Abstract
Neural drive originating in higher brain areas reaches exercising limb muscles through the corticospinal-motoneuronal pathway which links the motor cortex and spinal motoneurones. The properties of this pathway have frequently been observed to change during fatiguing exercise in ways that could influence the development of central fatigue, i.e. the progressive reduction in voluntary muscle activation. However, based on differences in motor cortical and motoneuronal excitability between exercise modalities (e.g. single-joint vs locomotor exercise), there is no characteristic response that allows for a categorical conclusion about the effect of these changes on functional impairments and performance limitations. Despite the lack of uniformity in findings during fatigue, there is strong evidence for marked ‘inhibition’ of motoneurones as a direct result of voluntary drive. Endogenous forms of neuromodulation, such as via serotonin released from neurones, can directly affect motoneuronal output and central fatigue. Exogenous forms of neuromodulation, such as brain stimulation, may achieve a similar effect, but the evidence is weak. Non-invasive transcranial direct current stimulation can cause transient or long-lasting changes in cortical excitability, however, variable results across studies cast doubt on its claimed capacity to enhance performance. Furthermore, with these studies it is difficult to establish a cause-and-effect relationship between brain responsiveness and exercise performance. This review briefly summarises changes in the corticomotoneuronal pathway during various types of exercise, considers the relevance of these changes for the development of central fatigue, and the potential of non-invasive brain stimulation to enhance motor cortical excitability, motoneuronal output, and, ultimately, exercise performance.
Graphcial Abstract
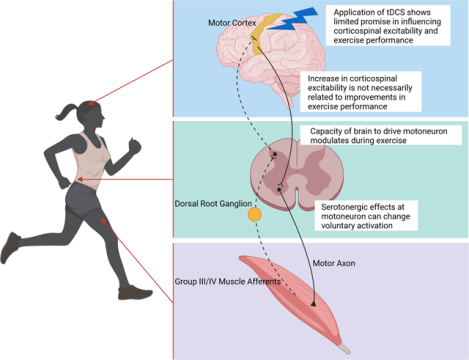
Abstract Figure illustrating main components of the corticospinal-motoneuronal pathway and factors influencing its properties, and potentially performance, during fatiguing exercise. This Topical Review summarizes previously observed changes in the excitability of the corticomotoneuronal pathway during exercise and critically discusses the role of these alterations in determining the development of central fatigue.
Introduction
We have moved beyond the era in which voluntary contractions are considered capable of eliciting the maximal possible force from the muscle during brief, and especially during sustained, maximal efforts. It is now recognized that the firing rates of motoneurones during maximal efforts are insufficient to fuse muscle fibre contractions and some motoneurones may not be recruited at all. Voluntary activation during such efforts is commonly high, but still incomplete, and the level of voluntary activation usually deteriorates with exercise, i.e. central fatigue develops (Gandevia, 2001).
This Topical Review aims to critically discuss our current understanding of the changes of the corticospinal-motoneuronal pathway during exercise and their relevance to the development of central fatigue. Our writing also considers the potential of a specific endogenous and a specific exogenous form of neuromodulation to alter and enhance motor cortical excitability and performance. We chose to emphasize intraspinal serotonin and transcranial direct current stimulation over other monoamines and brain stimulation strategies as existing evidence allows for a relatively clear message with little room for interpretation. The corticomotoneuronal pathway, which includes the motor cortex, descending corticospinal axons, and spinal motoneurones, is a major means by which neural drive originating in higher brain areas reaches exercising limb muscles. Changes within components of this pathway during fatiguing muscle contractions mean that they have the potential to influence the development of central fatigue, and thus performance, during exercise. However, the pathway is part of a more complex set of multiple corticofugal, propriospinal, and spinal influences (both excitatory and inhibitory) acting on motoneurones. This is illustrated in Figure 1A.
Figure 1. Diagrams to show the inputs to the motoneurones (A) and some of the ways by which serotonin may modify motoneuronal output.
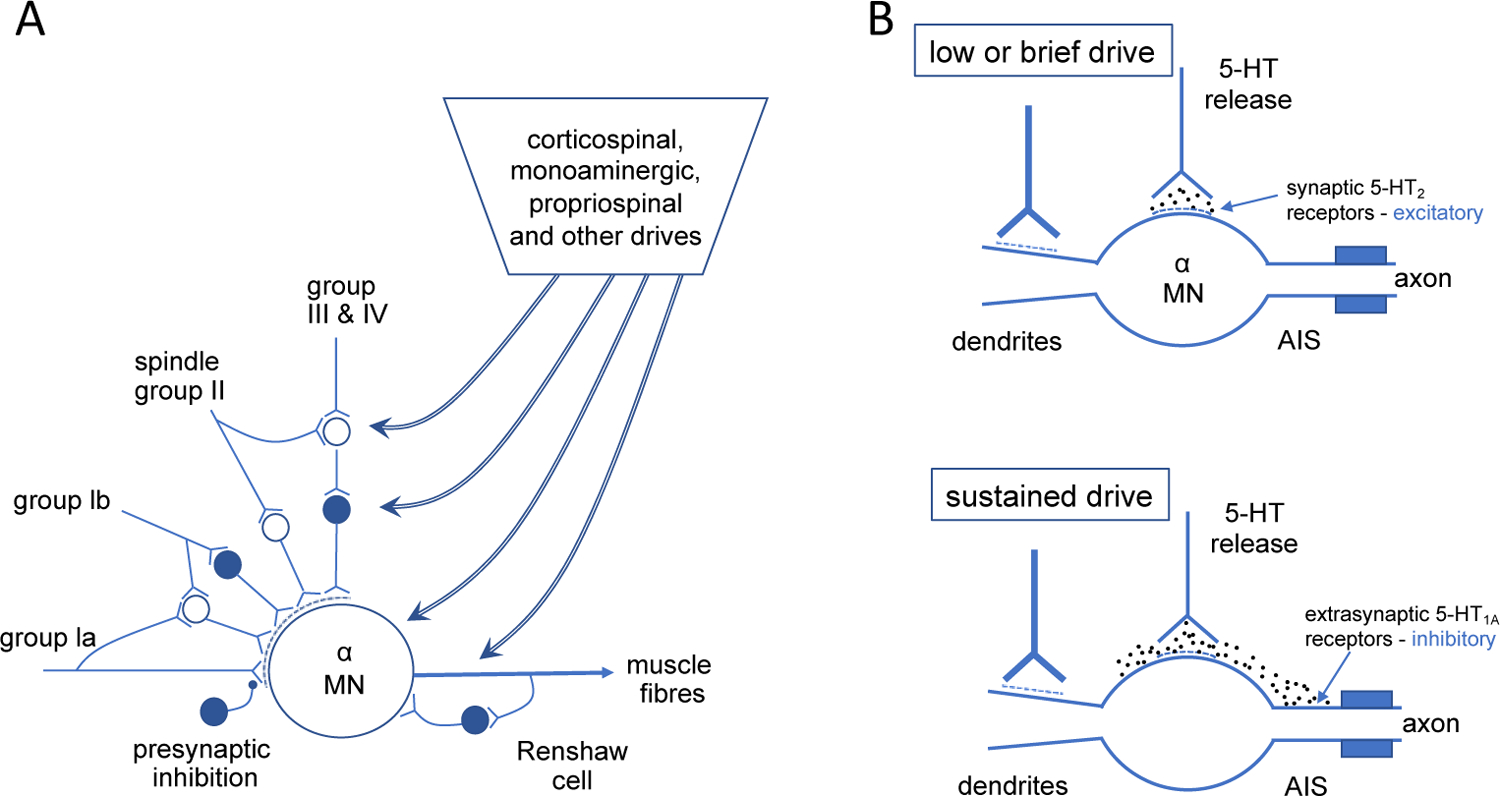
(B). A: Summary of descending and other inputs to alpha motoneurones for an agonist muscle. Cells with solid circles are inhibitory. Dashed curved regions at premotoneuronal terminals denote presynaptic inhibition acting selectively on the afferent paths to the motoneurone. Inputs to gamma motoneurones are not included. Modified from Gandevia (2001) B: Two schematics to show the potential effects of different levels of voluntary drive on the motoneuronal output as modified by release of serotonin (5-HT) from descending monoaminergic paths. Above: at low levels of voluntary drive, motoneuronal output can be facilitated via 5-HT acting on intrasynaptic receptors in the soma-dendritic region. Below: at higher sustained levels of drive, the local concentration of 5-HT increases to such an extent that it spreads to activate inhibitory receptors at the axon initial segment (AIS) and can thus reduce the firing frequency of the motoneurone.
