Abstract
Fluorescent indicators that respond to changes in biological membrane potentials provide a powerful complement to existing methods for monitoring neuronal activity. Indicators that absorb and emit in the near infrared window are especially attractive, since lower energy wavelengths excite fewer biological molecules and can penetrate more deeply into biological tissues. In this work, we incorporate sulfone rhodamine chromophores into a voltage-sensitive scaffold in order to generate voltage sensitive fluorophores which absorb and emit above 700 nm. These Sulfone Rhodamine Voltage Reporters (SuRhoVRs) partition into cell membranes and display good sensitivity to membrane potential changes. The most sensitive SuRhoVR derivative also displays excellent photostability and can track membrane potential changes in dissociated rat hippocampal neurons.
Keywords: fluorescence, fluorescent probes, membrane potential, responsive indicators
Graphical Abstract
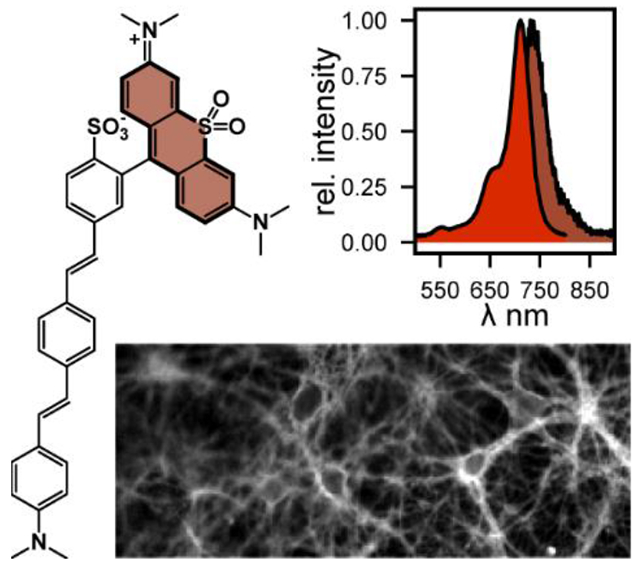
Sulfone-containg rhodamines integrated with phenylenevinylene molecular wires enable voltage imaging from mammalian neurons with excitation and emission profiles in the far-red to near-infrared range.
Introduction
Cells expend a tremendous amount of energy to maintain an unequal distribution of ions across their membranes and establish potentials across their cell membranes (Vmem). Excitable cells such as neurons and cardiomyocytes rapidly change their Vmem, coordinating their activity across vast, complex cellular networks. Measuring changes in Vmem is critical for understanding cellular physiology both in healthy systems and in disease states. Physical measurements of Vmem such as patch-clamp electrophysiology make direct contact with cell membranes and are highly reliable reporters of Vmem changes.[1,2] However, because direct cellular contact is required, patch-clamp electrophysiology is highly invasive and low-throughput which can limit its effectiveness.[1,2] As a result, optical monitoring of Vmem changes has emerged as an attractive complement to patch clamp electrophysiology.[3]
Our lab has undertaken a program to develop voltage sensitive dyes which allow for less invasive and higher throughput imaging of Vmem changes. These voltage-sensitive fluorophores, called VoltageFluors (VF dyes), sense Vmem changes through a photoinduced electron transfer (PeT) mechanism[4] from an aniline group connected to highly conjugated molecular wire that is sensitive to transmembrane potential.[3] Importantly, the chemical structures of the VF dye chromophore and voltage sensing regions can be precisely modified for a variety of applications. In previous studies, we have altered the sensitivity of VF dyes through modification of the molecular wire and tuned the optical properties of VF dyes through the incorporation of different chromophores.[5–11] However, our previous VF dyes have primarily absorbed and emitted within the visible region of light.[5–10,12][13] Highly red-shifted VF dyes will be essential for the application of these probes to more complex biological systems as lower energy light can penetrate more deeply into biological tissue and reduce background from autofluorescence.[14] Therefore, synthesizing VF dyes with absorbance and emission maxima in the near-infrared region has been a long-standing goal.
