Abstract
Objectives:
Examine the associations of sleep measures with kidney function changes over time among individuals from a community-based study.
Methods:
The sample includes 1657 participants (287 with chronic kidney disease [CKD]) in Multi-Ethnic Study of Atherosclerosis Sleep Cohort (mean age: 57.7 years, male: 46.0%). We examined the associations of a large set of sleep variables (polysomnography [PSG], actigraphy, and questionnaires) and cardiovascular disease (CVD) risk factors with changes in estimated glomerular filtration rate (eGFR) and urinary albumin to creatinine ratio (UACR) over approximately 5 years using high-dimensional regression. The modifying effect of sleep on the associations between CVD risk factors and kidney function was investigated.
Results:
Sleep metrics predicted kidney function decline only among individuals with baseline CKD. Among this group, eGFR decline was associated with decreased stage N3 sleep (0.32 ml/min/1.73m2/year per 10% decrease in N3, p<.001); increased actigraphy napping frequency (beta: −0.20 [−0.30, −0.07]), and the actigraphy sleep midpoint trajectory in early morning (ref: midnight, beta: −0.84 [−1.19, −0.50]). UACR increase was associated with high wake bouts trajectory (ref: low, beta: 0.97 [0.28, 1.67]) and increased sleep-related hypoxemia (oxygen saturation %time<90 [≥5%], beta: 2.17 [1.26, 3.08]). Sleep metrics – N3 sleep, naps, and midpoint trajectory - significantly modified associations between HbA1c and eGFR decline.
Conclusions:
Reduced deep sleep, daytime napping, increased wake bouts, delayed sleep rhythms, and overnight hypoxemia are associated with longitudinal kidney function decline, with effects most apparent in CKD individuals. Deep sleep, napping, and sleep timing modified the association between HbA1c and kidney function.
Keywords: diabetes, albuminuria, chronic kidney disease, sleep apnea, sleep duration, sleep health
Introduction
Chronic kidney disease (CKD) is a worldwide public health problem that is increasing in incidence and prevalence.1 Prior research suggests that various aspect of sleep, such as sleep with reduced duration and quality, obstructive sleep apnea (OSA), and restless leg syndrome, influence kidney health.2–5 However, findings from these studies are inconsistent. For example, the significant association between short sleep duration and decreased estimated glomerular filtration rate (eGFR) found by Guo et al (2015)4 was not replicated in other studies.2,6 Although Jackson et al (2021) recently found sleep apnea associated hypoxia was associated with higher CKD prevalence, the relationship between sleep apnea and the development and progression of CKD is not addressed.7–8 The literature is therefore limited by either focus on one or only a few metrics of sleep or their cross-sectional designs. With increasing recognition that sleep is multi-dimensional, it is important to consider multiple potential sleep exposures that may contribute to kidney disease development and utilize longitudinal data to better identify potential cause associations.
Prior studies also have not systematically addressed how baseline CKD may modify associations between sleep and kidney disease progression. Patients with CKD generally experience poor sleep compared with those without CKD, and sleep apnea is more prevalent in advanced stages of CKD.9–10 In addition, fatigue is common in individuals with advanced CKD, which may increase the number or duration of naps.11 Therefore, the relationship between sleep and kidney function may vary by baseline CKD status.
Cardiovascular disease (CVD) risk factors are important risk factors for CKD.12–13 While most research has examined individual risk factors, recent studies suggest that several CVD risk factors may have synergistic influence on the risk of CKD.14 CVD risk factors, such as obesity, diabetes, and hypertension, also are associated with various sleep measures, and may be on the pathways linking sleep disturbances to CKD.15–16 However, the causal pathways and potential interactions among CVD risk factors and sleep, and their effects on kidney function, are not well understood. Although clinical trials are the gold standard for causal inference, they are not practical for studying multiple exposures.
The last years have witnessed a revolution of methodological and computational advances which allow for analyzing high-dimensional data, characterized by a larger number of variables than typically considered in classical multiple regression analyses.17–18 High-dimensional regression has been widely applied for simultaneously selecting important predictors and estimating their effects from a large set of variables. The number of variables included in high-dimensional regression is proportional to or can be much larger than the sample size of the data. Analyzing data from observational longitudinal studies using modern statistical techniques provides opportunities to examine multiple sleep and CVD risk factors simultaneously in relationship to kidney function.
The objective of this study is to examine the associations between sleep measures and changes in kidney function over time, as assessed by longitudinal decline in eGFR or by increase in urinary albumin to creatinine ratio (UACR). A large set of sleep variables (i.e., polysomnography [PSG], actigraphy, and questionnaires data) and CVD risk factors were simultaneously assessed in high-dimensional regression analyses. Our specific goals were to identify: whether sleep characteristics significantly associated with changes in kidney function and to identify which relationships differed by CKD status (with vs. without CKD), specifically, we assessed standard and novel measures of sleep that predict kidney function change; and which sleep measures modified the associations between baseline CVD risk factors and changes in kidney function.