Reductions in the responsiveness of the corticomotoneuronal pathway to voluntary drive, or artificial stimulation, can result from the net decreases in the excitability of the primary motor cortex and/or spinal motoneurones. Compromised excitability requires increased synaptic input into (i.e., activation of) the motor cortex and/or spinal motoneurones to maintain muscle activation at the level needed to sustain a given task (Fornal et al., 2006). However, if an increase in (voluntary) neural drive to the motor cortex and from it to motoneurones is not possible, or is insufficient (e.g., near exhaustion) to overcome reduced excitability or increased inhibition, activation of motor units by the central nervous system decreases and compromises muscle output, i.e. central fatigue increases (Petersen et al., 2003; Martin et al., 2006; Klass et al., 2008; Taylor et al., 2016). However, during some types of exercise, such as maximal intensity single-joint contractions, the excitability of the corticomotoneuronal pathway is typically increased (Gandevia, 1996). This could, in theory, mean that a given degree of synaptic input into the motor cortex might lead to greater motor unit activation and thus offset the development of central fatigue during exercise. However, the actual impact of fatigue-induced changes in corticomotoneuronal pathway excitability on the capacity of an exercising human remains elusive.
Evaluating the excitability of the corticomotoneuronal pathway
Exercise-induced alterations in the ‘excitability’ of the corticomotoneuronal pathway can be quantified using non-invasive stimulation techniques which evoke compound responses recorded in the surface electromyography (EMG) signal. Fatigue-related changes to the magnitude of a response indicate the change to neural excitability between the stimulation and recording sites (i.e., an increase in size reflects greater excitability, a decrease reflects reduced excitability).
When transcranial magnetic stimulation (TMS) is applied to the contralateral hemisphere of the motor cortex, a short-latency EMG response, termed a motor-evoked potential (MEP), appears in a target muscle. With the use of TMS alone, it is not possible to distinguish between fatigue-related changes in the excitability of the motor cortex, spinal motoneurones, or muscle fibres. To decipher changes in the excitability of the components of the corticomotoneuronal pathway and muscle, it is, in addition to TMS, necessary to also apply cervicomedullary [CMS; eliciting a cervicomedullary motor evoked potential (CMEP)] and peripheral nerve [eliciting a maximal compound muscle action potential (Mmax)] stimulations. Sidenote: An alternative to CMS is thoracic electrical stimulation which excites corticospinal axons innervating leg muscles (Martin et al., 2008). The Mmax is essential because 1) it allows one to target an equivalent proportion of the motoneurone pool with CMS and TMS, both within and across participants; 2) it enables tracking of muscle sarcolemmal excitability; 3) it can be used to account for the influence of peripheral excitability on fatigue-related changes to CMEP and MEP size; e.g., normalizing the CMEP to Mmax reveals fatigue-related changes likely attributable to motoneuronal excitability (Butler et al., 2003). To isolate the cortical contributions to changes in MEP size (i.e., account for motoneuronal and peripheral influences), the MEP can be normalized to the CMEP (Taylor et al., 2002; Martin et al., 2008). The CMEP is the optimal spinal-level control for the MEP because, when the responses are matched in size, CMS and TMS activate many of the same corticospinal axons (Gandevia et al., 1999; Taylor et al., 2002). Further, the CMEP is not influenced by conventional presynaptic inhibition (Nielsen & Petersen, 1994; Jackson et al., 2006) and has a large monosynaptic component (Petersen et al., 2002), which makes it the most direct measure of motoneuronal excitability in humans (McNeil et al., 2013).
In addition to the MEP, delivery of TMS during a voluntary contraction causes a brief pause (typically 100–300 ms) in volitional EMG activity, termed the silent period (SP). Exercise-induced prolongations of the SP have been considered to reflect an increase in cortical inhibition (Inghilleri et al., 1993; Chen et al., 1999). However, the SP, particularly when short, is also influenced by motoneuronal excitability (Yacyshyn et al., 2016). Thus, it is likely unwise to attribute an exercise-induced prolongation of the SP solely to intracortical inhibition. Finally, several paired-pulse paradigms have been used to quantify intracortical excitatory or inhibitory processes. Details on these techniques can be found elsewhere (Valls-Sole et al., 1992; Kujirai et al., 1993).
Motor cortex excitability and fatigue
As with nearly all indices of fatigue, the MEP and SP are influenced strongly by the parameters of the fatiguing task. Accordingly, there is not a characteristic response that allows a categorical statement about the influence of fatiguing exercise on motor cortical excitability, particularly as few studies have controlled for both spinal and peripheral influences on the MEP. Even with an isometric task, which is the predominant mode of exercise studied because it allows the most experimental control, but suffers from a lack of functional relevance, the findings are mixed for the MEP. For example, regardless of the muscle, MEP size typically increases as a percentage of Mmax during both fatiguing submaximal (Hoffman et al., 2009) and maximal (e.g., (Taylor et al., 1999) tasks (Note: motoneuronal excitability is reduced and so cannot enhance MEP size). However, data are equivocal for intermittent tasks. Specifically, non-normalized MEPs were previously reported to increase during repeated maximal voluntary contractions (MVCs) of the dorsiflexors (Mileva et al., 2012), remain unchanged during intermittent plantar flexor MVCs (Iguchi & Shields, 2012) and, when normalized to Mmax, remain unaltered from before to immediately after an intermittent submaximal quadriceps contraction protocol (Hilty et al., 2011). Even when one accounts for subcortical influences, it is not clear if motor cortical excitability is increased, or unaffected, by a fatiguing isometric task. There is a more stereotypical response for the SP than the MEP because, regardless of the specifics of the single-joint task (e.g., sustained vs. intermittent, upper vs. lower limb), SP duration increases, which is usually attributed to increased cortical inhibition (Taylor et al., 1996; Taylor et al., 2000; Hilty et al., 2011; Otieno et al., 2022). Interestingly, based on a pharmacological approach (lumbar intrathecal fentanyl) attenuating approximately 60% of group III/IV muscle afferent feedback from the lower limbs (Hureau et al., 2018) during submaximal intermittent quadriceps contractions, it was suggested that, without affecting MEPs normalized for Mmax, the central projection of these sensory neurons determines the exercise-induced increase in SP, at least during this exercise modality (Hilty et al., 2011).
