Abstract
Clostridium novyi alpha-toxin belongs to the family of large clostridial cytotoxins which act on cells through the modification of small GTP-binding proteins. We present here an analysis of the catalytic domain of alpha-toxin. A NH2-terminal 551-amino-acid fragment, α551, was found to contain the full enzyme activity of the holotoxin, whereas a slightly shortened fragment encompassing 509 amino acids showed no detectable enzyme activity. Further characterization of the enzymatically active fragment α551 revealed a substrate specificity for both UDP–N-acetylglucosamine and UDP-glucose. A Michaelis-Menten constant of 17 μM was determined for the substrate UDP–N-acetylglucosamine, while that for UDP-glucose was about 20 times higher, indicating a weaker affinity of the toxin for the latter substrate. Mutation of the aspartic acids of a conserved motif DXD within α551 reduced enzyme activity >700-fold and inhibited cytotoxicity after microinjection in cells. Inhibition of enzyme activity of the DXD mutant could be partially overcome by increased concentrations of manganese ions, suggesting the involvement of these aspartic acids in Mn2+ binding. By construction of chimeras of enzymatically active fragments of C. sordellii lethal toxin and C. novyi alpha-toxin, we located the region involved in nucleotide-sugar specificity to amino acids 133 through 517.
Type A strains of Clostridium novyi are causative agents of gas gangrene infections in humans and animals (7). Among a variety of different toxins produced by this pathogen is the alpha-toxin, a large 250-kDa protein which exhibits lethal and edematizing activity in vivo (7). In vitro, alpha-toxin is a potent cytotoxin, causing pronounced changes of cell morphology (1, 2). Sharing a considerable sequence homology with the toxins A and B of C. difficile and the lethal toxin from C. sordellii, the alpha-toxin has been grouped into the family of large clostridial cytotoxins (10). All of these toxins are glycosyltransferases, modifying and thereby inactivating different members of the Rho and Ras subfamily of small GTP-binding proteins (14–16). In consequence, several signal transduction pathways are inhibited (19), resulting in the breakdown of cytoskeletal structures (17). While the lethal toxin from C. sordellii modifies Rac, Cdc42, Ras, and the Ras-related GTPases Ral and Rap (11, 14, 18), the other toxins glucosylate Rho, Rac, and Cdc42 (20). Unlike the other members of this toxin family which transfer the glucose moiety from UDP-glucose to their protein substrates, the alpha-toxin uses UDP-N-acetylglucosamine (20).
The large clostridial cytotoxins are thought to consist of three distinct functional domains (21). While repetitive sequences at the C-terminal end of the toxins appear to mediate the receptor binding, a hydrophobic domain in the central region appears to be involved in the translocation from endosomal vesicles into the cytosol (21). Recent publications have shed some light on the enzymatic domain of large clostridial cytotoxins. N-terminal fragments of C. difficile toxin B and C. sordellii lethal toxin exhibit the full glucosyltransferase activity of their respective holotoxins (8, 9). Furthermore, studies with chimeric toxin fragments have delineated the region responsible for the difference in substrate specificity of C. difficile toxin B and C. sordellii lethal toxin within these N-terminal fragments (8). Lastly, a conserved amino acid motif DXD has been shown to be essential for enzyme activity (4). Recent structural data support the notion that this motif is involved in the binding of the nucleotide moiety of the UDP-glucose via coordination of a manganese ion (5).
Here we studied the enzyme domain of C. novyi α-toxin and present a detailed characterization of its catalytic properties especially with respect to nucleotide-sugar specificity, which separates alpha-toxin from the other members of the family of large clostridial cytotoxins. Our study localizes the region within the active domain of C. novyi alpha-toxin, which defines the difference in nucleotide-sugar specificity.
MATERIALS AND METHODS
Materials.
14C-labeled UDP-hexoses were obtained from DuPont NEN (Dreieich, Germany). PCR primers were from MWG Biotech (Ebersberg, Germany). All other reagents were of analytical grade and were purchased from commercial sources.
Purification of large clostridial cytotoxins.
The lethal toxin of C. sordellii 6018 was purified as described for C. difficile toxin B (9). The alpha-toxin of C. novyi was purified as reported previously (20).
PCR amplification.
Amplification of lethal toxin fragments and construction of the truncated fragment LT546 were done as described previously (8).
