Abstract
Biofilm could be defined as a complex communities of microorganisms seen affixed to surfaces, they form clusters without sticking to any surface and buried firmly in an extracellular matrix (ECM). This matrix is formed by microorganisms in the formation of either extracellular polymeric substances (EPSS) or extracellular polymer. Many reviews have addressed the negative consequences of biofilm production in the food industry, among which we talk about biofilms being responsible for spoilage microorganisms and foodborne pathogens such as Listeria monocytogenes, Bacillus cereus etc. These contamination could be linked to biofilms presence in the processing plant. Although researches have tried conferring solutions to these challenges in the food industry, however, in this review we have tried to focus on the positive impact of biofilms formed in the food industry. It is critically expedient while trying to find the solution to the challenges of biofilm in the food industry to develop and give a major focus on the advantages and positive impact biofilm has in the food industry, which has been greatly neglected. Hence in this article, we have highlighted some positive impacts of biofilms formed in the food industry, like enhancing plant health and productivity of food products, as an agent of water and wastewater treatment in the food industry, as a tool in reducing the amount of excess sludge in the wastewater treatment plant. The development of edible biofilms, fermented food products and the production of biodegradable food packaging are also part of biofilms beneficial roles in the food industries.
Keywords: Biofilm, Food industry, Positive impacts, Extracellular polymeric substances
1. Introduction
1.1. Biofilm definition
Biofilms are historically believed to have come to light in the primitive period on earth as a protective system for prokaryotic organisms during that period. This occurs consequently as a result of the harshness of the earth’s condition for the survival of the prokaryotes. Therefore, the production of biofilms protects their cells by supplying them homeostasis, thereby, stimulating complex production of interconnection between the cells of the biofilm (Hall-Stoodley, 2004).
Biofilm as stated in 1978 by Bill Costerton, is a heterogeneous structure made up of various microbial populations covered by matrix (mainly of exopolysaccharides) which enables them attach to inert (e.g., plastic, glass) or organic (e.g., mucosa, skin) surfaces.
Biofilms can also be said to be a collection of one or more kinds of microorganisms which can possibly grow on various surfaces. They include organisms such as bacteria, fungi and protists (Vidyasagar, 2016).
Biofilm can also be termed as the complex communities of microbes affixed to a surface or which probably forms aggregates without attaching to a surface, such as observed in Staphylococcus aureus, Pseudomonas aeruginosa, as well a few other bacteria (Alhede et al., 2011, Haaber et al., 2012) and hid securely in an extracellular matrix (ECM).
Biofilms can be further defined as bacterial populations enclosed in a matrix and attached to each other or a surface with an organic matrix protecting these organisms that are sessile. This definition encompases aggregate of microorganisms, flocculates and population adhering to pores of membrane (Marshall, 1992, Costerton et al., 1995, Genigeorgis, 1995).
López (2010) stated that a biofilm is made up of any syntrophic collective microorganisms whereby their cells adhere to each other also frequently to a surface. Hence these attached cells get embedded in a slimy extracellular matrix that is made of collection of extracellular polymeric substances (EPS) (Hall-Stoodley et al., 2004, López et al., 2010). Furthermore, these cells within the biofilm generate the EPS components, which typically are a polymeric combination of proteins, extracellular polysaccharides, lipids and DNA (Hall-Stoodley et al., 2004, López et al., 2010, Aggarwal et al., 2016). This have been metaphorically reported as “cities for microbes” by reason of their structure (three-dimensional) which portray a lifestyle of community for microorganisms (Watnick and Kolter, May 2000, Quanta Magazine, 2017).
Biofilms are known to comprise primarily of microorganisms that are viable and nonviable fixed in polyanionic extracellular polymeric substances which is surface tied (Carpentier and Cerf, 1993, Wimpenny et al., 1993). The extracellular polymeric substances (EPS), which protect the biofilm inhabitants could possibly consist of polysaccharides, proteins, phospholipids, teichoic and nucleic acids, and other polymeric substances hydrated to 85 to 95 % water (Costerton and Irvin, 1981, Sutherland, 1983).
Biofilm refers to microorganisms of complex, sessile communities seen affixed to a surface or fixed firmly in an extracellular matrix aggregates (Raniti et al., 2018). They are a microbial mass on the interfaces, and they remain as microbial contamination origin in medical field and other industries, especially in food processing industry (Furukawa, 2015). Usually they are accepted as contamination source of microorganisms and are also regarded as attachment of microbial colonies on interfaces used in the industries, especially in the instance of high drug resistance of biofilms which is considered as a signficant challenge in the medical field (Costerton et al., 1999; Davey and O’toole, 2000; Ghannoum and O’toole, 2004; Kolter and Greenberg, 2006; Furukawa et al., 2006). Interestingly most microorganisms potentially have capacity to form a biofilm, and they all have their contributing factors to the food industry just as they have been contributing to biological water clarification (Furukawa et al., 2006).
1.2. Biofilm formation/development
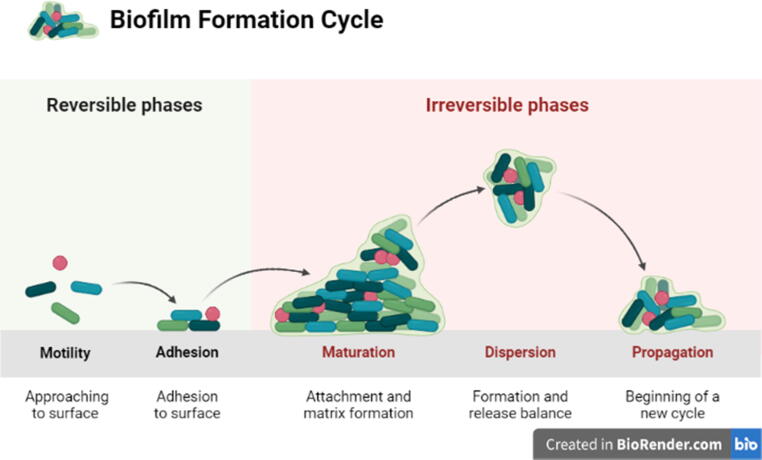
Biofilm is an assemblage cells of microorganisms usually embedded in a matrix of bacterial self-produced extracellular polymeric substances (EPSs), which is permanently connected with a surface. The stages of biofilms development are highlighted in Fig. 1 below:
Fig. 1.
Biofilm development stages: (1) Approaching to surface/motility; (2) Adhesion to surface; (3) Attachment and matrix formation/maturation; (4) Formation and release balance/dispersion and (5) Beginning of a new cycle/propagation.
(1) Planktonic microorganisms are initially approaching a surface in an aqueous medium for attachment (Donlan, 2002);
(2) permanent sticking based on the generation of microorganism-mediated EPSs, as polyhydroxy groups in EPSs colonize bacteria to the surface via hydrogen bonding (Kjelleberg et al., 2007);
(3) monolayer microcolonies formation on the surfacess that are fixed as a result of replication by initial colonizers (Guzmán-Soto et al., 2021) and advancement of the biofilm into a three-dimensional disposition by affixing debris from the environment that is adjacent and by exerting new planktonic bacteria (Guzmán-Soto et al., 2021);
(4) lastly expansion or dispersion in which sessile, matrix-encased biofilm cells change to planktonic bacteria that freely swim through quorum sensing (QS) or a cell-to-cell signaling mechanism by active and passive processes (Webb, 2007);
(5) the cycle begins again.