In this study, we were inspired by recently developed sulfone rhodamines which replace the oxygen atom in the prototypical rhodamine scaffold with a sulfone group to red-shift the absorbance and emission maxima by about 150 nm to above 710 nm.[15] We modified these sulfone rhodamine chromophores in order to incorporate them into VF dye scaffolds. This modification results in VF dyes with absorbance and emission maxima above 700 nm that retained high voltage sensitivity. We then characterized the derivative with the highest voltage sensitivity, m-SuRhoVR (10), in dissociated rat hippocampal neurons and found that it was highly photostable and could faithfully report on both evoked and spontaneous activity from multiple neurons simultaneously. We believe that these sulfone rhodamine voltage reporters represent an important step forward for VF dyes
Results and Discussion
In order to incorporate sulfone rhodamine chromophores into VoltageFluor scaffolds, we developed a novel synthetic route to access sulfone rhodamine chromophores containing both
sulfonate groups for proper VF dye localization and bromides on the pendant aryl ring for subsequent cross coupling. Our route is a significant departure from the previously published synthetic route to access sulfone rhodamine chromophores which utilizes a nucleophilic attack on a xanthene scaffold to incorporate the pendant ring[15][16] and is analogous to an acid-mediated condensation for the synthesis of 2-methyl substituted sulfone rhodamines, which provided the dye in low yield (0.4%).[17]
Briefly, 3-iodoaniline was methylated by reductive amination to generate compound 1 in 80% yield (Scheme 1). Compound 1 was reacted with sodium sulfide in the presence of catalytic copper iodide to generate compound 2 in 67% yield (Scheme 1). Compound 2 was then condensed in propionic acid with benzaldehyde derivatives containing a sulfonate and a bromine for further synthetic elaboration to yield sulfide rhodamines 3 (p-SRho) and 4 (m-SRho) in 34% and 61% yields respectively (Scheme 1). Sulfide rhodamines 3 and 4 were then oxidized with tert-butylammonium oxone in a 1:1 mixture of dichloromethane and methanol to yield sulfone rhodamines 5 (p-SuRho) and 6 (m-SuRho) in 8% and 12% yields respectively (Scheme 1). The overall yields for 5 and 6 from compound 2 are 3% and 7%, which is comparable to previous acid-catalyzed condensations (0.4%)[17] or aryl-lithium addition of a protected sulfonate into a ketone (9%).[6]
