Abstract
Aims
Islet autoantibody screening of infants and young children in the Northern Hemisphere, together with semi-annual metabolic monitoring, is associated with a lower risk of ketoacidosis (DKA) and improved glucose control after diagnosis of clinical (stage 3) type 1 diabetes (T1D). We aimed to determine if similar benefits applied to older Australians and New Zealanders monitored less rigorously.
Methods
DKA occurrence and metabolic control were compared between T1D relatives screened and monitored for T1D and unscreened individuals diagnosed in the general population, ascertained from the Australasian Diabetes Data Network.
Results
Between 2005 and 2019, 17,105 relatives (mean (SD) age 15.7 (10.8) years; 52% female) were screened for autoantibodies against insulin, glutamic acid decarboxylase and insulinoma-associated protein 2. Of these, 652 screened positive to a single and 306 to multiple autoantibody specificities, of whom 201 and 215, respectively, underwent metabolic monitoring. Of 178 relatives diagnosed with stage 3 T1D, 9 (5%) had DKA, 7 of whom had not undertaken metabolic monitoring. The frequency of DKA in the general population was 31%. After correction for age, sex and T1D family history, the frequency of DKA in screened relatives was >80% lower than in the general population. HbA1c and insulin requirements following diagnosis were also lower in screened relatives, consistent with greater beta cell reserve.
Conclusions
T1D autoantibody screening and metabolic monitoring of older children and young adults in Australia and New Zealand, by enabling pre-clinical diagnosis when beta cell reserve is greater, confers protection from DKA. These clinical benefits support ongoing efforts to increase screening activity in the region and should facilitate the application of emerging immunotherapies.
Keywords: Adolescent, Australasian Diabetes Data Network, beta cell, child, diabetic ketoacidosis, HbA1c, IDAA1c, insulin dose, Intranasal Insulin Trial, islet autoimmunity, screening, TrialNet, type 1 diabetes
Introduction
Insulin therapy for type 1 diabetes (T1D) carries a large treatment burden and does not fully alleviate the risks of vascular disease and premature death (1). To improve outcomes, immune therapies targeting islet autoimmunity to prevent progression from pre-clinical (stage 1 and 2) to clinical (stage 3) disease are being developed (2). Prevention is critically dependent on identifying high-risk individuals with circulating islet autoantibodies. This presents a challenge requiring a paradigm shift in clinical practice, given that the disease has traditionally been diagnosed in its end-stage as a metabolic disorder and a rationale for early pre-clinical diagnosis is not generally recognised (3).
Autoantibodies against insulin (IAA), glutamic acid decarboxylase (GADA), and insulinoma-associated protein-2 (IA-2A) reliably identify children and young adults destined to develop insulin-requiring (stage 3) T1D (4, 5). In birth cohort studies of genetically at-risk European and North American children, early diagnosis through autoantibody screening and metabolic monitoring every 6–12 months is associated with a significantly lower frequency of diabetic ketoacidosis (DKA) at diagnosis of stage 3 disease (~5% compared to 30–50% in the general population) and, over the first year, with better glucose control and a lower insulin requirement (6–9). Studies of young children (age 2 to 6 years) in Germany have also shown that islet autoantibody screening affords protection from DKA irrespective of familial risk (10, 11).
In older populations, the effect of screening on DKA, glycaemia and insulin requirement is less certain. The Diabetes Prevention Trial – Type 1 (DPT-1) demonstrated a low DKA frequency of 4% in antibody-positive children and young adults (mean age 10 years) who were screened and then monitored closely for progression to stage 3 disease (12). However, it is not known whether screening older children without mandating rigorous clinical trial monitoring protects against DKA. Demonstration of such a clinical benefit is particularly relevant to older children and young adults, whose ongoing participation in screening programs and immunotherapy trials will be essential for the development of more effective interventions (13).
In Australia and New Zealand, autoantibody screening for families was introduced in 1988 in Melbourne (14) and expanded in 2005 to cover Australian and New Zealand sites participating in international studies administered by TrialNet (15) and the Intranasal Insulin Trials (INITs) (16–18). In 2019, in response to the closure of INIT screening and funding cuts to TrialNet, Type1Screen (ACTRN12620000510943) was established to provide ongoing autoantibody screening for Australasian families. In the present study, we aimed to determine the frequency of DKA at diagnosis and the subsequent trajectories of HbA1c, insulin use and beta cell function of children and young adults screened by TrialNet and INIT, compared to these outcomes in the individuals with T1D in the general population enrolled in the Australasian Diabetes Data Network (ADDN) (19).
Methods
Adult participants and guardians gave informed consent and children gave assent to join TrialNet, INIT and ADDN. Study protocols were approved by the relevant human research ethics committees and carried out in accordance with the Declaration of Helsinki as revised in 2008. In 2021, the Melbourne Health Human Research Ethics Committee approved the study protocol and a waiver of consent to allow linkage of date of birth, sex and postcode information between TrialNet, INIT and ADDN.
Study populations
Clinical sites that contributed to TrialNet, INIT and ADDN are listed in the Table S1. Autoantibody assays used for TrialNet and INIT are included in the Islet Autoantibody Standardisation Program (20).
Established in 2005, TrialNet enrolled first-, second- and third-degree relatives aged 1 to 45 years in Australia and New Zealand to be screened for IAA, GADA and IA-2A by a central laboratory based in the U.S.A. Data for 8,769 screened relatives were obtained in September 2019. Participants who screened negative and were aged under 18 years were, until 2017, offered follow-up with annual telephone contact to ask about hyperglycaemia symptoms and to offer repeat autoantibody testing. Participants who tested positive to one or more autoantibodies and a small subgroup who screened negative were also offered metabolic monitoring by semi-annual HbA1c and oral glucose tolerance testing.
