Abstract
Introduction
Here, the discussion focused on the function and possible mechanism of cancer stem cell-like cells (CSCs)-derived exosomal CDKN2B-AS1 in thyroid cancer.
Methods
Specifically, the bioinformatics analysis, dual-luciferase reporter assay and RT-qPCR were conducted to obtain the expression and regulation of CDKN2B-AS1, and the downstream miR-122-5p/P4HA1 axis. Exosomes were identified by transmission electron microscopy. The uptake of exosome by recipient cells was observed by PKH67 labeling. Functional experiments and western blot were adopted to detect the effects of exosomal CDKN2B-AS1/miR-122-5p/P4HA1 axis on thyroid cancer cells. Tumor xenograft and in vivo metastasis model combined with RT-qPCR, western blot and hematoxylin-eosin staining verified the role of CDKN2B-AS1.
Results
Exosomal CDKN2B-AS1 up-regulated P4HA1 expression through miR-122-5p. CDKN2B-AS1 and P4HA1 expressions were up-regulated, and miR-122-5p expression was down-regulated in thyroid cancer. Silent CDKN2B-AS1 reduced cell viability and stemness. CDKN2B-AS1 was found to be abundant in CSCs and CSCs-derived exosomes. Exosomal CDKN2B-AS1 silencing could transfer to thyroid cancer cells to elevate E-cadherin level, and diminish P4HA1, N-cadherin and Vimentin levels, thus impeding cell migration and invasion. MiR-122-5p inhibitor reversed the function of exosomal CDKN2B-AS1, while P4HA1 silencing attenuated the effect of miR-122-5p inhibitor. Exosomal CDKN2B-AS1 affected the growth and metastasis of thyroid cancer through the miR-122-5p/P4HA1 axis.
Conclusion
CSCs-derived exosomal CDKN2B-AS1 acts as an oncogene in thyroid cancer through miR-122-5p/P4HA1 axis.
Keywords: Thyroid cancer, Long non-coding RNA, Exosomes, Cancer stem cell-like cells, CDKN2B-AS1
1. Introduction
Thyroid cancer is the most common endocrine malignancy [1]. Most thyroid cancers are differentiated thyroid cancers accompanied by a good prognosis, but a small percentage will develop into aggressive thyroid cancers, leading to invasive and distant metastasis and a poor prognosis [1,2]. In spite of substantial efforts to treat patients with aggressive thyroid cancer, including tumor resection, drug targeted therapy, chemotherapy and radiotherapy, some problems such as poor prognosis during the treatment process still exist [3,4]. Therefore, further comprehending and revealing the mechanism of occurrence and development of thyroid cancer, as well as putting forward more specific molecular markers and therapeutic targets are of great significance to clinical diagnosis and treatment.
Long non-coding RNA (lncRNA) has now been proved to be associated with a variety of cancer behaviors [5,6], some of which are involved in the carcinogenic or tumor suppressor pathway of thyroid cancer [7]. For example, Yuan et al. found that LncRNA SLC26A4-AS1 promotes DDX5 degradation through the ubiquitin-proteasome pathway to attenuate MRN complex-mediated DNA repair signals and thyroid cancer metastasis [8]. Liu et al. pointed out that LncRNA XIST, as the ceRNA of miR-34a, competes with MET for miR-34a binding, thereby regulating the proliferation of thyroid cancer cells and tumor growth [9]. According to our analysis regarding the lncRNA related to thyroid cancer through lncRNADisease, we confirmed that the relationship between CDKN2B-AS1 and thyroid cancer is yet to be verified. Interestingly, CDKN2B-AS1 has been proven to be an indispensable long-chain non-coding RNA in multiple diseases, which can promote the migration and invasion of assorted malignant tumor cells [10]. The above findings provide certain theoretical supports for us to further study the mechanism of CDKN2B-AS1 in thyroid cancer.
In recent years, exosomes have been regarded as “messengers” for the communication between cells [11,12]. A study described that CDKN2B-AS1 can be detected in exosomes [13]. According to reports, many exosomal lncRNAs transmit signals and phenotypes between cancer cells, including thyroid cancer cells [14,15]. Exosomes can be secreted into the microenvironment through exocytosis by diverse cells such as tumor cells and macrophages [16,17]. Also, the existing literature reports that the exosomal lncRNA secreted by cancer stem cell-like cells (CSCs) plays a pivotal biological role in the occurrence and development of tumors [18]. CSCs, a subgroup of self-renewing cells, are more likely to trigger resistance to chemotherapy and radiation therapy, as well as recurrence and metastatic disease [19]. Therefore, we intended to explore whether CDKN2B-AS1 functioned as an exosomal lncRNA in CSCs of thyroid cancer in the tumor microenvironment. In addition, we initially explored the molecular mechanism of CDKN2B-AS1 regulating the occurrence and development of thyroid cancer, contributing to a theoretical basis for molecular targeted therapy against thyroid cancer.
2. Materials and methods
2.1. Bioinformatics analysis
The starBase (http://www.sysu.edu.cn) was adopted to analyze the expression and the target miRNA of CDKN2B-AS1 in thyroid cancer. The miRDB (http://mirdb.org/mirdb/index.html) and TargetScan (http://www.targetscan.org/vert_72/) database were utilized to predict the target genes of miR-122-5p.
2.2. Cell and culture
Nthy-ori3-1 cells (90011609, European Collection of Authenticated Cell Cultures (ECACC), UK), as well as thyroid cancer cell lines TPC-1 (0397, Rio de Janeiro Cell Bank (BCRJ), Brazil), HTH83 (MZ-2194, Ningbo Mingzhou Biological Technology Co., LTD, China) and FTC-133 (94060901, ECACC, UK) were cultured in RPMI-1640 (PM150110, Procell, China) containing 10% fetal bovine serum (FBS; 164210-500, Procell, China). SW579 cells (HTB-107, American Type Culture Collection (ATCC), USA) were incubated in Leibovitz's L-15 Medium (30-2008, ATCC, USA) blended with 10% FBS at 37 °C with 5% CO2.
2.3. Transfection
For studying the effect of CDKN2B-AS1 in thyroid cancer, we transfected short hairpin RNA (shRNA)-targeted CDKN2B-AS1 plasmid (sh-CDKN2B-AS1 group) (C01001, GenePharma, China) and negative control (sh-NC group) (C03002, GenePharma, China) into TPC-1 and SW579 cells. On the other hand, in order to confirm the molecular mechanism of CDKN2B-AS1 in thyroid cancer, miR-122-5p inhibitor (I) (miR20000421-1-5, RIBOBIO, China) and shP4HA1 (sh-P4HA1 group) (C01001, GenePharma, China) were separately transfected or co-transfected into TPC-1 and SW579 cells, with the inhibitor control (IC) (miR2N0000001-1-5, RIBOBIO, China) and sh-NC as the corresponding control. All these plasmids and oligonucleotides were transfected into TPC-1 and SW579 cells using Lipofectamine 3000 (L3000075) purchased from Invitrogen (USA).