C. novyi type A 19402 (kindly donated by G. Schallehn, Bonn, Germany) was used as a source of chromosomal DNA. Amplification of the N-terminal 900-amino-acid fragment CN1 was performed by the PCR System 2400 (Perkin-Elmer) using the primer pair CN1C-CN1N (5′-AGATCTATGCTTATAACAAGAGAACAA-3′ and 5′-GGATCCATCTTTTTCTTTTATTATACT-3′). The reaction was carried out with 300 nmol of primers and 125 ng of chromosomal DNA for 25 cycles (denaturation: 94°C, 10 s; annealing: 48°C, 30 s; and elongation: 68°C, 3 min) in a total volume of 100 μl. The amplified DNA fragment was cloned into pCR2.1 (Invitrogen BV). After mobilization with BamHI, the gene fragment was cloned into pGEX-2T (pGEX-2T–CN1).
Cloning of truncated alpha-toxin fragments.
The toxin fragment CN1 was used for subcloning the truncated fragments α551 and α509. α509 was constructed as follows: pGEX-2T–CN1 was digested with BstBI, leading to the mobilization of a truncated fragment of 509 amino acids together with a short stretch of the pGEX-2T sequence adjacent to the 5′ end of the alpha-toxin fragment. This gene fragment was treated with the Klenow fragment of DNA polymerase I to generate blunt ends, followed by a digestion with BamHI. The resulting fragment was cloned into the pGEX-2T vector which had been digested with BamHI-SmaI.
The fragment α551 was constructed using the Seamless Cloning Kit (Stratagene) as previously described (8). Briefly, toxin fragment CN1 was used as a template for the amplification of the truncated fragment with primers SC9 (5′-GGGGCTCTTCATTCATAATTGAGAGTTCTTCCTATATAAGT-3′) and SC10 (5′-GGGGCTCTTCAGAATTCATCGTGACTGACTGACGATC-3′). The amplicon was digested with Eam1104I and relegated.
Site-directed mutagenesis of toxin fragment α551.
The QuikChange Kit (Stratagene) was used for mutating one nucleotide in pGEX-2T-α551, according to the manufacturer's instructions. The primers were constructed as follows: for α551.D284A, primer pair alphaD284Asen-alphaD284Aanti (5′-GTGGTGTATATTGTGCTTTAGATTTTCTTC-3′ and 5′-GAAGAAAATCTAAAGCACAATATACACCAC-3′); and for α551.D286A, primer pair alphaD286Asen-alphaD286Aanti (5′-TATATTGTGATTTAGCTTTTCTTCCTGGAG-3′ and 5′-CTCCAGGAAGAAAAGCTAAATCACAATATA-3′).
Construction of alpha-toxin–lethal toxin chimeras. (i) LT132.α551.
Chimera LT132-α551 was constructed using the Seamless Cloning technique (Stratagene). Primers SC10 (5′-GGGGCTCTTCAGAATTCATCGTGACTGACTGACGATC-3′) and SC14 (5′-GGGGCTCTTCAATCATAAAAAACTTTAACTGTATAATCGCT-3′) were employed to amplify a fragment containing the pGEX-2T vector and a gene fragment consisting of the N-terminal 132 amino acids of lethal toxin. Lethal toxin fragment LT546 in pGEX-2T served as a template for this reaction. The alpha-toxin portion of the chimera was amplified using the primers SC9 (5′-GGGGCTCTTCATTCATAATTGAGAGTTCTTCCTATATAAGT-3′) and SC13 (5′-GGGGCTCTTCAGATAAGAATTCATTGCTAGTAAATACATTA-3′) and the fragment α551 in pGEX-2T as a template. Following Eam1104I digestion, the fragments were ligated to constitute the chimera. The procedures were carried out according to the manufacturer's instructions with minor modifications.
(ii) LT517.α551. Chimera LT517.α551 was constructed using the splicing-by-overlap extension technique as described elsewhere (12). For the generation of the lethal toxin portion, primers SOE1 (5′-AGATCTATGAACTTAGTTAACAAAGCC-3′) and SOE5 (5′-AGCCAACTACTAGTTATTTCCTGTTCTGA-3′) were employed in an amplification using LT546 in pGEX-2T as a template. The alpha-toxin portion was amplified with the primers SOE6 (5′-CAGGAAATAACTAGTAGTTGGCTTTTAAGA-3′) and SOE4 (5′-CCATTGCTGCAGGCATC-3′).
Sequencing.
Sequencing of CN1 and all its truncated derivatives was done with the ABI PRISM Dye Terminator Cycle Sequencing Ready Reaction Kit (Perkin-Elmer) to check both for correct cloning and mutations due to PCR amplification, sequencing was performed at least twice with overlapping DNA fragments.
Expression of recombinant proteins.