The INIT II randomised trial showed that a year of treatment with intranasal insulin did not decrease the risk of disease progression from stage 1 to stage 3 T1D (18). Screening for INIT was performed for first- or second-degree relatives aged 4 to 30 years between April 2006 and May 2016. Autoantibodies were measured by the Royal Melbourne Hospital Endocrine Laboratory, which participates in the Islet Autoantibody Standardization Program. Data for 9,715 individuals screened in Australia and New Zealand were obtained in January 2020. Participants who tested positive to a single autoantibody were offered repeat autoantibody testing annually and those with multiple autoantibodies were invited to join the INIT intervention study and undertake eligibility testing. Ninety-four participants enrolled in the intervention (nasal insulin or placebo) and were monitored with semi-annual HbA1c and oral glucose tolerance test (OGTT).
The ADDN database collates routine clinic data from people with T1D attending clinical centers in Australia and New Zealand, including most of the sites that enrolled for TrialNet and INIT (Table S1) (19). All participants provided consent for their data to be used for unspecified research. ADDN data were current up to October 2019.
Data linkage
To identify individuals who participated in both TrialNet and INIT, we considered those with matching sex, date of birth, geographical region (Australian State/Territory or New Zealand North or South Island) and date of sample collection as the same individual (N=1379 duplicate screens). TrialNet/INIT participants were linked to ADDN on the basis of matching sex, date of birth, geographical region and, if available, postcode. The accuracy of data linkage was assessed for 25 relatives screened for TrialNet/INIT at Royal Melbourne Hospital and registered with ADDN through the hospital clinic after diagnosis of stage 3 T1D; all were correctly matched.
Diagnosis of diabetes, ketoacidosis and insulin dose-adjusted HbA1c
TrialNet and INIT used the standard definition of stage 3 T1D (21), namely fasting glucose ≥7.0 mmol/L or random glucose ≥11.1 mmol/L or glucose two hours following oral glucose challenge (1.75g/kg to a maximum of 75g) ≥11.1 mmol/L or HbA1c ≥48mmol/mol (6.5%). In the absence of symptoms of hyperglycaemia, two or more abnormal measures were required to confirm the diagnosis. Individuals who had been diagnosed with diabetes by an external practitioner were identified by telephone or email follow-up as part of TrialNet and INIT, or through data linkage with ADDN. DKA in TrialNet, INIT and ADDN was defined according to ISPAD criteria (22): venous blood pH<7.3 or venous bicarbonate <15 mmol/L and blood beta-hydroxybutyrate >3 mmol/L or moderate to large ketonuria on urinalysis. The DKA status of 105 screened relatives was also recorded in ADDN. In all cases, the DKA classifications in TrialNet/INIT agreed with those in ADDN.
Insulin dose-adjusted HbA1c (IDAA1c) was calculated as 4 × daily insulin dose per kilogram body weight added to HbA1c (in percentage units) (23).
Statistical methods
To compare outcomes between TrialNet/INIT and ADDN, ADDN data were restricted to participants who were diagnosed in either Australia or New Zealand at a comparable age (2 to 48 years) and during the same epoch (2006 to 2019).
The DKA analysis compared 6627 ADDN registrants to 175 relatives screened in TrialNet/INIT for whom DKA status (2 missing) and sex (1 missing) were known. Data for relatives screened in TrialNet/INIT were removed from the ADDN dataset before the two groups were compared. To compare frequencies of DKA at diagnosis, a generalised linear model with a binomial distribution and a log link (log-binomial model) was fitted. The exposure variable was participant group (TrialNet/INIT versus ADDN) and the response was presence or absence of DKA, with adjustment for age at diagnosis and sex. To determine an effect of family history of T1D, DKA frequency in a subset of 142 ADDN registrants identified as having a first-degree relative with T1D (and who were not screened in TrialNet/INIT) was compared to that of the 175 TrialNet/INIT participants.
To determine HbA1c, insulin use and IDAA1c outcomes over the three years following diagnosis of stage 3 T1D we used data from 7395 ADDN registrants, 91 of whom had been screened for autoantibodies in TrialNet or INIT. Where an individual had two or more visits within a single month, values were averaged to ensure no more than one record in each visit period (Table S2). To compare groups over time, a linear mixed model was fitted. The outcome was HbA1c, insulin dose or IDAA1c and the exposure variables were participant group (TrialNet/INIT or ADDN) and month since diagnosis both fitted as factors, and their interaction. A random term for participant ID was included to allow for correlation between visits for the same participant. Outcomes were adjusted for age at diagnosis, sex and age at visit. If a significant interaction was found, comparison of trial means at a specific month were made using a Tukey test.
A total of 3378 relatives were re-screened for autoantibodies in TrialNet and INIT. In this group, the incidence of stage 3 T1D, stratified for autoantibody number (zero, one, two or more) at the initial screen was compared using logrank tests.
R software (v4.0.4; www.r-project.org) was used for all analyses except for logrank tests performed with Prism Software (v9.0.0; Graphpad, San Diego, CA). A significance level of 5% was used for all analyses.