Scheme 1.
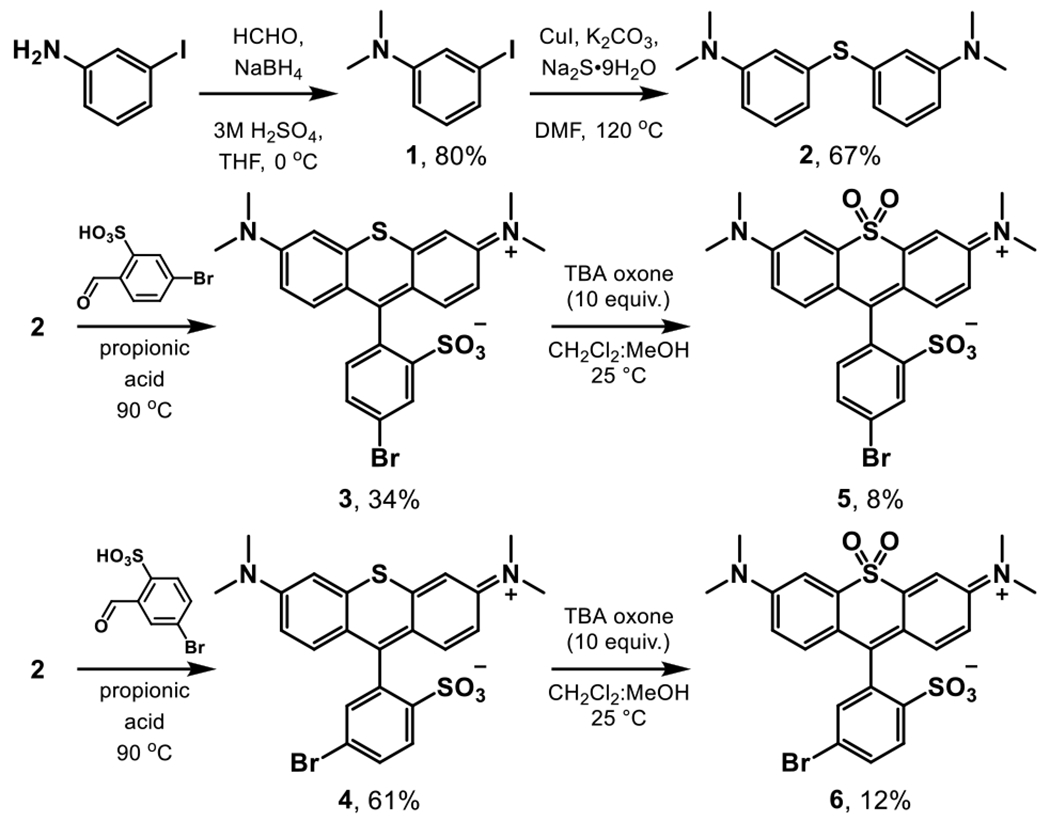
Synthesis of brominated sulfone rhodamines
Initial attempts were made to couple sulfone rhodamines 5 and 6 to a previously published phenylene vinylene molecular wire scaffold to generate the sulfone rhodamine voltage reporters 9 and 10.[18] Unfortunately, 5 and 6 were unstable under these reaction conditions. However, sulfide rhodamines 3 and 4 were stable under the same Heck conditions. Compounds 3 and 4 could be coupled to the phenylene vinylene scaffold to generate sulfide rhodamine voltage reporters 7 (p-SRhoVR) and 8 (m-SRhoVR) in 11% and 12% yields respectively (Scheme 2). Compounds 7 and 8 were then oxidized with tert-butylammonium oxone in a 1:1 mixture of dichloromethane and methanol to yield sulfone rhodamine voltage reporters 9 (p-SuRhoVR) and 10 (m-SuRhoVR) in 8% and 2% yields respectively (Scheme 2).
Scheme 2.
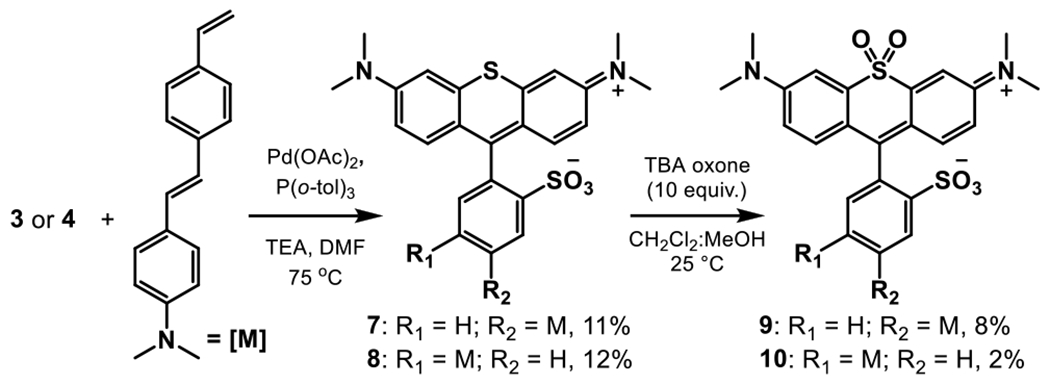
Synthesis of sulfide and sulfone RhoVRs
Spectroscopic Characterization
Previously synthesized sulfone rhodamine dyes displayed spectral shifts of about 130 nm relative to corresponding sulfide rhodamines.[15,17,19] To evaluate the effect of oxidizing the bridgehead atom in these newly synthesized rhodamine scaffolds, we measured the absorbance and emission spectra of rhodamines 3-10. Oxidizing sulfide rhodamines 3 and 4 to sulfone rhodamines 5 and 6 results in bathochromic shifts in the absorbance maxima (128 nm and 131 nm respectively, Figure 1, Table 1) and emission maxima (133 nm and 137 nm respectively, Figure 1, Table 1). Likewise, oxidizing sulfide rhodamine voltage reporters 7 and 8 to sulfone rhodamine voltage reporters 9 and 10 also results in bathochromic shifts in the absorbance maxima (130 nm and 131 nm respectively, Figure 2, S1, Table 1) and emission maxima (134 nm and 134 nm respectively, Figure 2,S1, Table 1). Thus, the absorbance and emission maxima of these novel xanthene scaffolds can be shifted by over 120 nm in one synthetic step.