If the fatiguing task involves locomotor exercise, changes in MEP and SP differ from those obtained during isometric, single-joint exercise (Weavil & Amann, 2018). Specifically, when recorded from the contracting quadriceps muscles, MEP, normalized to Mmax, remains unchanged during exhaustive cycling exercise (Weavil et al., 2016; Sidhu et al., 2017; Sidhu et al., 2018). However, when one accounts for the fatigue-related increase in neural drive (Sidhu et al., 2012; Weavil et al., 2016), or changes at the spinal level (Sidhu et al., 2017; Sidhu et al., 2018), MEP size actually decreases. Furthermore, the duration of the SP recorded from the quadriceps is not affected by exhaustive cycling exercise (Sidhu et al., 2017; Sidhu et al., 2018). Interestingly, SP duration is reduced and the exercise-induced decrease in motor cortical excitability (MEP normalized for CMEP) prevented when a given fatiguing cycling task is performed after pharmacological blockade of group III/IV muscle afferent feedback from locomotor muscles (Sidhu et al., 2017; Sidhu et al., 2018). These findings suggest a considerable impact of these sensory neurons on corticomotoneuronal excitability during locomotor exercise. A more detailed discussion of the influence of group III/IV muscle afferent feedback on corticomotoneuronal excitability during locomotor exercise can be found elsewhere (Weavil & Amann, 2018; Weavil & Amann, 2019). Regardless, the obvious discrepancies in exercise-induced changes in corticomotoneuronal excitability with single-joint compared to locomotor exercise may be related to the larger systemic changes that occur with whole-body tasks, including greater input from group III/IV muscle afferents (Weavil & Amann, 2018). Furthermore, there are methodological differences between studies of cycling and single-joint exercise that may also be important.
Paired-pulse TMS protocols have been used to try to localize fatigue-related changes in MEP size to intracortical sources; however, even interstimulus intervals (ISIs) as brief as 10 ms may include spinal effects on the MEP (Ni et al., 2007), with spinal mechanisms completely dominating fatigue-related reductions in the MEP at an ISI of 100 ms (McNeil et al., 2009; McNeil et al., 2011). Irrespective of the ISI, there is no consistent effect of fatiguing exercise on intracortical inhibition or facilitation because, regardless of the type of exercise, the paired-pulse MEP has been reported to decrease (Hunter et al., 2016), increase (Mason et al., 2019) or remain unchanged (Sidhu, 2021).
The disparate results described in the preceding paragraphs reveal challenges in terms of relating indices of motor cortical excitability to function, which is invariably impaired by fatiguing exercise. It is straightforward to conclude that a reduction in MEP size could contribute to impaired function, but the interpretation is complex when the MEP increases with fatiguing exercise. In this scenario, it is attractive to interpret this enhanced cortical excitability as a necessary adaptation to preserve performance. However, given that increased MEP size commonly coincides with marked impairments of voluntary activation and maximal force (Gandevia et al., 1996), one must question if MEP size directly relates to function. If it does, the corollary is that impairments of voluntary activation and maximal force would be greater if not for the increased cortical excitability. Unfortunately, it is not possible to clamp cortical excitability to test this hypothesis. Further, MEP size during the SP argues against a relationship between MEP size and voluntary muscle output. For example, the MEP 100 ms into the SP was much larger than the one elicited from a truly relaxed muscle, even though motoneuronal excitability, as estimated by CMEP, was not different (McNeil et al., 2009). Hence, the motor cortex is markedly facilitated when voluntary activation of motoneurones is impossible, which would lead one to conclude that MEP size has negligible functional relevance. Although this interpretation might be unappealing because TMS provides the best indirect measure of motor cortical excitability in humans, such a scenario is entirely plausible because the response to synchronous activation of corticomotoneuronal outputs via an external stimulus (i.e., the MEP) may be a poor proxy for neuronal excitability as it relates to volitional activation of motor cortical output to motoneurones. Perhaps future experimental or technological advances may allow to better probe the functional relevance of fatigue-related changes in the MEP and SP.
Motoneurones and fatigue
We are repeatedly reminded that the motoneurone is the Sherringtonian final common pathway which commands the force in all muscle contractions, whether they are elicited by voluntary drive, a reflex, or a combination of inputs to the motoneurone pool (see Figure 1A). Importantly, the sign (excitation or inhibition), and the size and effectiveness of these inputs can modify motoneuronal output during contractions of an unfatigued, and, especially, a fatiguing muscle. These processes are affected by reflex inputs and their pathways to motoneurones as well as a variety of presynaptic mechanisms which alter the effectiveness of inputs to motoneurones, with a well-known example being classical presynaptic effects on group Ia terminals.
In the light of newer developments and recent studies, the impact of spinal neuromodulatory systems is becoming more important to consider in the operation of human limb motoneurones. The system for which there is increasing electrophysiological evidence is the serotonergic system (5-HT), which descends from raphe nuclei and synapses on motoneurones. The discharge frequency of raphe-spinal cells can parallel limb motor activity during, for example, natural walking (e.g. Jacobs et al., 2002; Fornal et al., 2006). Recent studies in animals and humans have provided strong evidence that serotonin modifies motor output through spinal mechanisms. Importantly, studies of turtle motoneurones have revealed the relevance of serotonin concentration and its receptor binding at the axon initial segment in regulating motoneuronal firing rate during simulated muscle fatigue (Cotel et al., 2013). Specifically, when these high-affinity receptors are activated by buspirone (a partial 5-HT1A receptor agonist), motoneuronal output is impaired as judged by reduced areas of F-waves (by 27%) in a hand muscle and CMEPs (by 31%) in the biceps brachii (D’Amico et al., 2017). Other pharmacological manipulations of the serotonergic system in humans alter motoneuronal output (‘gain’) in interlimb tasks (Wei et al., 2014) and reveal complex serotonergic actions on the different compartments of motoneurones (Thorstensen et al., 2022). For review of this system see Kavanagh and Taylor (Kavanagh & Taylor, 2022).