Results
Between 2005 and 2019, TrialNet and INIT performed islet autoantibody screening on 17,105 T1D relatives aged between 1 and 45 years living in Australia and New Zealand (Figure 1). The mean (SD) age of participants at screening was 15.7 (10.8) years, 52% were female, and their geographical distribution reflected that of the general population (Figure S1). An autoantibody against either insulin, GAD or IA-2 was detected in 652 (3.8%) participants and another 306 (1.8%) were positive for multiple autoantibodies. Follow-up with repeat autoantibody testing was performed for most multiple autoantibody-positive participants (224 of 306; 73%), and for a lower proportion of those with a single autoantibody (332 of 652; 51%) or who were autoantibody-negative (2,822 of 16,147; 17%). Repeat autoantibody testing identified 100 participants who developed multiple autoantibodies subsequent to their first screening test. However, most individuals with multiple autoantibodies (306/406 or 75%) were identified at their first screening visit (Figure 1).
Figure 1. Participant flow diagram.
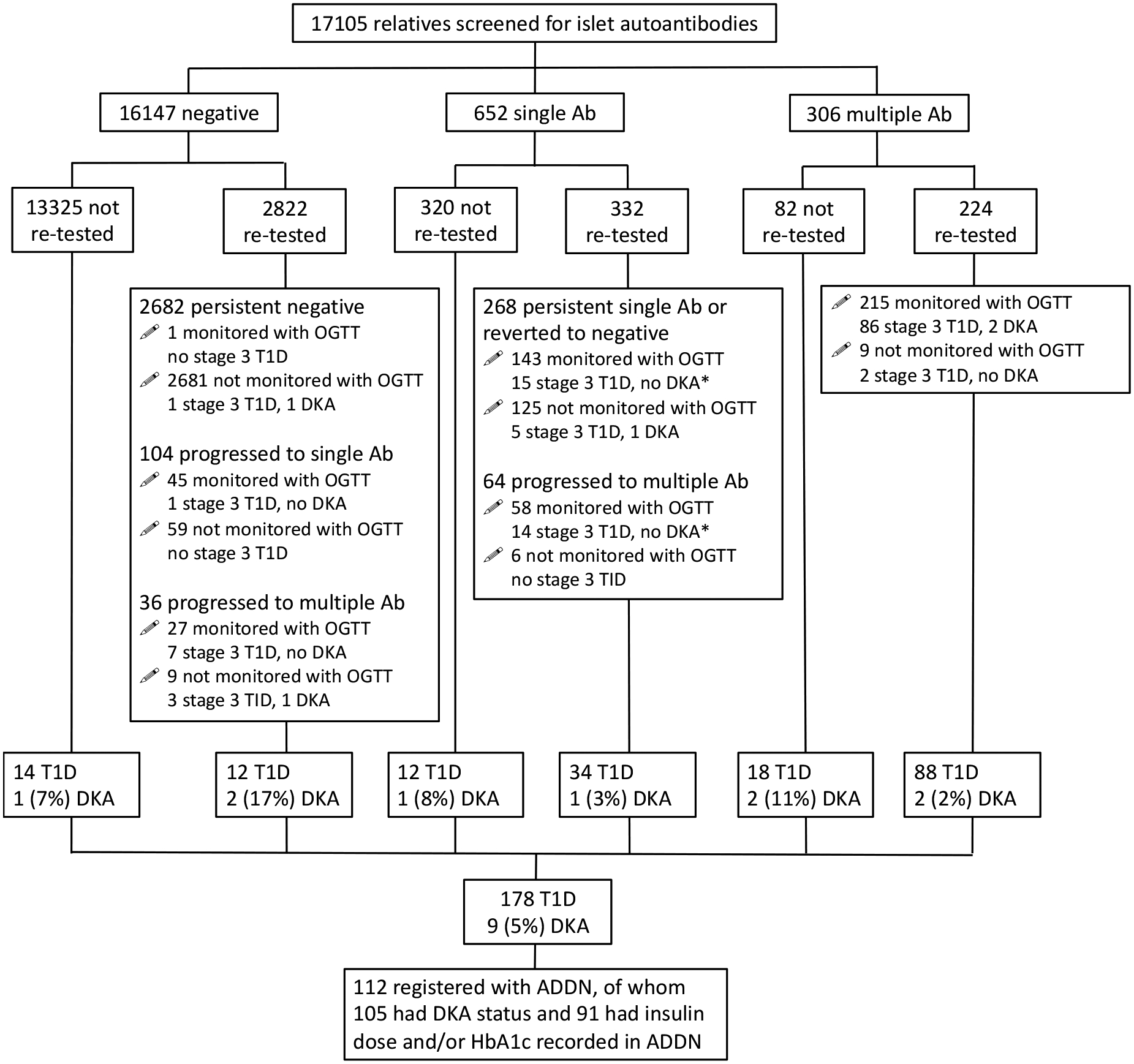
Ab: autoantibody; T1D: diagnosed with stage 3 type 1 diabetes; DKA: presence of diabetic ketoacidosis at diagnosis; ADDN: Australasian Diabetes Data Network; OGTT: oral glucose tolerance test; * DKA status unknown for 1 individual.
A total of 178 screened relatives were observed to develop clinical (stage 3) T1D, most of whom had been re-tested for autoantibodies and subsequently monitored with one or more OGTTs (Figure 1). Twenty-six cases, comprising just 0.02% of 16,147, occurred in registrants who had initially screened negative to autoantibodies. A higher frequency of stage 3 T1D was observed in those who screened positive for a single autoantibody (46; 7%) or for multiple autoantibodies (106; 35%).