Figure 1.
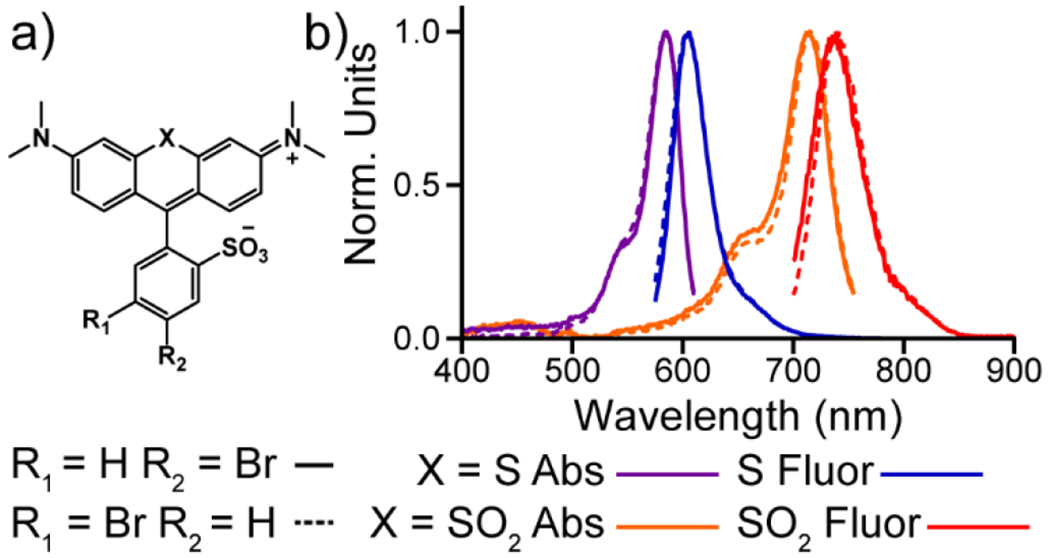
Absorbance and emission spectra for sulfide and sulfone rhodamines. a) Structures of sulfide and sulfone rhodamines. b) Normalized absorbance (purple, orange) and emission (blue and red) spectra for sulfide (3 and 4) and sulfone (5 and 6) rhodamines. All spectra were obtained at 1 μM dye concentration in a TBS (50 mM Tris-HCl, pH 7.4, 0.1 M NaCl) solution containing 0.1 % (w/w) SDS. All spectra were normalized to their respective maxima.
Table 1.
Spectral properties of sulfide- and sulfone-containing rhodamine dyes and RhoVRs.
| dye | λabs (nm) | λem (nm) | Φfl | ε (M−1•cm−1) |
|---|---|---|---|---|
| p-SRho (3) | 585 | 606 | 0.52[a] | 23,000 |
| m-SRho (4) | 585 | 605 | 0.42[a] | 26,000 |
| p-SuRho (5) | 713 | 739 | 0.21[b] | 34,000 |
| m-SuRho (6) | 716 | 742 | 0.20[b] | 34,000 |
| p-SRhoVR (7) | 582 | 603 | 0.33[a] | 29,000 |
| m-SRhoVR (8) | 581 | 603 | 0.07[a] | 23,000 |
| p-SuRhoVR (9) | 712 | 737 | 0.09[b] | 36,000 |
| m-SuRhoVR (10) | 712 | 737 | 0.03[b] | 31,000 |
All spectral properties were determined at 1 μM dye concentration in a TBS (50 mM Tris-HCl, pH 7.4, 0.1 M NaCl) solution containing 0.1 % (w/w) SDS. Quantum yields were determined by comparison to
Rhodamine 101 or
Cy5.5 standards.
