kidney explant.
Supporting data on the accumulation of polyreactive B cells in rejecting allografts came from studies of heart allograft explants with cardiac allograft vasculopathy (CAV), which is characterized by intimal thickening and lumen narrowing of the main coronary arteries. CAV is a major cause of heart graft loss, and the majority of these grafts present with immune infiltrates of T and B cell clusters together with plasma cells and macrophages. Chatterjee et al. generated 102 EBV-immortalized B-cell clones from three explanted heart grafts with CAV and reported that while none were HLA-reactive, approximately half of the clones were polyreactive, namely reactive to apoptotic cells, MDA, insulin, dsDNA, LPS, cardiolipin, and/or apoptotic cells70. Overall, the rates of polyreactivity were considerably higher than observed in the blood of healthy individuals and thus provided convincing evidence of an accumulation of polyreactive B cells around the coronary arteries of allografts with CAV. Approximately half of the clones were IgM and the rest were IgG, and the percentage of mutated Ig sequences was significantly higher in B cells from the graft compared to the blood. The authors characterized these as “natural” antibodies, although it is unclear if those polyreactive IgM and IgG antibodies were in fact produced by innate B1 cells infiltrating the graft or by B-2 cells. This uncertainty is due to the lack of consensus on definitive phenotypes of B1 B cells in humans, and the inability to lineage trace in a way that is possible in mice.
Because of the technical challenges involved in studying innate B cells in human transplant recipients and linking their presence to graft outcomes, clinical studies have focused on investigating the presence of polyreactive antibodies and correlating them with graft outcomes71,72. The caveat of these studies is that serum polyreactivity may be the result of a wide repertoire of antigen-specific antibodies or a limited repertoire of broadly reactive antibodies. Nevertheless, judicious selection of antigenic targets, by using apoptotic cells or the oxidized epitope, malondialdehyde, allowed See et al. to conclude increased levels of natural polyreactive antibodies in the serum from patients on ventricular assist devices (VAD) compared to those who were not73. More recently, Zorn and colleagues assessed natural antibodies from a retrospective cohort of 635 patients who received a kidney transplant72. Defining natural antibodies as a 50% increase in reactivity to malondialdehyde, they showed that the presence of anti-malondialdehyde antibodies is a significant risk factor for graft loss (hazard ratio, 2.68; 95% confidence interval, 1.49 to 4.82; P=0.001). While the authors label these as natural antibodies based on reactivity, whether anti-malondialdehyde IgG detected in transplant recipients are the product of innate B cells, or whether B2 cells can also produce antibodies with these reactivities, remains to be definitively demonstrated.
Innate-like B cells driving immunity in kidney allografts undergoing AMR
The role of innate B cells and autoantibodies in allograft rejection was recently assessed by Asano et al. by applying single-cell transcriptomics to B cells isolated from fresh kidney biopsies diagnosed with active or chronic AMR, and their BCRs were expressed as human IgG1 mAbs and specificity assessed 74. In their single-cell transcriptomics dataset, they compared intrarenal B cells with activated tonsillar B cells from tonsillectomy samples. Overall, intrarenal and tonsillar B cells had similar ratios of class-switched (IgA, IgE, IgG) and unswitched (IgD and IgM) B cells. Unswitched IgM+ B cells in rejected renal allograft and tonsil tissue were transcriptionally similar, whereas, class-switched intrarenal B cells were transcriptionally distinct from class-switched tonsillar B cells.