Kavanagh and colleagues (Kavanagh et al., 2019; Thorstensen et al., 2020) recently used oral paroxetine to increase endogenous intraspinal concentrations of 5-HT during brief isometric maximal voluntary contractions of the elbow flexors in healthy subjects. This intervention increased maximal voluntary activation by 1.5% and torque by ~4.5%, suggesting that, during brief maximal efforts, combined raphe-spinal drive and reuptake inhibition allows 5-HT to facilitate motoneuronal output (likely via 5-HT2 receptors in the soma-dendritic compartment and activation of persistent inward currents; Fig 1B upper panel). However, during brief isometric efforts after sustained maximal voluntary contractions, which reduced torque by 40%, maximal torque and voluntary activation were lower with paroxetine. This is consistent with strong sustained 5-HT drive combined with reuptake inhibition causing 5-HT concentrations to rise sufficiently to inhibit motoneuronal output at the axon initial segment, likely via 5-HT1A receptors.
The dynamics of the raphe-spinal output and its effectiveness in activation of the many 5-HT receptors (both facilitatory and inhibitory) depends on the duration and intensity of voluntary contractions. This will determine the curtailment of motoneuronal firing rate imposed by the axon initial segment. These recent insights are important for the quantification of voluntary activation via TMS as compared to peripheral nerve stimulation. Specifically, these limits mean that voluntary activation assessed by peripheral nerve stimulation can be well below 100%, while voluntary activation assessed by TMS may appear complete. These serotonin-related effects on the motoneurone significantly challenge the adequacy of TMS to quantify voluntary muscle activation. Further studies are needed to quantify these dynamic 5-HT neuromodulatory processes at both spinal and supraspinal levels and to define their operating limits under different fatiguing conditions.
A corroborative observation on the importance of descending inputs is that voluntary activation of a motoneurone pool is needed to evoke the intensity-linked neuromodulatory effects on the pool as these effects are absent when the motoneurones are driven by antidromic stimulation. Such stimulation will also influence the motoneurones via direct afferent activation as well as via inputs produced secondarily by the evoked contraction (Khan et al., 2016; see also D’Amico et al., 2020). Further, the effects of volitional exercise depend on the intensity of the voluntary contraction and which motoneurones within the pool are activated during the task (Figure 2). Finally, the many changes in electrophysiological behaviour of human motoneurones in fatigue, such as the reduction in response to a corticospinal stimulus, are not inconsequential. They produce changes in voluntary output when matching voluntary contractions are performed (Petersen et al., 2003) (see also Taylor & Martin, 2009).
Figure 2. Reductions in motoneuronal excitability induced by maximal and submaximal isometric exercise.
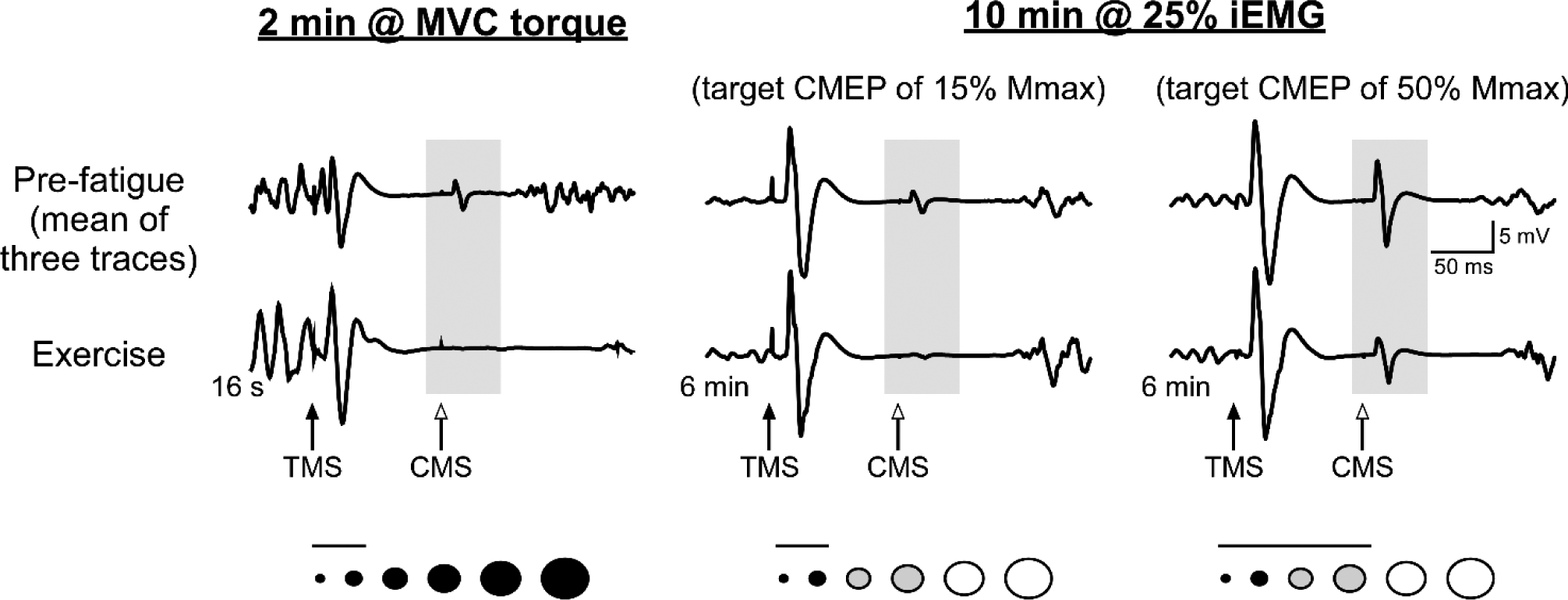
Raw traces of biceps brachii CMEPs recorded from a single participant during a sustained maximal (McNeil et al., 2009) or submaximal (McNeil et al., 2011) isometric contraction of the elbow flexors. CMEPs are recorded during the SP following TMS (100 ms ISI between TMS and CMS), and the reduction in CMEP size reflects a decrease in motoneuronal excitability because biceps brachii Mmax increases during these tasks. Beneath each set of traces is a schematic representation of the biceps brachii motoneurone pool. Circles represent motoneurones of different size, whereas the colour indicates the presumed activation during the fatiguing contraction (black = active throughout, grey = active part of the time, white = not active at any point). The horizontal line above them indicates the motoneurones that would likely contribute to the CMEP considered in each set of traces (i.e., a small CMEP would involve only small, low-threshold motoneurones). Left traces: During a sustained 2-min maximal voluntary contraction (MVC), the reduction in motoneuronal excitability was so rapid that the CMEP was virtually abolished after 16 s. Middle and right traces: After six minutes of a sustained 10-min contraction at the level of integrated EMG activity produced at 25% MVC torque (25% iEMG), there was a marked decrease of the small CMEP (~15% Mmax) but a modest decrease of the large CMEP (~50% Mmax). This indicates that impairment of motoneuronal excitability is limited to the parts of the pool that have been repetitively activated. For comparable findings in the lower limb see (Finn et al., 2018).
Neuromodulation with non-invasive brain stimulation: a way to enhance the corticospinal-motoneuronal pathway?
