Abstract
A novel member of the transforming growth factor β (TGF-β) family has been identified in the filarial nematode parasite Brugia malayi by searching the recently developed Expressed Sequence Tag (EST) database produced by the Filarial Genome Project. Designated tgh-2, this new gene shows most similarity to a key product regulating dauer larva formation in Caenorhabditis elegans (DAF-7) and to the human down-modulatory cytokine TGF-β. Homology to DAF-7 extends throughout the length of the 349-amino-acid (aa) protein, which is divided into an N-terminal 237 aa, including a putative signal sequence, a 4-aa basic cleavage site, and a 108-aa C-terminal active domain. Similarity to human TGF-β is restricted to the C-terminal domain, over which there is a 32% identity between TGH-2 and TGF-β1, including every cysteine residue. Expression of tgh-2 mRNA has been measured over the filarial life cycle. It is maximal in the microfilarial stage, with lower levels of activity around the time of molting within the mammal, but continues to be expressed by mature adult male and female parasites. Expression in both the microfilaria, which is in a state of arrested development, and the adult, which is terminally differentiated, indicates that tgh-2 may play a role other than purely developmental. This is consistent with our observation that TGH-2 is secreted by adult worms in vitro. Recombinant TGH-2 expressed in baculovirus shows a low level of binding to TGF-β-receptor bearing mink lung epithelial cells (MELCs), which is partially inhibited (16 to 39%) with human TGF-β, and activates plasminogen activator inhibitor-1 transcription in MELCs, a marker for TGF-β-mediated transduction. Further tests will be required to establish whether the major role of B. malayi TGH-2 (Bm-TGH-2) is to modulate the host immune response via the TGF-β pathway.
Transforming growth factor β (TGF-β) is a stable, multifunctional extracellular growth factor with an extremely wide range of biological activities in metazoan animals. In vertebrates, nearly all cells have surface receptors for, and are stimulated or inhibited by, TGF-β. The nature and polarity of the response depends on the cell lineage, its state of differentiation and proliferation, and its environment, particularly with respect to the presence of other growth factors (49). TGF-β plays a crucial role in the coordination of morphogenesis and remodeling of mesenchymal tissues during embryological development. In Drosophila melanogaster, the decapentaplegic (dpp) gene is involved in the specification of the dorsal-ventral axis (16), in gut morphogenesis, and in wing development and patterning (27, 37). In Xenopus embryos, dpp homologs ventralize cells, while more distantly related activin proteins induce mesoderm (53). In vertebrates, TGF-β-related molecules have been found that control sexual development (Müllerian inhibiting substance [11]), pituitary hormone production (inhibins α and β [30, 55]), skeletal muscle growth (myostatins [34]), and the creation of bone and cartilage (bone morphogenetic proteins [BMPs] [44]).
TGF-β is a particularly important modulator of the growth, differentiation, and activities of cells of the immune system (28), and multiple members of the superfamily are now associated with immune inhibition (10). The most commonly reported effects of TGF-β on leukocytes are inhibitory, suppressing lymphocyte proliferation, although in certain contexts TGF-β exerts stimulatory effects, as in isotype switching in B lymphocytes (50). In parasitic infections, TGF-β has emerged as one of the key cytokines (48), together with interleukin 4 (IL-4) and IL-10, which down-regulate cellular response and compromise immunity to a spectrum of intracellular infections, including those caused by Leishmania species (7, 8, 29, 46, 59), Mycobacterium tuberculosis (23, 24), Plasmodium chabaudi (54), Toxoplasma gondii (25), and Trypanosoma cruzi (17). Similar findings have been reported for infections with extracellular helminths, such as Schistosoma mansoni (39, 57). In keeping with the pleiotropic functions of TGF-β, there are also reported examples of a protective role for this cytokine against some pathogens (36, 38), and indeed it is highly likely to reduce the severity of immunopathogenic reactions (56). It is intriguing to consider the possible role of TGF-β in long-lived chronic infections, such as filariasis, caused by nematodes of the genera Brugia and Wuchereria, in which immune down-regulation is a prominent feature (4, 31). As we discuss below, the production of TGF-β-family cytokines by filariae holds out the possibility that these molecules, as well as related host effectors, may be directly involved in the immunosuppression which characterizes some filarial infections.