Extinction coefficients were determined at the respective dye absorbance maxima.
Figure 2.
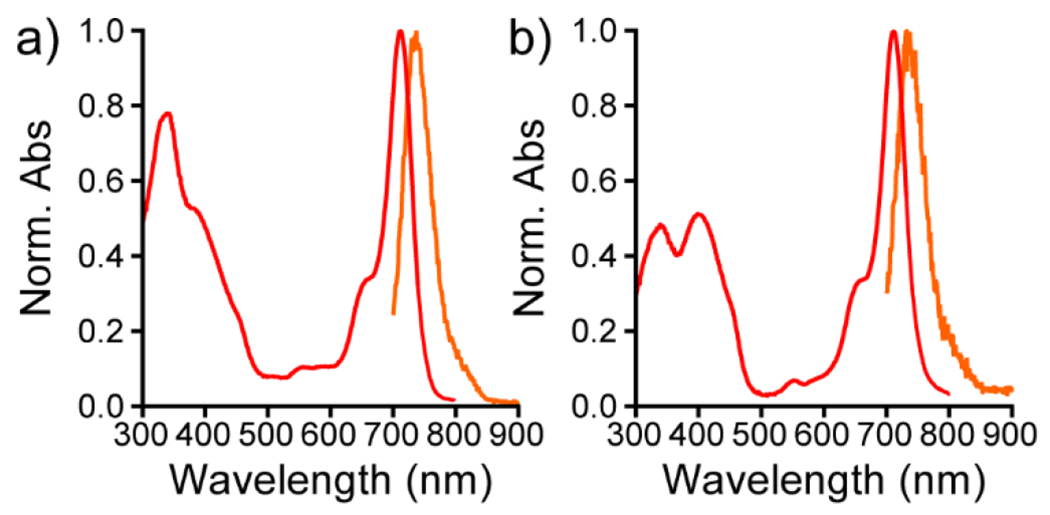
Absorbance and emission spectra for sulfone RhoVRs. Normalized absorbance (red) and emission (orange) spectra for a) SuRhoVR 9 and b) SuRhoVR 10. All spectra were obtained at 1 μM dye concentration in a TBS (50 mM Tris-HCl, pH 7.4, 0.1 M NaCl) solution containing 0.1 % (w/w) SDS. All spectra were normalized to their respective maxima.
All of the newly synthesized dyes display similar extinction coefficients at their respective absorbance maxima, but there are clear differences in the fluorescence quantum yields (Table 1). Dyes containing a bromine atom or molecular wire region meta to the chromophore region (m-dyes: 4,6,8,10) all have lower quantum yields than their para isomers (p-dyes: 3,5,7,9) perhaps due to decreased distance between the chromophore and quenching groups (Table 1). Also, the voltage sensitive dyes (7-10) display much lower quantum yields than their respective parent fluorophores (3-6) as the voltage sensitive scaffold largely quenches the fluorescent chromophore in the absence of an electrical potential across the dye molecule (Table 1).
Cellular Characterization of Sulfone Rhodamine Voltage Reporters
After analyzing the newly synthesized dyes spectroscopically, we loaded p-SuRhoVR and m-SuRhoVR onto HEK 293T cells where they primarily localized to the cell membranes (Figure 3a,g). The dyes show similar cellular brightness; m-SuRhoVR (10) is about 15% brighter in cell membranes than p-SuRhoVR in HEK cells (rel. brightness 1.0 vs. 0.86, Table 2). We did not evaluate SRhoVRs in cells – preliminary experiments showed they bleached rapidly. Previous work shows that the S-rhodamine core of SRhoVRs generates substantial 1O2[20] and is used as a photosensitizer to kill cells in photodynamic therapy,[21] which we wanted to avoid.
Figure 3.