Methods
Study design and participants
The Multi-Ethnic Study of Atherosclerosis (MESA) is a multisite prospective study to investigate the prevalence and progression of subclinical CVD and to identify risk factors for incident CVD in racially/ethnically diverse sample.19 At MESA Exam 5 (2010–2013), 10 years after the initial examination, all participants not reporting regular use of oral appliance devices, nocturnal oxygen, or nightly positive airway pressure devices were invited to participate in the MESA Sleep Ancillary Study (MESA SLEEP), which collected sleep measures from PSG, actigraphy, and sleep questionnaires.20 Participants underwent two repeated measures of kidney function at MESA Exam 5 and Exam 6 (2016-2018). The median time interval was 301 days (range: 0-1024) between MESA Exam 5 and MESA Sleep, and was 1971 days (range: 1317-2674) between the MESA Sleep and MESA Exam 6.
Variables
CKD at baseline was defined as eGFR <60 ml/min/1.73m2 or UACR ≥30 mg/g.21 eGFR was calculated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.21 UACR was determined from a spot midstream urine sample. The annual eGFR/UACR change was eGFR (or UACR) at MESA Exam 6 – eGFR (or UACR) at Exam 5)/follow-up years.
We classified the variables into four domains: 29 actigraphy or PSG sleep measures, 4 self-reported sleep variables, 12 demographics variables, and 38 clinical measures (See Supplemental Table 1 for details). In addition to sleep duration and quality, PSG measures provided information on sleep architecture.15 The sleep architecture refers to the basic structural organization of normal sleep, which shifts between non-rapid eye movement (NREM) and rapid eye movement (REM) sleep. NREM sleep is divided into stages N1, N2, and N3, representing a continuum of relative depth. The clinical domain includes CVD risk factor measures (BMI, glucose, HbA1C, total cholesterol, triglycerides, systolic blood pressure, hypertension medication, and smoking). We examined the correlations among variables for multicollinearity.
Two variables, average oxygen saturation (SAO2) and minutes of SAO2 during NREM sleep, were removed from analyses because they are highly correlated with the same variables measured during REM sleep (r>0.80). In addition to the traditional summary sleep measures, actigraphy derived weekly sleep trajectories (Supplemental Figures 2–4) for sleep duration, wake after sleep onset, intranight instability index, sleep midpoint (clock time of the epoch that is midway in the sleep interval), wake bouts (number of continuous periods of wake, at least 30 seconds long, during the sleep interval), and daytime nap duration were identified using latent class growth models (LCGM). The technical details about applying LCGM to identify the weekly sleep trajectories has been previously described.15 The weekly sleep trajectories characterize sleep patterns changing by day over a course of one week, which are novel measures of night-to-night pattern of sleep variation. Intra-night instability index derived from actigraphy data was shown to be an informative marker for nightly sleep quality, see Chen et al for details.15
Statistical analyses
As datasets get larger and more complex, there is a growing interest in using high-dimensional regression where the number of features or covariates is much larger than that in traditional regression analyses in low dimension. In this paper, we included all variables from four domains introduced in the previous section into high-dimensional models, and study their associations with the outcomes of kidney function changes.
A three-step analytical scheme was designed to address the aims proposed in Introduction.
Step 1:
Identification of interactions between sleep and baseline CKD status: we first tested the interactions between all sleep variables and baseline CKD status in their relationships to kidney function changes, conditioning on the remaining variables in high dimension.
Step 2:
Identification of characteristics associated with kidney function changes: we then applied high-dimensional regression analyses to estimate associations, including all variables in the four domains and significant interaction terms from Step 1. Only significant associations were retained in the final model and reported in the Result section.
Step 3:
Examination of effect modification: for significant sleep and CVD risk factors associated with kidney function change identified in Step 2, we first tested their interactions. For those CVD risk factors identified to have significant interactions with sleep, we re-evaluated their relationships to changes in kidney function through high dimensional analyses stratifying the sample by the selected sleep measures.
The analyses described in Steps 1 and 2 utilized the entire sample data, regardless of CKD status. For those sleep features with evidence of significant interaction with baseline CKD status, we report their association by CKD status in the Result section.
We used R software MICE package to generate 20 imputed datasets following the guidance to multiple imputation of missing data in nephrology by Blazek et al.22 Excluding the participants with missing kidney function outcomes from analyses, we imputed missing values for the remaining variables. Because the distribution of annual UACR change is heteroscedastic with heavy tails (supplemental figure 1), the high-dimensional Huber regression was used for all the analyses for the outcome of UACR change.23 All other associations were estimated using LASSO implemented in R software glmnet package.24 The SAS MIANALYZE procedure was used to combine the results from the analyses of 20 imputed datasets. Importantly, associations of interest, we provided confidence interval and p-value, and the significance level was determined using a Bonferroni adjustment for multiple comparisons. Details on the statistical analyses can be found in Supplemental Materials.