The recombinant GTP-binding proteins RhoA, Rac, Cdc42, and Ha-Ras were prepared as previously described (13). The recombinant toxin fragments were expressed and purified as glutathione S-transferase (GST) fusion proteins in accordance with the manufacturer's instructions. GST fusion proteins from the Escherichia coli expression vector pGEX-2T were isolated by affinity chromatography with glutathione-Sepharose (Amersham Pharmacia Biotech), followed by cleavage of the toxin fragment proteins from the GST fusion protein by thrombin treatment (100 μg/ml for 30 min at 22°C). Thrombin was removed via binding to benzamidine-Sepharose.
Glucosylation reaction.
Recombinant GTP-binding proteins (50 μg/ml) were incubated with alpha-toxin, recombinant toxin fragments, or chimeric fragments of alpha-toxin and lethal toxin at the indicated concentrations in a buffer containing 50 mM HEPES (pH 7.5), 100 mM KCl, 2 mM MgCl2, 1 mM MnCl2, 100 μg of bovine serum albumin (BSA) per ml, and 10 to 30 μM UDP–[14C]N-acetylglucosamine or UDP-[14C]glucose for 30 min at 37°C. The total volume was 20 μl. Labeled proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and subsequently by phosphorimaging (Molecular Dynamics).
Glucohydrolase reaction.
Toxin fragments or mutants were incubated with 20 μM UDP–[14C]N-acetylglucosamine and 100 μM unlabeled UDP–N-acetylglucosamine in a buffer containing 50 mM HEPES (pH 7.5), 100 mM KCl, 2 mM MgCl2, 100 μM BSA, and 1 mM MnCl2. The total volume was 20 μl. In a time course experiment, samples (1.5 μl) were taken at each time point and subjected to thin-layer chromatography with PI-Cellulose plates (Merck), and 0.2 M LiCl was used as the mobile phase to separate hydrolyzed glucose from UDP-glucose. The plates were dried and analyzed by phosphorimaging.
Kinetic experiments.
Initial rate data for the glucosyltransferase reaction were determined with regard to UDP–N-acetylglucosamine or UDP-glucose binding by varying the concentration of the respective nucleotide-sugar from about 0.2 to 2 × Km. Experiments were performed at 7.5 μM GST-Rac. Kinetic values were obtained by analysis of Lineweaver-Burk plots of initial velocities from three independent experiments.
RESULTS
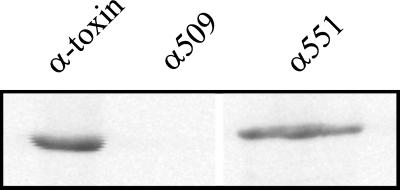
N-terminal fragments of large clostridial cytotoxins contain the full enzyme activity of their respective holotoxins (8, 9). To obtain functional data for the alpha-toxin from C. novyi, we constructed fragments of the toxin and expressed them in E. coli as GST fusion proteins. Figure 1 shows an SDS-PAGE analysis of the cleaved recombinant proteins α551 and α509, which comprise the toxin's N-terminal 551 and 509 amino acids, respectively. The 551-amino-acid fragment is of analogous size to the enzymatically active 546-amino-acid fragments of C. difficile toxin B and C. sordellii lethal toxin described previously (8, 9). These proteins were tested for their enzyme activity in an in vitro glucosylation assay using 14C-labeled UDP–N-acetylglucosamine. The large fragment (α551) catalyzed the modification of the substrate GST-Rac as intensively as did the holotoxin (Fig. 2). Fragment α509, however, did not express detectable enzyme activity. Fragments consisting of the central or C-terminal part of the toxin also did not express enzyme activity (not shown).
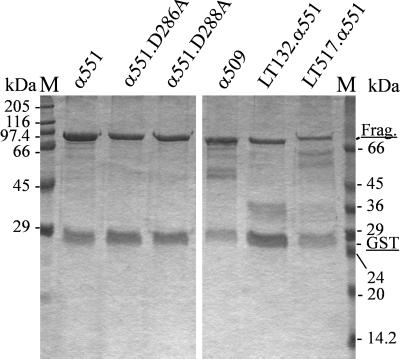
FIG. 1.
Purified recombinant toxin fragments and chimeras. N-terminal toxin fragments, mutated fragments, and chimeric toxin fragments were constructed as GST fusion proteins, expressed in E. coli, and purified by affinity chromatography. Toxin fragments and mutants (Frag.) are shown as GST fusion proteins, α551 (lane 1), α551.D286A (lane 2), α551.D288A (lane 3), α509 (lane 4), LT132.α551 (lane 5), and LT517.α551 (lane 6); approximately 3 μg was loaded in lane 1, 2 μg was loaded in lanes 2 to 5, and 1 μg was loaded in lane 6.
FIG. 2.