2.4. Cell viability assay
The cell counting kit-8 (CCK-8) analysis was performed to determine cell viability. TPC-1 and SW579 cells that were transfected or co-cultured with exosomes were harvested and seeded into 96-well plates at a density of 1 × 102 cells/well for 24 or 48 h, followed by the detection on viability with CCK-8 kit (CA1210, Solarbio, China). Briefly, 10 μL of CCK-8 solution was mixed with cells to further culture at 37 °C for 2 h. The absorption at 450 nm was recorded using an HBS-1096C microplate reader (E0229, Beyotime, China), with each set of three replicate wells.
2.5. Sphere-forming assay
Transfected TPC-1 and SW579 cells (1 × 103 cells/well) were initially placed in 6-well ultra-low attachment plates (CLS3471, Corning, USA) and cultured in serum-free sphere medium for 12–14 days [20]. Cell clusters were observed under a microscope (DMi1, Leica, Germany) (Magnification ×100). Moreover, CSCs of the transfected TPC-1 and SW579 cells were harvested for later assays.
2.6. Exosome isolation and identification
Exosomes were successfully extracted from CSCs of the transfected TPC-1 and SW579 cells in culture medium using total exosome separation reagents (4478359, Invitrogen, USA). Simply put, CSCs were cultured in RPMI-1640 or Leibovitz's L-15 medium without FBS at 37 °C to collect culture media. After that, culture media were centrifuged (200 g, 30 min (min)) to remove cells and debris. The supernatant containing cell-free medium obtained in the previous step was transferred to a new tube. Then, 0.5 volume of total exosome separation reagents was added and mixed for additional incubation at 4 °C overnight. Subsequently, the sample was centrifuged at 10,000 g for 1 h and the supernatant was discarded. The resulting pellet encompassing exosomes was resuspended in phosphate buffered saline (PBS, 10010049, Gibco, USA) for subsequent analyses.
In order to identify the morphology of exosomes, the exosomes fixed in 2% paraformaldehyde (158127, Sigma–Aldrich, USA) were transferred to the Formvar/Carbon Supported Copper Grids (TEM-FCF100CU, Sigma–Aldrich, USA) and placed at room temperature for 30 min. Afterwards, 1% glutaraldehyde (G5882, Sigma–Aldrich, USA) was added, and then the morphology of the exosomes was observed under a transmission electron microscope (TEM) (JEM-2000EXII, Jeol Ltd, Japan).
2.7. Co-culture system
To study the role of exosomal CDKN2B-AS1 derived from CSCs of thyroid cancer, we constructed a co-culture system. Specifically, CSCs formed by TPC-1 and SW579 cells transfected with sh-NC or sh-CDKN2B-AS1 were collected to extract exosomes (sh-CDKN2B-AS1-EXO also named sh-CDKN2B-AS1 (CSCs), sh-NC-EXO), and then co-cultured with recipient TPC-1 and SW579 cells, respectively. Subsequently, the recipient thyroid cancer cells were subjected to functional analysis.
2.8. Real-time quantitative PCR (RT-qPCR)
The total exosomal RNAs and protein isolation kit (4478545, Invitrogen, USA) could be used to purify the total RNAs in exosomes. Total RNAs from tumor tissues or cells were harvested by Triquick reagent (R1100, Solarbio, China) and tissue/cell miRNA extraction kit (R2220, Solarbio, China). Then, the collected RNAs were inversely transcribed into cDNAs according to One Step SuperRT-PCR Mix Kit (T2240, Solarbio, China) or miRNA First-Strand cDNA Synthesis Kit (KR211, TIANGEN, China). Thereafter, qPCR was conducted with FastFire qPCR PreMix (FP207, TIANGEN, China) and miRNA qRT-PCR Kit (FP411, TIANGEN, China) under the Thermal Cycler Dice Real Time System III (Takara, China). Oligo (dT)-Universal Tag universal reverse transcription primer was used as the reverse primer of miRNA and U6. The sequence information of primers was exhibited as follows: CDKN2B-AS1 (5′-3′): AGTTAGGGTGTGGTATGTGCC, ACATCCAAGACAGCAAGTGGT; P4HA1 (5′-3′): AGTACAGCGACAAAAGATCCAG, CTCCAACTCACTCCACTCAGTA; GAPDH (5′-3′): TGCACCACCAACTGCTTAGC, CATGCACTGTGGTCATGAG; MiR-122-5p forward primer (5′-3′): TGGAGTGTGACAATGGTGTTTG; U6 forward primer (5′-3′): CTCGCTTCGGCAGCACATATACT.
2.9. Exosome labeling
The isolated exosomes were mixed with PKH67 with PKH67 Green Fluorescent Cell Linker Midi Kit (MIDI67, Sigma–Aldrich, USA) and incubated at room temperature for 4 min. The labeling of exosomes was terminated by adding 1% bovine serum albumin (A1933, Sigma–Aldrich, USA). The stained exosomes were then obtained utilizing the exosome isolation kit. The exosomes were incubated with TPC-1 and SW579 cells respectively in basal medium at 37 °C for 3 h. After the nuclei were colored with 4′, 6-diamidine-2-phenylindole dihydrochloride (DAPI; D9542, Sigma–Aldrich, USA), the staining was observed with a fluorescence microscope (LSM710, Carl Zeiss, Germany).
2.10. Wound healing assay
Cells were plated in 6-well plates at a density of 2 × 103 cells/well and cultured to 90–100% confluence. Then, cell layer was scratched using sterile pipette tip to create wounds, followed by washing to remove floating cells and debris. The wound sizes at 0 h and 48 h were measured utilizing a microscope (Magnification ×100) to assess cell migration.
2.11. Transwell assay
Transwell cell culture inserts loaded into 24-well plates (CLS3399, Corning, USA) were pre-coated with Matrigel (E6909, Sigma–Aldrich, USA). In a nutshell, 1 × 104 thyroid cancer cells in serum-free medium were seeded in the upper chamber. The complete medium containing 10% FBS was added to the bottom chamber. After 48-h incubation, invading cells were fixed and stained with crystal violet solution (C0121, Beyotime, China), the number of which was counted under a microscope (Magnification ×250).
2.12. Western blot
The expressions of P4HA1 and epithelial-mesenchymal transition (EMT)-related proteins in transfected TPC-1 and SW579 cells or tumor tissues were detected by western blot. Cell protein samples were obtained via total protein extraction kit (V900854, Merck, Germany), and quantified by bicinchoninic acid kit (PC0020, Solarbio, China). Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, and subsequently transferred to polyvinylidene fluoride membranes (IPVH00010, Millipore, USA). The membranes were blocked with 5% non-fat milk, and then probed with primary antibodies against E-cadherin (#14472, 135 kDa, 1/1000, Cell Signaling Technology (CST), USA), N-cadherin (#13116, 140 kDa, 1/1000, CST, USA), Vimentin (#5741, 57 kDa, 1/1000, CST, USA), and P4HA1 (ab244400, 61 kDa, 0.2 μg/ml, Abcam, UK) at 4 °C overnight. Then, the membranes were reacted with horseradish peroxidase (HRP)-coupled goat anti-rabbit or anti-mouse secondary antibody (ab205718, ab205719, 1/5000, Abcam, UK) at room temperature for 2 h. The specific bands were visualized via enhanced chemiluminescence western blotting substrate (32132X3, Thermo Scientific, USA), and then semi-quantified using ImageJ software (v1.8.0, National Institutes of Health, USA). GAPDH (#2118, 37 kDa, 1/1000, CST, USA) was the control protein.