Pathway enrichment analysis of the differentially expressed genes by intrarenal compared to tonsillar B cells was related to innate receptors and signaling pathways which included the pattern recognition receptors, NLRP1, NOD1, TLR2, and TLR7, the interferon (IFN)-related pathways, and several cytokine ligands and receptors, including IL15, TNFRSF1B, and TNFRSF13B74. Interestingly, the transcriptional repressors, BCL6 and BACH2, that are critical to the differentiation into GC B cells were preferentially expressed in class-switched tonsillar B cells but downregulated in intrarenal B cells. One gene that was notably upregulated in intrarenal B cells was AHNAK, a scaffolding protein that is upregulated in murine peritoneal B1a and B1b cells 75. By conducting a more detailed analysis of the human counterparts of murine AHNAK covariate genes, the authors concluded that AHNAK covariate genes were enriched in intrarenal B cells. Furthermore, by analyzing 2,855 human genes that were orthologs to murine genes enriched in peritoneal B1 cells, they additionally demonstrated an enrichment of this gene set in intrarenal B cells. Thus the majority of intrarenal B cells accumulating in the context of human kidney transplant rejection have a transcriptome that is enriched for mouse orthologs of innate B cells from mouse, and were classified as a unique subset of human innate “Bin” cells.
Consistent with the innate-like transcriptional phenotype, class-switched intrarenal B cells preferentially expressed the innate cytokine IL-15. Immunofluorescence microscopy confirmed that infiltrating B and other immune cells in rejected renal allografts expressed IL-15, while IL-15RA was moderately expressed in both tonsil and renal graft tissue. These data suggested that IL-15 secreted by B cells might be captured by tubular cells for presentation to immune cells; indeed, IL-15 has been reported to upregulate activation molecules and the costimulatory molecule CD80 on B-1a cells, and to prompt an anti-inflammatory to pro-inflammatory shift in the B-1a cells76. Furthermore, the abundance of IL-15 in rejected renal allografts and improved graft survival following the antagonization of IL-15 have been recognized in previous studies 77,78.
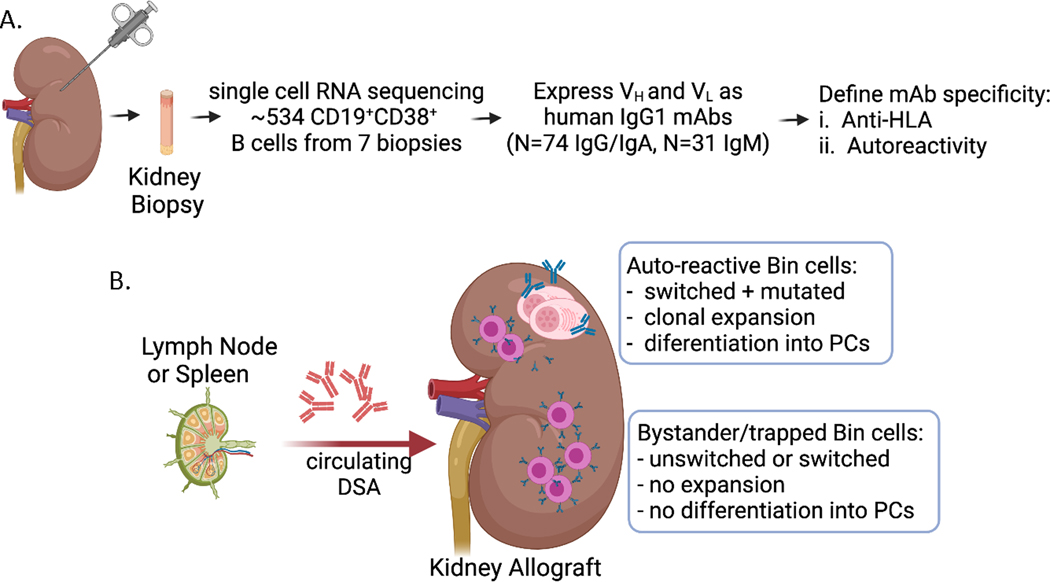
The specificity of the innate-like B cells was also examined by cloning the BCRs from intrarenal and tonsillar B cells and expressing them HEK293 as human IgG1 mAbs (Fig 1A). A total of 105 BCRs expressed as recombinant mAbs was initially assessed for HLA reactivity. Although 15% of mAbs showed multiple HLA reactivity, predominantly towards HLA-C, they were not donor-specific even in patients with circulating donor-specific antibodies. Furthermore, epitope sharing could not explain the broad HLA reactivity, and instead the mAb binding to HLA was shown to be due to low-affinity polyreactivity, as addition of serum completely abrogated HLA-binding. Thus, mAb binding to HLA was likely a technical artifact, and their low affinity binding unlikely to have physiological relevance.