Glucosylation of Rac by alpha-toxin and fragments. Recombinant GST-Rac (1 μg) was glucosylated by alpha-toxin, α509, or α551 (100 nM each) in the presence of UDP–[14C]N-acetylglucosamine for 30 min. Labeled proteins were then analyzed by SDS-PAGE and phosphorimaging (shown).
To characterize further the active toxin fragment α551, we determined its nucleotide-sugar specificity. For this purpose, the toxin fragment and GST-Rac were incubated at 37°C in the presence of UDP-glucose or UDP–N-acetylglucosamine, respectively. As shown in Fig. 3, the fragment accepted both nucleotide-sugars as substrates. However, UDP-glucose resulted in a weaker labeling than UDP–N-acetylglucosamine. Similar data were obtained for the holotoxin (not shown). Kinetic experiments provided further insight into the nucleotide-sugar specificity of α551. The relative specific activities for the glycosyltransferase reaction (kcat and kcat/Km) of UDP-glucose and UDP–N-acetylglucosamine, as well as the Michaelis constants (Km), were compared by varying the UDP-glucose concentrations from 0.2 to 2 × Km at a fixed GST-Rac concentration of 7.5 μM. The values obtained by Lineweaver-Burk plots are summarized in Table 1. We observed an overall reduced efficiency of the glycosyltransferase reaction in the presence of UDP-glucose compared with UDP–N-acetylglucosamine, which was caused both by an increase in Km, and a decrease in kcat for the reaction involving UDP-glucose as a substrate.
FIG. 3.
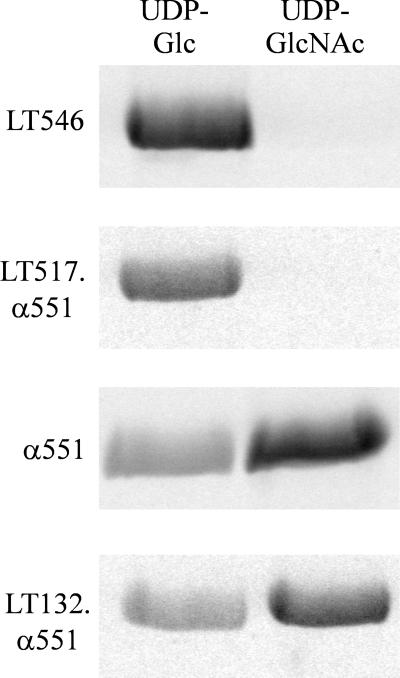
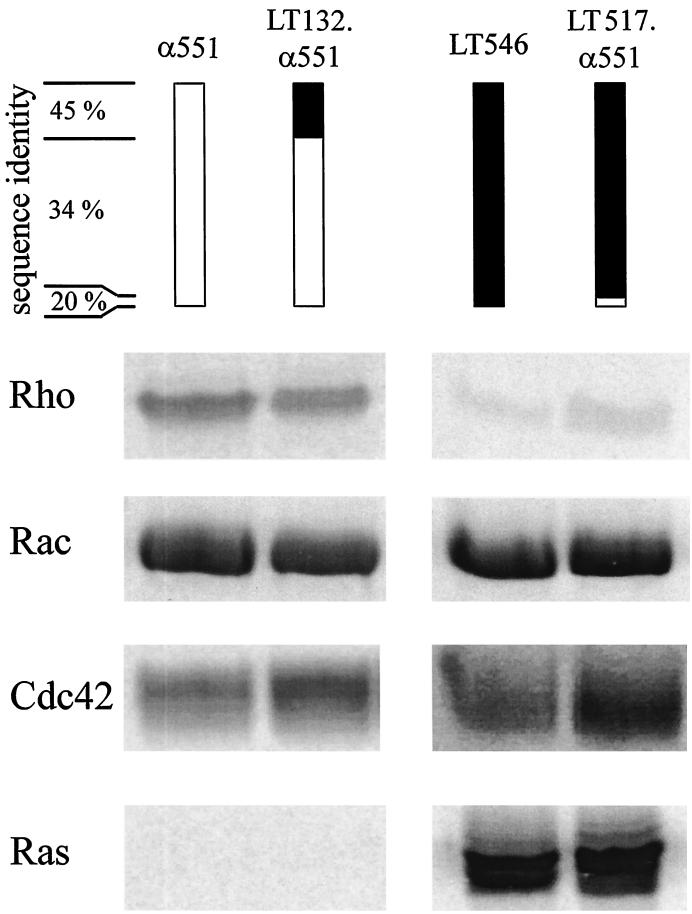
Nucleotide-sugar specificity of alpha-toxin fragments and chimeric toxin fragments. Recombinant GST-Rac (2 μg) was glucosylated by α551, LT546, LT132.α551, or LT517.α551 in the presence of UDP-[14C]glucose or UDP–[14C]N-acetylglucosamine as indicated. Labeled proteins were then analyzed by SDS-PAGE and phosphorimaging (shown).