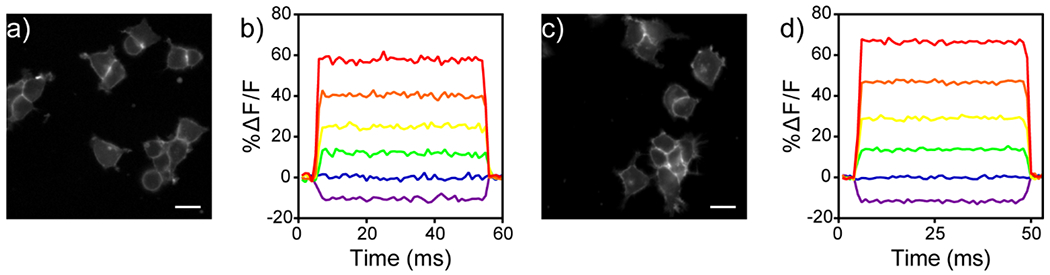
Cellular characterization of SuRhoVRs. Widefield fluorescence images of HEK cells stained with either 1 μM p-SuRhoVR (a) or 1 μM m-SuRhoVR (c). Representative plots of ΔF/F (%) vs time for a single HEK cell under patch-clamp electrophysiological control and stained with either 1 μM p-SuRhoVR (b) or 1 μM m-SuRhoVR (d). Data are from a holding potential of −60 mV to −100 (purple), −60 (blue), −20 (green), +20 (yellow), +60 (orange), or +100 mV (red). Scale bars are 20 μm. A 631 nm LED was used as the excitation light.
Table 2.
Voltage sensititivty of SuRhoVRs
| dye | cell brightness | voltage sensitivity[f] | signal to noise[f] |
|---|---|---|---|
| p-SuRhoVR (9) | 0.86 ± 0.01[a][d] | 32 ± 1[a][d] | 26:1 ± 2[a][d] |
| m-SuRhoVR (10) | 1.0 ± 0.09[b][d] | 37 ± 1[b][d] 37 ± 1[c][d] |
46:1 ± 9[b][d] 87:1 ± 25[c][e] |
All data are determined in HEK 293T cells. All values are mean ± standard error of the mean for
n = 5,
n = 4, or
n = 3 cells.
Excitation at 631 nm, 77 mW/mm2.
Excitation at 660 nm, 51 mW/mm2.
per 100 mV.
We then determined the voltage sensitivities of p-SuRhoVR (9) and m-SuRhoVR (10) by measuring the relative fluorescence changes in HEK 293T cells under patch clamp control of membrane potential (Figure 3b,d; Figure S2). m-SuRhoVR (10) displays higher sensitivity to membrane potential changes than p-SuRhoVR (9) (37% ∆F/F vs. 32% ∆F/F, Figure 3, Table 2) and a higher signal-to-noise ratio (SNR) for a 100 mV step than p-SuRhoVR (46:1 vs. 26:1, Table 2). Since m-SuRhoVR displayed higher cellular brightness, voltage sensitivity, and SNR in HEK 293T cells, we selected it for further characterization in dissociated rat hippocampal neurons. However, before conducting neuronal experiments, we also characterized m-SuRhoVR in HEK cells with 660 nm excitation light (Figure S3). Under these imaging conditions, m-SuRhoVR displays the same voltage sensitivity (37% ∆F/F, Figure S3) but nearly twice the SNR observed with 631 nm excitation light (87:1 vs. 46:1, Figure S3, Table 2). Therefore, we used 660 nm excitation light for all subsequent experiments in dissociated rat hippocampal neurons.
m-SuRhoVR also localizes to the cell membranes of dissociated rat hippocampal neurons (Figure 4a–c, e–f).
Figure 4.
