Poultry meat and eggs are among the most common sources of animal protein for humans worldwide. The global poultry market was valued at $322.55 billion USD in 2020 and will reach $422.97 billion USD in 2025 at a compound annual growth rate of 7%. Asia Pacific was the largest region in the global poultry market in 2020, contributing to 32% of the total market. In China, 15.57 billion poultry were slaughtered in 2020 with a year-over-year increase of 6.35%.
A growing demand for raw poultry and poultry products worldwide has put forward new requirements for sustainable poultry production. Antimicrobials have been widely used in animal farming ever since the addition of low doses of antibiotics to the animal diet was discovered to promote animal growth in the 1950s. It is estimated that in the United States, 70% of antibiotics (24.5 million pounds per year) are used in animals, while only 30% are consumed by humans. The heavy use of antimicrobials for both therapeutic and growth-promoting purposes in farm animals fuels the development and dissemination of antimicrobial resistance among animals, humans, and environments.1 Fortunately, an increasing number of countries have banned the use of antimicrobials as growth promoters in recent years. China banned colistin in 2017 and all antimicrobial growth promoters in 2020. These actions are beneficial for preventing the development of antimicrobial resistance and reducing drug residues in animal foods; however, they pose challenges for maintaining the efficiency and sustainability of food animal production.
The basic question of why antimicrobials promote animal growth is still unanswered, which impedes the effectiveness of finding antimicrobial alternatives. However, accumulated evidence has suggested that the growth-promoting effects of antimicrobials were due to their influences on animal gut microbiota. A moderate shift in microbiome composition (limited or no loss of population size) of the animals treated with antimicrobials may result in an optimal microbiota that can change the host physiology and metabolism and reduce intestinal defense, thus enabling the animals to reach their genetic potential.2 Currently, antimicrobial alternatives showing growth-promoting effects, including probiotics, prebiotics, organic acids, enzymes, phytogenics, etc., have all been proven to modulate the gut microbiome.
Similar to humans and other animals, the poultry gut is inhabited by a great number of microbes involved in host immune modulation, nutrient metabolism, and pathogen exclusion, etc.3 An optimal and balanced chicken gut microbiome is necessary for a healthy animal and is a prerequisite for better production performance. In addition to using the well-recognized antimicrobial alternatives, it has also been found to be reasonable and feasible to directly manipulate the poultry gut microbiome, e.g., through fecal microbiota transplantation. However, although reconstructing a new microecosystem and/or transferring a desired trait to farm animals by fecal microbiota transplantation can be achieved in laboratory experiments, it is not easy to perform this in practical production processes, especially under modern intensive farming conditions. To overcome the limitations, the construction of artificial bacterial communities, i.e., synthetic microbiomes or communities, has received substantial interest. Using defined microbes from the donor feces to restore the gut ecosystem or transfer an expected phenotype to the receivers guarantees controllability, reproducibility, and safety.