Fig 1.
(A) Experimental approach taken by Asano et al. to isolate intrarenal B cells from biopsies taken from kidneys diagnosed with active or chronic AMR. (B) Cartoon depicting the two major groups of Bin cells infiltrating kidney allografts, while donor-specific antibodies are likely to be generated in the lymph nodes and spleen.
Asano et al. then focused on assessing the clonal relationships among the sequenced BCRs where they noted a limited number of shared clonal families in Bin cells from most patients, and that many of the plasma cells were clonally related to each other and to intrarenal B cells74. These findings raised the possibility that local self-antigens were driving in situ selection and differentiation of intrarenal B cells into antibody-secreting cells and plasma cells, instead of low-affinity polyreactivity74,79–81. Consistent with this notion, 76% of mAbs from clonally expanded plasma cells had HEp-2 reactivity. Because the differentiation into plasma cells requires high-affinity interactions between the BCR and their ligand, Asano et al. went on to identify their potential antigenic targets. Three mAbs with antinuclear reactivity and from different clonal families were used in immunoprecipitation assays with HEp-2 cell lysates. Tandem mass spectrometry of the immunoprecipitates identified that the nucleolar antigens, Ki67 and HEATR as the top hits targeted by the innate-like B cells. Collectively those observations showed that a breach of Bin cell self-tolerance and strong selection for self-antigens can occur in the kidney of renal allografts during rejection.
In a subsequent analysis of 28 highly mutated mAbs expressed from Bin cells isolated from 6 patients, 21% (from 5 different patients) showed reactivity to inflamed kidney tissue. Importantly, these mAbs expressed with a FLAG tag to reduce non-specific detection showed specific nuclear or perinuclear binding, and the majority of mAbs bound to limited cell types, often tubules74. Taken together, the extensive analysis by Asano et al. showed that kidney infiltrating B cells have innate B cell phenotypes, secrete the pro-inflammatory cytokine, IL-15, and exhibit a Type 1 interferon signature74. Importantly, a small fraction of these cells respond to local antigens and undergo clonal expansion and differentiation into plasma cells, whereas the majority of graft-infiltrating B cells were likely to have been trapped in bystander B cells (Fig 1B). Future studies are needed to clarify how autoreactive B cells and antibodies contribute to rejection.
CONCLUSION
There are substantial gaps in our understanding of the immunobiology biology of innate B cells in solid organ transplantation, including what drives their loss of self-tolerance and whether their roles extend beyond the antibodies they produce. The function of the antibodies in transplantation produced by these innate B cells requires clarification, but are likely to differ from donor-specific antibodies since the former tends to recognize intracellular targets whereas the latter recognizes cell surface HLA molecules. Finally, while innate B cells have long been considered as a bridge that integrate innate and adaptive immunity, new data suggest that they may play a role in sustaining pro-inflammatory states and thus serve as a biomarker for tissue injury and chronic rejection. While clinical studies may provide associative insights into potential role, ultimately, answers to the fundamental questions of the roles innate B cells and the antibodies they produce in allograft rejection will require ex vivo experimentation, as well as in vivo investigations in pre-clinical models where innate B cell subsets are better defined.
Acknowledgments
Grant Support: This work was supported in part by grants from the National Institutes of Health (AI142747 and AI148705) to ASC.
Footnotes
Disclosure: The authors declare no conflicts of interest.