Four TGF-β family members are encoded in the genome of the free-living nematode Caenorhabditis elegans (41, 47). One homolog, DAF-7, controls entry to and exit from developmental arrest represented by the dauer larva and acts via a well-characterized TGF-β-like signaling pathway (15, 18, 41, 45). Expression of DAF-7 is highest in L1 larvae committed to non-dauer development, is low in L2 larvae, and is almost undetectable in L3 and pheromone-induced L2d larvae. Another homolog, UNC-129, acts as a guide for axon growth (12), while DBL-1 (also named CET-1) affects body size in hermaphrodite and male worms as well as tail formation in males alone (35, 51). The precise function of the fourth gene, designated tig-2, remains to be determined (51). The Brugia malayi life cycle contains a series of developmental steps and arrest points which may be governed by TGF-β homologs, and we postulated that the parasitic mode of life may select variants able to mimic the host cytokine TGF-β. We have previously described a member of the TGF-β family from B. malayi, designated transforming growth factor homolog-1 (tgh-1), which is expressed during parasite growth and development within the mammal and which bears closest similarity with the dpp/DBL-1 family of signaling molecules (19). We now report on a second gene, tgh-2, which more closely resembles C. elegans daf-7 and human TGF-β and which is expressed at high levels in stages which are either in a state of arrested development (microfilariae) or have completed their developmental program (adult worms).
MATERIALS AND METHODS
Parasites.
Male adult jirds (Meriones unguiculatus) infected intraperitoneally with B. malayi organisms were purchased from TRS Labs (Athens, Ga.) and used as a source of adult parasites and microfilariae. Vector stage parasites (infective third-stage larvae) were obtained from Aedes agypti mosquitoes infected with B. malayi parasites via membrane feeding with infective blood containing 16,000 microfilariae/ml.
Isolation of the tgh-2 cDNA.
A B. malayi expressed sequence tag (EST), MBAFCE6E01, was found to bear homology to the 3′ end of C. elegans daf-7. The full-length protein sequence of DAF-7 was then used to search the database of approximately 11,000 ESTs from B. malayi deposited in the EST database (dbEST) in February 1997 by the Filarial Genome Project (58). Two additional ESTs were identified with TGF-β-like sequences, one which represented the 3′ terminus of a novel gene and one (SWAFCA78) which corresponded to a partially truncated N-terminal sequence. Similarity searching of the same data set with SWAFCA78 identified three further ESTs, two of which appeared to code for the full-length transcript. These ESTs were obtained from microfilarial, adult female, and adult male conventionally constructed cDNA libraries in the laboratories of M. Blaxter and R. Ramzy (University of Cairo, Cairo, Egypt) and S. Williams (Smith College, Northampton, Mass.). On the supposition, later verified, that the two termini represented a single gene, primers were made corresponding to the 5′ and 3′ coding termini as follows: 5′-ATGACGTTCATTGCGGTGTCG-3′ (sense) and 5′-AGCACAGGCACACCGCCG-3′ (antisense). These primers were used to amplify full-length cDNA from a B. malayi microfilarial library constructed in the laboratory of S. Williams and provided by the Filarial Genome Project. The PCR was cycled between 94, 55, and 72°C (1 min each) for 35 rounds, followed by 1 round of 72°C for 10 min. The reaction product was purified and cloned into the pMOSblue T-vector (Amersham). Two clones were picked and sequenced in both directions, with identical results, using ABI PRISM Dye Terminator cycle sequencing kits (Perkin-Elmer).
RNA extraction and reverse transcription-PCR (RT-PCR).
Mosquitoes were collected at various time points after feeding with infected blood. Total RNA was extracted from individual mosquitoes using either TRIZOLV or RNAZOL (Biotex Inc.) according to the manufacturer's instructions. RNA from adult worms was extracted as described previously (19). First-strand cDNA was synthesized from mosquitoes by using the GeneAmp RNA PCR Kit (Perkin-Elmer) using oligo(dT) as the primer. Infected mosquitoes were detected by amplifying each first-strand cDNA with primers specific for cystatin gene, cpi-2, and positive samples were pooled (W. F. Gregory and R. M. Maizels, submitted for publication). First-strand cDNA from adult worms of the closely related species Brugia pahangi was synthesized by Emma Lewis (Veterinary Parasitology, University of Glasgow) as previously described (19).