Using data for the 3,378 screened relatives who underwent autoantibody re-testing with or without metabolic monitoring, the risk of disease progression to stage 3 T1D progressively increased with detection of 0, 1 or ≥2 autoantibodies on the first screening test (p <0.001 for each comparison, Figure 2), the five-year frequencies being 0.3%, 8.5% and 33%, respectively. Disease progression was not different between TrialNet and INIT (data not shown).
Figure 2. Incidence of stage 3 type 1 diabetes after the first screening test.
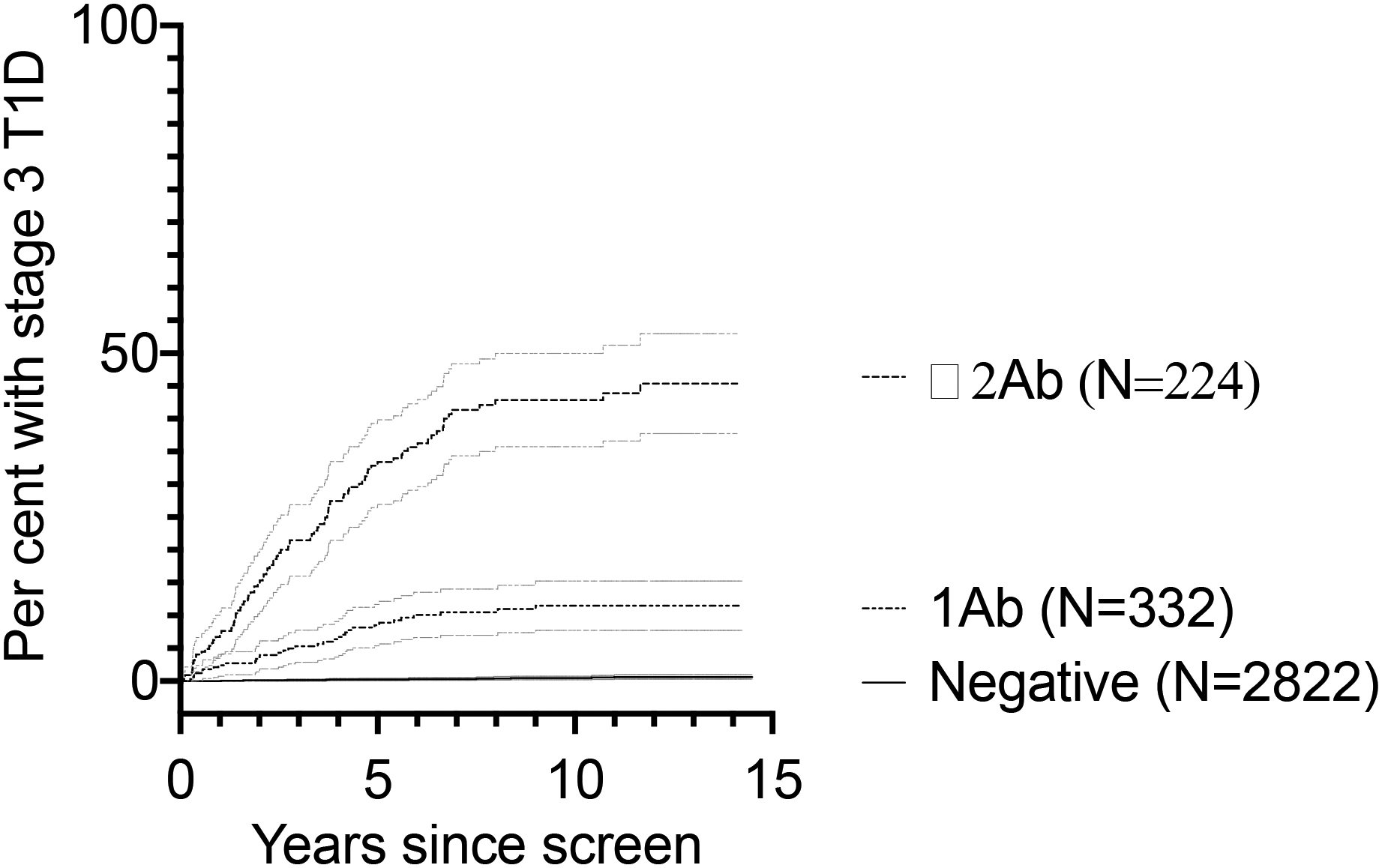
Data are for 3378 participants who were re-tested for autoantibodies in TrialNet and INIT, stratified for autoantibody status at the initial screen, with grey lines indicating 95% confidence boundaries.
Information on DKA at diagnosis was available for 176 of the 178 relatives who developed stage 3 T1D after being screened; nine (5%) had DKA (Figure 1). Three were female and all were under 16 years of age, their mean (SD) age of diabetes diagnosis with DKA being 13.5 (3.1) years. DKA occurred more frequently in the 55 relatives who had not undertaken metabolic monitoring by OGTT compared to the 123 who did (7 [13%] vs 2 [2%]; p=0.0042).
In screened relatives, the mean (SD) age of diagnosis was 13.4 (7.3) years compared to 10.0 (5.8) years in the general population, with 44 and 48% females respectively. The frequency of DKA in the ADDN general population was 31%. After adjustment for age of diagnosis and sex, the risk of DKA remained markedly lower at 5.4 (95%CI 2.9 to 10.3) per cent in screened relatives compared to 30.7 (95%CI 29.6 to 31.8) per cent in the general population, or a decrease in DKA frequency of 82% (p<0.001).