1.3. Why biofilm formation
There are environments that are termed harsh, where relatively few species of microorganisms are capable of multiplying and, therefore, can only exist for a significant period. However, microorganisms that survive any of these environmental extremes are called extremophiles. Examples of some environmental and adaptive features of microorganisms are bacteria in Low-Nutrient environment microbial growth in these environments are mostly in biofilms. Microbes generally form a biofilm in reaction to different factors, (O'Toole and Kolter, 1998)b such as cellular realization of specific or non-specific affixing sites on a surface, nutritional cues, or by subjection of planktonic cells to sub-inhibitory concentrations of antibiotics (Karatan and Watnick, 2009, Hoffman et al., 2005).
If a cell reverses to the biofilm mode of growth it usually goes through a shift known as phenotypic shift, its behaviour is such that large suites of genes are distinctively regulated (An and Parsek, 2007). Low nutrient availability, which is an environmental stress also cause changes in the phenotypic structure of planktonic cells to the sessile (affixed) form (Carpentier and Cerf, 1993). Substratum composition, fluid flow and surface chemistry and topography, also influence biofilm formation (Mittelman, 1998a).
With microorganisms co-habiting as a part of a biofilm, it confers some privileges as “Communities of microbes are usually more resilient to stress” (Van Houdt and Michiels, 2010). It is generally known that natural ecosystems are usually short of nutrient availability and hence formation of biofilm is a very key adaptive factor for continuity under these conditions (Mittelman, 1998).
It has been observed that autotrophic and heterotrophic microbes stay together in biofilms and benefit from their various community members. Producers termed autotrophs, such as algae or photosynthetic bacteria, these generate their own food organic compounds form, whereas heterotrophs are unable to generate their food, hence there is the need for external carbon sources. “In these multi-organismal communities, it is observed they often crossfeed” (Vidyasagar, 2016).
Some species of microorganisms cannot affix to a surface however they can attach themselves directly to the initial colonies or to the matrix. Small signaling molecules assisted by the cell–cell systems of communication bring about this establishment or colonization, this fact is termed quorum sensing (Fuqua et al., 1994), hence formation of biofilm is a very significant quorum-sensing controlled phenotype (Huber et al., 2003).
Biofilm formation has been seen in various aspects of nature: Dental plaque which is a slimy bacteria buildup which is formed on the exterior of pond scum; teeth; they have been usually found growing on metals and minerals; on tissues of plant and animal; underground and above the ground, underwater; on medical devices such as pacemakers and catheters and food contact surfaces, food industry infrastructures as well as food matrixes.
2. Formation of biofilm in the food industry
In food industries, usually the surfaces and equipment with food and non-food-contact are regularly annexed by microorganisms that can form biofilms (Alvarez-Ordonex et al., 2019). The extracellular matrix formed during biofilm production is mainly a collection of polysaccharides, such as exogenous DNA or proteins, cellulose (Kumar and Anand, 1998). This matrix plays an important role in adhering to hard surfaces (meat, fruits, bones, food industry equipment, etc.) (Flemming et al., 2016). The powerful tenaciousness of these biofilms in the food industry is as a result of the structural role of the extracellular matrix which produces complex gradients regarding oxygen diffusion and nutrients, having enzymes (extracellular) which is used for the purpose of nutrition (Galie et al., 2018). This shields the cells embedded against compounds that are toxic and permits for the movement of cell communication molecules (Galie et al., 2018).
It is worth noting that food associated bacteria or resident members of the flora that attach to and take over food processing environments interact with major foodborne pathogenic bacteria both in dual- and multiple-species biofilm models (Yuan et al., 2022), this has been studied considerably in vitro in contemporary years. The symbiotic interactions in a number of instances have been seen in foodborne bacterial pathogens which are poor formers of biofilm, this take opportunity of their relationship with very potent biofilm producers in order to be able to affix or attach to food-contact equipment/materials (Dass and Wang, 2022). Hence organisms such as Listeria monocytogenes have shown synergistic interaction with several strains of Enterococcus faecium and Enterococcus faecalis (da Silva Fernandes et al., 2015) while Escherichia coli on the other hand has demonstrated combined synergistic relationships with Burkholderia caryophylli and Ralstonia insidiosa strains which were isolated from the produce of freshly-cut product in food processing plant (Liu et al. 2014, 2015b).
Although, there are several advantages conferred by biofilm formation to the cells of microbes in a food industry environments, this includes resistances such as mechanical (against liquid streams in pipelines), physical (against desiccation) and chemical protection (against chemicals, antimicrobials and disinfectants used in the industry) (Flemming et al., 2016). In most cases, however, this represents a challenge that is of great concern, since biofilms formed by pathogenic or spoilage microorganisms could be origin of food cross-contamination, thereby diminishing the competency of food processing procedures and eventually compromising the quality of food and its safety standard (Coughlan et al., 2016).
2.1. Consequences of biofilm formation in food industries
2.1.1. Spoilage microorganisms and foodborne pathogens in biofilms
Several of these contaminations may be ascribed to the existence of biofilms in the processing plant, some examples are briefly highlighted.
2.1.1.1. Listeria monocytogenes
One of the most common foodborne pathogens is Listeria monocytogenes, it actually cause formation of biofilms on equipment such as plastic, stainless steel, and polycarbonate surfaces and other numerous surface materials with food contact (Mafu et al., 1990; Nelson, 1990; Frank and Koffi, 1990; Helke et al., 1993; Jeong and Frank, 1994; Dhir and Dodd, 1995 and Kim and Frank, 1995). Hence, species of Listeria are suitable for survival and growth in many micro-niches usually found in food processing facilities.
2.1.1.2. Pseudomonas spp.
Pseudomonads are ubiquitous and contribute greatly to food spoilage. Environments where food is processed such as on fruits, vegetables, meat surfaces, drains and floors, and in dairy products (with low acidity), such are habitat for Pseudomonads (Brocklehurst et al., 1987; Piette and Idziak, 1991; Criado et al., 1994 and Hood et al., 1997). Pseudomonas spp. generate abundant amounts of EPS which has been known to affix and form biofilms on surfaces of stainless steel (Barnes et al., 1999).
2.1.1.3. Bacillus spp.
Species of Bacillus can be seen all through the processing plants of dairy products (Oosthuizen et al., 2001). They survive heat processing most likely due to their ability to form spores, it’s been documented that these genera accumulate on joints and pipelines in the processing environment (Jeong and Frank, 1994). With situation where hot fluid flows continuously over a surface for more than 16 h, Organisms such as Bacillus and other heat resistant bacteria might likely form a biofilm (Frank, 2000).
A serious food safety concern in the food industry has been with B. cereus biofilm formation because of its ability to behave as a probable means of product contamination as well as recontamination (Srey et al., 2013). Since their spores are present everywhere in the environment, they find their way into final food products and food processing plants, including dairy products, vegetables, rice, and meat (Fratamico et al., 2005). B. cereus has been known to cause formation of biofilm on surfaces like storage tanks, stainless steel, and pipes conveyor belts with food contact (Christison et al., 2007).