2.13. Dual-luciferase reporter assay
The wild-type (WT) luciferase reporter plasmid (CDKN2B-AS1-WT or P4HA1-WT) and mutant (MUT) reporter plasmid (CDKN2B-AS1-MUT or P4HA1-MUT) were constructed using psiCHECK-2 vector (C8021, Promega, USA). The sequences of miR-122-5p mimic (M) (miR10000421-1-5) and mimic control (MC) (miR1N0000001-1-5) were purchased from RIBOBIO (China). Subsequently, the luciferase reporter recombinant plasmid and miR-122-5p mimic or mimic control were co-transfected into HEK293 cells (CL-0001, Procell, China) (which were used as tool cells), and then cultured in minimum essential medium (PM150467, Procell, China) at 37 °C. After transfection, the luciferase activity in cell lysates was measured using the dual-luciferase reporter system (D0010, Solarbio, China), and normalized to Renilla luciferase activity.
2.14. Animal experiment
The nude mouse xenograft experiment was used to verify the effect of CSCs-derived exosomal CDKN2B-AS1 on the growth of thyroid cancer tumors. A total of 48 male BALB/c nude mice (6 weeks old) were ordered from Hangzhou Medical College (China) and preserved in the specific pathogen free-grade animal laboratory. The animal experiments were approved by the Zhejiang Baiyue Biotechnology Co., Ltd. Laboratory Animal Welfare Ethics Committee (ZJBYLA-IACUC-20220716). The exosomes isolated from CSCs of TPC-1 cells without or with transfection of sh-NC or sh-CDKN2B-AS1 were incubated with TPC-1 cells to form CSCs-EXO, CSCs-shNC-EXO, and CSCs-sh-CDKN2B-AS1-EXO groups, respectively, with PBS as Sham group. Nude mice were randomly divided into 4 groups (n = 6), then subcutaneously injected with TPC-1 cells and bred for 28 days. The tumor volume was recorded at 0, 7, 14, 21, and 28 days. After the measurement on the 28th day, all mice were euthanized via cervical dislocation after anesthesia (80 mg/kg pentobarbital sodium, P0500000, Merck, Germany). Then the tumors were collected and photographed, and the weight was recorded.
In order to construct a metastasis model in vivo, different groups of TPC-1 cells were injected intravenously into mice through tail (n = 6). Six weeks later, the lung tissues of all mice were harvested, and the metastatic nodules secondary to the lung were observed by hematoxylin and eosin (H&E) staining. Lung tissues were embedded in paraffin (1496904, Merck, Germany) to perform H&E staining. Paraffin-embedded sections were stained with hematoxylin solution for 5 min and eosin solution for 1 min using H&E staining kit (C0105) purchased from Beyotime (China). At last, the pathological condition of lung tissues was observed under a microscope (Magnification ×100).
2.15. Statistical analysis
The data analyzed by Graphpad 8.0 software were described by the mean ± standard deviation. The independent sample t test was used for the comparison between two groups, and the one-way analysis of variance was for the comparison among multiple groups. The difference was statistically significant with a value of p < 0.05.
3. Results
3.1. CDKN2B-AS1 was highly expressed in thyroid cancer and affected cell viability and stemness
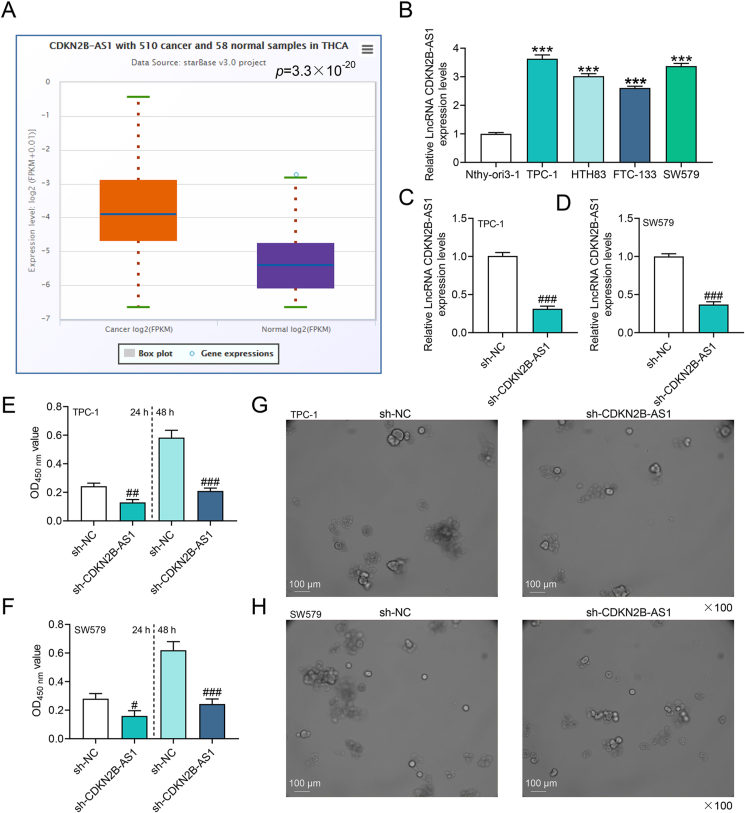
In order to fathom out the role of CDKN2B-AS1 in thyroid cancer, starBase was applied, which predicted that CDKN2B-AS1 was highly expressed in thyroid cancer (p = 3.3 × 10−20, Fig. 1A). Cell experiments were in favor of this prediction, unveiling that the expression of CDKN2B-AS1 was prominently higher in TPC-1, HTH83, FTC-133 and SW579 cells than in Nthy-ori3-1 cells (p < 0.001, Fig. 1B). The CDKN2B-AS1 content was the most abundant in TPC-1 and SW579 cells among other cells, so these two cells were selected for further experiments. The transfection of CDKN2B-AS1 silencing plasmid reduced the level of CDKN2B-AS1 in TPC-1 and SW579 cells (p < 0.001, Fig. 1C and D). CCK-8 assay results indicated that sh-CDKN2B-AS1 impeded the viability of thyroid cancer cells (p < 0.05, Fig. 1E and F). Similarly, sh-CDKN2B-AS1 hindered tumor sphere formation (Fig. 1G and H).
Fig. 1.
CDKN2B-AS1 was highly expressed in thyroid cancer and affected cell viability and tumor sphere formation (A) StarBase (http://www.sysu.edu.cn) analyzed the expression of CDKN2B-AS1 in thyroid cancer.(B) The level of CDKN2B-AS1 in thyroid cancer cells was assessed by RT-qPCR.(C–D) Short hairpin RNA (shRNA)-targeted CDKN2B-AS1 plasmid was transfected into TPC-1 and SW579 cells, and the level of CDKN2B-AS1 was detected by RT-qPCR.(E–F) The effect of CDKN2B-AS1 silencing on the viability of thyroid cancer cells was tested by cell counting kit-8 (CCK-8).(G–H) Sphere-forming assay was performed to detect the effect of CDKN2B-AS1 silencing on the stemness of thyroid cancer cells. ∗∗∗p < 0.001 vs. Nthy-ori3-1; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. shRNA targeted NC (sh-NC).