TABLE 1.
Kinetic values of the glucosyltransferase reaction of α551 for UDP-glucose and UDP–N-acetylglucosamine
| Nucleotide-sugar | Kinetic valuesa
|
||||
|---|---|---|---|---|---|
| Km (μM) | Relative Km (%) | kcat (h−1) | Relative kcat (%) | Relative kcat/Km (%) | |
| UDP–N-acetylglucosamine | 17 | 100 | 2,800 | 100 | 100 |
| UDP-glucose | 307 | 5.5 | 282 | 10.0 | 0.6 |
Values were all determined to be statistically accurate to a standard deviation of not greater than ±20%. The kinetic parameters were determined from Lineweaver-Burk plots of initial rates of reaction as a function of the UDP-glucose or UDP–N-acetylglucosamine concentration. Assays were performed at a fixed GST-Rac concentration.
C. novyi alpha-toxin is the only member of the large clostridial cytotoxin family to accept UDP–N-acetylglucosamine as a nucleotide-sugar substrate, the other toxins exclusively use UDP-glucose. To localize the region defining nucleotide-sugar specificity, chimeric toxin fragments of alpha-toxin and C. sordellii lethal toxin were tested for their enzyme activity. A chimera consisting of the NH2-terminal 132 amino acids of lethal toxin and amino acids 133 through 551 of alpha-toxin possessed the protein substrate and the nucleotide-sugar specificity of the alpha-toxin fragment α551. On the other hand, a chimera comprising the first 517 amino acids of lethal toxin and amino acids 518 through 551 of alpha-toxin possessed the same substrate and cosubstrate specificity as lethal toxin (Fig. 3 and 4).
FIG. 4.
Substrate specificity of α551 and lethal toxin–alpha-toxin chimeras. Recombinant Rho, Rac, Cdc42, and Ras (2 μg each) were glucosylated by α551 or LT132.α551 in the presence of UDP–[14C]N-acetylglucosamine or by LT546 or LT517.α551 in the presence of UDP-[14C]glucose for 30 min. Labeled proteins were analyzed by SDS-PAGE and phosphorimaging (shown).
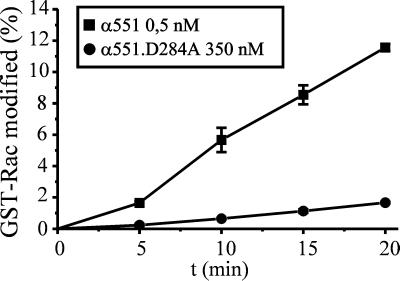
Mutation of the conserved aspartic acids of the amino acid motif DXD in the large clostridial cytotoxins C. difficile toxin B and C. sordellii lethal toxin drastically reduces enzyme activity (4). To probe the significance of the DXD motif in alpha-toxin, the aspartic acids of this motif in α551 were replaced individually by alanine. α551.D284A was about 1,000-fold less efficient for modification of GST-Rac protein relative to the wild type (Fig. 5). Similar results were obtained with α551.D286A (not shown).
FIG. 5.
Time course of the glucosylation of GST-Rac by α551 and α551.D284A. GST-Rac (1 μg) was incubated with purified NH2-terminal toxin fragment α551 (0.5 nM, ■) or mutant α551.D284A (350 nM, ●) in the presence of UDP–[14C]N-acetylglucosamine (10 μM) for the indicated times. Labeled proteins were analyzed by SDS-PAGE and phosphorimaging.
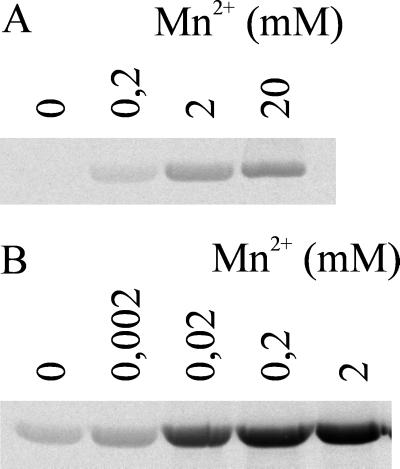
Given the impact of manganese on enzyme activity of lethal toxin DXD mutants (4), we measured the manganese ion dependency of the glucosyltransferase activity of α551.D286A. As shown in Fig. 6, wild-type fragment activity was only moderately enhanced by increasing concentrations of manganese ions, e.g., no increase in labeling was observed by increasing the Mn2+ concentration from 0.2 to 2 mM. In contrast, the activity of α551.D286A was dramatically increased with the addition of Mn2+ from 0.2 to 2 mM.