Results
A total of 1935 participants had complete sleep measures from PSG, actigraphy, and sleep questionnaires, of which 1657 had longitudinal measures of change in eGFR (CKD at baseline: 287), and 1552 had longitudinal change in UACR (CKD at baseline: 268). Overall, the mean age is 58, and 46% of sample is male (Table 1). 21% of the sample was classified with severe OSA (Apnea-Hypopnea Index [AHI] ≥30). The mean percentage of PSG stage N3 sleep is 10%. There were four classes of actigraphy weekly trajectory for wake bouts during sleep (Supplemental Figure 2): Low: 41.2% of participant had low wake bouts; moderate: 38.5% of participants had moderate wake bouts; high: 16.4% of participants had high wake bouts; very high: 3.8% of participant had very high wake bouts. The low, moderate, and high classes had relatively stable pattern over a week. The very-high class had an increasing pattern from Monday to Sunday. In the analyses, we combined the classes of very high and high into one class.
Table 1:
Baseline characteristics for individuals included in the analyses. Chronic kidney disease (CKD) at baseline was defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73m2 or urinary albumin to creatinine ratio (UACR) ≥30 mg/g. Mean (s.e.) is presented for continuous variable, and N (%) for categorical variable. Polysomnography (PSG), hemoglobin A1C (HbA1c).
| All Mean (s.e.)/N (%) | No CKD Mean (s.e.)/N (%) | CKD Mean (s.e.)/N (%) | |
|---|---|---|---|
| N= | 1657 | 1370 | 287 |
| Demographic and Clinical Characteristics | |||
| Age (years) | 57.69 (0.21) | 56.56 (0.22) | 63.1 (0.53) |
| Male | 762 (45.99) | 623 (45.47) | 139 (48.43) |
| Hypertension | 890 (53.71) | 657 (47.96) | 233 (81.18) |
| Diabetes | 314 (18.95) | 213 (15.55) | 101 (35.19) |
| Systolic blood pressure (mm Hg) | 121.82 (0.48) | 120.07 (0.49) | 130.21 (1.37) |
| HbA1c (%) | 5.96 (0.02) | 5.86 (0.02) | 6.44 (0.08) |
| Medication biguanides | 192 (11.59) | 141 (10.29) | 51 (17.77) |
| Health insurance (public) | 506 (30.85) | 354 (26.11) | 152 (53.52) |
| eGFR baseline (ml/min/1.73m2) | 82.2 (0.42) | 85.95 (0.35) | 64.31 (1.29) |
| UACR baseline (mg/g, median [IQR]) | 5.40 (3.40, 10.50) | 4.90 (3.20, 7.80) | 31.65 (6.10, 69.50) |
| Sleep Characteristics | |||
| AHI≥30 | 327 (21.21) | 258 (20.14) | 69 (26.44) |
| PSG oxygen--%time < 90 (>= 5%) | 271 (17.57) | 208 (16.24) | 63 (24.14) |
| PSG percentage of N3 sleep | 10.29 (0.23) | 10.51 (0.25) | 9.18 (0.56) |
| Actigraphy number of naps | 0.82 (0.02) | 0.77 (0.02) | 1.09 (0.06) |
| Actigraphy midnight weekly sleep midpoint trajectory | 354 (22.22) | 303 (23.01) | 51 (18.48) |
| Actigraphy wee-hours weekly sleep midpoint trajectory | 932 (56.25) | 775 (56.57) | 157 (54.7) |
| Actigraphy high number of wake bouts trajectory | 322 (20.21) | 264 20.05) | 58 (21.01) |
Sleep Characteristics Associated with Kidney Function Changes
We present the variables associated with eGFR decline in Table 2. There were significant interactions between three sleep measures (PSG stage N3 sleep, actigraphy number of naps, and actigraphy sleep midpoint trajectory) and baseline CKD. The marginal associations for these three measures were not statistically significant, and consistent with no statistically significant associations between eGFR decline and these sleep measures in individuals without CKD. In contrast, for participants with CKD only: lower percentage of PSG stage N3 sleep (beta: −0.32 [−0.33, −0.30]), higher actigraphy number of naps (beta: −0.20 [−0.30, −0.07]), and the actigraphy sleep midpoint trajectory in early morning (ref: in midnight, beta: −0.84 [−1.19, −0.50]), were associated with eGFR decline.