Non-invasive brain stimulation paradigms, including transcranial direct current stimulation (tDCS) have been used as a potential tool to augment human brain function, improve exercise performance and reduce fatigability. They have gained popularity in recent years, probably because tDCS is low cost and easy to use. It involves application of low-intensity electrical currents that can modulate neuronal excitability in targeted brain regions (Nitsche & Paulus, 2001; Di Lazzaro et al., 2013), the effects of which are mediated by polarity-specific modification of the resting membrane potential (Nitsche & Paulus, 2001). For example, anodal tDCS is thought to augment cortical excitability by facilitating subthreshold depolarization, whereas cathodal tDCS may decrease cortical excitability by causing subthreshold hyperpolarization (Liebetanz et al., 2002). tDCS can cause transient or long lasting changes in cortical excitability, the latter being comparable to persistent forms of neuroplasticity known as long-term potentiation and long-term depression (Nitsche et al., 2003). tDCS-induced neuroplasticity depends on calcium channels (Monte-Silva et al., 2013) and glutamatergic synapses influenced by NMDA receptor-dependent mechanisms (Liebetanz et al., 2002; Nitsche et al., 2003); although, GABAergic inhibitory mechanisms may also play a role (Cengiz et al., 2013).
As discussed above, fatigue during upper or lower limb single-joint and cycling exercise influences the excitability of the corticomotoneuronal pathway, including intracortical circuitry mediated by GABAergic mechanisms (McNeil et al., 2011; Sidhu et al., 2018). As these mechanisms are common to both exercise-induced fatigue and tDCS (Nitsche & Paulus, 2001; Cengiz et al., 2013), it is possible that tDCS-mediated changes in intracortical circuitry can influence how the brain responds to fatiguing exercise and therefore modulate descending cortical drive and exercise performance (Sidhu, 2021). However, in addition to the problem that some studies using tDCS have not measured corticomotoneuronal excitability (Cogiamanian et al., 2007; Iacob et al., 2016), a true cause-and-effect relationship between brain responsiveness and exercise performance is difficult to establish often because there is a lack of parallel between exercise performance and corticomotoneuronal excitability (Figure 3) (Williams et al., 2013; Sidhu, 2021).
Figure 3. Examples of variability in outcomes reported between exercise performance (time-to-task failure; TTF) and corticomotoneuronal excitability.
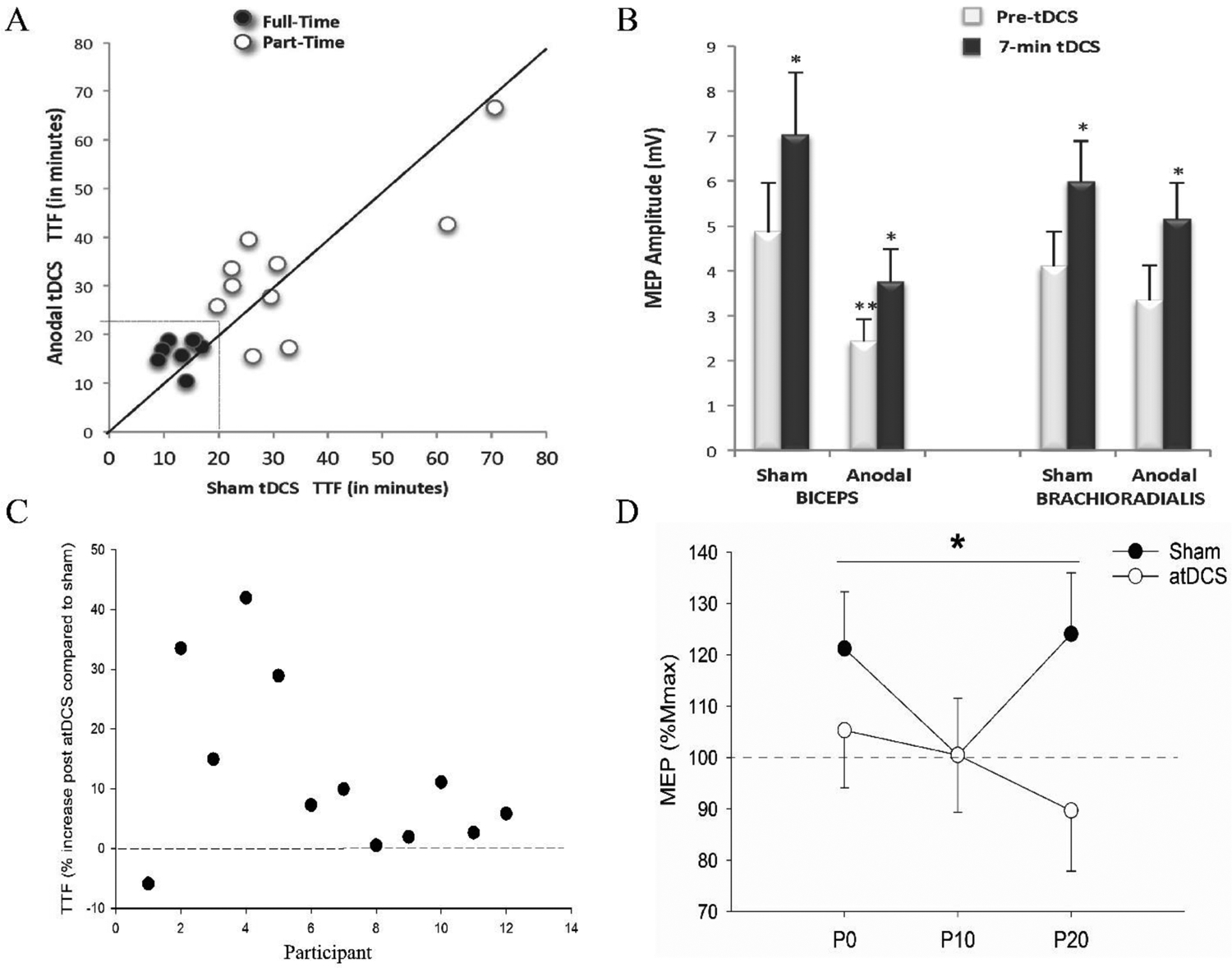
Preliminary data from single joint elbow flexion exercise (panels A and B; figures taken from Williams et al., 2013) demonstrating that (A) an increase in exercise performance with anodal tDCS (atDCS) applied during exercise is not accompanied by (B) changes in corticomotoneuronal excitability. In A, dark circles represent participants who received tDCS through to task failure in both conditions (Full-Time; n=8), whereas open circles represent participants for whom tDCS terminated before they reached task failure for one or both stimulation conditions (Part-Time; n=10). In B, MEP amplitude (mean ± SEM) increases to a similar extent from pre-tDCS to 7 min of delivering tDCS measured during the 20% maximum voluntary contraction task in participants who reached task failure before tDCS was discontinued (i.e., Full-Time; n= 8 out of 18). *denotes difference from “Pre-tDCS”; P< 0.05. **denotes main effect of stimulation condition since MEP prior to applying tDCS were significantly lower in Anodal compared to Sham session P < 0.05. Data from cycling study (panel C and panel D, from (Sidhu, 2021)) demonstrating (C) an increase in TTF, but (D) an attenuation in corticomotoneuronal excitability measured via resting MEP in a hand muscle (% Mmax; normalized to values post tDCS; mean ± SEM) in the condition when anodal tDCS was applied prior to cycling exercise compared to sham condition at 0 min (P0), 10 min (P10) and 20 min (P20) post cycling exercise. *main effect of session; P < 0.05.