To measure the levels of expression of tgh-2 in the mosquito stages, PCR was performed with gene-specific primers for tgh-2 and β-tubulin by using 5 μl of pooled cDNA. In both cases, the presence of introns ensured that contaminating DNA could not yield false positive results. The PCR was cycled between 94, 55, and 72°C (1 min each) for 35 rounds, followed by 1 round of 72°C for 10 min. The levels of expression of tgh-2 in the mammalian stages were measured as previously described (19). The oligonucleotides used were as follows: for tgh-2, 5′-GGTCGCCGCAAACGTAGCTAT-3′ (sense) and 5′-AGCACAGGCACACCGCCG-3′ (antisense); and for β-tubulin, 5′-AATATGTGCCACGAGCAGTC-3′ (sense) and 5′-GCCATACTCCTCACGAATTT-3′ (antisense).
Expression of recombinant tgh-2 and production of antisera.
Primers were designed to amplify the entire coding region of tgh-2 minus the putative signal peptide from a B. malayi microfilarial cDNA library. The 5′ sequence was taken from ESTs SWAFCA54 and SWMFCA851 and the 3′ untranslated region (UTR) from MBAFCE6E01. The fragment was digested with NdeI-BamHI and cloned into the pET-15b vector (Novagen), which produces proteins containing an N-terminal six-histidine tag. The oligonucleotides used were 5′-GGCAGCCATATGCCATCGACACACGGAACCACC-3′ (sense) and 5′-CGCGGATCCTTAAGCACAGGCACACCGCCG-3′ (antisense). Clones were fully sequenced on both strands. The constructs were transformed into BL21 (DE3) cells, and protein production was induced by 1 mM IPTG (isopropyl-β-d-thiogalactopyranoside) for 3 h at 37°C. Recombinant TGH-2 was obtained by purifying the fusion by following the manufacturer's protocol. Cells were sonicated in the presence of 6 M urea. After removal of insoluble material by centrifugation at 12,000 × g for 15 min, the expressed protein was bound to HIS-Bind resin (Novagen). Bound proteins were eluted in the presence of 6 M urea, which was removed by overnight dialysis in phosphate-buffered saline in the presence of 100 mM EDTA to avoid precipitation.
Antisera were produced by subcutaneous immunization of BALB/c and CBA mice with 20 μg of purified recombinant protein in complete Freund's adjuvant, followed 1 month later by a booster with 10 μg of protein in incomplete Freund's adjuvant. A second boosting was carried out a week later, and sera were collected 1 week after the last boosting.
Western blotting.
Approximately 50 mixed adult worms of B. malayi were recovered from jirds and cultured overnight at 37°C in medium supplemented with 25 mM HEPES, 1% glucose, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. The culture supernatant containing the excretory-secretory (ES) products was centrifuged at 2,000 × g for 20 min to remove any microfilariae present in the culture. The supernatant was then precipitated overnight at −20°C by adding an equal volume of cold acetone. The following day, the proteins were centrifuged, washed once with cold 50% acetone, and resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.4 M Tris-HCl [pH 6.3], 2.3% SDS, 10% glycerol, and 5% 2-mercaptoethanol). For immunoblot analysis, SDS-PAGE-separated proteins were electrophoretically transferred to a nitrocellulose membrane in 39 mM glycine, 48 mM Tris, 0.375% (wt/vol) SDS, and 20% methanol and then were probed with a 1/200 dilution of anti-TGH-2 serum or normal mouse serum, followed by an incubation with a horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G diluted 1/2,000. Bound anti-TGH-2 antibodies were visualized using the ECL detection method (Amersham), followed by autoradiography.
Expression of tgh-2 in Sf21 cells.
Sf21 cells (kindly provided by A. Alcami, Cambridge University) were cultured at 28°C in TC100 medium (Gibco) containing 10% (vol/vol) fetal calf serum. The full-length tgh-2 cDNA was cloned into pBAC-1 (Novagen), which contains a C-terminal six-histidine tag. Two clones were fully sequenced on both strands to confirm fidelity. SF21 cells were cotransfected with tgh-2 and BacPAK6 viral DNA (Clontech) using Lipofectin (Life Technologies, Inc.). Recombinant viruses were plaque purified three times on monolayers of Sf21 cells. The expression of the recombinant viruses was confirmed by metabolic labeling, and one of these viruses was selected for further experiments.
Recombinant proteins and radiolabeling.