3.2. Exosomes derived from CSCs carried CDKN2B-AS1 to the recipient thyroid cancer cells
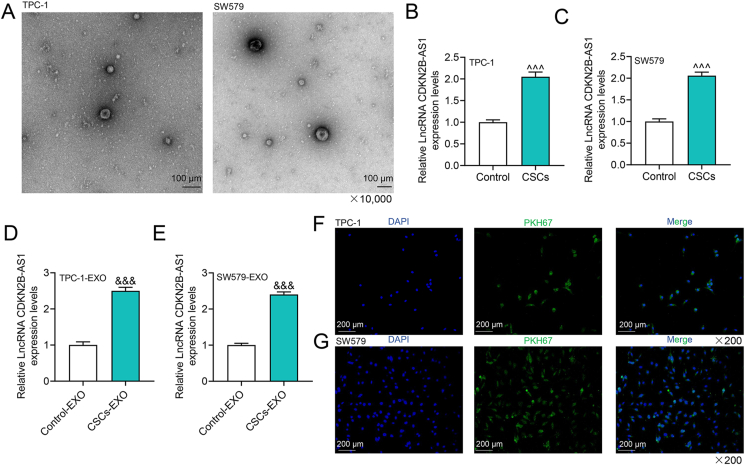
Exosomes have communication functions between cells by carrying diverse “goods” such as lncRNA [14,13]. We successfully extracted exosomes derived from CSCs formed by TPC-1 and SW579 cells, as shown in Fig. 2A. Compared with that in parental thyroid cancer cells, the expression of CDKN2B-AS1 in CSCs was up-regulated (p < 0.001, Fig. 2B and C). In the same way, CDKN2B-AS1 was more ample in exosomes derived from CSCs than in parental cells (p < 0.001, Fig. 2D and E). Using PKH67-labeled CSCs-derived exosomes to co-culture with receptor TPC-1 and SW579 cells, respectively, we identified that PKH67 was localized in the cytoplasm of the recipient cells, signifying that the exosomes were transported to the recipient cells (Fig. 2F and G).
Fig. 2.
The characterization of exosomes and the expression of CDKN2B-AS1 in cancer stem cell-like cells (CSCs) and their exosomes.(A) Observation and identification of exosomes under a transmission electron microscope (TEM).(B–C) The expression of CDKN2B-AS1 in CSCs and their parental cells.(D–E) The expression of CDKN2B-AS1 in exosomes derived from CSCs.(F–G) CSCs-derived exosomes were co-cultured with TPC-1 and SW579 cells respectively to observe the localization of PKH67 (green fluorescent label) in the cells. ˆˆˆp < 0.001 vs. Control (TPC-1 or SW579 cells); &&&p < 0.001 vs. Control-EXO (exosomes derived from TPC-1 or SW579 cells).
3.3. Effect of CSCs-derived exosomal CDKN2B-AS1 silencing on recipient thyroid cancer cells
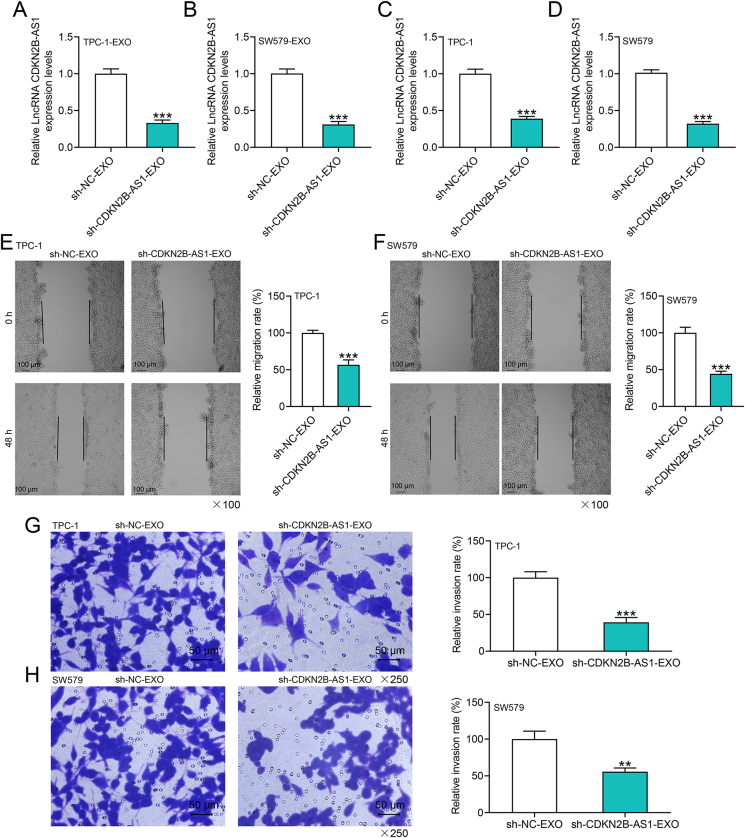
To further comprehend how CDKN2B-AS1 affects the progression of thyroid cancer through exosomes, we transfected CDKN2B-AS1 silencing plasmid into CSCs and extracted exosomes, revealing that the level of CDKN2B-AS1 in exosomes was notably reduced (p < 0.001, Fig. 3A and B). Subsequently, the exosomes extracted from CSCs with different transfection were co-cultured with the recipient tumor cells, and the sh-CDKN2B-AS1-EXO group presented reduced level of CDKN2B-AS1 in the recipient tumor cells (p < 0.001, Fig. 3C and D). Functional experiments indicated that the migration and invasion of thyroid cancer cells were hampered in sh-CDKN2B-AS1-EXO group (p < 0.01, Fig. 3E–H).
Fig. 3.
The effects of exosomes derived from CSCs with CDKN2B-AS1 silencing on the migration and invasion of thyroid cancer cells.(A–B) RT-qPCR detection found that sh-CDKN2B-AS1 reduced the level of CDKN2B-AS1 in exosomes.(C–D) RT-qPCR was performed to examine the effects of exosomes derived from CSCs with CDKN2B-AS1 silencing on the level of CDKN2B-AS1 in thyroid cancer cells.(E–F) Exosomes from CSCs transfected with sh-CDKN2B-AS1 reduced the migration of parental cells, which was tested by wound healing experiments.(G–H) Transwell assay tested the effects of exosomes derived from CSCs with CDKN2B-AS1 silencing on cell invasion in each group. ∗∗p < 0.01, ∗∗∗p < 0.001 vs. sh-NC-EXO (exosomes derived from TPC-1 or SW579 cells-CSCs transfected with sh-NC).