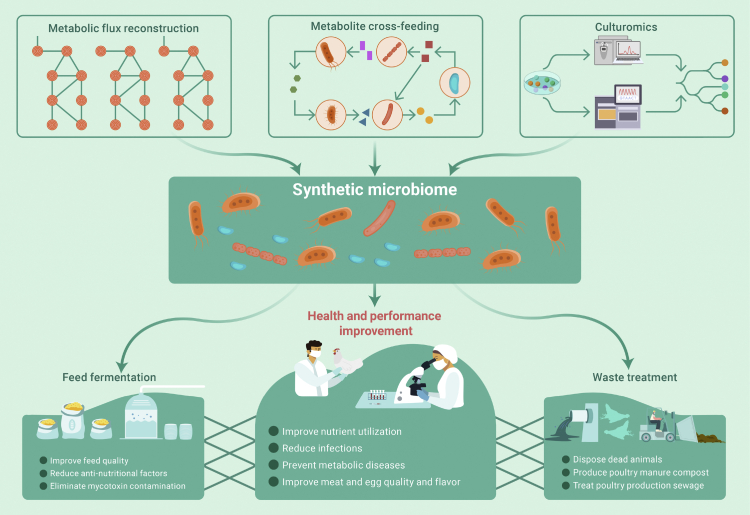
Currently, two strategies, “top down” and “bottom up,” are proposed and applied in designing synthetic microbiomes (also known as microbiome engineering).4 To date, the effectiveness of designed synthetic microbiomes has been demonstrated with specific goals, ranging from environmental remediation, microbiome-associated disease treatment, immune regulation, and protection and treatment of pathogen infection in plants, humans, and animals. In fact, compound probiotics that have been widely used in poultry production as antimicrobial alternatives can be regarded as a prototype for a synthetic microbiome. However, unlike a simple mixing of different probiotic strains, the synthetic microbiome functions as a whole and features an engineered structure and activity. Given the important role of the gut microbiome in poultry physiology and metabolism, a synthetic microbiome with diverse functions can be expected and designed, including but not limited to the following considerations: (1) reducing antinutritional factors to improve nutrient utilization. For example, insoluble non-starch polysaccharides are a common antinutritional factor in cereal-based poultry feeds, which can be digested using a designed cellulolytic bacterial consortium for fermentation in vitro or as feed additive to function in vivo. (2) Increasing energy supply to improve epithelial absorption and barrier function. Short-chain fatty acid, one of the major products of gut microbes, is an important energy source for gut epithelial cells. A synthetic microbiome with enhanced short-chain fatty acid production is crucial for promoting poultry gut health. (3) Modulating the immune system or inhibiting the colonization of pathogens to reduce infections. Recently, a nine-member synthetic microbial community was designed and demonstrated to effectively promote the maturation of the chicken immune system.5 (4) Regulating host metabolic signals to prevent metabolic diseases. For instance, in laying hens, fatty liver hemorrhagic syndrome is very similar to the human non-alcoholic fatty liver disease, which can be ameliorated by modulating the gut microbiota and thereby improving the host lipid metabolism. (5) Changing host metabolism to improve the quality and flavor of meat and eggs. Increasing evidence has shown that the quality or flavor of animal products can be regulated by the gut microbiota. It is therefore highly anticipated to use a synthetic microbiome consisting of key gut taxa from better-performing poultries to improve the production traits in receiver animals. In addition to modulating the gut microbiome during poultry rearing, the synthetic microbiome can be applied in other poultry production processes, such as the elimination of mycotoxin contamination in poultry feed; disposal of dead animals (using a designed microbial community to ferment); production of poultry manure compost; and treatment of poultry production sewage. All these possible applications of the synthetic microbiome in poultry production contribute to a sustainable poultry industry (Figure 1).
Figure 1.
Applications of the synthetic microbiome in poultry production
Top-down and bottom-up approaches are currently proposed to design a synthetic microbiome. In the top-down design, a functional microbial community is first determined, and then the microbiome is optimized or minimized based on the structural and functional stability to achieve better performance. The bottom-up design starts from characterizing the function and metabolic pathways of individual microorganisms and then combines complementary functions or reconstructs metabolic networks in a synthetic community to generate a desired output.4 In both approaches, microbial metabolite cross-feeding and metabolic flux reconstruction should be considered. Individual microorganisms in a synthetic microbiome can be genetically engineered strains with metabolic flux modification or directly obtained from a naturally selected microbiome through culturomic method. A well-designed synthetic microbiome can be used in the preparation of poultry feed, the animal raising process, and the disposal of poultry production waste.