To determine if a family history of T1D may have contributed to the lower frequency of DKA in TrialNet/INIT participants, we calculated DKA frequency in a subgroup of 142 within the ADDN general population group (46% female) who had a first-degree relative with T1D but were not screened in TrialNet/INIT. The mean (SD) age of this group of 9.6 (5.5) years. In this comparison, the risk of DKA after adjusting for age of diagnosis and sex was 4.5 (95%CI 2.3 to 8.9) per cent in TrialNet/INIT and 26.3 (95%CI 19.0 to 36.5) per cent for ADDN, equivalent to an 83% risk reduction (p<0.001).
A total of 7,395 ADDN registrants had data for HbA1c and/or insulin use recorded on at least one occasion during the three years and one month following diagnosis, including 91 who had been screened for T1D in TrialNet/INIT. To compare their HbA1c, insulin requirement and beta cell function (IDAA1c) following diagnosis of stage 3 T1D, linear mixed models were fitted (Figure 3 and supplemental Table S2). Significant interactions between visit and participant groups were found for HbA1c (p <0.001), insulin dose (p<0.001) and IDAA1c (p<0.001). At one month following diagnosis, HbA1c in mmol/mol (68 (95%CI 63 to 73) c.f. 97 (95%CI 96 to 97)) and daily insulin dose in units/kg (0.33 (95%CI 0.21 to 0.44) c.f. 0.74 (95%CI 0.73 to 0.75)) were significantly lower in screened relatives compared to the general population. IDAA1c was significantly lower in screened relatives at months 1 and 2 following diagnosis of stage 3 T1D, consistent with greater residual beta cell function early after diagnosis of stage 3 T1D. However, no significant differences in either HbA1c, insulin requirement or IDAA1c were observed beyond the first two months.
Figure 3. Insulin requirement, HbA1c and beta cell function over three years following diagnosis of stage 3 type 1 diabetes.
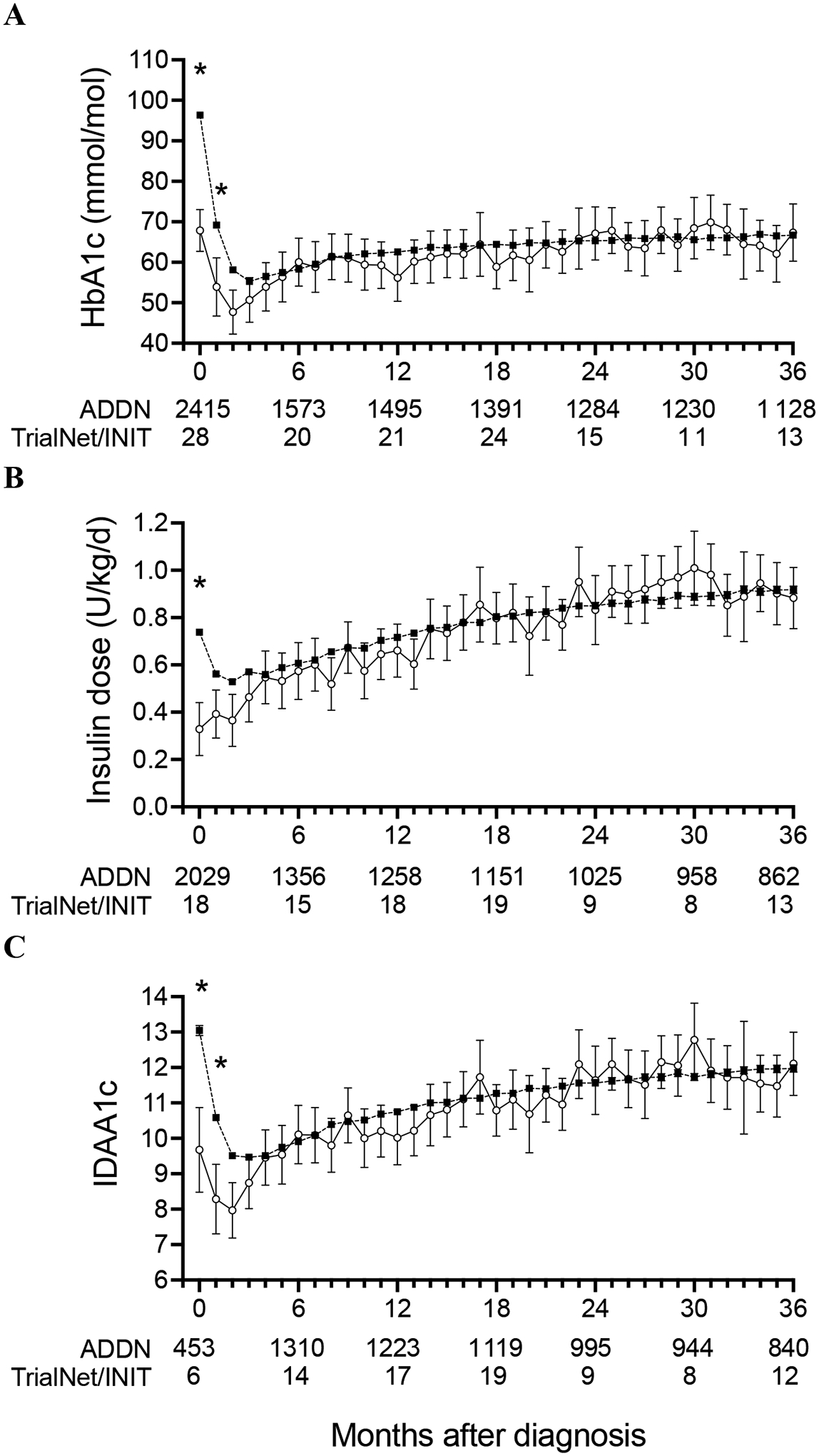
Monthly measures of HbA1c (A), insulin dose (B) and IDAA1c (C) for relatives screened in TrialNet/INIT (open circles) and the general population registered in ADDN (filled squares) are shown as predicted mean and 95%CI. The number of individuals contributing to each 6-month data point is provided, with values for each month provided in Supplemental Table S2, with different individuals potentially contributing to different time points. * indicates significant difference (Tukey<0.05) between ADDN and TrialNet/INIT groups at that time point.