2.1.1.4. Salmonella spp.
Salmonella is known to be isolated from slaughter and evisceration area such as poultry processing equipment (Helke et al., 1994; Joseph et al., 2001). The environment is usually wet hence, very ideal for biofilm formation. Although very little is known of the occurrence of Salmonella biofilms in environments such as food processing, however (Helke and Wong, 1994, Jones and Bradshaw 1997, Joseph et al., 2001) has shown and proven Salmonella to be able to affix and form biofilms on surfaces like as found in cement, plastic, and the stainless steel in the food processing plants.
2.1.1.5. Staphylococcus aureus
Staphylococcus aureus being a major foodborne pathogen and with the ability to form biofilms is the cause for outbreaks of foodborne diseases connected with the ingestion of products such as milk and other dairy products (Maria-Guadalupe et al., 2018). Biofilms formation usually occurs on virtually all sorts of surfaces of technological systems in the dairy industry.
2.1.2. Biofilm formation and impedance of heat transfer and metal surfaces Corrosion.
Sandu and Singh (1991), stated that mechanical blockage in fluid handling systems and the impairement of heat transfer could be possibly caused as a result of biofilm formation, furthermore corrosion to metal surfaces has also been attributed to biofilm formation (Bryers, 1987), although these challenges are not usual in the food industry.
In essence, surfaces and equipment of food and non-food-contact in food industries are regularly populated by biofilm forming microorganisms. More often than not, this typifies a serious problem, because there is the possibility of cross-contamination in foods as a result of biofilms established by pathogenic or spoilage microbes thereby decreasing the efficacy of food processing procedure, hence compromise to food safety and quality (Coughlan et al. 2016).
While adequate measures are taken to guide against the negative impact of biofilms on the food industry, we cannot afford like the saying goes to “throw the baby away with the bad water”. Far too many reviews have centered on the negative consequences of biofilm production in the food industry, in this review, we have tried to focus on some of the positive effects of biofilms formed in the food industry. Regulated biofilms formed by beneficial microorganisms signifies that they could be beneficial therefore creating an opportunity, since they could be enhanced to improve the quality and yield of fermented food or advance applications in biotechnology, centered on ameliorating food quality and safety (Berlanga and Guerrero, 2016).
2.2. Impact on the food industry
The positive impacts or roles of biofilm formation are little-known as it has been stated, however, some roles are discussed in this review. Seixas et al. (2015), stated that “positive applications of biofilm depend on the flexibility and strength of biofilm, solubility in water, and biofilm permeability against water vapour”. It is worth knowing that biofilm could possibly protect food from lipid oxidation, gas, water and odour (Kim et al., 2012). Biofilms can be used for water and wastewater treatment. They enhance plant health and productivity of food products, they are seen to be an agent of water and wastewater treatment in food industries as well as help in reducing the amount of excess sludge in wastewater. The development of edible biofilms and for production of biodegradable food packaging is also one of its beneficial roles in the food industry.
3. Biofilm: Production of better yield of plant product
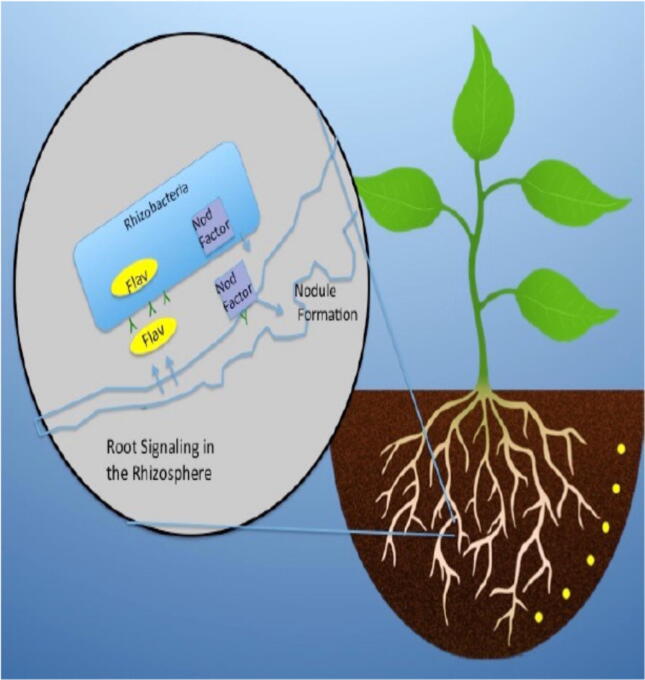
Rinaudi and Giordano, 2010 gave a data summary on formation of biofilm by rhizobacteria. Generally large number of beneficial soil bacteria species, such as rhizobia, produce microcolonies or biofilms when they attach to root cells. Chemotaxis is a very a paramount attribute during attachment as bacteria are usually drawn in the direction of nutrients, this could be from the metabolites as a result of the abiotic surface or conditioning film. Zhang et al. (2015) studied and substantially reported this as shown in Fig. 2 describing the rhizosphere of plants. In such an environment, so many organic compounds are generated by the roots of plants and these are called exudates which have been related with the enrollment and growth of rhizosphere microbiome which can shield the host plant from pathogens, supplying the plant often with nitrogen, phosphorous and other nutrients (Chaparro et al., 2013). Researchers have made available growing facts stating plants to be able to control the community of microbiome composition, their physiology, and their microbial gene expression through the root exudates (Chaparro et al., 2013, Zhang et al., 2015). Plants can also control biofilms and communities of microbes on leaves and some other surfaces. A famous example of biofilm control has actually been reported in some species of macroalgae (Egan et al., 2013). Some macroalgae like Delisea pulchra inhabit environments such as the marine habitat having possibly elevated biofouling pressure, still this plant is populated by a small number of bacteria. Researchers from countries like Australia and Denmark revealed that a vital cause for the absence of colonization by bacteria was due to the generation of some compounds of quorum-inhibiting furanone by D. pulchra (Givskov et al., 1996). D. pulchra is not distinctive in this circumstances because some quorum- and biofilm-inhibiting compounds have been reported in some invertebrates as well as some other marine algae (Egan et al., 2013). Nonetheless, one mostly uninvestigated area is the part of bacterial metabolism and signalling in the course of succession of microbes in biofilm development.
Fig. 2.
The Rhizosphere
Source: Zhang et al. (2015).
The rhizosphere is generally termed as the straight away adjoining to the roots of plant and typifies that environment in which plants can enroll and control the physiology and expression of gene by communities of beneficial biofilm producers, though conceptualization appear here, however contemporary studies of transcriptome by Zhang et al. (2015) reveal that rhizosphere interrelationship between the plant-growth-promoting bacterium, Bacillus amyloliquefaciens SQR9 and maize are somewhat large; and could constitute a model for furthering growth, development and functioning of biofilms which is of great benefit.
Biofilms, therefore, provide survival sites for bacteria that are beneficial like Rhizobium, by giving defenses as seen above and increasing the possibility of the survival of the bacteria and consequently develop in the environment of the plant after their usage in the food industry. It has been revealed that biofilms increase.
(i) fitness/wellness of the individual or single bacterium and.
(ii) apparently the health of the plant, efficiency and productivity due to the progressive selective edge of the specific bacterium.
Hence this can be advantageous to agriculture and as an extension to the food-producing industry, when you have a better yield of plant products like beans, peas, clover etc, you end up having a variety of food processing in food industries which stands as a great benefit to man.