Table 2:
Measures associated with annual estimated glomerular filtration rate (eGFR) change. Any association for a variable was adjusted for all other variables in the four domains listed in the supplemental materials. All associations are significant under Bonferroni adjustment, i.e., p-value<0.003.
| Outcome: annual eGFR change (N=1657) | ||
|---|---|---|
| Predictors | Beta (95% CI) | |
| Sleep variables | ||
| 10 percentage decrease in PSG N3 sleep *** | Non-CKD | −0.00 (−0.01, 0.01) |
| CKD | −0.32 (−0.33, −0.30) | |
| Average number of naps (actigraphy) *** | Non-CKD | −0.03 (−0.12,0.06) |
| CKD | −0.20 (−0.30, −0.07) | |
| Early morning sleep midpoint trajectory (ref: midnight) *** | Non-CKD | 0.11 (−0.03,0.24) |
| CKD | −0.84 (−1.19, − 0.50) | |
| Clinical variables | ||
| HbA1c | −0.19 (−0.21, −0.17) | |
| Systolic blood pressure | −0.01 (−0.01, −0.01) | |
| Baseline eGFR | −0.02 (−0.02, −0.02) | |
| Baseline log (UACR) | −0.17 (−0.20, −0.14) | |
| Medication Biguanides | −0.37 (−0.41, −0.33) | |
| Demographic variables | ||
| Age | −0.02 (−0.02, −0.01) | |
the sleep characteristic with significant interaction with baseline chronic kidney disease (CKD) status. The associations with changes in kidney function for these sleep characteristics are further shown for participants with and without CKD at baseline;
non-CKD: estimate for participants without CKD at baseline;
CKD: estimate for participants with CKD at baseline.
Polysomnography (PSG), hemoglobin A1C (HbA1c), urinary albumin to creatinine ratio (UACR).
The variables significantly associated with UACR change are described in Table 3. There were significant interactions between two sleep measures (actigraphy wake bouts trajectory and PSG Oxygen--%time<90[>=5%]) and baseline CKD, such that they were only associated with UACR increase in participants with baseline CKD (ref: low wake bouts trajectory, beta: 0.97 [0.28, 1.67]), and PSG Oxygen--%time<90(>=5%) (beta: 2.17 [1.26, 3.08]).
Table 3:
Measures associated with annual urinary albumin to creatinine ratio (UACR) change. Any association for a variable was adjusted for all other variables in the four domains listed in the supplemental materials. All associations are significant under Bonferroni adjustment, i.e., p-value<0.003.
| Outcome: annual UACR change (N=1552) | ||
|---|---|---|
| Predictors | Beta (95% CI) | |
| Sleep variables | ||
| PSG oxygen--%time < 90 (>= 5%) *** | Non-CKD | 0.15 (−0.08,0.38) |
| CKD | 2.17 (1.26,3.08) | |
| Actigraphy high number of wake bouts trajectory (ref: low) *** | Non-CKD | −0.27 (−0.57,0.04) |
| CKD | 0.97 (0.28,1.67) | |
| Clinical variables | ||
| HbA1c | 0.27 (0.17, 0.37) | |
| Demographic variables | ||
| Health insurance public (ref: no) | 0.26 (0.09, 0.43) | |
the sleep characteristic with significant interaction with baseline chronic kidney disease (CKD) status. The associations with changes in kidney function for these sleep characteristics are further shown for participants with and without CKD at baseline;
Non-CKD: estimate for participants without CKD at baseline;
CKD: estimate for participants with CKD at baseline.
Polysomnography (PSG), hemoglobin A1C (HbA1c).
In addition, the severe OSA (AHI≥30) was not significantly associated with changes in kidney function in this study. For example, the association between AHI≥30 and eGFR decline was small and not statistically significant (beta=0.02, p=0.453). In an early version of this paper, we also modeled OSA using AHI as a continuous variable, which was not significantly associated with changes in kidney function.
Sleep Modifies the Association between HbA1c and eGFR Decline
Among all CVD risk variables, only HbA1c and systolic blood pressure were significantly associated with the change in eGFR, and HbA1c was also associated with the change in UACR. Furthermore, only the association between HbA1c and change in eGFR was significantly modified by sleep. Figure 1 shows the results of analyses stratified by the moderating sleep metrics. A one unit increase in baseline HbA1c was associated with: 0.04 (p=0.204) and 0.25 (p<.001) (ml/min/1.73m2/year) eGFR decline for the participants with PSG stage N3 sleep above and below median; 0.06 (p=0.056) and 0.21 (p<.001) (ml/min/1.73m2/year) eGFR decline for the participants with actigraphy number of naps below and above median; and 0.02 (p=0.560) and 0.23 (p<.001) (ml/min/1.73m2/year) eGFR decline for the participants having actigraphy sleep midpoint trajectory in midnight and the participants having trajectory in early morning, respectively.