3.4. Exosomal CDKN2B-AS1 may function by targeting miR-122-5p/P4HA1 axis
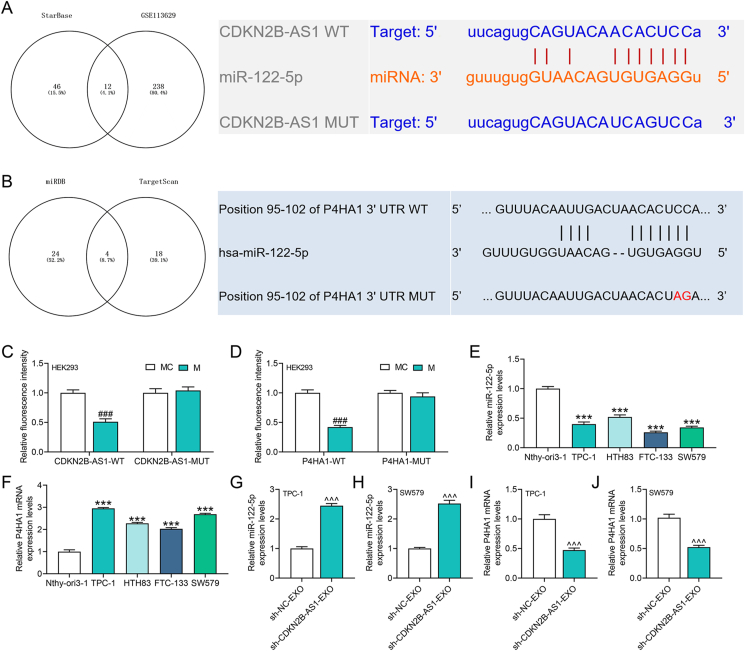
Bioinformatics analysis explored the downstream miRNAs of CDKN2B-AS1 which were intersected with thyroid cancer-related miRNAs in the GSE113629 dataset, and 12 candidate miRNAs were thus obtained (Fig. 4A). The literature suggested that miR-122-5p can inhibit thyroid cancer, which aroused our interest (Fig. 4A). Subsequently, we analyzed the target gene protein of miR-122-5p and screened P4HA1 that was related to EMT (Fig. 4B). Dual-luciferase reporter assay verified that CDKN2B-AS1 targeted miR-122-5p that bound to P4HA1 (p < 0.001, Fig. 4C and D). Next, we uncovered that miR-122-5p expression was down-regulated, while P4HA1 level was markedly up-regulated in thyroid cancer cells (p < 0.001, Fig. 4E and F). In addition, we tested the effects of exosomes derived from CSCs with CDKN2B-AS1 silencing on the levels of miR-122-5p and P4HA1 in recipient cells, validating that sh-CDKN2B-AS1 promoted miR-122-5p expression but suppressed P4HA1 level (p < 0.001, Fig. 4G–J).
Fig. 4.
CDKN2B-AS1 in exosomes targeted miR-122-5p/P4HA1 axis in recipient thyroid cancer cells.(A) The starBase and GSE113629 data were applied to analyze the miRNAs targeted by CDKN2B-AS1 and acting on thyroid cancer, the results of which showed the targeted binding sites of CDKN2B-AS1 and miR-122-5p.(B) The miRDB (http://mirdb.org/mirdb/index.html) and TargetScan database (http://www.targetscan.org/vert_72/) predicted the target genes of miR-122-5p, and the TargetScan database predicted the targeted binding sites of miR-122-5p and P4HA1.(C–D) The binding relationship between miR-122-5p with CDKN2B-AS1 and P4HA1 was verified by dual-luciferase reporter assay.(E–F) The expressions of miR-122-5p and P4HA1 in thyroid cancer cells were detected by RT-qPCR.(G–J) RT-qPCR was performed to detect the effects of exosomes derived from CSCs with CDKN2B-AS1 silencing on the expressions of miR-122-5p and P4HA1. ∗∗∗p < 0.001 vs. Nthy-ori3-1; ###p < 0.001 vs. MC (mimic control); ˆˆˆp < 0.001 vs. sh-NC-EXO.
3.5. The effects of CDKN2B-AS1/miR-122-5p/P4HA1 axis on the viability, invasion and EMT-related proteins of thyroid cancer cells
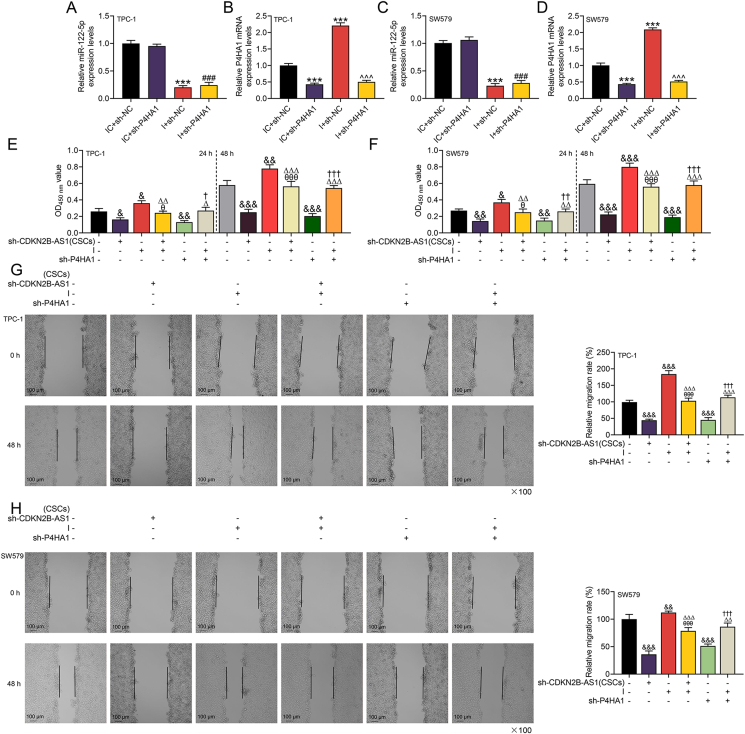
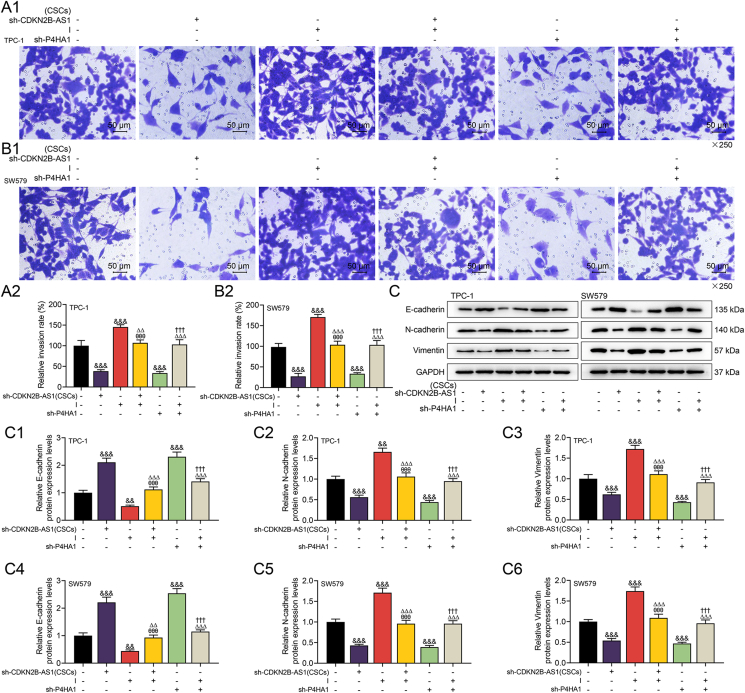
To further reveal the potential mechanism of CDKN2B-AS1 from CSCs-derived exosomes affecting the biological functions of thyroid cancer cells, we conducted relevant rescue experiments. Prior to the functional experiment, we detected that miR-122-5p inhibitor dwindled the expression of miR-122-5p and promoted that of P4HA1, while sh-P4HA1 reversed the level of P4HA1 enhanced by miR-122-5p inhibitor (p < 0.001, Fig. 5A–D). Functional experiments affirmed that CDKN2B-AS1 silencing and P4HA1 silencing attenuated the biological functions of thyroid cancer cells, while miR-122-5p inhibitor had the opposite effect (p < 0.05, Fig. 5E–H, 6A1-2, 6B1-2). Specifically, CDKN2B-AS1 silencing inhibited receptor thyroid cancer cell viability, migration and invasion, while miR-122-5p inhibitor generated promoting effects to the same aspects. Also, P4HA1 silencing reversed the malignant phenotype of cells enhanced by miR-122-5p inhibitor (p < 0.05, Fig. 5E–H, 6A1-2, 6B1-2). Later, we learned that sh-CDKN2B-AS1 and sh-P4HA1 both up-regulated expression of epithelial marker E-cadherin, but down-regulated those of N-cadherin and Vimentin, while miR-122-5p inhibitor generated the opposite regulatory effects (p < 0.01, Fig. 6C, C1-6). Importantly, the effect of sh-CDKN2B-AS1 on the aforementioned proteins was reversed by miR-122-5p inhibitor, and sh-P4HA1 also offset the effect of miR-122-5p inhibitor (p < 0.01, Fig. 6C, C1-6).