FIG. 6.
Manganese ion dependency of enzyme activity of α551 and α551.D286A. GST-Rac (1 μg) was incubated with α551 (1 nM; B) or α551.D286A (500 nM; A) in the presence of UDP–[14C]N-acetylglucosamine (10 μM) with the indicated concentrations of MnCl2 for 30 min. Labeled proteins were then analyzed by SDS-PAGE and phosphorimaging (shown).
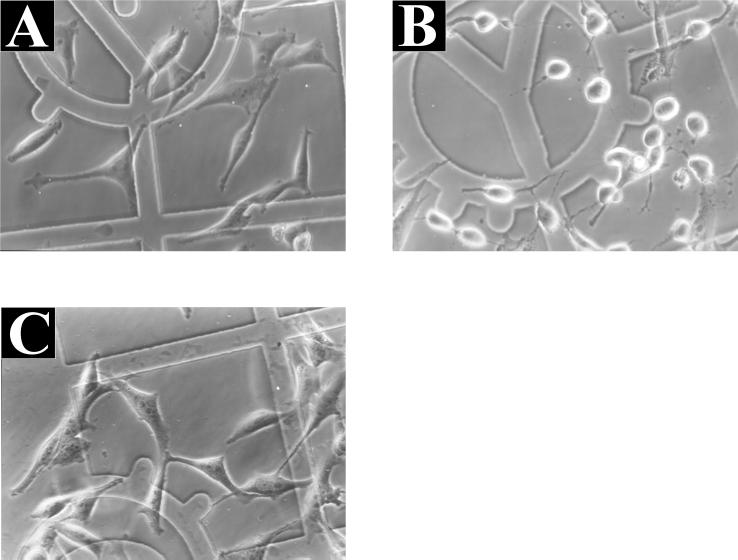
Finally, we tested the toxin fragment α551 and α551.D286A for cellular activity by microinjection into HeLa cells. The wild-type fragment α551 elicited the characteristic morphological changes observed with the holotoxin. The cells rounded up about 30 min after microinjection. In contrast, α551.D286A had no effect on the morphology of HeLa cells (Fig. 7). Moreover, the truncated fragment α509 was inactive when introduced into cells (not shown).
FIG. 7.
Microinjection of α551 and α551.D286A in HeLa cells. HeLa cells were injected with control buffer (A) or with buffer containing 1 μM α551 (B) or α551.D284A (C). Photographs were taken 30 min (B) or 4 h (A and C) after microinjection.
DISCUSSION
In this study, we present an analysis of the catalytic domain of C. novyi alpha-toxin. The data were obtained by investigating the enzymatic properties of recombinant alpha-toxin fragments. As observed for other clostridial cytotoxins, an N-terminal fragment of about 550 amino acids was necessary and sufficient for full enzyme activity, whereas a truncated fragment consisting of the N-terminal 509 amino acids possessed no detectable glucosyltransferase activity (Fig. 2). Both UDP–N-acetylglucosamine and UDP-glucose served as substrates for alpha-toxin, a finding that had not been observed in earlier publications. This discrepancy might be explained by improvement of conditions to purify and store the toxins. Kinetic data clearly show that UDP–N-acetylglucosamine is a preferred substrate compared to UDP-glucose. Alpha-toxin exhibits both an increased affinity (ca. 20-fold) and an increased turnover rate (ca. 10-fold) with UDP–N-acetylglucosamine resulting in a 180-fold increase in transferase efficiency with UDP–N-acetylglucosamine relative to UDP-glucose. The data could be explained by the fact that the two nucleotide-sugars share the same general structure and similar electronic properties apart from the bulky side chain of UDP–N-acetylglucosamine. Therefore, UDP-glucose might be bound in the cleft for UDP–N-acetylglucosamine, but without forming the interactions mediated by the side chain with the enzyme which might result in the decreased affinity.
Alpha-toxin is the only large clostridial cytotoxin to use UDP–N-acetylglucosamine as a substrate. To elucidate the molecular background of this special feature, chimeric toxin fragments were constructed to locate the region involved in nucleotide-sugar recognition. The size of the regions swapped between the toxin fragments was chosen in analogy to the chimeric proteins of C. difficile toxin B and C. sordellii lethal toxin described previously (8). From the data obtained by the characterization of two lethal toxin–alpha-toxin fragment chimeras, we propose amino acids 133 through 517 contain the region determining the different nucleotide-sugar specificity of the two toxins. Because the nucleotide moieties of UDP-glucose and UDP–N-acetylglucosamine are unchanged, this region is most likely involved in the binding of the sugar moiety of the substrate.