Figure 1:
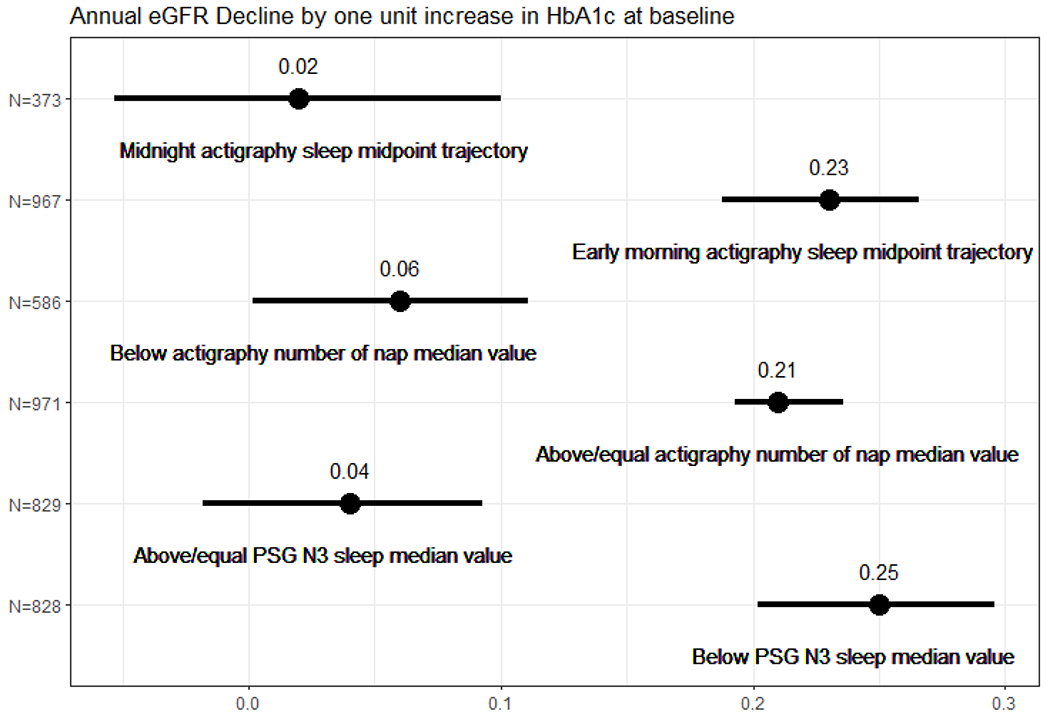
Associations between hemoglobin A1C (HbA1c) and estimated glomerular filtration rate (eGFR) decline stratified by selected sleep metrics, evaluating if these sleep characteristics (stage N3 sleep, napping frequency, and sleep timing) modify the relationship between HbA1c and changes in kidney function, by high-dimensional regression analyses. The dot with bar represents the association estimate with 95% confidence interval. For example, a one unit increase in baseline HbA1c was associated with: 0.04 (p=0.204) and 0.25 (p<.001) (ml/min/1.73m2/year) estimated glomerular filtration rate decline for the participants with polysomnography (PSG) stage N3 sleep above or equal to the median value and the participants below median
Discussion
Applying advanced high-dimensional statistical methods, we identified several sleep characteristics related to 5-year longitudinal decline in kidney function assessed by eGFR or UACR. Among individuals with CKD, baseline decreases in PSG stage N3 sleep, actigraphy daytime napping frequency, and actigraphy sleep midpoint in early morning were associated with decline in eGFR; actigraphy trajectories of increased wake bouts and increased sleep-related hypoxemia were associated with increase in UACR. These results suggest that individuals with existing CKD may be especially vulnerable to the effects of disturbed sleep architecture, delayed timing, and overnight hypoxemia.5
To the best of our knowledge, this is the first study to find that stage N3 sleep, delayed timing, and wake bouts during sleep are associated with a decline in kidney function among individuals with CKD. Among participants with CKD, each ten percent decrease in stage N3 sleep was associated with an eGFR decline of 0.32 ml/min/1.73m2 per year. In addition, the sleep pattern with high wake bouts was associated with an increase in albuminuria of 0.84 mg/g per year. Stage N3 sleep is characterized by high parasympathetic and low sympathetic nervous system activity, associated with restorative functions. The mechanisms linking reduced stage N3 sleep to kidney function are not clear, but experimental data suggest that stage N3 sleep deprivation leads to abnormalities in sympathetic nervous system activity and corticotrophin pathways, which may affect kidney function directly or in-directly.25 Animal studies have demonstrated that progressive stimulation of the renal sympathetic nervous system leads to stepwise activation of renin release, renal sodium absorption, and decrease of GFR.26 Furthermore, reduced stage N3 sleep has been associated with increased incidence of hypertension, coronary artery calcium, and atrial fibrillation, as well as visceral obesity.27 High wake bouts during sleep reflect less consolidated sleep, sleep disturbance, and sleep-wake rhythm disorder, which have been linked to systemic inflammation and nocturnal blood pressure profile.28–29 Nocturnal and non-dipping blood pressure are independent risk factors for mortality (over and beyond daytime blood pressure).30 The negative effects of a delayed midpoint trajectory is consistent with literature implicating altered circadian rhythms in cardiometabolic and vascular diseases.5 Although ambulatory blood pressure measures were not available in our study, it is plausible that poorly controlled nocturnal hypertension may have contributed to worsening of kidney function over time.31–32 Our findings highlight the potential benefits of evaluating sleep quality and ambulatory blood pressure monitoring in high risk populations, such as those with CKD.