Although fascinating, more efforts are required for designing and effectively applying synthetic microbiomes in poultry production. Challenges and research directions are summarized below. First, the role of gut microbes and mechanisms involved in poultry health and disease is not fully understood. For instance, which microbes regulate growth speed, food intake, and disease resistance in certain poultry individuals and through what mechanisms? How do poultry genetics interact with gut microbes and thus codetermine animal phenotypes? Can the host select specific microbes to colonize and have beneficial effects? What are the keystone taxa (or core microbiome), and how do they interact with other microbes in a balanced poultry gut microbiome to exert beneficial effects on the host? Are there “epidemic probiotics” in different types of poultry? Answering these questions is essential for synthesizing microbial communities with defined functions. Additionally, revealing causal relationships, but not associations, in poultry microbiome studies is highly expected. Second, obtaining pure bacterial cultures and exploring the function of the microbiome at the strain level is key to synthesizing the microbiome. Recently, culturomics, an approach combining high-throughput cultivation (multiple culture conditions) and identification (MALDI-TOF mass spectrometry and 16S rRNA sequencing) of bacteria in a community, has been performed in both human and animal microbiome studies. The culturomic approach enables the culture of hundreds of new microbes that can be utilized as the basic units in the synthetic microbiome. However, in poultry, microbial culturomics is just at the beginning. Large-scale cultivation studies and new microbial culture and identification techniques, e.g., a microfluidic-based cell separation and cultivation method, are continuously needed in poultry-associated microbial communities. Third, a stable synthetic microbiome requires smart design by taking full consideration of the microbial interactions. Different relationships exist among microbial individuals, including mutualism, commensalism, ammensalism, and competition, among others. Cooperative metabolite cross-feeding among microbes prioritizes other interactions in designing synthetic microbiomes. Additionally, when needed, metabolic/genetic engineering and metabolic modeling combined with in silico metabolic flux reconstruction should be fully considered in a well-designed microbiome. Fourth, more efforts should be made with respect to how to use a synthetic microbiome in production practices, especially how to introduce it into animal guts. As gut microbes may have a colonization “priority effect,” we suggest an early-life inoculation of the designed consortium, for example through an in ovo injection technique that has been successfully used for administering probiotics in chicken gut. Alternatively, after hatching, a synthetic microbiome can be introduced to the birds through drinking water or be directly gavaged into the crop. Lastly, applying a synthetic microbiome in poultry production is a typical interdisciplinary activity; cooperation among experts in microbiology, animal science, computational biology, and engineering science is highly needed.
Acknowledgments
This work was supported by the Hainan Provincial Natural Science Foundation of China (2021JJLH0084); the National Key Research and Development Program of China (2022YFA1304201); and the 2115 Talent Development Program of China Agricultural University and Chinese Universities Scientific Fund.
Declaration of interests
The authors declare no competing interests.
Published Online: November 26, 2022
Contributor Information
Yongfei Hu, Email: huyongfei@cau.edu.cn.
Yuming Guo, Email: guoyum@cau.edu.cn.
References
- 1.Hu Y., Gao G.F., Zhu B. The antibiotic resistome: gene flow in environments, animals and human beings. Front. Med. 2017;11:161–168. doi: 10.1007/s11684-017-0531-x. [DOI] [PubMed] [Google Scholar]
- 2.Cox L.M., Blaser M.J. Antibiotics in early life and obesity. Nat. Rev. Endocrinol. 2015;11:182–190. doi: 10.1038/nrendo.2014.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y., Wang Y., Zhu B., et al. Metagenome-assembled genomes and gene catalog from the chicken gut microbiome aid in deciphering antibiotic resistomes. Commun. Biol. 2021;4:1305. doi: 10.1038/s42003-021-02827-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawson C.E., Harcombe W.R., Hatzenpichler R., et al. Common principles and best practices for engineering microbiomes. Nat. Rev. Microbiol. 2019;17:725–741. doi: 10.1038/s41579-019-0255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zenner C., Hitch T.C.A., Riedel T., et al. Early-Life Immune System maturation in chickens using a synthetic community of cultured gut bacteria. mSystems. 2021;6:e01300–e01320. doi: 10.1128/mSystems.01300-20. [DOI] [PMC free article] [PubMed] [Google Scholar]