A tryptophan conserved at position 102 within all the clostridial cytotoxins has been recently shown to be important for the nucleotide-sugar affinity of lethal toxin fragments (3). Located outside of the above-mentioned region, this residue should interact with the nucleotide moiety of the substrates. This conclusion is consistent with data obtained from recently published crystal structures of two glycosyltransferases (5, 6). In both of these studies, an aromatic amino acid residue was proposed to be involved in a stacking interaction with the uracil ring of the nucleotide. This residue is conserved throughout the members of the respective glycosyltransferase families. Likewise, a tryptophan 102 analogue is conserved among all proteins related to the catalytic subunit of large clostridial cytotoxins (3).
Another characteristic feature of a variety of glycosyltransferases is the amino acid motif DXD, which has been shown to coordinate a manganese ion in one of the above-mentioned crystal structures (6). Site-directed mutagenesis of the alpha-toxin fragment's DXD motif resulted in mutants exhibiting a drastically reduced enzyme activity in glycosyltransferase assays and a lack of intracellular activity (Fig. 5 and 7). In the presence of high concentrations of manganese ions, the activity of the mutants was considerably increased, a finding which is consistent with the view that the DXD motif is involved in the coordination of a manganese ion. However, we cannot exclude that manganese ions increase enzyme activity independently of the DXD motif and that the observation of the enhancement was possible because the basal activity was low. The increase in activity of the wild-type fragment by low amounts of manganese ions can be explained by the general dependency of large clostridial cytotoxins on manganese ions (Fig. 6).
Investigating chimeric toxin fragments of C. difficile toxin B and C. sordellii lethal toxin, we have shown recently that a region of amino acids 271 through 516 defines the substrate specificity of the cytotoxins (8). Corroborating these data, amino acids 133 through 551 were found to be sufficient to define the substrate specificity of α-toxin as shown by the lethal toxin–alpha-toxin chimera LT132.α551 (Fig. 4). In addition to this active chimera, we constructed several fusion proteins of alpha-toxin and lethal toxin (LT270.α551, LT132.α408.LT546, and LT408.α551). However, all of these chimeric proteins were inactive. On the other hand, the addition of amino acid residues 518 to 551 of alpha-toxin to the inactive fragment (residues 1 to 517) of lethal toxin restored the activity of lethal toxin. Thus far it is not clear whether amino acids 518 to 551 harbor catalytic important residues or have a structure-stabilizing effect on the transferase.
ACKNOWLEDGMENTS
We thank K. Thoma for excellent technical assistance.
This study was financially supported by the Deutsche Forschungsgemeinschaft and the Fonds der Chemischen Industrie.
REFERENCES
- 1.Bette P, Frevert J, Mauler F, Suttorp N, Habermann E. Pharmacological and biochemical studies of cytotoxicity of Clostridium novyi type A alpha-toxin. Infect Immun. 1989;57:2507–2513. doi: 10.1128/iai.57.8.2507-2513.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bette P, Oksche A, Mauler F, von Eichel-Streiber C, Popoff M R, Habermann E. A comparative biochemical, pharmacological and immunological study of Clostridium novyi α-toxin, C. difficile toxin B, and C. sordellii lethal toxin. Toxicon. 1991;29:877–887. doi: 10.1016/0041-0101(91)90224-f. [DOI] [PubMed] [Google Scholar]
- 3.Busch C, Hofmann F, Gerhard R, Aktories K. Involvement of a conserved tryptophan residue in the UDP-glucose binding of large clostridial cytotoxin glycosyltransferases. J Biol Chem. 2000;275:13228–13234. doi: 10.1074/jbc.275.18.13228. [DOI] [PubMed] [Google Scholar]
- 4.Busch C, Hofmann F, Selzer J, Munro J, Jeckel D, Aktories K. A common motif of eukaryotic glycosyltransferases is essential for the enzyme activity of large clostridial cytotoxins. J Biol Chem. 1998;273:19566–19572. doi: 10.1074/jbc.273.31.19566. [DOI] [PubMed] [Google Scholar]