Fig. 5.
The effects of CDKN2B-AS1/miR-122-5p/P4HA1 axis on the viability and migration of thyroid cancer cells.(A–D) RT-qPCR was used to detect the transfection efficiency of miR-122-5p inhibitor (I) and shRNA-targeted P4HA1 (sh-P4HA1), with U6 and GAPDH serving as internal controls.(E–F) After 24 or 48 h of co-culture of exosomes and recipient cells, the effect of CDKN2B-AS1/miR-122-5p/P4HA1 on cell viability was assessed by CCK-8 assay.(G–H) Cell migration in each group was measured by wound healing experiment. ∗∗∗p < 0.001 vs. IC (inhibitor control) + sh-NC; ###p < 0.001 vs. IC + sh-P4HA1; ˆˆˆp < 0.001 vs. I + sh-NC; &p < 0.05, &&p < 0.01, &&&p < 0.001 vs. control (No transfection); θp < 0.05, θθθp < 0.001 vs. sh-CDKN2B-AS1 (CSCs) (transfected into CSCs); △p < 0.05, △△p < 0.01, △△△p < 0.001 vs. I (transfected into recipient thyroid cancer cells); †p < 0.05, ††p < 0.01, †††p < 0.001 vs. sh-P4HA1 (transfected into recipient thyroid cancer cells).
Fig. 6.
CDKN2B-AS1/miR-122-5p/P4HA1 axis altered the thyroid cancer cell invasion and epithelial-mesenchymal transition (EMT)-related proteins.(A1, A2, B1, B2) MiR-122-5p inhibitor reversed the invasion of thyroid cancer cells inhibited by exosomes derived from CSCs with CDKN2B-AS1 silencing, and P4HA1 silencing reversed miR-122-5p inhibitor-promoted cell invasion as determined by Transwell.(C, C1-6) The expression changes of epithelial markers E-cadherin, mesenchymal markers N-cadherin and Vimentin were detected by western blot, with GADPH serving as the internal control. &&p < 0.01, &&&p < 0.001 vs. control (No transfection); θθθp < 0.001 vs. sh-CDKN2B-AS1 (CSCs); △△p < 0.01, △△△p < 0.001 vs. I; †††p < 0.001 vs. sh-P4HA1.
3.6. Exosomal CDKN2B-AS1 from thyroid cancer CSCs induced tumorigenesis and metastasis of thyroid cancer in vivo
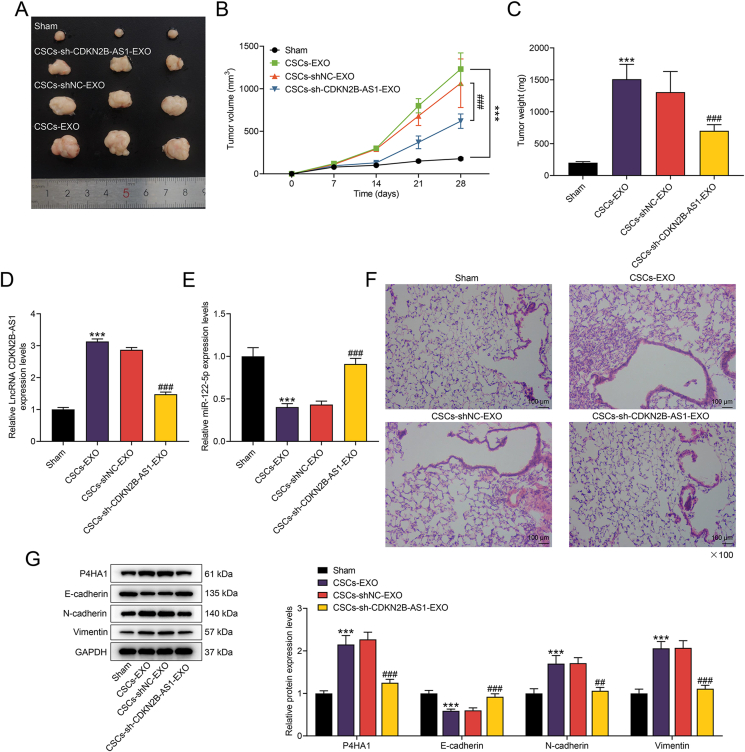
The exosomes derived from CSCs promoted the growth of thyroid cancer tumors, including the increment in volume and weight (p < 0.001, Fig. 7A–C). The exosomes from CSCs with CDKN2B-AS1 silencing alleviated the tumor growth (p < 0.001, Fig. 7A–C). We identified up-regulated CDKN2B-AS1 and down-regulated miR-122-5p in CSCs-EXO group, while the inverse trends appeared in CSCs-sh-CDKN2B-AS1-EXO group (p < 0.001, Fig. 7D and E). In addition, metastatic nodules were increased in CSCs-EXO group, and such increase was partially confronted with suppression in CSCs-sh-CDKN2B-AS1-EXO group (Fig. 7F). Finally, the alleviated downregulation of E-cadherin and up-regulation of P4HA1, N-cadherin and Vimentin caused by CSCs-derived exosomes could be noticed in CSCs-sh-CDKN2B-AS1-EXO group (p < 0.01, Fig. 7G).
Fig. 7.
The effect of CDKN2B-AS1 derived from CSCs in thyroid cancer was verified in vivo.(A–C) In vivo transplantation tumor experiment determined the effects of exosomes extracted from CSCs transfected with or without sh-CDKN2B-AS1 on the volume and weight of transplanted tumors.(D–E) The expressions of CDKN2B-AS1 and miR-122-5p in tumor tissues were evaluated by RT-qPCR.(F) An in vivo metastasis model was constructed, and hematoxylin and eosin (H&E)-stained lung tissues were used to detect the effect of CDKN2B-AS1 on metastatic nodules.(G) The expressions of P4HA1 and EMT-related proteins in tumor tissues of each group were detected by western blot. ∗∗∗p < 0.001 vs. Sham; ##p < 0.01, ###p < 0.001 vs. CSCs-shNC-EXO (exosomes derived from TPC-1 or SW579 cells-CSCs transfected with sh-NC).