4. Water and wastewater treatment by biofilms in a food industry
Food industries generally need a great deal of water as a constituent of what is being produced, water is also needed in large amounts for production processes in the food industries. Furthermore water is also used for cleaning of equipment, machines and raw materials (Bhagwat, 2019). Hence, it is expedient to adequately treat water for food processes, which is a prerequisite for food safety, as well as processed wastewater to avoid environmental pollution. So many investigations have centered on biofilms in water and wastewater systems (Asri et al., 2019, Mahapatra et al., 2015), however, the role of microbial diversity in biofilms produced in the food industry has not yet been effectively known. Although some operational plant biofilms are formed in environments that is expected to have known elevated microbial diversity as seen in a floor drain, however, other biofilms on the other hand are formed in environments possibly controlled only by one or a limited number of species of microorganisms as seen in a plate heat exchanger.
Generally, food industry biofilms could possibly have an excessive residue of food and mineral content that begin with process water and product. These components also protect the microorganisms that are being held within the biofilm. Hence, the implication of formation of biofilm in the food industry is its usage as a treatment agent.
Methods of treatment such as biological treatment have proven to be the foremost solution for the food industry and agriculture (Roy and Saha, 2021). Biological wastewater treatment systems makes the removal of organic wastewater, ammonium and nitrate contaminate possible (Roy and Saha, 2021). Engineers in food industries take hold of natural biofilm environmental task in advancing water-treatment procedures (Cunningham et al., 2010). It is worth noting that biofilms over a century have been successfully utilized in water and wastewater treatment. Treatment method known as sand filter treatment was first used in the 1860 s for both water and wastewater treatment and was developed by English engineers (Cunningham et al., 2010). Wastewater treatment normally takes place in stages, namely the primary treatment which is absolutely physical, and requires the disposition of objects that are floating followed by sedimentation, a procedure that disposes up to one-third of the BOD value (Cunningham et al., 2010). This is immediately followed by the secondary treatment which entail microbial oxidation, bringing about a considerable further depletion in BOD. Usually it could take one of two forms, the traditional trickling filter and the more recent activated sludge process both are aerobic processes. The first is frequently via a bulky bed of sand and gravel, this give rise to biofilm comprising bacteria, protozoans, fungi and algae (Sehar and Naz, 2016), with the outcome of the treated water having its BOD decreased by about 80–85 %. Activated sludge facilities furthermore attain greater degree in reduction of the BOD. In this stage the wastewater is oxygenated in tanks which have been previously planted with a diverse microbial sludge. The principal constituent of this is the bacterium Zoogloea, this organism usually secretes slime, thereby resulting into aggregates known as flocs, about which some other microorganisms like the protozoans attach to (Cunningham et al., 2010). However part of the organic content of the water is not straight away oxidised but it becomes absorb into the flocs. Eventually after some hours’ residence in the tank, the sludge is permitted to settle out, and the residual water that has been treated comes out of the system.
It should be noted that organic chemicals which could possibly be deleterious or pass on unpleasant tastes and odours could be eliminated by further filtration via an activated charcoal filter that adsorbs soluble chemicals (Cunningham et al., 2010). Filtration does not only physically eliminate numerous particles, those microbes on the filter materials growing in biofilms also do use carbon gotten from the water as it moves in a specified direction. This reduces the content of the organic carbon in water, giving rise to lower microbial growth in the pipes supplying the water. It is worth noting that the consequent of biological filtration is the change of organic carbon in the water into bacterial biomass. This biomass ideally is immobilized on the filter media and eliminated in the process of the backwash cycle (Cunningham et al., 2010).
General-purpose water that has been put through microbial activity in an ordered procedure in the plant used for treatment is usually more “biologically stable” and hence probably less expected to add to microbial increase during its usage.
4.1. Biofilm in wastewater treatment
The system of biofilm technology whereby solid media are included into growth suspended reactors in order to secure adhesive surfaces to give opportunity for biofilms attachment, so as to cause a rise in the concentration of microorganisms as well as the rates of degradation of contaminants by the by the biofilms (Sehar and Naz, 2016). This is made possible as a result of several removal mechanisms, such as bioaccumulation, biodegradation, biomineralization and biosorption that could be possibly undertaken by the community of the biofilm (Pal et al., 2010). Different nutrients, including nitrogen-containing compounds and phosphorus, carbonaceous materials as well as pathogens that are trapped from the wastewater are broken down by the communities of microbes in the biofilm (Sehar and Naz, 2016). Biofilter treated water could be used for agricultural and other recreational purposes or discharged to the environment once the pollutants are removed (Tripathi and Hussain, 2022). Advantages of wastewater treatment with biofilm systems includes excessive active biomass concentration, increased ability of recalcitrant degradation, increased biomass residence time, enhanced operational flexibility, ability to endure environmental changes, low space requirements, decrease in hydraulic retention time as well as reduced growth rate of microorganism, resulting in reduced sludge production (Sehar and Naz, 2016).
4.2. Excess sludge reduced by the biofilm process
The Moving Bed Biofilm Reactor (MBBR), a technological biological wastewater treatment indistinguishable to other types of biological process for organic carbon compounds degradation where microorganisms which are necessary usually grow on a carrier medium method as biofilm, which produces excess sludge (Barwal and Chaudhary 2014). Although it’s been observed that, there is significant reduction in the amount occurring during the process of biofilm as compared with the processes of the activated sludge of similar capacity (Sehar and Naz, 2016, Shahot et al., 2014). However, after treatment, within the MBBR, there is the need to separate the purified wastewater arising from the sludge through sedimentation. Peradventure during wastewater treatment, there is direct release of the wastewater into another treatment plant, the separating sludge stage could be omitted especially when the capacity and facility’s design gives room for subsequent separation, hence allowing the exclusion of unwanted process of sedimentation during transportation.
Though operators of water treatment have continuous challenge against biofilms, the quality of water could be improved by the communities of bacteria (Sehar and Naz, 2016). The use of biofilms is beneficial especially in sand filters. Bacteria usually feed on organic material as water trickles through the sand grains which subsequently is colonized by these bacteria that feed on the organic material in water forming colonies of biofilm. The biofilms are feed by constant stream of nutrient hence removing unwanted organic matter from the water (Wang et al., 2019). Ultimately water treated with biofilm usually forms fewer disinfection byproducts and less disinfectant.
5. Biofilms and food processing: Sources of fermented food
It has often been postulated that those microorganisms that can trigger niche-specific functions, are most especially involved in biofilm formation with respect to the environmental cues or food component existing in industrial settings and they can also be microbes that are most persistent in the food processing environments, by attaching to surfaces and equipment (Alvarez-Ordonez et al., 2019). Quite a lot of authors have evaluated formation of biofilm using food-related microorganisms and have affirmed that it is mostly influenced by the food constutients present in the medium or as a consequence of diverse environmental conditions which usually are predominant during food processing. Research has shown that several biofilm formation in bacteria could be modulated by simple carbohydrates such as glucose, discovered in Aeromonas hydrophila to control acyl-homoserine lactone quorum-sensing molecules (Jahid et al., 2013). Xue et al. (2014) showed that milk lactose increase biofilm formation in Staphylococcus aureus, mainly by bringing about the formation of polysaccharide intercellular adhesin protein and Bacillus subtilis, through activation of the LuxS-mediated quorum-sensing system (Duanis-Assaf et al., 2016). It is worth noting that ribose has been recognized as a quorum-sensing inhibitor in the autoinducer-2 producing microorganism Lactobacillus paraplantarum (Liu et al., 2017b). Food components have also been described to advance biofilm formation such as l-leucine in L. monocytogenes (Skovager et al., 2013) as well as butyric acid, expelled in the process of milk lipolysis, found in Bacillus spp. (Pasvolsky et al., 2014). Moreover, Bassi et al., 2017 pointed out that biofilm formation by Streptococcus thermophilus on stainless steel relies on the availability of milk proteins.