For participants with CKD, actigraphy high nap frequency was associated with longitudinal decrease in eGFR and increase in UACR. Increasing napping has been linked to cognitive decline, reduced nightly sleep quality, and mortality.15,33 The napping measures in this study were from actigraphy, which may be more sensitive as a marker of excessive daytime sleepiness or circadian disturbance, compared to subjective measures such as the Epworth Sleepiness Scale. Questionnaires assessing sleepiness are prone to measurement error. On the other hand, it is difficult to discern napping from quiet, sedentary behaviors from actigraphy, and the procedures for identifying napping in the MESA SLEEP Exam (use both diary and actigraphy data to annotate naps) has not been validated. The negative relationship between napping and kidney function may be partially explained by poor nightly sleep. In addition, reverse causality is possible, compared to individuals without CKD, people with CKD experience more fatigue and sedentary behavior, which may result in more napping. 11,34 Moreover, more napping may be a marker of severe chronic health problems, which may not be captured by adjusting for measured confounders.
An unexpected finding was a lack of association between severe OSA (defined by AHI) and kidney function. Although prior reports identified associations between OSA with kidney disease,35–36 their study populations, and definitions of sleep and kidney function, are different from those in this article. The methodological differences may also explain inconsistency in findings. The traditional analyses utilized in those studies did not adjust for other sleep traits. For example, AHI and sleep stages co-vary, but time in NREM and REM sleep were not adjusted in the analyses by Adams et al and Canales et al.35–36 The high-dimensional analyses is less prone to the influences of unselected confounders.
In contrast to the null association with OSA defined by AHI, we found that overnight hypoxemia was significantly associated with longitudinal increase in UACR among individuals with CKD in this study. Various measures of hypoxemia have been implicated with kidney disease measured in cross-sectional studies.5,36 Measures of overnight hypoxemia are relatively readily obtained in clinic settings, and may be particularly useful for screening for sleep disordered breathing-related risk.
Inflammation, increased vascular endothelial growth factor (VEGF), and endothelial cell proliferation have been found to closely associated with kidney function in diabetic kidney disease.37 Poor sleep may accelerate the negative effects of diabetes on kidney function. Consistent with this hypothesis, we observed stronger associations between HbA1c and eGFR decline in the participants with lower stage N3 sleep, higher frequency of naps, and later sleep timing. While diabetes is one of the most important risk factors for CKD, research examining the impact of sleep on the development and progression of diabetic nephrology is sparse, and limited by cross-sectional design and small sample size. Our findings suggest that individuals with CKD with both glucose impairments and sleep disorders may be particularly vulnerable to decline in kidney function. Sleep fragmentation and intermittent hypoxia can active the sympathetic nervous system and renin-angiotensin-aldosterone system, and contributing to systemic inflammation, glomerular hypertrophy, increased VEGF, and endothelial dysfunction.38–39 These may contribute to the pathways that sleep and diabetes affecting kidney health simultaneously. It is possible that maintenance of sleep quality may prevent or retard the diabetic kidney disease. Further research is needed to better understand the mechanisms underlying the effect of sleep on diabetic nephrology.
A strength of this study is the availability of both objective and subjective sleep measures, and the longitudinal design which provided data on change in kidney function over an approximately 5 year period, allowing a comprehensive examination of the temporal associations between baseline measures and kidney function. Another strength is the statistical methodology. In traditional analyses, researchers select a few sleep variables and adjust for several covariates, which might ignore important sleep characteristics, confounders, and their interactions. In contrast, we applied a high-dimensional statistical technique with a data-driven selection of sleep variables associated with kidney function. The high-dimensional analysis is a novel approach to examine how multiple exposures simultaneously affect kidney function. A limitation of this study is that many mechanisms linking CVD risk factors and sleep were not measured (such as nocturnal hypertension, sympathetic tone, and data at cellular and molecular levels). The findings may also reflect confounding due to the effects of medications not specifically characterized (e.g., SGLT2 inhibitors) or to subclinical cardiac disease. Future studies with more completed cardiovascular variables are wanted to elucidate the cardiovascular confounding effects. Over approximately 5 years, a total of 278 participants were not assessed for eGFR in the follow-up MESA visit (1657 participants were assessed), and a total of 383 participants’ UACR were not measured in the follow-up visit (1552 were measured). The second limitation is that the follow-up time for change in kidney function started before the sleep was measured. However, the time lag between MESA Exam 5 and MESA SLEEP was modest, and an individual’s sleep habit would not likely change significantly over this period.40 The third limitation is the relatively small sample size for participants with baseline CKD. Although the associations between certain sleep measures and kidney function were found to be statistically significant among individuals with CKD, a generalization of these findings require validation in future studies with a larger CKD sample. Moreover, these associations were relatively small in terms of estimated regression coefficients, which may restrict extension of findings to clinical practice; however, it is plausible that even small changes in kidney function can lead to significant public health impact at the population level and provide insight into the mechanisms by which sleep may affect kidney function.