References:
- 1.Kearney JF. B cell subpopulations and secondary lymphoid organ architecture. Semin Immunol. 2008;20(1):1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Viau M, Zouali M. B-lymphocytes, innate immunity, and autoimmunity. Clin Immunol. 2005;114(1):17–26. [DOI] [PubMed] [Google Scholar]
- 3.Charmetant X, Bachelet T, Dechanet-Merville J, et al. Innate (and Innate-like) Lymphoid Cells: Emerging Immune Subsets With Multiple Roles Along Transplant Life. Transplantation. 2021;105(12):e322–e336. [DOI] [PubMed] [Google Scholar]
- 4.Milner EC, Anolik J, Cappione A, Sanz I. Human innate B cells: a link between host defense and autoimmunity? Springer Semin Immunopathol. 2005;26(4):433–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendelac A, Bonneville M, Kearney JF. Autoreactivity by design: innate B and T lymphocytes. Nat Rev Immunol. 2001;1(3):177–186. [DOI] [PubMed] [Google Scholar]
- 6.Reyneveld GI, Savelkoul HFJ, Parmentier HK. Current Understanding of Natural Antibodies and Exploring the Possibilities of Modulation Using Veterinary Models. A Review. Front Immunol. 2020;11:2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasare C, Medzhitov R. Control of B-cell responses by Toll-like receptors. Nature. 2005;438(7066):364–368. [DOI] [PubMed] [Google Scholar]
- 8.Adler LN, Jiang W, Bhamidipati K, et al. The Other Function: Class II-Restricted Antigen Presentation by B Cells. Front Immunol. 2017;8:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lund FE. Cytokine-producing B lymphocytes-key regulators of immunity. Current opinion in immunology. 2008;20(3):332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shen P, Fillatreau S. Antibody-independent functions of B cells: a focus on cytokines. Nat Rev Immunol. 2015;15(7):441–451. [DOI] [PubMed] [Google Scholar]
- 11.Palm AK, Friedrich HC, Kleinau S. Nodal marginal zone B cells in mice: a novel subset with dormant self-reactivity. Sci Rep. 2016;6:27687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011;11(1):34–46. [DOI] [PubMed] [Google Scholar]
- 13.Panda S, Ding JL. Natural antibodies bridge innate and adaptive immunity. J Immunol. 2015;194(1):13–20. [DOI] [PubMed] [Google Scholar]
- 14.Sanz I, Wei C, Jenks SA, et al. Challenges and Opportunities for Consistent Classification of Human B Cell and Plasma Cell Populations. Front Immunol. 2019;10:2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasseau A, Boudigou M, Le Pottier L, et al. Innate B Cells: the Archetype of Protective Immune Cells. Clin Rev Allergy Immunol. 2020;58(1):92–106. [DOI] [PubMed] [Google Scholar]
- 16.Marinkovic D, Marinkovic T. Putative role of marginal zone B cells in pathophysiological processes. Scand J Immunol. 2020;92(3):e12920. [DOI] [PubMed] [Google Scholar]
- 17.Palm AE, Kleinau S. Marginal zone B cells: From housekeeping function to autoimmunity? J Autoimmun. 2021;119:102627. [DOI] [PubMed] [Google Scholar]
- 18.Zouali M, Richard Y. Marginal zone B-cells, a gatekeeper of innate immunity. Front Immunol. 2011;2:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dammers PM, Visser A, Popa ER, et al. Most marginal zone B cells in rat express germline encoded Ig VH genes and are ligand selected. J Immunol. 2000;165(11):6156–6169. [DOI] [PubMed] [Google Scholar]
- 20.Hendricks J, Bos NA, Kroese FGM. Heterogeneity of Memory Marginal Zone B Cells. Crit Rev Immunol. 2018;38(2):145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balazs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17(3):341–352. [DOI] [PubMed] [Google Scholar]
- 22.MacLennan IC, Toellner KM, Cunningham AF, et al. Extrafollicular antibody responses. Immunol Rev. 2003;194:8–18. [DOI] [PubMed] [Google Scholar]