4. Discussion
In the context of extensive illustrations on the roles of genetic abnormalities and environmental factors in the development of thyroid cancer, what should be underscored lies in the exact molecular mechanism that affects the development of thyroid cancer [1,4]. A number of studies have proposed that proteins, miRNA and lncRNA contained in exosomes may be potential biomarkers of cancer [11,17]. Here, we have identified the up-regulation of CDKN2B-AS1 in thyroid cancer and explained the correlation between CDKN2B-AS1 and thyroid cancer. Importantly, we uncovered abundant CDKN2B-AS1 in thyroid cancer CSCs-derived exosomes, and revealed that CDKN2B-AS1 accelerated cancer growth and metastasis by targeting miR-122-5p/P4HA1 axis in receptor thyroid cancer cells.
A previous study has confirmed that lncRNA, as a biological marker, plays an indispensable role in the proliferation, metastasis, invasion and other biological processes of thyroid cancer cells [7]. At present, CDKN2B-AS1 has been proved to participate in the regulation of disease progression [[21], [22], [23]]. For example, CDKN2B-AS1 affects the biological phenotype of lung cancer cells by regulating miR-378b/NR2C2 [21]. Similar phenomena have also been demonstrated in hepatocellular carcinoma and laryngeal squamous cell carcinoma [22,23]. Here, we identified that CDKN2B-AS1 expression was elevated in thyroid cancer, and CDKN2B-AS1 silencing reduced the viability and stemness of thyroid cancer cells, manifesting that CDKN2B-AS1 may function as oncogenes in thyroid cancer.
CSCs are perceived as essential participants in tumor progression, and exosomes derived from CSCs are considered to be pivotal signal transmitters for chemoresistance and tumor metastasis [19,18]. We affirmed that CDKN2B-AS1 derived from thyroid cancer CSCs can be packaged into exosomes and further affect the migration and invasion of recipient thyroid cancer cells, which explains the relationship between CDKN2B-AS1 and the development of thyroid cancer. In the past, Dai et al. proposed that the exosomal LncRNA DOCK9-AS2 derived from CSCs promoted the stemness, proliferation, migration and invasion of papillary thyroid carcinoma via activating the Wnt/β-catenin pathway [24]. A previous study showed that CSCs-derived exosomes carry linc-ROR to induce EMT and impinge upon the local tumor microenvironment and distant metastatic niche [18]. These denoted an important regulatory role of exosomal lncRNA from thyroid cancer CSCs in the progression of thyroid cancer, and yielded a new direction for a better understanding of thyroid cancer in the future.
In order to further determine the mechanism of the influence from CSCs-derived exosomes on tumor progression, we predicted the downstream targets of CDKN2B-AS1. Among them, miR-122-5p had a binding site with CDKN2B-AS1, and it has been proven to inhibit the progression of thyroid cancer [25]. MiR-122-5p is related to a variety of cancers, including gastric cancer, colorectal cancer, hepatocellular carcinoma, etc. [[26], [27], [28]]. Here, functional analyses unveiled the tumor suppressor effect of miR-122-5p in thyroid cancer, which was consistent with previous studies. Importantly, we revealed that exosomal CDKN2B-AS1 derived from thyroid cancer CSCs can inhibit the level of miR-122-5p in thyroid cancer cells and affect the EMT process.
MiRNA usually acts on mRNA to advance or retard the progression of the disease [29,30]. By means of the prediction and verification, P4HA1 was identified as a downstream target gene of miR-122-5p. Consistently, miR-122 also inhibits EMT by regulating P4HA1 in ovarian cancer [31]. The effect of P4HA1 on various cancers has been reported. For example, it is highly expressed in lung cancer, breast cancer as well as head and neck cancer and is associated with poor prognosis [32]. Nevertheless, the role of P4HA1 in thyroid cancer has not been revealed. In this study, we provided the first evidence that P4HA1 made impacts on accelerating the malignant phenotype of thyroid cancer cells, and partially reversed the tumor suppressing effect of miR-122-5p, further enriching the mechanism of CDKN2B-AS1 in thyroid cancer. Besides, we have corroborated through in vivo experiments that exosomal CDKN2B-AS1 derived from thyroid cancer CSCs induced tumorigenesis and metastasis of thyroid cancer through modulating the miR-122-5p/P4HA1 axis.
5. Conclusion
The study takes the first step to reveal that CDKN2B-AS1 is an exosomal lncRNA derived from thyroid cancer CSCs and highly expressed in thyroid cancer. Functionally, thyroid cancer CSCs transfer CDKN2B-AS1 to recipient thyroid cancer cells through exosomes to promote the malignant progression of thyroid cancer. In mechanism, CDKN2B-AS1 acts as the ceRNA of miR-122-5p, thereby up-regulating the expression of P4HA1 to affect the EMT process. These findings authenticated that CDKN2B-AS1 is a promising target for the treatment of thyroid cancer. Nevertheless, we should further explore the role of CDKN2B-AS1/miR-122-5p/P4HA1 axis in the growth and metastasis of thyroid cancer tumors at the animal level, which will be an aspect of our future research.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of the Japanese Society for Regenerative Medicine.