Several studies have shown that, compared to some sort of sham condition, the application of anodal tDCS increases single joint (Cogiamanian et al., 2007) and locomotor exercise performance (e.g. Lattari et al., 2018; Sidhu, 2021). This has typically been documented as an increase in time-to-task failure. However, these outcomes are challenged by studies that have shown no influence of tDCS on endurance (e.g. Muthalib et al., 2013; Baldari et al., 2018). The variable results between studies cast some doubt on the ability of tDCS to enhance athletic performance and fatigability.
The variability in the outcomes reported between tDCS studies may be attributed to a multitude of factors, including monocephalic (Muthalib et al., 2013) versus bicephalic (Vitor-Costa et al., 2015; Sidhu, 2021) montages, differences in tDCS current and duration, application prior to vs during exercise, application on motor (Williams et al., 2013) versus non-motor pre-frontal (Vitor-Costa et al., 2015) areas, and history of prior synaptic activity (Hulme et al., 2014). Additionally, the effectiveness of tDCS may be influenced by variations in anatomical (e.g., skull thickness) and physiological (e.g., neurotransmitter availability and number of receptors) factors (Jamil et al., 2017).
The heterogeneity between studies is also influenced by the fact that not all studies report MEPs normalized for muscle dependant changes (e.g. Figure 3B). Although we have some, albeit limited, understanding of the effective stimulation intensities and durations of tDCS application across the general population (Jamil et al., 2017), it is unclear how these might interact to influence individual tDCS responses with exercise. It is likely that standardised approaches to tDCS application will not work in all individuals. To establish tDCS as a potential tool to ameliorate the effects of fatigue and augment performance in health and disease, more comprehensive work needs to be done to allow for individualised tDCS dosing based on intrinsic and extrinsic parameters. Trials will need large sample sizes, randomised designs and ideally pre-registration.
Finally, we draw attention to the frequently discussed, widespread problem with the replication and reproducibility of research findings (e.g. Ioannidis, 2005; Goodman et al., 2016). Given that our current understanding of exercise-induced changes in the excitability of the corticomotoneuronal pathway is based on a relatively small number of studies focusing on a specific exercise task, caution should be attached to findings from these few (sometimes solitary) studies, especially when there is no corroboration from other studies, or support from triangulation.
Summary
The capacity of the corticomotoneuronal pathway to relay neural signals from the motor cortex to exercising limb muscle changes depending on the exercise task that induces fatigue. Reductions in corticomotoneuronal pathway excitability require increases in synaptic input into the motor cortex and the motoneurones to maintain muscle activation at the level needed for a given task. If this increase is not possible, or insufficient, the activation of motor units by the central nervous system decreases, i.e. central fatigue develops. In contrast, during some exercise modalities, the excitability of the motor pathway can increase, which might, in theory, mean that a given level of motor cortical activity results in greater motor unit activation and potentially offsets the development of central fatigue. However, it needs to be emphasised that, largely due to the task-dependence of the changes and the current dearth of sufficient task-specific literature, the actual impact of fatiguing exercise on corticomotoneuronal pathway excitability and the associated consequences for the development of central fatigue remain elusive.
Based on recent animal and human studies, it is now clear that endogenous intraspinal serotonin can modify motoneurone excitability and motor output through spinal mechanisms, with the effects on voluntary muscle activation and torque depending on the exercise intensity and duration. Specifically, it appears that serotonergic inhibition of the motoneurones and motor output only occurs during high serotonergic drive to the motoneurones (i.e. fatiguing muscle contractions), whereas overall excitation and facilitation of voluntary muscle activation occurs at lower levels of drive (i.e. non-fatiguing muscle contractions). These observations suggest that intraspinal serotonin contributes to central fatigue by modifying motoneuronal excitability.
Finally, non-invasive brain stimulation paradigms, such as tDCS, have been suggested to augment human brain function and improve exercise performance. However, based on limited current insights, a true cause-and-effect relationship between brain responsiveness and exercise performance is difficult to establish, variable results between studies cast doubt on the ability of tDCS to affect corticospinal excitability and enhance athletic performance and fatigability.
Supplementary Material
KEY POINTS.
The capacity of motor cortical systems to drive voluntary motoneuronal output changes during fatiguing exercise and thus influences the development of central fatigue and performance.
It is too simplistic to conclude that decreases in motor cortical or motoneuronal excitability may contribute to central fatigue and impaired performance as some studies show increased corticomotoneuronal excitability in some exercise modalities.
Endogenous forms of neuromodulation, such as serotonin released from neurones, can alter central fatigue and motoneuronal output.
Exogenous forms of neuromodulation, such as non-invasive brain stimulation, may also do this, but current evidence is not convincing.
Further well-controlled studies and replications are needed to expose the cause-and-effect relationship between brain responsiveness and exercise performance
Funding
M.A. was supported by the National Heart, Lung, and Blood Institute (HL-116579 and HL-139451), and the U.S. Veterans Affairs Rehabilitation Research and Development (E3343-R).
Biography

Dr. Markus Amann is a Professor of Anesthesiology at the University of Utah, Theodore H. Stanley Presidential Endowed Chair in Anesthesiology, and Adjunct Professor in the Departments of Internal Medicine and Biomedical Engineering. His research focuses on autonomic cardiovascular control mechanisms, the neural control of breathing, and the etiology of central nervous system fatigue.
Footnotes
Competing interests
The authors declare no conflict of interest.
Data availability statement
This is a Topical Review, all data discussed in this writing have been published previously. References to these data are included throughout.