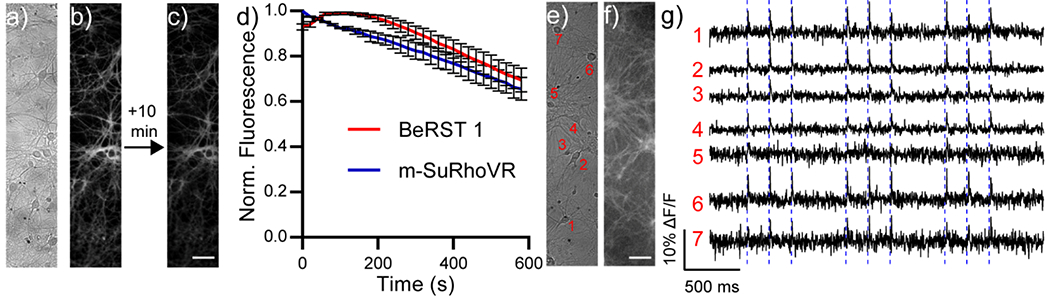
Imaging with m-SuRhoVR (10) in neurons. a-d) Photostability of 10 in neurons. a) Initial transmitted light and b) fluorescence image of rat hippocampal neurons stained with 500 nM m-SuRhoVR (10). c) Fluorescence image of the same neurons after 10 minutes of continuous illumination at 660 nm (51 mW/mm2). Exporsure time is 100 ms. Scale bar is 10 μm. d) Plot of normalized fluorescence intensity vs time for neurons stained with either BeRST 1 (500 nM, red) or m-SuRhoVR (10, 500 nM, blue). Data are mean fluorescence values normalized to t = 0. Error bars are standard error of the mean for n = 3 areas (26 neurons for m-SuRhoVR; 16 neurons for BeRST 1). e-g) Imaging membrane potential dynamics with m-SuRhoVR (10). e) Transmitted light image and f) fluorescence image of rat hippocampal neurons stained with m-SuRhoVR (10, 500 nM) were stimulated with a field electrode. Exposure time was 2 ms (1/50th of images in panels (b) and (c) to enable optical detection of action potentials. Scale bar is 10 μm. g) Plots of relative change in fluorescence (ΔF/F) vs time for seven different cell bodies. Dashed blue lines indicate approximate timing of external electrode stimulus.
m-SuRhoVR shows good photostability, comparable to a previously published near-infrared silicon rhodamine VF dye, BeRST 1.[6] After 10 minutes of continuous exposure to 660 nm excitation light, m-SuRhoVR retains 66% of its initial fluorescence intensity in dissociated rat hippocampal neurons (Figure 4a–d), while BeRST 1 retained 69% of its maximum fluorescence intensity (under matched illumination power at 631 nm). We then evaluated whether m-SuRhoVR could faithfully report both evoked and spontaneous action potentials in dissociated rat hippocampal neurons. Observing action potentials is challenging as a sampling rate comparable to the timescale of an action potential (1 ms) must be used, limiting the amount of time for photon collection. Even under these photon-starved conditions (2 ms exposure time), m-SuRhoVR can still faithfully report evoked activity (Figure 4e–f) and spontaneous activity (Figure S4) from multiple neurons simultaneously. Although the nominal voltage sensitivity of m-SuRhoVR (10), at 37% ΔF/F per 100 mV is higher than BeRST (24%), the signal to noise ratio for both evoked and spontaneous action potentials is lower.[22] This is most like a result of the mis-match in LED excitation wavelength and maximum absorbance of m-SuRhoVR (10) and the lower QE of sCMOS detectors at the longer emission wavelengths of m-SuRhoVR (10).[23]
Conclusion
In this work, we developed a novel oxidation route to synthesize sulfone rhodamine chromophores. We then incorporated these sulfone rhodamine chromophores into a voltage sensitive scaffold in order to generate voltage sensitive dyes which have absorbance and emission maxima in the near-infrared region. These dyes retain good sensitivity to Vmem changes and stain cell membranes effectively. Furthermore, the most sensitive sulfone rhodamine voltage reporter displays excellent photostability and can faithfully report evoked and spontaneous activity in dissociated rat hippocampal neurons. A current challenge is the lack of high-powered LEDs centered at 700 nm, which results in underestimation of the true brightness of SuRhoVR dyes. Additionally, the quantum efficiency of sCMOS detectors falls off rapidly beyond 700 nm.[23] Advances in detector efficiency at longer wavelengths will further improve the utility of far-red SuRhoVR dyes.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health – National Institute of Neurological Disorders and Stroke (NIH – NINDS, R01NS098088). BKR was supported, in part, by a training grant from NIH – National Institute of General Medical Science (NIGMS, T32GM066698).