References
- 1.Cabanillas M.E., McFadden D.G., Durante C. Thyroid cancer. Lancet. 2016;388(10061):2783–2795. doi: 10.1016/S0140-6736(16)30172-6. [DOI] [PubMed] [Google Scholar]
- 2.Seib C.D., Sosa J.A. Evolving understanding of the epidemiology of thyroid cancer. Endocrinol Metab Clin N Am. 2019;48(1):23–35. doi: 10.1016/j.ecl.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Cabanillas M.E., Ryder M., Jimenez C. Targeted therapy for advanced thyroid cancer: kinase inhibitors and beyond. Endocr Rev. 2019;40(6):1573–1604. doi: 10.1210/er.2019-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laha D., Nilubol N., Boufraqech M. New therapies for advanced thyroid cancer. Front Endocrinol. 2020;11:82. doi: 10.3389/fendo.2020.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao J., Zhang M., Zhang L., Lou J., Zhou F., Fang M. Non-coding RNA in thyroid cancer - functions and mechanisms. Cancer Lett. 2021;496:117–126. doi: 10.1016/j.canlet.2020.08.021. [DOI] [PubMed] [Google Scholar]
- 6.Uhlig J., Case M.D., Blasberg J.D., Boffa D.J., Chiang A., Gettinger S.N., et al. Comparison of survival rates after a combination of local treatment and systemic therapy vs systemic therapy alone for treatment of stage IV non-small cell lung cancer. JAMA Netw Open. 2019;2(8) doi: 10.1001/jamanetworkopen.2019.9702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedaghati M., Kebebew E. Long noncoding RNAs in thyroid cancer. Curr Opin Endocrinol Diabetes Obes. 2019;26(5):275–281. doi: 10.1097/MED.0000000000000497. [DOI] [PubMed] [Google Scholar]
- 8.Yuan J., Song Y., Pan W., Li Y., Xu Y., Xie M., et al. LncRNA SLC26A4-AS1 suppresses the MRN complex-mediated DNA repair signaling and thyroid cancer metastasis by destabilizing DDX5. Oncogene. 2020;39(43):6664–6676. doi: 10.1038/s41388-020-01460-3. [DOI] [PubMed] [Google Scholar]
- 9.Liu H., Deng H., Zhao Y., Li C., Liang Y. LncRNA XIST/miR-34a axis modulates the cell proliferation and tumor growth of thyroid cancer through MET-PI3K-AKT signaling. J Exp Clin Cancer Res. 2018;37(1):279. doi: 10.1186/s13046-018-0950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song C., Qi Y., Zhang J., Guo C., Yuan C. CDKN2B-AS1: an indispensable long non-coding RNA in multiple diseases. Curr Pharmaceut Des. 2020;26(41):5335–5346. doi: 10.2174/1381612826666200806102424. [DOI] [PubMed] [Google Scholar]
- 11.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu F., Li F., Lin X., Xu F., Cui R.R., Zhong J.Y., et al. Exosomes increased angiogenesis in papillary thyroid cancer microenvironment. Endocr Relat Cancer. 2019;26(5):525–538. doi: 10.1530/ERC-19-0008. [DOI] [PubMed] [Google Scholar]
- 13.Poulet C., Njock M.S., Moermans C., Louis E., Louis R., Malaise M., et al. Exosomal long non-coding RNAs in lung diseases. Int J Mol Sci. 2020;21(10) doi: 10.3390/ijms21103580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wen D., Liu W.L., Lu Z.W., Cao Y.M., Ji Q.H., Wei W.J. SNHG9, a papillary thyroid cancer cell exosome-enriched lncRNA, inhibits cell autophagy and promotes cell apoptosis of normal thyroid epithelial cell Nthy-ori-3 through YBOX3/P21 pathway. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.647034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qian X., Qu H., Zhang F., Peng S., Dou D., Yang Y., et al. Exosomal long noncoding RNA AGAP2-AS1 regulates trastuzumab resistance via inducing autophagy in breast cancer. Am J Cancer Res. 2021;11(5):1962–1981. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Hardin H., Zhang R., Helein H., Buehler D., Guo Z., Lloyd R.V. The evolving concept of cancer stem-like cells in thyroid cancer and other solid tumors. Lab Invest. 2017;97(10):1142–1151. doi: 10.1038/labinvest.2017.41. [DOI] [PubMed] [Google Scholar]
- 17.Pegtel D.M., Gould S.J. Exosomes. Annu Rev Biochem. 2019;88:487–514. doi: 10.1146/annurev-biochem-013118-111902. [DOI] [PubMed] [Google Scholar]
- 18.Hardin H., Helein H., Meyer K., Robertson S., Zhang R., Zhong W., et al. Thyroid cancer stem-like cell exosomes: regulation of EMT via transfer of lncRNAs. Lab Invest. 2018;98(9):1133–1142. doi: 10.1038/s41374-018-0065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grassi E.S., Ghiandai V., Persani L. Thyroid cancer stem-like cells: from microenvironmental niches to therapeutic strategies. J Clin Med. 2021;10(7) doi: 10.3390/jcm10071455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jian X., He H., Zhu J., Zhang Q., Zheng Z., Liang X., et al. Hsa_circ_001680 affects the proliferation and migration of CRC and mediates its chemoresistance by regulating BMI1 through miR-340. Mol Cancer. 2020;19(1):20. doi: 10.1186/s12943-020-1134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo H., Feng Y., Yu H., Xie Y., Luo F., Wang Y. A novel lncRNA, loc107985872, promotes lung adenocarcinoma progression via the notch1 signaling pathway with exposure to traffic-originated PM2.5 organic extract. Environ Pollut. 2020;266(Pt 1) doi: 10.1016/j.envpol.2020.115307. [DOI] [PubMed] [Google Scholar]
- 22.Shen X., Li Y., He F., Kong J. LncRNA CDKN2B-AS1 promotes cell viability, migration, and invasion of hepatocellular carcinoma via sponging miR-424-5p. Cancer Manag Res. 2020;12:6807–6819. doi: 10.2147/CMAR.S240000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui X., Yu T., Shang J., Xiao D., Wang X. Long non-coding RNA CDKN2B-AS1 facilitates laryngeal squamous cell cancer through regulating miR-497/CDK6 pathway. OncoTargets Ther. 2019;12:8853–8862. doi: 10.2147/OTT.S221620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dai W., Jin X., Han L., Huang H., Ji Z., Xu X., et al. Exosomal lncRNA DOCK9-AS2 derived from cancer stem cell-like cells activated Wnt/beta-catenin pathway to aggravate stemness, proliferation, migration, and invasion in papillary thyroid carcinoma. Cell Death Dis. 2020;11(9):743. doi: 10.1038/s41419-020-02827-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu N., Tian Y., Song Y., Zang L. miR1225p suppresses the oncogenesis of PTC by inhibiting DUSP4 expression. Mol Med Rep. 2021;23(5) doi: 10.3892/mmr.2021.12007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meng L., Chen Z., Jiang Z., Huang T., Hu J., Luo P., et al. MiR-122-5p suppresses the proliferation, migration, and invasion of gastric cancer cells by targeting LYN. Acta Biochim Biophys Sin. 2020;52(1):49–57. doi: 10.1093/abbs/gmz141. [DOI] [PubMed] [Google Scholar]
- 27.Yin W., Xu J., Li C., Dai X., Wu T., Wen J. Circular RNA circ_0007142 facilitates colorectal cancer progression by modulating CDC25A expression via miR-122-5p. OncoTargets Ther. 2020;13:3689–3701. doi: 10.2147/OTT.S238338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang Y., Zhang D., Zheng T., Yang G., Wang J., Meng F., et al. lncRNA-SOX2OT promotes hepatocellular carcinoma invasion and metastasis through miR-122-5p-mediated activation of PKM2. Oncogenesis. 2020;9(5):54. doi: 10.1038/s41389-020-0242-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liang W., Sun F. Identification of pivotal lncRNAs in papillary thyroid cancer using lncRNA-mRNA-miRNA ceRNA network analysis. PeerJ. 2019;7 doi: 10.7717/peerj.7441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ye M., Dong S., Hou H., Zhang T., Shen M. Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer progression through regulation of the miR-204/IGF2BP2/m6A-MYC signaling. Mol Ther Nucleic Acids. 2021;23:1–12. doi: 10.1016/j.omtn.2020.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Duan Y., Dong Y., Dang R., Hu Z., Yang Y., Hu Y., et al. MiR-122 inhibits epithelial mesenchymal transition by regulating P4HA1 in ovarian cancer cells. Cell Biol Int. 2018;42(11):1564–1574. doi: 10.1002/cbin.11052. [DOI] [PubMed] [Google Scholar]
- 32.Li M., Wu F., Zheng Q., Wu Y., Wu Y. Identification of potential diagnostic and prognostic values of P4HA1 expression in lung cancer, breast cancer, and head and neck cancer. DNA Cell Biol. 2020;39(5):909–917. doi: 10.1089/dna.2019.5170. [DOI] [PubMed] [Google Scholar]