- 5.Charnock S J, Davies G J. Structure of the nucleotide-diphospho-sugar transferase, SpsA from Bacillus subtilis, in native and nucleotide-complexed forms. Biochemistry. 1999;38:6380–6385. doi: 10.1021/bi990270y. [DOI] [PubMed] [Google Scholar]
- 6.Gastinel L N, Cambillau C, Bourne Y. Crystal structures of the bovine β4galactosyltransferase catalytic domain and its complex with uridine diphosphogalactose. EMBO J. 1999;18:3546–3557. doi: 10.1093/emboj/18.13.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hatheway C L. Toxigenic clostridia. Clin Microbiol Rev. 1990;3:66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hofmann F, Busch C, Aktories K. Chimeric clostridial cytotoxins: identification of the N-terminal region involved in protein substrate recognition. Infect Immun. 1998;66:1076–1081. doi: 10.1128/iai.66.3.1076-1081.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hofmann F, Busch C, Prepens U, Just I, Aktories K. Localization of the glucosyltransferase activity of Clostridium difficile toxin B to the N-terminal part of the holotoxin. J Biol Chem. 1997;272:11074–11078. doi: 10.1074/jbc.272.17.11074. [DOI] [PubMed] [Google Scholar]
- 10.Hofmann F, Herrmann A, Habermann E, von Eichel-Streiber C. Sequencing and analysis of the gene encoding the α-toxin of Clostridium novyi proves its homology to toxins A and B of Clostridium difficile. Mol Gen Genet. 1995;247:670–679. doi: 10.1007/BF00290398. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann F, Rex G, Aktories K, Just I. The Ras-related protein Ral is monoglucosylated by Clostridium sordellii lethal toxin. Biochem Biophys Res Commun. 1996;227:77–81. doi: 10.1006/bbrc.1996.1470. [DOI] [PubMed] [Google Scholar]
- 12.Horton R M, Hunt H D, Ho S N, Pullen J K, Pease L R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989;77:61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- 13.Just I, Fritz G, Aktories K, Giry M, Popoff M R, Boquet P, Hegenbarth S, von Eichel-Streiber C. Clostridium difficile toxin B acts on the GTP-binding protein Rho. J Biol Chem. 1994;269:10706–10712. [PubMed] [Google Scholar]
- 14.Just I, Selzer J, Hofmann F, Green G A, Aktories K. Inactivation of Ras by Clostridium sordellii lethal toxin-catalyzed glucosylation. J Biol Chem. 1996;271:10149–10153. doi: 10.1074/jbc.271.17.10149. [DOI] [PubMed] [Google Scholar]
- 15.Just I, Selzer J, Wilm M, von Eichel-Streiber C, Mann M, Aktories K. Glucosylation of Rho proteins by Clostridium difficile toxin B. Nature. 1995;375:500–503. doi: 10.1038/375500a0. [DOI] [PubMed] [Google Scholar]
- 16.Just I, Wilm M, Selzer J, Rex G, von Eichel-Streiber C, Mann M, Aktories K. The enterotoxin from Clostridium difficile (ToxA) monoglucosylates the Rho proteins. J Biol Chem. 1995;270:13932–13936. doi: 10.1074/jbc.270.23.13932. [DOI] [PubMed] [Google Scholar]
- 17.Müller H, von Eichel-Streiber C, Habermann E. Morphological changes of cultured endothelial cells after microinjection of toxins that act on the cytoskeleton. Infect Immun. 1992;60:3007–3010. doi: 10.1128/iai.60.7.3007-3010.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Popoff M R, Chaves O E, Lemichez E, Von Eichel-Streiber C, Thelestam M, Chardin P, Cussac D, Chavrier P, Flatau G, Giry M, Gunzburg J, Boquet P. Ras, Rap, and Rac small GTP-binding proteins are targets for Clostridium sordellii lethal toxin glucosylation. J Biol Chem. 1996;271:10217–10224. doi: 10.1074/jbc.271.17.10217. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Rümenapp U, Bienek C, Keller J, Von Eichel-Streiber C, Jakobs K H. Inhibition of receptor signaling to phospholipase D by Clostridium difficile toxin B—role of Rho proteins. J Biol Chem. 1996;271:2422–2426. doi: 10.1074/jbc.271.5.2422. [DOI] [PubMed] [Google Scholar]
- 20.Selzer J, Hofmann F, Rex G, Wilm M, Mann M, Just I, Aktories K. Clostridium novyi α-toxin-catalyzed incorporation of GlcNAc into Rho subfamily proteins. J Biol Chem. 1996;271:25173–25177. doi: 10.1074/jbc.271.41.25173. [DOI] [PubMed] [Google Scholar]
- 21.Von Eichel-Streiber C, Boquet P, Sauerborn M, Thelestam M. Large clostridial cytotoxins: a family of glycosyltransferases modifying small GTP-binding proteins. Trends Microbiol. 1996;4:375–382. doi: 10.1016/0966-842X(96)10061-5. [DOI] [PubMed] [Google Scholar]