In summary, applying the state of art high-dimensional statistical techniques, we identified novel sleep characteristics associated with kidney function decline using a large set of sleep measures from actigraphy, PSG, and sleep questionnaires. Reduced deep sleep (stage N3), increased wake bouts, delayed timing, hypoxemia, and higher napping frequency appear to be associated with adverse effects on kidney function for individuals with CKD. Moreover, deep sleep, napping, and sleep timing may play an effect-modifying role in the relationship between HbA1c and kidney function. It implies the potential susceptibility of diabetic individuals to sleep disturbances.
Supplementary Material
Funding
MESA is conducted and supported by the National Heart, Lung, and Blood Institute (NHLBI) in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001881, and DK06349. Funding support for the MESA Sleep Exam was provided by grant HL56984. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org. Dr. Susan Redline is partly funded through the National Heart, Lung, and Blood Institute (1R35 HL135818-01).
T.Huang is supported by K01 HL143034.
Declaration of Competing Interest
Dr. Patel reports grants and personal fees from Philips Respironics, grants and personal fees from Bayer Pharmaceuticals, personal fees from NovaResp Technologies, grants from Sommetrics, grants from Respicardia, outside the submitted work. Dr. Martha Daviglus reports grants from National Institutes of Health (NIH), during the conduct of the study. Dr. Susan Redline reports grants from NIH, during the conduct of the study; personal fees from Apnimed Inc, grants and personal fees from Jazz Pharma, personal fees from Eli Lilly, outside the submitted work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jha V, Garcia-Garcia G, Iseki K, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013; 382: 260–272. [DOI] [PubMed] [Google Scholar]
- 2.Franke FJ, Arzt M, Kroner T, et al. Daytime napping and diabetes-associated kidney disease. Sleep Med. 2019; 54: 205–212. [DOI] [PubMed] [Google Scholar]
- 3.Petrov ME, Kim Y, Lauderdale DS, et al. Objective sleep, a novel risk factor for alterations in kidney function: the CARDIA study. Sleep Med. 2014;15: 1140–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo X, Yu S, Li Z, et al. Self-reported sleep duration is associated with reduced glomerular filtration rate among adults with hypertension: a population based study from rural northeast China. J Sleep Res. 2015; 24:351e8. [DOI] [PubMed] [Google Scholar]
- 5.Nigam G, Camacho M, Chang E, et al. Exploring sleep disorders in patients with chronic kidney disease. Nature and Science of Sleep. 2018; 10: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ohkuma T, Fujii H, Iwase M, et al. Association between sleep duration and urinary albumin excretion in patients with type 2 diabetes: the Fukuoka diabetes registry. PLoS One. 2013; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jackson CL, Umesi C, Gaston SA, et al. Multiple, objectively-measured sleep dimensions including hypoxic burden and chronic kidney disease: finding from the Muti-Ethic Study of Atherosclerosis. Thorax. 2021; 76: 704–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Full KM, Jackson CL, Rebholz CM, et al. Obstructive sleep apnea, other sleep characteristics, and risk of CKD in the atherosclerosis risk in Communities Sleep Heart Health Study. Clin J Am Soc Nephrol. 2020; 31: 1859–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. Chronic Kidney Disease Surveillance System—United States, website. http://www.cdc.gov/ckd
- 10.Huang Z, Tang X, Zhang T, et al. Prevalence of sleep apnoea in non-dialysis chronic kidney disease patients: a systematic review and meta-analysis. NEPHROLOGY. 2019; 24: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 11.Artom M, Moss-Morris R, Caskey F, et al. Fatigue in advanced kidney disease. Kidney Int. 2014; 86: 497–505. [DOI] [PubMed] [Google Scholar]
- 12.Zelnick LR, Weiss NS, Kestenbaum BR, et al. Diabetes and CKD in United States population, 2009-2014. Clin J Am Soc Nephrol. 2017; 12:1984–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xia J, Wang L, Ma ZH, et al. Cigarette smoking and chronic kidney disease in the general population: a systematic review and meta-analysis of prospective cohort studies. Nephrol Dial Transplant. 2017; 32: 475–487. [DOI] [PubMed] [Google Scholar]
- 14.Shi WR, Zhou YP, Wang HY, et al. Synergistic interaction of hypertension and hyperhomocysteinemia on chronic kidney disease: findings from the National Health and Nutrition Examination Survey 1999-2006. J Clin Hypertens. 2019; 21:1567–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Patel S, Redline S, et al. Weekly sleep trajectories and their association with obesity and hypertension in the Hispanic/Latino population. SLEEP. 2018; 10.1093/sleep/zsy150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalil M, Power N, Gramham E, et al. The associations between sleep and diabetes outcomes – A systematic review. Diabetes Res Clin Pract. 2020; 10.1016/j.diabres.2020.108035. [DOI] [PubMed] [Google Scholar]