Several researches have examined the capability of diverse microorganisms to form biofilms when food extracts are available. It has been shown that Campylobacter jejuni and Campylobacter coli formed more biofilm on glass, stainless steel and polystyrene when the medium of growth was augmented with a chicken meat exudate, being a supplementary source of nutrients and covering as well as conditioning the abiotic surfaces (Brown et al., 2014). Akin results has been attained for Salmonella spp. and Campylobacter spp. using chicken juice and pork in the presence of glass surfaces and polystyrene (Li et al., 2017b) also in the case of Salmonella spp. when catfish mucus extract and several food-contact materials were used (Dhowlaghar et al., 2018). Several researchers such as Dimakopoulou-Papazoglou et al., 2016; Iliadis et al., 2018 have recently observed and modelled the way how disparate conditions in the environment prevalent during food processing effect the formation of biofilm, so as to gain useful clue for the prevention or control of biofilm. However, it is worth noting that few microorganisms have demonstrated high capabilities of biofilm formation even under adverse or hostile environmental conditions prevalent in some specific processing system. A notable example is in the case of Alicyclobacillus acidoterrestris where lowering medium pH has triggered biofilm formation, in such a manner that at pH values lower than 3.6 robust cell adhesion and convergent formation of biofilm was seen (Shemesh et al., 2014). It is expedient that food producers should be well informed on the fact that biofilms generated in such environments as food processing could actually shield cells individually from diverse processing and disinfectant agents.
Furthermore, research has established that few combinations have demonstrated increase in the formation of biofilm such as in the consortia of yeast and Lactic acid bacteria (LAB) (Furukawa et al., 2010). It has also been established that there were tremendious increase in the formation of biofilm with the consortium of Lactobacillus casei and Saccharomyces cerevisiae (Kawarai et al., 2007). Lactic acid bacteria and yeast usually co-exist in several traditional food fermentations across the world since they both live in anoxygenic environments and they require monosaccharides like glucose for growth (Furukawa et al., 2010; 2011; 2014). For instance, lactic acid bacteria and yeast have been isolated from Fukuyama pot vinegar, which is capable of increased biofilm formation in consortium, as well as isolates of Lactobacillus plantarum ML11- 11 and S. cerevisiae Y11, which are of a higher mixed-species biofilm-forming amalgamation (Furukawa et al., 2008). In East Asia including Japan fukuyama pot vinegar is one of the primeval types of rice vinegar which been produced without any artificial maneuver in the course of fermentation (Okazaki et al., 2010; Haruta et al., 2006).
Furukawa, 2015, observed that Lactobacillus. plantarum ML11-11 and Saccharomyces cerevisiae cells formed evident co-aggregates in a couple of minutes as they co-aggregated so well and co-aggregation is essential for biofilm formation of mixed species which was seen using using SEM and FISH, from the observation it was obvious that the underpart of biofilm was filled with Lactic acid bacteria biofilm. Hence, lactic acid bacteria cells adhere abiotic surface firstly and then secondly co-aggregates with yeast after which, thirdly, co-aggregates sediment on the affixed LAB cells, afterwards the LAB and yeast cells grow in the biofilm bringing about completed and fully grown biofilm mixed-species.
The association between an unnamed lectin-like protein on Lactobacillus plantarum ML11-11 cell and mannan on S. cerevisiae cell was expedient for the joint aggregation. Non-co– aggregative mutants were isolated by repeatedly culturing the non-co-aggregative cells (Furukawa et al., 2012) and the mannan build up expedient for the co-aggregation was made clear (Hirayama et al., 2012). It was observed that Lactobacillus plantarum ML11-11 produced more thickened biofilm mixed-species with the mutant strains of S. cerevisiae yeasts which are non-foaming than with its foaming parent strain, also Lactobacillus plantarum ML11-11 formed greater co-aggregation with mutant strains of yeasts that are non-foaming than with its own parent strain (Furukawa, 2015). The biofilm formed by these mixed species have been used in the production of ethanol (Abe et al., 2013; Morinaga and Furukawa 2013). It was observed that the biofilm mixed-species can withstand physical stress and are good producers of ethanol, especially when the pH is reduced as a result of the lactic acid produced, this significantly decrease the biofilm fermentation system from contamination (Abe et al., 2013; Morinaga and Furukawa 2013). This biofilm system would be subsequently used for the biomass fermentation process.
Fakhouri et al. (2004) stated the possibility of development of edible biofilm as substitute for preservation of food in order to lengthen the shelf-life of vegetables, fruits as well as improve the quality of food. Darmasiwi et al. (2018) carried out research on biofilm formation ability from guava seed via fermentation of the seed, it is worth noting that there is great potential in guava seed waste using the fermentation method. Solid-state fermentation was used to prepare the fermented guava seed which was wrapped with banana leaves at 37 °C for 72 h, this process was prefaced by isolation and screening of bacteria from the fermentation products, bacteria culture preparation for biofilm formation, and biofilm formation by broth cultures and glass slides methods. This glass slide edible biofilm formation was detected using light microscopy with 0.5 % crystal violet dye, on the other hand formation biofilm by the method of broth cultures was spotted by transmission electron microscopy (TEM) by the use of phosphotungstic acid 2 % dye. From a fermented guava seed waste product three strains of lactic acid bacteria (LAB) were isolated. The strains all gave the potential for biofilm formation in varied stages by the method of broth cultures except one. Thus, this demonstrated the ability of guava seed fermentation to generate edible biofilm.
Another source of edible biofilm is Kimchi a recognized Korean fermented vegetable food. The taste and health benefits of this fermented food has increased its marketing worldwide. This fermented products in addition have been noted by few researchers as being a major source of lactic acid bacteria, including the species of Lactococcus sp., Lactobacillus sp., Leuconostoc sp. and Weissella sp. (Jang et al. 2015).
Strains of LAB, such as Weissela viridescens 113 bac-, L. lactis 69 bac+, L. lactis 94 bac+, L. casei 40 bac-, L. helveticus 352 bac-, L. casei Y1, L. plantarum KF and L. brevis BBE-Y52 have also been reported of having ability for biofilm production with the anti-bacterial pathogen (Gómez et al., 2016, Kumar et al., 2017, Fang et al., 2018). Sapalina et al., 2020 found out that kimchi, a Korea fermented food with good health benefits is a good source of LAB. They noted the production of high biofilms from isolates KA2, KA5, KB1, and KC4 from kimchi at 48 h and subsequently all were identified as Lactobacillus brevis.
5.1. Development of edible biofilms and food packaging
Aggregation of microorganisms releases substances like lipids, proteins, polysaccharides or combinations from the various substances comprising around 85 % volume, this substances are extra polymeric substances (EPS) matrix, they produce layers that are flexible over the surfaces. Furthermore, the constitution of the various components corresponds with the age of biofilm formation, the type of species of microorganism and the environmental factors (Vu et al., 2009). Capitani et al. (2016) started that diverse polymers are currently being used for edible films synthesis such as lipids, polysaccharides and proteins or a combination of any two of the polymers.