Discussion
In T1D relatives in Australia and New Zealand, we demonstrate that islet autoantibody screening is associated with a lower risk of DKA, particularly when combined with metabolic monitoring by OGTT. Screening was also associated with lower HbA1c and insulin requirement at diagnosis of stage 3 T1D, consistent with greater beta cell function. To our knowledge, this is the first time these benefits of early pre-clinical diagnosis have been demonstrated in an older population screened for autoantibodies and monitored infrequently by OGTT. This population will continue to be critically important for the development of preventative immunotherapies by contributing the majority of participants to phase II clinical trials.
DKA at T1D diagnosis is a potentially life-threatening complication that has increased in frequency over the last decade (24–26) to affect around one in three children with newly-diagnosed T1D (27). We found that participation in islet autoantibody screening and metabolic monitoring programs in our region was associated with >80% lower risk of this serious complication irrespective of family history of T1D. This degree of benefit is similar to that observed in genetically at-risk children followed from birth with autoantibody testing and metabolic monitoring, whose DKA frequencies ranged from 3–15% compared to 17–36% for age-matched children who were not screened (7, 10, 28). In contrast to these birth cohort studies, the participants in our study were first screened for islet autoantibodies at a much older age (mean (SD) 15.7 (10.8) years) and were less frequently re-tested and monitored for progression to stage 3 disease. Although autoantibody screening and metabolic monitoring is not currently considered cost-effective (29), our demonstration that less intense follow-up can provide comparable protection from DKA suggests that even simpler, less costly care pathways could be developed to provide economic justification for T1D screening, particularly if combined with cheaper autoantibody assays (30) and more effective immunotherapy (13).
Because beta cell function declines rapidly during progression from stage 2 to stage 3 T1D (31), pre-clinical diagnosis by islet autoantibody screening is expected to identify incipient stage 3 individuals who have relatively high beta cell reserve. This ‘lead time’ effect has been directly confirmed by the demonstration that C-peptide concentrations are higher in children who developed stage 3 disease in the TEDDY birth cohort study compared to age-matched children diagnosed in the community (6) and is supported by several other studies of young children which show that screening is associated with lower HbA1c and insulin requirements at diagnosis (8–10). We extend this finding to an older population by demonstrating in our cohort that HbA1c, insulin requirement and the IDAA1c measure of beta cell function are superior at diagnosis in screened populations. Within the first two months from diagnosis, a majority of screened individuals and only a minority of those in the general population entered partial disease remission, defined as an IDAA1c value of less than 9 (23). Although this metabolic benefit did not endure beyond two months, this time window is sufficiently wide to provide immunotherapy, which is currently most effective when there is significant residual beta cell function to rescue from ongoing autoimmune attack (31). Early diagnosis to identify individuals with preserved beta cell function also has important implications for future trials of immunotherapy in stage 3 T1D. Previous trials, summarised in (2), have mostly recruited individuals presenting with symptomatic hyperglycaemia and limited beta cell function. None of the immunotherapies that met the primary outcome of preserving C-peptide in stage 3 T1D actually delivered sufficient clinical benefit to justify regulatory approval. It is interesting to speculate whether these trials might have been more successful had they been conducted in individuals diagnosed with asymptomatic (‘silent’) stage 3 T1D through a screening program. Preservation of beta cell function with a delay in the need to initiate insulin therapy would provide stronger evidence to support regulatory approval of immune therapy and might also provide opportunities to use adjunctive therapies such as SGLT inhibitors (32) or GLP-1 agonists (33) to achieve acceptable glucose control in the longer term without the complexity and risks of insulin therapy.
Our data identified many individuals who were not followed-up with metabolic testing after their initial screen, even if it returned multiple islet autoantibodies. Such individuals were more likely to develop DKA than their counterparts who underwent monitoring. This highlights a deficiency in care pathways that must be rectified to maximise the ability of screening to protect from DKA and to ensure most at-risk individuals have opportunities to access timely immunotherapy. The Type1Screen program in our region aims to improve retention and follow-up of screened relatives by employing dedicated nurse educators and communications personnel.
For the 224 multi-antibody individuals followed in TrialNet or INIT, the 5-year incidence of stage 3 T1D of 33% was comparable to that observed in the entire TrialNet Pathway to Prevention Study cohort, which mostly comprises children and young adults from North America (32). This rate of disease progression is somewhat lower than the rate of ~40% observed in younger children (4) and for individuals who enrolled in the TrialNet oral insulin study, which involved a slightly younger population (mean age 8 years) who had tested positive for IAA (34). The lower rate of progression in older multi-autoantibody children has implications for the design of future immunotherapy trials, which for reasons of safety and feasibility will continue to recruit individuals who are screened as adolescents or adults.