References
- Baldari C, Buzzachera CF, Vitor-Costa M, Gabardo JM, Bernardes AG, Altimari LR & Guidetti L. (2018). Effects of Transcranial Direct Current Stimulation on Psychophysiological Responses to Maximal Incremental Exercise Test in Recreational Endurance Runners. Front Psychol 9, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler JE, Taylor JL & Gandevia SC. (2003). Responses of human motoneurons to corticospinal stimulation during maximal voluntary contractions and ischemia. J Neurosci 23, 10224–10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cengiz B, Murase N & Rothwell JC. (2013). Opposite effects of weak transcranial direct current stimulation on different phases of short interval intracortical inhibition (SICI). Exp Brain Res 225, 321–331. [DOI] [PubMed] [Google Scholar]
- Chen R, Lozano AM & Ashby P. (1999). Mechanism of the silent period following transcranial magnetic stimulation. Evidence from epidural recordings. Exp Brain Res 128, 539–542. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Marceglia S, Ardolino G, Barbieri S & Priori A. (2007). Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci 26, 242–249. [DOI] [PubMed] [Google Scholar]
- Cotel F, Exley R, Cragg SJ & Perrier JF. (2013). Serotonin spillover onto the axon initial segment of motoneurons induces central fatigue by inhibiting action potential initiation. Proc Natl Acad Sci U S A 110, 4774–4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico JM, Butler AA, Heroux ME, Cotel F, Perrier JM, Butler JE, Gandevia SC & Taylor JL. (2017). Human motoneurone excitability is depressed by activation of serotonin 1A receptors with buspirone. The Journal of physiology 595, 1763–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amico JM, Rouffet DM, Gandevia SC & Taylor JL. (2020). Unlike voluntary contractions, stimulated contractions of a hand muscle do not reduce voluntary activation or motoneuronal excitability. J Appl Physiol (1985) 128, 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Ranieri F, Profice P, Pilato F, Mazzone P, Capone F, Insola A & Oliviero A. (2013). Transcranial direct current stimulation effects on the excitability of corticospinal axons of the human cerebral cortex. Brain Stimul 6, 641–643. [DOI] [PubMed] [Google Scholar]
- Finn HT, Rouffet DM, Kennedy DS, Green S & Taylor JL. (2018). Motoneuron excitability of the quadriceps decreases during a fatiguing submaximal isometric contraction. J Appl Physiol (1985) 124, 970–979. [DOI] [PubMed] [Google Scholar]
- Fornal CA, Martin-Cora FJ & Jacobs BL. (2006). “Fatigue” of medullary but not mesencephalic raphe serotonergic neurons during locomotion in cats. Brain Res 1072, 55–61. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. (1996). Insights into motor performance and muscle fatigue based on transcranial stimulation of the human motor cortex. Clin Exp Pharmacol Physiol 23, 957–960. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. (2001). Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81, 1725–1789. [DOI] [PubMed] [Google Scholar]
- Gandevia SC, Allen GM, Butler JE & Taylor JM. (1996). Supraspinal factors in human muscle fatigue: evidence for suboptimal output from the motor cortex. The Journal of physiology 490, 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC, Petersen N, Butler JE & Taylor JL. (1999). Impaired response of human motoneurones to corticospinal stimulation after voluntary exercise. The Journal of physiology 521 Pt 3, 749–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman SN, Fanelli D & Ioannidis JP. (2016). What does research reproducibility mean? Sci Transl Med 8, 341ps312. [DOI] [PubMed] [Google Scholar]
- Hilty L, Lutz K, Maurer K, Rodenkirch T, Spengler CM, Boutellier U, Jancke L & Amann M. (2011). Spinal opioid receptor-sensitive muscle afferents contribute to the fatigue-induced increase in intracortical inhibition in healthy humans. Exp Physiol 96, 505–517. [DOI] [PubMed] [Google Scholar]
- Hoffman BW, Oya T, Carroll TJ & Cresswell AG. (2009). Increases in corticospinal responsiveness during a sustained submaximal plantar flexion. J Appl Physiol (1985) 107, 112–120. [DOI] [PubMed] [Google Scholar]
- Hulme SR, Jones OD, Raymond CR, Sah P & Abraham WC. (2014). Mechanisms of heterosynaptic metaplasticity. Philos Trans R Soc Lond B Biol Sci 369, 20130148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter SK, McNeil CJ, Butler JE, Gandevia SC & Taylor JL. (2016). Short-interval cortical inhibition and intracortical facilitation during submaximal voluntary contractions changes with fatigue. Exp Brain Res 234, 2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hureau TJ, Weavil JC, Thurston TS, Broxterman RM, Nelson AD, Bledsoe AD, Jessop JE, Richardson RS, Wray DW & Amann M. (2018). Identifying the role of group III/IV muscle afferents in the carotid baroreflex control of mean arterial pressure and heart rate during exercise. The Journal of physiology 596, 1373–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacob E, Light AR, Donaldson GW, Okifuji A, Hughen RW, White AT & Light KC. (2016). Gene Expression Factor Analysis to Differentiate Pathways Linked to Fibromyalgia, Chronic Fatigue Syndrome, and Depression in a Diverse Patient Sample. Arthritis Care Res (Hoboken) 68, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iguchi M & Shields RK. (2012). Cortical and segmental excitability during fatiguing contractions of the soleus muscle in humans. Clin Neurophysiol 123, 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inghilleri M, Berardelli A, Cruccu G & Manfredi M. (1993). Silent period evoked by transcranial stimulation of the human cortex and cervicomedullary junction. The Journal of physiology 466, 521–534. [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JP. (2005). Why most published research findings are false. PLoS Med 2, e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Baker SN & Fetz EE. (2006). Tests for presynaptic modulation of corticospinal terminals from peripheral afferents and pyramidal tract in the macaque. The Journal of physiology 573, 107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs BL, Martin-Cora FJ & Fornal CA. (2002). Activity of medullary serotonergic neurons in freely moving animals. Brain Res Brain Res Rev 40, 45–52. [DOI] [PubMed] [Google Scholar]
- Jamil A, Batsikadze G, Kuo HI, Labruna L, Hasan A, Paulus W & Nitsche MA. (2017). Systematic evaluation of the impact of stimulation intensity on neuroplastic after-effects induced by transcranial direct current stimulation. The Journal of physiology 595, 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh JJ, McFarland AJ & Taylor JL. (2019). Enhanced availability of serotonin increases activation of unfatigued muscle but exacerbates central fatigue during prolonged sustained contractions. The Journal of physiology 597, 319–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh JJ & Taylor JL. (2022). Voluntary activation of muscle in humans: does serotonergic neuromodulation matter? The Journal of physiology 600, 3657–3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SI, Taylor JL & Gandevia SC. (2016). Unexpected factors affecting the excitability of human motoneurones in voluntary and stimulated contractions. The Journal of physiology 594, 2707–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M, Levenez M, Enoka RM & Duchateau J. (2008). Spinal mechanisms contribute to differences in the time to failure of submaximal fatiguing contractions performed with different loads. J Neurophysiol 99, 1096–1104. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P & Marsden CD. (1993). Corticocortical inhibition in human motor cortex. The Journal of physiology 471, 501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattari E, de Oliveira BS, Oliveira BRR, de Mello Pedreiro RC, Machado S & Neto GAM. (2018). Effects of transcranial direct current stimulation on time limit and ratings of perceived exertion in physically active women. Neurosci Lett 662, 12–16. [DOI] [PubMed] [Google Scholar]
- Liebetanz D, Nitsche MA, Tergau F & Paulus W. (2002). Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 125, 2238–2247. [DOI] [PubMed] [Google Scholar]