Lactic acid bacteria (LAB) produces edible biofilms which can be consumed by humans and animals (Sapalina et al., 2020). It could therefore be employed in the reduction of pathogenic microorganisms in such places as the food and health industry. Biofilms can also be formed by lactic acid bacteria (LAB) on diverse surfaces commonly used in food industries (Gomez et al., 2016). This type of biofilm formed by LAB are known to be edible since the source is safe for consumption by human. Hence some microbes form edible biofilms that are hidden by a matrix. Guerrieri et al. (2009), stated that in the area of food packaging LAB biofilms could evolve as novel technologies. The systems involved in packaging of the food is such that LAB producing biofilms is being added to food. These days, bacterial biofilms are generally explored to generate environmentally friendly packaging due to various deliberations. According to Valdes et al., 2017, these biofilms were structured coatings to regenerated mechanical vigour, water vapour, oxidative stability and packaged food through increasing its antioxidant and antimicrobial capabilities.
Hence, biofilms have been approved for the storage of beverages and foods. This is due to the fact that biofilms reduce the pollution effects from commercial products composed primarily of polymers which are non-biodegradable (Malathi et al., 2014). Another benefit of biofilms is that they are able to direct moisture gases and migration of lipids, it is also beneficial during the process of the addition of nutrients and additives.
Researchers have looked at various means of formulating edible biofilms, such as Dianin et al., 2019, their study was structured to generate and characterize biofilms that are edible from whey protein isolate (WPI). They prepared two compositions: the first being without the probiotic Lactobacillus casei while the second was with the probiotic. It was observed that the addition of the probiotic culture did not affect water vapour permeation (0.28–0.35 g.mm/m2.day.kPa) as well as the density (1.272–1.303 g/cm3) of the films. Although, the probiotic containing film culture had more solubility (42.8 vs 34.8 %), was more resistance (higher tensile strength, 23.3 vs 12.6 N), thicker (16.18 vs 13.15 µm), lower flexibility (lower elongation at break, 5.27 vs 45.4 %) and yellowish. The probiotic biofilm showed images of agglomerates in all superficial extensions as well as a higher number of orifices on Scanning Electron Microscopy. The Lactobacillus casei stayed viable (5.70 to 7.77 log cfu/g) for the entire period of films storage (25 °C/28 days), although, obvious counts (greater than6 log cfu/g) were recorded to the 14th day. They concluded that the use of WPI originated films with satisfactory characteristics could extend grapes shelf life (Dianin et al., 2019).
6. Conclusion
There are so many advantages that formation of biofilms exerts on microbial cells from being destroyed especially in the environment of the food industry, such as physical, chemical and mechanical resistance, even to resistance of antimicrobials and the disinfectants that are being used in the food industry.
Emphasis needs to be placed on biofilms produced by microorganisms that are absolutely beneficial, causing no harm, particularly of great importance in the food industry. Generally, it is likely that what the food industry gains from microorganisms that form biofilms is usually their fermentative, biochemical, biotechnological and antimicrobial characteristics. Such microbes in the biofilms matrix most likely can affect positively the characteristics quality of food products like biochemical composition, texture and sensorial properties through the produce of certain secondary metabolites.
Biofilm scientists and engineers observe a startling characteristic of bacteria in a biofilm; bacteria that are very much alike show great dissimilarity in biofilm form than when isolated as single cells floating in water (planktonic form). Hence it is paramount to have a good understanding and knowledge of the genetic activity of biofilm forming bacteria, this will help in current and future researches in the development of new methods in improving the beneficial roles of these biofilms in the food industry.
However, in the future their might be the challenge of refining this technology and developing very good protocols for supporting the persistence and growth of desirable and beneficial biofilms as against undesirable biofilms in the food industry.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
A.A. Olanbiwoninu, Email: aa.olanbiwoninu@acu.edu.ng.
B.M. Popoola, Email: bm.popoola@acu.edu.ng.
References
- Aggarwal S., Stewart P., Hozalski R. Biofilm cohesive strength as a basis for biofilm recalcitrance: are bacterial biofilms overdesigned? Microbiol. Insights. 2016;8(2):29–32. doi: 10.4137/MBI.S31444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhede, M., Kragh, K.N., Qvortrup, K., Allesen-Holm, M., van Gennip, M., Christensen, L.D., Jensen, P.Ø., Nielsen, A.K., Parsek, M. and Wozniak, D. 2011. Phenotypes of non-attached Pseudomonas aeruginosa aggregates resemble surface-attached biofilm. PloS One 2011; 6:e27943; PMID:22132176; https://doi.org/10.1371/journal.pone.0027943 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]. [DOI] [PMC free article] [PubMed]
- An D., Parsek M.R. The promise and peril of transcriptional profiling in biofilm communities. Curr. Opin. Microbiol. June 2007;10(3):292–296. doi: 10.1016/j.mib.2007.05.011. [DOI] [PubMed] [Google Scholar]
- Asri M., Elabed S., Ibnsouda Koraichi S., El Ghachtouli N. In: Handbook of Environmental Materials Management. Hussain C., editor. Springer; Cham: 2019. Biofilm-Based Systems for Industrial Wastewater Treatment. [DOI] [Google Scholar]
- Barwal A., Chaudhary R. To study the performance of biocarriers in moving bed biofilm reactor (MBBR) technology and kinetics of biofilm for retrofitting the existing aerobic treatment systems: a review. Rev. Environ. Sci. Biotechnol. 2014;13:285–299. doi: 10.1007/s11157-014-9333-7. [DOI] [Google Scholar]
- Bhagwat, V.R. 2019. Food Safety and Human Health. ScienceDirect: 219-247. Doi. 10.1016/B978-0-12-816333-7.00009-6.
- Bryers J.D. Biologically active surfaces: processes governing the formation and persistence of biofilms. Biotechnol. Prog. 1987;3(2):57–68. [Google Scholar]
- Capitani M.I., Matus-Basto A., Ruiz-Ruiz J.C., Santiago-Garcia J.L., Betancur-Ancona D.A., Nolasco S.M., Tomas M.C., Segura-Campos M.R. Characterization of biodegradable films based on Salvia hispanica L. Protein and Mucilage. Food Bioprocess Technol. 2016;1:1. [Google Scholar]
- Carpentier B., Cerf O. Biofilm and their consequences with particular reference to hygiene in the food industry. J. Appl. Bacteriol. 1993;75:499–511. doi: 10.1111/j.1365-2672.1993.tb01587.x. [DOI] [PubMed] [Google Scholar]
- Chaparro J.M., Badri D.V., Bakker M.G. Root exudation of phytochemicals in Arabidopsis follows specific patterns that are developmentally programmed and correlate with soil microbial functions. PLoS One. 2013;8:e55731. doi: 10.1371/journal.pone.0055731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christison C.A., Lindsay D., von Holy A. Cleaning and handling implements as potential reservoirs for bacterial contamination of some ready-to-eat foods in retail delicatessen environments. J. Food Prot. 2007;70:2878–2883. doi: 10.4315/0362-028x-70.12.2878. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Irvin R.T. The bacterial glycocalyx in nature and disease. Ann Rev Microbiol. 1981;83:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Leweandowski Z., Caldwell D.E., Korber D.R., Lappin-Scott H.M. Microbial biofilms. Annual Reviews of. Microbiology. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Costerton J.W., Stewart P.S., Greenberg E.P. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- Cunningham, A. B., Lennox, J. E. and Ross, R. J. 2010. The Hypertext: A Unique Teaching and Learning Resource Based on Web Technologies, Version 4.2, Eds. 2001-2010. www.cs.montana.edu/webworks/projects/steresbook/index.html.