Footnotes
Supporting information for this article is given via a link at the end of the document.
References
- [1].Armstrong CM, Gilly WF, Methods Enzymol. 1992, 207, 100–122. [DOI] [PubMed] [Google Scholar]
- [2].Williams SR, Mitchell SJ, Nat. Neurosci 2008, 11, 790–798. [DOI] [PubMed] [Google Scholar]
- [3].Miller EW, Curr. Opin. Chem. Biol 2016, 33, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Li L, Nano Lett. 2007, 7, 2981–2986. [DOI] [PubMed] [Google Scholar]
- [5].Boggess SC, Gandhi SS, Siemons BA, Huebsch N, Healy KE, Miller EW, ACS Chem. Biol 2019, 14, 390–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Huang YL, Walker AS, Miller EW, J. Am. Chem. Soc 2015, 137, 10767–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Deal PE, Kulkarni RU, Al-Abdullatif SH, Miller EW, J. Am. Chem. Soc 2016, 138, 9085–9088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Kulkarni RU, Kramer DJ, Pourmandi N, Karbasi K, Bateup HS, Miller EW, Proc. Natl. Acad. Sci 2017, 201610791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kulkarni RU, Vandenberghe M, Thunemann M, James F, Andreassen OA, Djurovic S, Devor A, Miller EW, ACS Cent. Sci 2018, 4, 1371–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ortiz G, Liu P, Naing SHH, Muller VR, Miller EW, J. Am. Chem. Soc 2019, 141, 6631–6638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Franke JM, Raliski BK, Boggess SC, Natesan DV, Koretsky ET, Zhang P, Kulkarni RU, Deal PE, Miller EW, J. Am. Chem. Soc 2019, DOI 10.1021/jacs.9b05912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Franke JM, Raliski BK, Boggess SC, Natesan DV, Koretsky ET, Zhang P, Kulkarni RU, Deal PE, Miller EW, J. Am. Chem. Soc 2019, 141, 12824–12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gonzalez MA, Walker AS, Cao KJ, Lazzari-Dean JR, Settineri NS, Kong EJ, Kramer RH, Miller EW, J. Am. Chem. Soc 2021, 143, 2304–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Weissleder R, Ntziachristos V, Nat. Med 2003, 9, 123–8. [DOI] [PubMed] [Google Scholar]
- [15].Liu J, Sun YQ, Zhang H, Shi H, Shi Y, Guo W, ACS Appl. Mater. Interfaces 2016, 8, 22953–22962. [DOI] [PubMed] [Google Scholar]
- [16].Dejouy G, Laly M, Valverde IE, Romieu A, Dye. Pigment 2018, 159, 262–274. [Google Scholar]
- [17].Deng F, Liu L, Huang W, Huang C, Qiao Q, Xu Z, Spectrochim. Acta - Part A Mol. Biomol. Spectrosc 2020, 240, 118466. [DOI] [PubMed] [Google Scholar]
- [18].Miller EW, Lin JY, Frady EP, a Steinbach P, Kristan WB, Tsien RY, Proc. Natl. Acad. Sci. U. S. A 2012, 109, 2114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Detty MR, Prasad PN, Donnelly DJ, Ohulchanskyy T, Gibson SL, Hilf R, Bioorganic Med. Chem 2004, DOI 10.1016/j.bmc.2004.03.029. [DOI] [PubMed] [Google Scholar]
- [20].Detty MR, Prasad PN, Donnelly DJ, Ohulchanskyy T, Gibson SL, Hilf R, Bioorganic Med. Chem 2004, 12, 2537–2544. [DOI] [PubMed] [Google Scholar]
- [21].Detty MR, Gibson SL, Wagner SJ, J. Med. Chem 2004, 47, 3897–3915. [DOI] [PubMed] [Google Scholar]
- [22].Huang YL, Walker AS, Miller EW, J. Am. Chem. Soc 2015, 137, 10767–10776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Fowler B, Liu C, Mims S, Balicki J, Li W, Do H, Appelbaum J, Vu P, in Proc. SPIE, 2010, p. 753607. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