- 23.Puga I, Cols M, Barra CM, et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2011;13(2):170–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerutti A, Cols M, Puga I. Marginal zone B cells: virtues of innate-like antibody-producing lymphocytes. Nat Rev Immunol. 2013;13(2):118–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Treml LS, Carlesso G, Hoek KL, et al. TLR stimulation modifies BLyS receptor expression in follicular and marginal zone B cells. J Immunol. 2007;178(12):7531–7539. [DOI] [PubMed] [Google Scholar]
- 26.Belperron AA, Dailey CM, Bockenstedt LK. Infection-induced marginal zone B cell production of Borrelia hermsii-specific antibody is impaired in the absence of CD1d. J Immunol. 2005;174(9):5681–5686. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Uduman M, Siu JHY, et al. Spatiotemporal segregation of human marginal zone and memory B cell populations in lymphoid tissue. Nat Commun. 2018;9(1):3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weill JC, Reynaud CA. IgM memory B cells: specific effectors of innate-like and adaptive responses. Curr Opin Immunol. 2020;63:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bagnara D, Squillario M, Kipling D, et al. A Reassessment of IgM Memory Subsets in Humans. J Immunol. 2015;195(8):3716–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Attanavanich K, Kearney JF. Marginal zone, but not follicular B cells, are potent activators of naive CD4 T cells. J Immunol. 2004;172(2):803–811. [DOI] [PubMed] [Google Scholar]
- 31.Belperron AA, Dailey CM, Booth CJ, et al. Marginal zone B-cell depletion impairs murine host defense against Borrelia burgdorferi infection. Infect Immun. 2007;75(7):3354–3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubtsov AV, Swanson CL, Troy S, et al. TLR agonists promote marginal zone B cell activation and facilitate T-dependent IgM responses. J Immunol. 2008;180(6):3882–3888. [DOI] [PubMed] [Google Scholar]
- 33.Nemazee D. Natural history of MZ B cells. J Exp Med. 2021;218(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lutfi F, Wu L, Sunshine S, Cao X. Targeting the CD27-CD70 Pathway to Improve Outcomes in Both Checkpoint Immunotherapy and Allogeneic Hematopoietic Cell Transplantation. Front Immunol. 2021;12:715909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agematsu K. Memory B cells and CD27. Histol Histopathol. 2000;15(2):573–576. [DOI] [PubMed] [Google Scholar]
- 36.Tierens A, Delabie J, Michiels L, et al. Marginal-zone B cells in the human lymph node and spleen show somatic hypermutations and display clonal expansion. Blood. 1999;93(1):226–234. [PubMed] [Google Scholar]
- 37.Tull TJ, Pitcher MJ, Guesdon W, et al. Human marginal zone B cell development from early T2 progenitors. J Exp Med. 2021;218(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kantor AB, Stall AM, Adams S, et al. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A. 1992;89(8):3320–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kantor AB, Herzenberg LA. Origin of murine B cell lineages. Annu Rev Immunol. 1993;11:501–538. [DOI] [PubMed] [Google Scholar]
- 40.Hayakawa K, Hardy RR, Parks DR, et al. The “Ly-1 B” cell subpopulation in normal immunodefective, and autoimmune mice. J Exp Med. 1983;157(1):202–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroese FG, Ammerlaan WA, Deenen GJ. Location and function of B-cell lineages. Ann N Y Acad Sci. 1992;651:44–58. [DOI] [PubMed] [Google Scholar]
- 42.Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7(3):293–301. [DOI] [PubMed] [Google Scholar]
- 43.Jensen CT, Kharazi S, Boiers C, et al. FLT3 ligand and not TSLP is the key regulator of IL-7-independent B-1 and B-2 B lymphopoiesis. Blood. 2008;112(6):2297–2304. [DOI] [PubMed] [Google Scholar]
- 44.Hardy RR. B-1 B cell development. J Immunol. 2006;177(5):2749–2754. [DOI] [PubMed] [Google Scholar]
- 45.Duber S, Hafner M, Krey M, et al. Induction of B-cell development in adult mice reveals the ability of bone marrow to produce B-1a cells. Blood. 2009;114(24):4960–4967. [DOI] [PubMed] [Google Scholar]