- 17.Meinshausen N, Bauhlmann P. High-dimensional graphs and variable selection with the Lasso. Ann Stat. 2006; 34:1436–1462. [Google Scholar]
- 18.van der Geer S, Buhlmann P, Ritov Y. On asymptoticaly optimal confidence regions and tests for high-dimensional models. Ann Stat. 2014; 42: 166–1202. [Google Scholar]
- 19.Bild DE, Bluemke DA, Burke GL, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002. 156:871–81. [DOI] [PubMed] [Google Scholar]
- 20.Chen X, Wang R, Zee P, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). SLEEP. 2015; 38:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ricardo AC, Knutson K, Chen J, et al. The association of sleep duration and quality with CKD progression. J Am Soc Nephrol. 2017; 28: 3708–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blazek K, Zwieten A, Saglimbene V, et al. A practical guide to multiple imputation of missing data in nephrology. Kidney Int. 2021; 99: 68–74. [DOI] [PubMed] [Google Scholar]
- 23.Fan J, Li Q, Wang Y: Estimation of high-dimensional mean regression in the absence of symmetry and light tail assymptions. Journal of the Royal Statistical Society, B. 2016; 79:247–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010; 33: 1–22. [PMC free article] [PubMed] [Google Scholar]
- 25.Javaheri S, Redline S. Sleep, slow-wave sleep and blood pressure. Curr Hypertens Rep. 2012; 214: 442–448. [DOI] [PubMed] [Google Scholar]
- 26.Johns EJ, Kopp UC, DiBona GF. Neural control of renal function 2011; 1: 731–767. [DOI] [PubMed] [Google Scholar]
- 27.Ro MN, Blackwell T, Redline S, et al. Association between sleep architecture and measures of body composition. SLEEP. 2009; 32: 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsumoto T, Tabara Y, Murase K, et al. Association between sleep disturbance and nocturnal blood pressure profiles by a linear mixed model analysis: the Nagahama study. Sleep Med. 2019; 61:104: 109. [DOI] [PubMed] [Google Scholar]
- 29.Silvani A. Sleep disorders, nocturnal blood pressure, and cardiovascular risk: a translational perspective. Auton Neurosci. 2019; 218: 31–42. [DOI] [PubMed] [Google Scholar]
- 30.Sturrock NDC, George E, Pound N, et al. Non-dipping circadian blood pressure and renal impairment are associated with increased mortality in diabetes mellitus. Diabet Med. 2000; 17: 360–364. [DOI] [PubMed] [Google Scholar]
- 31.Kado H, Kusaba T, Matoba S, et al. Normotensive non-dipping blood pressure does not predict the risk of chronic kidney disease progression. Hypertens Res. 2019; 42: 354–361. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Li Y, Zhang J, et al. Prognostic effect of isolated nocturnal hypertension in Chinese patients with nondialysis chronic kidney disease. J Am Heart Assoc. 2016; 10.1161/JAHA.116.004198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang CS, Bangdiwala SI, Rangarajan S, et al. Association of estimated sleep duration and naps with mortality and cardiovascular events: a study of 116 632 people from 21 countries. Eur Heart J. 2019; 40: 1620–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson N, Giri A, Wei G, et al. Sedentary behavior in individuals with diabetic chronic kidney disease and maintenance hemodialysis. J Ren Nutr. 2015; 25: 364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adams RJ, Appleton SL, Vakulin A, et al. Chronic kidney disease and sleep apnea association of kidney disease with obstructive apnea in a population study of men. SLEEP. 2017; 40:1–9. [DOI] [PubMed] [Google Scholar]
- 36.Canales MT, Hagen EW, Barnet JH, et al. Sleep apnea and kidney function trajectory: results from a 20-year longitudinal study of healthy middle-aged adults. SLEEP. 2018; 10.1093/sleep/zsx181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fineburg D, Jandeleit-Dahm KAM, Cooper ME. Diabetic nephropathy: diagnosis and treatment. Nat Rev Endocrinol. 2013; 9: 713–723. [DOI] [PubMed] [Google Scholar]
- 38.Tamura A, Kawano Y, Watanabe T, et al. Obstructive sleep apnea increases hemoglobin A1c levels regardless of glucose tolerance status. Sleep Med. 2012; 13: 1050–1055. [DOI] [PubMed] [Google Scholar]
- 39.Zhang XB, Jiang XT, Cai FR, et al. Vascular endothelial growth factor levels in patients with obstructive sleep apnea: a meta-analysis. Eur Arch Otorhinolaryngol. 2017; 274: 661–670. [DOI] [PubMed] [Google Scholar]
- 40.Gilmour H, Stranges S, Kaplan M, et al. Longitudinal trajectories of sleep duration in the general population. Health Reports. 2013; 24: 14–20. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