- Martin PG, Butler JE, Gandevia SC & Taylor JL. (2008). Noninvasive stimulation of human corticospinal axons innervating leg muscles. J Neurophysiol 100, 1080–1086. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC & Taylor JL. (2006). Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci 26, 4796–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J, Howatson G, Frazer AK, Pearce AJ, Jaberzadeh S, Avela J & Kidgell DJ. (2019). Modulation of intracortical inhibition and excitation in agonist and antagonist muscles following acute strength training. Eur J Appl Physiol 119, 2185–2199. [DOI] [PubMed] [Google Scholar]
- McNeil CJ, Butler JE, Taylor JL & Gandevia SC. (2013). Testing the excitability of human motoneurons. Frontiers in human neuroscience 7, 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Giesebrecht S, Gandevia SC & Taylor JL. (2011). Behaviour of the motoneurone pool in a fatiguing submaximal contraction. The Journal of physiology 589, 3533–3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil CJ, Martin PG, Gandevia SC & Taylor JL. (2009). The response to paired motor cortical stimuli is abolished at a spinal level during human muscle fatigue. The Journal of physiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileva KN, Sumners DP & Bowtell JL. (2012). Decline in voluntary activation contributes to reduced maximal performance of fatigued human lower limb muscles. Eur J Appl Physiol 112, 3959–3970. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo MF, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W & Nitsche MA. (2013). Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul 6, 424–432. [DOI] [PubMed] [Google Scholar]
- Muthalib M, Kan B, Nosaka K & Perrey S. (2013). Effects of transcranial direct current stimulation of the motor cortex on prefrontal cortex activation during a neuromuscular fatigue task: an fNIRS study. Adv Exp Med Biol 789, 73–79. [DOI] [PubMed] [Google Scholar]
- Ni Z, Gunraj C & Chen R. (2007). Short interval intracortical inhibition and facilitation during the silent period in human. The Journal of physiology 583, 971–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J & Petersen N. (1994). Is presynaptic inhibition distributed to corticospinal fibres in man? The Journal of physiology 477 (Pt 1), 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Fricke K, Henschke U, Schlitterlau A, Liebetanz D, Lang N, Henning S, Tergau F & Paulus W. (2003). Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J Physiol (Lond) 553, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA & Paulus W. (2001). Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 57, 1899–1901. [DOI] [PubMed] [Google Scholar]
- Otieno LA, Semmler JG, Smith AE & Sidhu SK. (2022). Submaximal isometric fatiguing exercise of the elbow flexors has no age-related effect on GABAB-mediated inhibition. J Appl Physiol (1985) 132, 167–177. [DOI] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL, Butler JE & Gandevia SC. (2003). Depression of activity in the corticospinal pathway during human motor behavior after strong voluntary contractions. J Neurosci 23, 7974–7980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen NT, Taylor JL & Gandevia SC. (2002). The effect of electrical stimulation of the corticospinal tract on motor units of the human biceps brachii. The Journal of physiology 544, 277–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu SK. (2021). Remote muscle priming anodal transcranial direct current stimulation attenuates short interval intracortical inhibition and increases time to task failure of a constant workload cycling exercise. Exp Brain Res 239, 1975–1985. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Cresswell AG & Carroll TJ. (2012). Motor cortex excitability does not increase during sustained cycling exercise to volitional exhaustion. J Appl Physiol 113, 401–409. [DOI] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Mangum TS, Jessop JE, Richardson RS, Morgan DE & Amann M. (2017). Group III/IV locomotor muscle afferents alter motor cortical and corticospinal excitability and promote central fatigue during cycling exercise. Clin Neurophysiol 128, 44–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu SK, Weavil JC, Thurston TS, Rosenberger D, Jessop JE, Wang E, Richardson RS, McNeil CJ & Amann M. (2018). Fatigue-related group III/IV muscle afferent feedback facilitates intracortical inhibition during locomotor exercise. The Journal of physiology 596, 4789–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Allen GM, Butler JE & Gandevia SC. (2000). Supraspinal fatigue during intermittent maximal voluntary contractions of the human elbow flexors. J Appl Physiol 89, 305–313. [DOI] [PubMed] [Google Scholar]
- Taylor JL, Amann M, Duchateau J, Meeusen R & Rice CL. (2016). Neural Contributions to Muscle Fatigue: From the Brain to the Muscle and Back Again. Med Sci Sports Exerc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE, Allen GM & Gandevia SC. (1996). Changes in motor cortical excitability during human muscle fatigue. The Journal of physiology 490 (Pt 2), 519–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Butler JE & Gandevia SC. (1999). Altered responses of human elbow flexors to peripheral-nerve and cortical stimulation during a sustained maximal voluntary contraction. Exp Brain Res 127, 108–115. [DOI] [PubMed] [Google Scholar]
- Taylor JL & Martin PG. (2009). Voluntary motor output is altered by spike-timing-dependent changes in the human corticospinal pathway. J Neurosci 29, 11708–11716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JL, Petersen NT, Butler JE & Gandevia SC. (2002). Interaction of transcranial magnetic stimulation and electrical transmastoid stimulation in human subjects. The Journal of physiology 541, 949–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen JR, Taylor JL & Kavanagh JJ. (2022). 5-HT2 receptor antagonism reduces human motoneuron output to antidromic activation but not to stimulation of corticospinal axons. Eur J Neurosci 56, 3674–3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorstensen JR, Taylor JL, Tucker MG & Kavanagh JJ. (2020). Enhanced serotonin availability amplifies fatigue perception and modulates the TMS-induced silent period during sustained low-intensity elbow flexions. The Journal of physiology 598, 2685–2701. [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Pascual-Leone A, Wassermann EM & Hallett M. (1992). Human motor evoked responses to paired transcranial magnetic stimuli. Electroencephalogr Clin Neurophysiol 85, 355–364. [DOI] [PubMed] [Google Scholar]
- Vitor-Costa M, Okuno NM, Bortolotti H, Bertollo M, Boggio PS, Fregni F & Altimari LR. (2015). Improving Cycling Performance: Transcranial Direct Current Stimulation Increases Time to Exhaustion in Cycling. PloS one 10, e0144916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavil JC & Amann M. (2018). Corticospinal excitability during fatiguing whole body exercise. Prog Brain Res 240, 219–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavil JC & Amann M. (2019). Neuromuscular fatigue during whole body exercise. Current Opinion in Physiology 10, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weavil JC, Sidhu SK, Mangum TS, Richardson RS & Amann M. (2016). Fatigue diminishes motoneuronal excitability during cycling exercise. J Neurophysiol 116, 1743–1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei K, Glaser JI, Deng L, Thompson CK, Stevenson IH, Wang Q, Hornby TG, Heckman CJ & Kording KP. (2014). Serotonin affects movement gain control in the spinal cord. J Neurosci 34, 12690–12700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PS, Hoffman RL & Clark BC. (2013). Preliminary evidence that anodal transcranial direct current stimulation enhances time to task failure of a sustained submaximal contraction. PloS one 8, e81418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yacyshyn AF, Woo EJ, Price MC & McNeil CJ. (2016). Motoneuron responsiveness to corticospinal tract stimulation during the silent period induced by transcranial magnetic stimulation. Exp Brain Res. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This is a Topical Review, all data discussed in this writing have been published previously. References to these data are included throughout.