A clear limitation of our study design was its inability to identify all known cases of T1D, which is particularly relevant for TrialNet/INIT participants who were not re-tested for autoantibodies or metabolically monitored after their initial screen, and for those whose diabetes was managed outside of the ADDN network. Such individuals may have been less attuned to symptoms of diabetes or less able to seek help if symptoms developed, which in turn may have placed them at greater risk of DKA. It is also possible that unmeasured differences between families that participated in TrialNet/INIT and those in the general ADDN population, such as T1D knowledge or access to medical care, could have accounted for the higher frequency of DKA in ADDN registrants who had a first degree relative. In addition, although screening is being extended into the general population (2), whether our findings from screening relatives also apply to screening individuals without a family history will require further study. In this regard, it is encouraging that the Fr1da general population screening study reported only two cases of DKA in 62 cases of stage 3 T1D (11).
In summary, in a large representative population of Australia and New Zealand, islet autoantibody screening of children and young adults, combined with metabolic monitoring, was associated with protection from DKA and greater beta cell function. These findings add support to recent efforts to offer screening to the Australian general population. Communicating these clinical benefits to clinicians, at-risk individuals and regulators should help encourage recruitment into screening programs and increase enrollment into clinical trials that will be needed to deliver prevention therapies to the clinic.
Supplementary Material
Acknowledgements
We are grateful to the many participants, study investigators and coordinators who contributed to data used for this work. INIT was supported by JDRF. TrialNet is supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). ADDN is supported by JDRF Australia, the recipient of the Australian Research Council Special Research Initiative in Type 1 Juvenile Diabetes. ADDN investigators are listed in Table S3. Data were supplied by the NIDDK Central Repositories and the ADDN data registry. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Funding statement
This work was supported by JDRF (1-SRA-2020-900 to JMW and PGC) and the Australian National Health and Medical Research Council (NHMRC) (Program Grant APP 1150425 and Leadership Investigator Grant APP 1173945 to LCH). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian National Health and Medical Research Council Research Institute Infrastructure Support Scheme.
We acknowledge the support of the Type 1 Diabetes TrialNet Study Group, which identified study participants and provided samples and follow-up data for this study. The Type 1 Diabetes TrialNet Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development, through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085453, U01 DK085461, U01 DK085465, U01 DK085466, U01 DK085476, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, UC4 DK11700901, U01 DK 106693-02, and the JDRF. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Footnotes
Conflict of interest statement
None of the authors reports a conflict of interest
Statement of Informed Consent
Informed consent was obtained from all individual participants and, for children, their parents or legal guardians.
Conflict of interest disclosure
None of the authors has a relevant conflict of interest to declare
Ethics approval statement
Study protocols were approved by the relevant human research ethics committees and carried out in accordance with the Declaration of Helsinki as revised in 2008. In 2021, the Melbourne Health Human Research Ethics Committee approved the study protocol and a waiver of consent to allow linkage of date of birth, sex and postcode information between TrialNet, INIT and ADDN.
Patient consent statement (if appropriate)
Adult participants and guardians gave informed consent and children gave assent to join TrialNet, INIT and ADDN.
References
- 1.Rawshani A, Sattar N, Franzen S, Rawshani A, Hattersley AT, Svensson AM, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018;392(10146):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenbaum CJ. A Key to T1D Prevention: Screening and Monitoring Relatives as Part of Clinical Care. Diabetes. 2021;70(5):1029–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couper JJ, Harrison LC. Controversies in medicine: redefining the diagnosis of type 1 diabetes. Med J Aust. 2019;211(4):157–9 e1. [DOI] [PubMed] [Google Scholar]
- 4.Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013;309(23):2473–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bingley PJ. Clinical applications of diabetes antibody testing. J Clin Endocrinol Metab. 2010;95(1):25–33. [DOI] [PubMed] [Google Scholar]
- 6.Steck AK, Larsson HE, Liu X, Veijola R, Toppari J, Hagopian WA, et al. Residual beta-cell function in diabetes children followed and diagnosed in the TEDDY study compared to community controls. Pediatr Diabetes. 2017;18(8):794–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elding Larsson H, Vehik K, Bell R, Dabelea D, Dolan L, Pihoker C, et al. Reduced prevalence of diabetic ketoacidosis at diagnosis of type 1 diabetes in young children participating in longitudinal follow-up. Diabetes Care. 2011;34(11):2347–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundgren M, Jonsdottir B, Elding Larsson H, DiPi Ssg. Effect of screening for type 1 diabetes on early metabolic control: the DiPiS study. Diabetologia. 2019;62(1):53–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barker JM, Goehrig SH, Barriga K, Hoffman M, Slover R, Eisenbarth GS, et al. Clinical characteristics of children diagnosed with type 1 diabetes through intensive screening and follow-up. Diabetes Care. 2004;27(6):1399–404. [DOI] [PubMed] [Google Scholar]