- Alfred B. Cunningham, John E. Lennox, and Rockford J. Ross, Eds. 2001-2010.
- Darmasiwi, S., Herawati, O. and Retnaningrum, E. 2018. Edible biofilm formation from guava seed waste fermentation. Physical Science and Engineering. 1: 39-43. https://doi.org/10.29037/digitalpress.11244.
- Dass, S. C. and Wang, R. 2022. Biofilm through the Looking Glass: A Microbial Food Safety Perspective. Pathogens. 11(3): 346. Doi: 0.3390/pthogens11030346. [DOI] [PMC free article] [PubMed]
- Dianin I., Oliveira J.A., Pimentel T., Hernandes N., Costa G. Edible biofilms formulated with whey proteins isolate and Lactobacillus casei probiotic culture: characterization and application in tomatoes or grapes. Chem. Eng. Trans. 2019;75:469–474. doi: 10.3303/CET1975079. [DOI] [Google Scholar]
- Donlan R.M. Biofilms: microbial life on surfaces. Emerg. Infect. Dis. [serial online] 2002;8(9):881–890. doi: 10.3201/eid0809.020063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan S., Harder T., Burke C. The seaweed holobiont: understanding seaweed-bacteria interactions. FEMS Microbiol. Rev. 2013;37:462–476. doi: 10.1111/1574-6976.12011. [DOI] [PubMed] [Google Scholar]
- Fakhouri F.M., Palmu P.S.T., Grosso C.R.F. Characterization of composite biofilms of wheat gluten and cellulose acetate phthalate. Braz. J. Chem. Eng. 2004;21(02):261–264. [Google Scholar]
- Fang F., Xu J., Li Q., Xia X., Du G. Characterization of a Lactobacillus brevis strain with potential oral probiotic properties. BMC Microbiol. 2018;18(221):1–9. doi: 10.1186/s12866-018-1369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fratamico P.M., Bhunia A.K., Smith J.L. Caister Academic Press; Norwich, England: 2005. Foodborne Pathogens: Microbiology and Molecular Biology; pp. 409–419. [Google Scholar] [Google Scholar]
- Fuqua W.C., Winans S.C., Greenberg E.P. Quorum sensing in bacteria: the LuxR-LuxI family of cell density-responsive transcriptional regulators. J. Bacteriol. 1994;176:269–275. doi: 10.1128/jb.176.2.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galié S., García-Gutiérrez C., Miguélez E.M., Villar C.J., Lombó F. Biofilms in the food industry: health aspects and control methods. Front. Microbiol. 2018;9:898. doi: 10.3389/fmicb.2018.00898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genigeorgis C. In: New Challenges in Meat Hygiene: Specific Problems in Cleaning and Disinfection. Burt S.A., Bauer F., editors. Ecceamst; European Consortium for Continuing Education in Advanced Meat Science and Technology: 1995. Biofilm: Their significance to cleaning in the meat sector; pp. 29–47. [Google Scholar]
- Givskov M., de Nys R., Manefield M. Eukaryotic interference with homoserine lactone-mediated prokaryotic signalling. J. Bacteriol. 1996;178:6618–6622. doi: 10.1128/jb.178.22.6618-6622.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez N.C., Ramiro J.M.P., Quecan B.X.V., de Melo Franco B.D.G. Use of potential probiotic lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes, Salmonella Typhimurium, and Escherichia coli O157:H7 biofilms formation. Front. Microbiol. 2016;7(863):1–15. doi: 10.3389/fmicb.2016.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri E., Niederhäusern S., Messi P., Sabia C., Iseppi R., Anacarso I., Bondi M. Use of lactic acid bacteria (LAB) biofilms for the control of Listeria monocytogenes in a small-scale model. Food Control. 2009;20:861–865. [Google Scholar]
- Irene Guzmán-Soto, Christopher McTiernan, Mayte Gonzalez-Gomez, Alex Ross, Keshav Gupta, Erik J. Suuronen, Thien-Fah Mah, May Griffith, and Emilio I. Alarcon. 2021. Mimiking biofilm formation and development: Recent progress in in vitro and in vivo biofilm models. iScience. 24(5): 102443. Doi. 10.1016/j.isci.2021.102443. [DOI] [PMC free article] [PubMed]
- Haaber, J., Cohn, M.T., Frees, D., Andersen, T.J. and Ingmer, H. 2012. Planktonic aggregates of Staphylococcus aureus protect against common antibiotics. PloS One 2012; 7:e41075; PMID:22815921; https://doi.org/10.1371/journal.pone.0041075 [Crossref], [PubMed], [Web of Science ®], [Google Scholar]. [DOI] [PMC free article] [PubMed]
- Hall-Stoodley, Luanne; Costerton, J. William; Stoodley, Paul (February 2004). “Bacterial biofilms: from the natural environment to infectious diseases”. Nature Reviews. Microbiology. 2 (2): 95–108. [DOI] [PubMed]
- Hoffman L.R., D'Argenio D.A., MacCoss M.J., Zhang Z., Jones R.A., Miller S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature. August 2005;436(7054):1171–1175. doi: 10.1038/nature03912. [DOI] [PubMed] [Google Scholar]
- Huber B., Eberl L., Feucht W., Polster J. Influence of polyphenols on bacterial biofilm formation and quorum-sensing. Zeitschrift fur Naturforschung C. J. Biosci. 2003;58:879–884. doi: 10.1515/znc-2003-11-1224. [DOI] [PubMed] [Google Scholar]
- Jang J.Y., Lee M.E., Lee H.W., Lee J.H., Park H.W., Choi H.J., Kim T.W. Extending the shelf life of kimchi with Lactococcus lactis strain as a starter culture. Food Sci. Biotechnol. 2015;24(3):1049–1053. [Google Scholar]
- Karatan E., Watnick P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. June 2009;73(2):310–347. doi: 10.1128/MMBR.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Jung C.-K., Kim D.-H., Kim S.-B. Properties of edible biofilm manufactured from yellowfin tuna (Thunnus albacares) skin gelatin. Korean J. Chem. Eng. 2012;29(6):786–791. [Google Scholar]
- Kjelleberg, S., Marshall, K.C. and Givskov, M. 2007. The biofilm mode of life. In: Kjelleberg S, Givskov M (ed.). The Biofilm Mode of Life: Mechanisms and Adaptations. Horizon Bioscience: Norfolk, UK. 2007. pp. 5–21.