- 46.Holodick NE, Tumang JR, Rothstein TL. Immunoglobulin secretion by B1 cells: differential intensity and IRF4-dependence of spontaneous IgM secretion by peritoneal and splenic B1 cells. Eur J Immunol. 2010;40(11):3007–3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gronwall C, Vas J, Silverman GJ. Protective Roles of Natural IgM Antibodies. Front Immunol. 2012;3:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Berland R, Wortis HH. Origins and functions of B-1 cells with notes on the role of CD5. Annu Rev Immunol. 2002;20:253–300. [DOI] [PubMed] [Google Scholar]
- 49.Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol. 2012;188(3):939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zimecki M, Whiteley PJ, Pierce CW, Kapp JA. Presentation of antigen by B cells subsets. I. Lyb-5+ and Lyb-5- B cells differ in ability to stimulate antigen specific T cells. Arch Immunol Ther Exp (Warsz). 1994;42(2):115–123. [PubMed] [Google Scholar]
- 51.Zhong X, Gao W, Degauque N, et al. Reciprocal generation of Th1/Th17 and T(reg) cells by B1 and B2 B cells. Eur J Immunol. 2007;37(9):2400–2404. [DOI] [PubMed] [Google Scholar]
- 52.Chousterman BG, Swirski FK. Innate response activator B cells: origins and functions. Int Immunol. 2015;27(10):537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baumgarth N. B-1 Cell Heterogeneity and the Regulation of Natural and Antigen-Induced IgM Production. Front Immunol. 2016;7:324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Griffin DO, Rothstein TL. A small CD11b(+) human B1 cell subpopulation stimulates T cells and is expanded in lupus. J Exp Med. 2011;208(13):2591–2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Zhurbenko N, Quach TD, Hopkins TJ, et al. , Hernandez AM. Human B-1 Cells and B-1 Cell Antibodies Change With Advancing Age. Frontiers in immunology. 2019;10:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cordero H, King RG, Dogra P, et al. Intrathymic differentiation of natural antibody-producing plasma cells in human neonates. Nat Commun. 2021;12(1):5761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cascalho M, Platt JL. TNFRSF13B Diversification Fueled by B Cell Responses to Environmental Challenges-A Hypothesis. Front Immunol. 2021;12:634544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsuji S, Cortesao C, Bram RJ, et al. TACI deficiency impairs sustained Blimp-1 expression in B cells decreasing long-lived plasma cells in the bone marrow. Blood. 2011;118(22):5832–5839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Salzer U, Chapel HM, Webster AD, et al. Mutations in TNFRSF13B encoding TACI are associated with common variable immunodeficiency in humans. Nat Genet. 2005;37(8):820–828. [DOI] [PubMed] [Google Scholar]
- 60.Castigli E, Wilson SA, Garibyan L, et al. TACI is mutant in common variable immunodeficiency and IgA deficiency. Nat Genet. 2005;37(8):829–834. [DOI] [PubMed] [Google Scholar]
- 61.de Mattos Barbosa MG, Lefferts AR, Huynh D, et al. TNFRSF13B genotypes control immune-mediated pathology by regulating the functions of innate B cells. JCI Insight. 2021;6(17). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li H, Limenitakis JP, Greiff V, et al. Mucosal or systemic microbiota exposures shape the B cell repertoire. Nature. 2020;584(7820):274–278. [DOI] [PubMed] [Google Scholar]
- 63.Bunker JJ, Erickson SA, Flynn TM, et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science. 2017;358(6361). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arthur CM, Sullivan HC, Gerner-Smidt C, et al. Microbial Exposure Regulates the Development of Anti-Blood Group Antibodies. Blood. 2016;128(22). [Google Scholar]