- 10.Winkler C, Schober E, Ziegler AG, Holl RW. Markedly reduced rate of diabetic ketoacidosis at onset of type 1 diabetes in relatives screened for islet autoantibodies. Pediatr Diabetes. 2012;13(4):308–13. [DOI] [PubMed] [Google Scholar]
- 11.Ziegler AG, Kick K, Bonifacio E, Haupt F, Hippich M, Dunstheimer D, et al. Yield of a Public Health Screening of Children for Islet Autoantibodies in Bavaria, Germany. JAMA. 2020;323(4):339–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triolo TM, Chase HP, Barker JM, Group DPTS. Diabetic subjects diagnosed through the Diabetes Prevention Trial-Type 1 (DPT-1) are often asymptomatic with normal A1C at diabetes onset. Diabetes Care. 2009;32(5):769–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.The Lancet Diabetes E. Type 1 diabetes research: poised for progress. Lancet Diabetes Endocrinol. 2019;7(1):1. [DOI] [PubMed] [Google Scholar]
- 14.Colman PG, McNair P, Margetts H, Schmidli RS, Werther GA, Alford FP, et al. The Melbourne Pre-Diabetes Study: prediction of type 1 diabetes mellitus using antibody and metabolic testing. Med J Aust. 1998;169(2):81–4. [DOI] [PubMed] [Google Scholar]
- 15.Bingley PJ, Wherrett DK, Shultz A, Rafkin LE, Atkinson MA, Greenbaum CJ. Type 1 Diabetes TrialNet: A Multifaceted Approach to Bringing Disease-Modifying Therapy to Clinical Use in Type 1 Diabetes. Diabetes Care. 2018;41(4):653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harrison LC, Honeyman MC, Steele CE, Stone NL, Sarugeri E, Bonifacio E, et al. Pancreatic beta-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care. 2004;27(10):2348–55. [DOI] [PubMed] [Google Scholar]
- 17.Fourlanos S, Perry C, Gellert SA, Martinuzzi E, Mallone R, Butler J, et al. Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes. 2011;60(4):1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison LC. A randomised controlled trial of intranasal insulin to prevent type 1 diabetes: Intranasal Insulin Trial II (INIT II). Immunology of Diabetes Society Internation Congress London 2018. [Google Scholar]
- 19.Clapin H, Phelan H, Bruns L Jr., Sinnott R, Colman P, Craig M, et al. Australasian Diabetes Data Network: Building a Collaborative Resource. J Diabetes Sci Technol. 2016;10(5):1015–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bingley PJ, Williams AJ. Validation of autoantibody assays in type 1 diabetes: workshop programme. Autoimmunity. 2004;37(4):257–60. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes A 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S15–S33. [DOI] [PubMed] [Google Scholar]
- 22.Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines 2018: Diabetic ketoacidosis and the hyperglycemic hyperosmolar state. Pediatr Diabetes. 2018;19 Suppl 27:155–77. [DOI] [PubMed] [Google Scholar]
- 23.Mortensen HB, Hougaard P, Swift P, Hansen L, Holl RW, Hoey H, et al. New definition for the partial remission period in children and adolescents with type 1 diabetes. Diabetes Care. 2009;32(8):1384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen ET, Stafford JM, Saydah S, D’Agostino RB Jr., Dolan LM, Lawrence JM, et al. Increase in Prevalence of Diabetic Ketoacidosis at Diagnosis Among Youth With Type 1 Diabetes: The SEARCH for Diabetes in Youth Study. Diabetes Care. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colman PG, Steele C, Couper JJ, Beresford SJ, Powell T, Kewming K, et al. Islet autoimmunity in infants with a Type I diabetic relative is common but is frequently restricted to one autoantibody. Diabetologia. 2000;43(2):203–9. [DOI] [PubMed] [Google Scholar]
- 26.Clapin H, Smith G, Vijayanand S, Jones T, Davis E, Haynes A. Moderate and severe diabetic ketoacidosis at type 1 diabetes onset in children over two decades: A population-based study of prevalence and long-term glycemic outcomes. Pediatr Diabetes. 2022. [DOI] [PubMed] [Google Scholar]
- 27.Cherubini V, Grimsmann JM, Akesson K, Birkebaek NH, Cinek O, Dovc K, et al. Temporal trends in diabetic ketoacidosis at diagnosis of paediatric type 1 diabetes between 2006 and 2016: results from 13 countries in three continents. Diabetologia. 2020;63(8):1530–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hekkala AM, Ilonen J, Toppari J, Knip M, Veijola R. Ketoacidosis at diagnosis of type 1 diabetes: Effect of prospective studies with newborn genetic screening and follow up of risk children. Pediatr Diabetes. 2018;19(2):314–9. [DOI] [PubMed] [Google Scholar]
- 29.McQueen RB, Geno Rasmussen C, Waugh K, Frohnert BI, Steck AK, Yu L, et al. Cost and Cost-effectiveness of Large-scale Screening for Type 1 Diabetes in Colorado. Diabetes Care. 2020;43(7):1496–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cortez FJ, Gebhart D, Robinson PV, Seftel D, Pourmandi N, Owyoung J, et al. Sensitive detection of multiple islet autoantibodies in type 1 diabetes using small sample volumes by agglutination-PCR. PLoS One. 2020;15(11):e0242049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogun MM, Bundy BN, Goland RS, Greenbaum CJ. C-Peptide Levels in Subjects Followed Longitudinally Before and After Type 1 Diabetes Diagnosis in TrialNet. Diabetes Care. 2020;43(8):1836–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobsen LM, Bocchino L, Evans-Molina C, DiMeglio L, Goland R, Wilson DM, et al. The risk of progression to type 1 diabetes is highly variable in individuals with multiple autoantibodies following screening. Diabetologia. 2020;63(3):588–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.von Herrath M, Bain SC, Bode B, Clausen JO, Coppieters K, Gaysina L, et al. Anti-interleukin-21 antibody and liraglutide for the preservation of beta-cell function in adults with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2021;9(4):212–24. [DOI] [PubMed] [Google Scholar]
- 34.Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study G, Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ. Effect of Oral Insulin on Prevention of Diabetes in Relatives of Patients With Type 1 Diabetes: A Randomized Clinical Trial. JAMA. 2017;318(19):1891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