- Kumar L.M., Saad W.Z., Mohammad R., Rahim R.A. Influence of biofilm-forming lactic acid bacteria against methicillin-resistant Staphylococcus aureus (MRSA S547) Asian Pac. J. Trop. Biomed. 2017;7(12):1107–1115. [Google Scholar]
- López D., Vlamakis H., Kolter R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010;2(7) doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahapatra A., Padhi N., Mahapatra D., Bhatt M., Sahoo D., Jena S., Dash D., Chayani N. Study of biofilm in bacteria from water pipelines. J. Clin. Diagn. Res. 2015;9(3):9–11. doi: 10.7860/JCDR/2015/12415.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malathi A.N., Santhosh K.S., Nidoni U. Recent trends of biodegradable polymer: biodegradable films for food packaging and application of nanotechnology in biodegradable food packaging. Curr. Trends Technol. Sci. 2014;3(2):1733–1738. [Google Scholar]
- Maria-Guadalupe, A., Maricarmen, I., Oscar-Alberto, S., Jean-Pierre, G., Pedro-Javier, G. and Melesio, G. 2018. Biofilm formation by Staphylococcus aureus isolated from food contact surfaces in the dairy industry of Jalisco, Mexico. Journal of food Quality.http://doi.org/10.1155/2018/1746139
- Marshall K.C. Biofilms: an overview of bacterial adhesion, activity, and control of surfaces. ASM News. 1992;58:202–207. [Google Scholar]
- Mittelman, M.W. 1998. Structure and functional characteristics of bacterial biofilms in fluid processing operations. 81:2760-4 [DOI] [PubMed]
- Mittelman M.W. Structure and functional characteristics of bacterial biofilms in fluid processing operations. J. Dairy Sci. 1998;81:2760–2764. doi: 10.3168/jds.S0022-0302(98)75833-3. [DOI] [PubMed] [Google Scholar]
- O'Toole, G. A.; Kolter, R. (October 1998)b. “Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development”. Molecular Microbiology. 30 (2): 295–304. [DOI] [PubMed]
- Pal S., Sarkar U., Dasgupta D. Dynamic simulation of secondary treatment processes using trickling filters in a sewage treatment works in Howrah, West Bengal, India. Desalination. 2010;253(1):135–140. [Google Scholar]
- “Building Codes for Bacterial Cities | Quanta Magazine”. Quanta Magazine. Retrieved 2017-07-25.
- Ranita R., Monalisa T., Gianfranco D., Vishvanath T. Strategies for combating bacterial biofilms: a focus on anti-biofilm agents and their mechanisms of action. Virulence. 2018;9(1):522–554. doi: 10.1080/21505594.2017.1313372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudi L.V. and Giordano W. 2010. An integrated view of biofilm formation in rhizobia. FEMS Microbiol. Lett. 304:1–11. [PubMed] [Google Scholar]. [DOI] [PubMed]
- Roy M., Saha R. 6 - Dyes and their removal technologies from wastewater: a critical review. Intell. Environ. Data Monit. Pollut. Manage. 2021:Pp.127-160. doi: 10.1016/B978-0-12-819671-7.00006-3. [DOI] [Google Scholar]
- Sandu C., Singh R.K. Energy increase in operation and cleaning due to heat-exchanger fouling in milk pasteurization. Food Technol. 1991;45(12):84–91. [Google Scholar]
- Sapalina F., Retnaningrum E. Molecular characterization of lactic acid bacteria producing edible biofilm isolated from kimchi. Biodiversitas. 2020;21:962–968. doi: 10.130557/biodio/d210315. [DOI] [Google Scholar]
- Sehar, S. and Naz, I. 2016. Role of the Biofilms in Wastewater Treatment. In D. Dhanasekaran, & N. Thajuddin (Eds.), Microbial Biofilms - Importance and Applications. IntechOpen. https://doi.org/10.5772/63499.
- Shama Sehar and Iffat Naz (July 13th 2016). Role of the Biofilms in Wastewater Treatment, Microbial Biofilms - Importance and Applications, Dharumadurai Dhanasekaran and Nooruddin Thajuddin, IntechOpen, DOI: 10.5772/63499. Available from: https://www.intechopen.com/books/microbial-biofilms-importance-and-applications/role-of-the-biofilms-in-wastewater-treatment.
- Seixas R., Varanda D., Bexiga R., Tavares L., Oliveira M. Biofilm-formation by Staphylococcus aureus and Staphylococcus epidermidis isolates from subclinical mastitis in conditions mimicking the udder environment. Pol. J. Vet. Sci. 2015;18(4):787–792. doi: 10.1515/pjvs-2015-0102. [DOI] [PubMed] [Google Scholar]
- Shahot A., Idris A., Omar R., Yusoff H.M. Review on biofilm processes for wastewater treatment. Life Sci. J. 2014;11:1–13. doi: 10.7537/marslsj111114.01. [DOI] [Google Scholar]
- Srey S., Jahid I.K., Ha S.D. Biofilm formation in food industries: a food safety concern. Food Control. 2013;31:572–585. [Google Scholar]
- Sutherland I.W. Microbial exopolysaccharides- their role in microbial adhesion in aqueous systems. Crit. Rev. Microbiol. 1983;10(2) doi: 10.3109/10408418209113562. [DOI] [PubMed] [Google Scholar]
- Tripathi, S. and Hussain, T. 2022. Biofiltration treatment of wastewater through microbial ecology-An Innovative Role of Biofiltration in Wastewater Treatment Plants (WWTPs). pp. 19-44. https://doi.org/10.1016/B978-0-12-823946-9.00005.
- Valdes A., Ramos M., Beltran A., Jimenez A., Garrigos M.C. State of the art of antimicrobial edible coatings for food packaging applications. Coatings. 2017;7(56):1–23. [Google Scholar]
- Van Houdt, R. and Michiels, 2010. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 109: 1117-1131. doi: 10.1111/j.1365-2672.2010.04756.x. [DOI] [PubMed]
- Vidyasagar, A. 2016. What are biofilms? https//www.livescience.com.
- Vu B., Chen M., Crawford R.J., Ivanova E.P. Bacterial extracellular polysaccharides involved in biofilm. Molecules. 2009;14:2535–2554. doi: 10.3390/molecules14072535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Parajuli, S., Sivalingam, V. and Bakke, R. 2019. Biofilm in Moving Bed Biofilm Process for Wastewater Treatment. In S. Dincer, M. S. Özdenefe, & A. Arkut (Eds.), Bacterial Biofilms. IntechOpen. https://doi.org/10.5772/intechopen.88520.
- Watnick P., Kolter R. Biofilm, city of microbes. J. Bacteriol. May 2000;182(10):2675–2679. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb, J.S. 2007. Differentiation and dispersal in biofilms. In: Kjelleberg S, Givskov M (eds.). The Biofilm Mode of Life: Mechanisms and Adaptations. Horizon Bioscience: Norfolk, UK. 7: pp. 167–174.
- Wimpenny J.W.T., Kinniment S.L., Scourfield M.A. In: Microbial Biofilms: Formation and Control. Denyer S.P., Gorman S.P., Sussman M., editors. Blackwell Scientific; London: 1993. The physiology and biochemistry of biofilm; pp. 51–94. [Google Scholar]
- Yaun L., Wang H., Liu W., Sadiq F.A., Zhao Y. Editorial: multi-species biofilms in the food industry. Font. Microbiol. 2022;13:1023428. doi: 10.3389/fmicb.2022.1023428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Yang D., Wang D. Whole transcriptomic analysis of the plant-beneficial rhizobacterium Bacillus amyloliquefaciens SQR9 during enhanced biofilm formation regulated by maize root exudates. BMC Genomics. 2015;16:685. doi: 10.1186/s12864-015-1825-5. [DOI] [PMC free article] [PubMed] [Google Scholar]