- 65.Gunti S, Notkins AL. Polyreactive Antibodies: Function and Quantification. J Infect Dis. 2015;212 Suppl 1:S42–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.See SB, Mantell BS, Clerkin KJ, et al. Profiling non-HLA antibody responses in antibody-mediated rejection following heart transplantation. Am J Transplant. 2020;20(9):2571–2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zorn E. New insights on innate B-cell immunity in transplantation. Xenotransplantation. 2018;25(3):e12417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Porcheray F, DeVito J, Helou Y, et al. Expansion of polyreactive B cells cross-reactive to HLA and self in the blood of a patient with kidney graft rejection. Am J Transplant. 2012;12(8):2088–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kinnunen T, Chamberlain N, Morbach H, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121(9):1595–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chatterjee D, Moore C, Gao B, et al. Prevalence of polyreactive innate clones among graft--infiltrating B cells in human cardiac allograft vasculopathy. J Heart Lung Transplant. 2018;37(3):385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gao B, Moore C, Porcheray F, et al. Pretransplant IgG reactivity to apoptotic cells correlates with late kidney allograft loss. Am J Transplant. 2014;14(7):1581–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.See SB, Aubert O, Loupy A, et al. Post-Transplant Natural Antibodies Associate with Kidney Allograft Injury and Reduced Long-Term Survival. J Am Soc Nephrol. 2018;29(6):1761–1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.See SB, Clerkin KJ, Kennel PJ, et al. Ventricular assist device elicits serum natural IgG that correlates with the development of primary graft dysfunction following heart transplantation. J Heart Lung Transplant. 2017;36(8):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Asano Y, Daccache J, Jain D, et al. Innate-like self-reactive B cells infiltrate human renal allografts during transplant rejection. Nat Commun. 2021;12(1):4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Heng TS, Painter MW, Immunological Genome Project C. The Immunological Genome Project: networks of gene expression in immune cells. Nat Immunol. 2008;9(10):1091–1094. [DOI] [PubMed] [Google Scholar]
- 76.Kanti Ghosh A, Sinha D, Mukherjee S, et al. IL-15 temporally reorients IL-10 biased B-1a cells toward IL-12 expression. Cell Mol Immunol. 2016;13(2):229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pavlakis M, Strehlau J, Lipman M, et al. Intragraft IL-15 transcripts are increased in human renal allograft rejection. Transplantation. 1996;62(4):543–545. [DOI] [PubMed] [Google Scholar]
- 78.Zheng XX, Gao W, Donskoy E, et al. An antagonist mutant IL-15/Fc promotes transplant tolerance. Transplantation. 2006;81(1):109–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paus D, Phan TG, Chan TD, et al. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. The Journal of experimental medicine. 2006;203(4):1081–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.O’Connor BP, Vogel LA, Zhang W, et al. Imprinting the fate of antigen-reactive B cells through the affinity of the B cell receptor. J Immunol. 2006;177(11):7723–7732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sciammas R, Shaffer AL, Schatz JH, et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity. 2006;25(2):225–236. [DOI] [PubMed] [Google Scholar]
- 82.Phan TG, Gardam S, Basten A, et al. Altered migration, recruitment, and somatic hypermutation in the early response of marginal zone B cells to T cell-dependent antigen. J Immunol. 2005;174(8):4567–4578. [DOI] [PubMed] [Google Scholar]
- 83.Tsay GJ, Zouali M. The Interplay Between Innate-Like B Cells and Other Cell Types in Autoimmunity. Front Immunol. 2018;9:1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaminski DA, Stavnezer J. Enhanced IgA class switching in marginal zone and B1 B cells relative to follicular/B2 B cells. J Immunol. 2006;177(9):6025–6029. [DOI] [PubMed] [Google Scholar]
- 85.Roy B, Shukla S, Lyszkiewicz M, et al. Somatic hypermutation in peritoneal B1b cells. Mol Immunol. 2009;46(8–9):1613–1619. [DOI] [PubMed] [Google Scholar]