Recombinant tgh-2 was obtained by purifying the fusion protein by following the manufacturer's protocol. SF21 cells were infected with the tgh-2 recombinant virus at a multiplicity of infection of 0.1 PFU/cell. After sonication and removal of the insoluble material, the recombinant protein was then purified as described above. Recombinant TGH-2 was activated by adding 10 μl of 1 M HCl to 100-μl samples, incubating at room temperature for 10 min, and neutralizing with 15 μl of 0.72 M NaOH–0.5 M HEPES. To produce recombinant protein for the luciferase assay, 2 × 105 SF21 cells/well seeded in a 24-well plate were infected with the recombinant virus at a multiplicity of infection of 20 PFU/cell for 2 h at 27°C. The inoculum was removed and replaced by 300 μl of fresh medium. After a 48-h incubation, the supernatant was removed and the cells were harvested in Dulbecco's modified Eagle's medium (DMEM) supplemented with 0.1% bovine serum albumin (BSA), 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Cells were then sonicated, and insoluble material was removed by centrifugation at 10,000 × g for 15 min. The supernatant from SF21 cells was diafiltrated in three changes of DMEM that were all supplemented with 0.1% BSA, 100 U of penicillin/ml, and 100 μg of streptomycin/ml according to the manufacturer's instructions (Vivaspin 6 concentrators; Vivascience Ltd.). The final concentration of the supernatants was 1.2 × 106 cell equivalents per ml. Recombinant human TGF-β2 (active domain; Boehringer Mannheim) and B. malayi TGH-2 (Bm-TGH-2) were iodinated by the Iodogen method (33).
Affinity labeling.
Mv-I-Lu cells (kindly donated by M. Yazdanbakhsh, Leiden University) were maintained in Eagle's minimum essential medium (Sigma) supplemented with 0.1 M nonessential amino acids, 1 mM sodium pyruvate, 2 mM l-glutamine, 100 U of penicillin/ml, 100 mg of streptomycin/ml, and 10% fetal calf serum (all from Sigma). Cells were detached from the culture flasks by incubation at 37°C with 1% trypsin–EDTA, washed, and resuspended in complete medium. Volumes of 1 ml containing 106 cells were aliquoted into a six-well plate and left overnight to adhere. The following day, Mv-I-Lu monolayers were incubated on ice for 3 to 4 h with 125I-labeled activated recombinant TGH-2 or TGF-β in binding buffer (33). Different concentrations of unlabeled inhibitor were added during this incubation period. After incubation, the cells were washed and lysed by incubation in solubilization buffer for 40 min on ice. Radioactivity was then counted in the soluble extracts.
Luciferase assay for TGF-β.
Modified mink lung epithelial cells (MLECs) (clone 32) were obtained (by kind donation from D. B. Rifkin, Department of Cell Biology, New York University Medical Center) which were stably transfected with an 800-bp fragment of the 5′ end of the human plasminogen activator inhibitor-1 (PAI-1) gene fused to the firefly luciferase reporter gene in a p19LUC-based vector containing the neomycin resistance gene from pMAMneo (1). Modified MLECs were maintained in DMEM supplemented with 10% fetal calf serum, 100 U of penicillin/ml, 100 μg of streptomycin/ml, 2 mM l-glutamine, and 200 μg of G418/ml. Confluent modified MLECs were trypsinized as described above and resuspended in complete media at 1.6 × 105 cells/ml. Volumes of 100 μl of cells/well were seeded in triplicate in an all-white 96-well tissue culture dish (BMG Biotechnologies). The cells were incubated at 37°C for 3 h for optimal attachment. Recombinant Bm-TGH-2 from SF21 cell lysates and from SF21 cell supernatants (100 μl) was added directly to attached cells after aspiratiration of the serum-containing medium. Similarly, recombinant human TGF-β dilutions (250 to 4 pg/ml; 100 μl/well) made in the same media as the test samples were added to the cells. Samples were incubated overnight at 37°C. After incubation, a luciferase assay was performed according to the manufacturer's instructions (Bright-Glo Luciferase Assay System; Promega). In brief, 100 μl of Bright-Glo reagent was added to the 100-μl samples in each well. Cells were incubated for 2 min at room temperature to allow complete lysis. Luciferase activity was assayed using a Luminometer (BMG Biotechnologies). Luciferase activity was reported as relative light units. Recombinant baculovirus expressing the vaccinia protein B15R (2), used as a control in these experiments, was the kind gift of Antonio Alcami, Department of Pathology, University of Cambridge, Cambridge, United Kingdom.
Nucleotide sequence accession number.
Sequence analyses were done with MacVector program Version 6.0. Trees reflecting evolutionary relationships were generated by phylogenetic analysis using parsimony (PAUP) (52). The cDNA sequence has been deposited in GenBank with the accession no. AF104016.
RESULTS
Identification of a new TGF-β homolog.
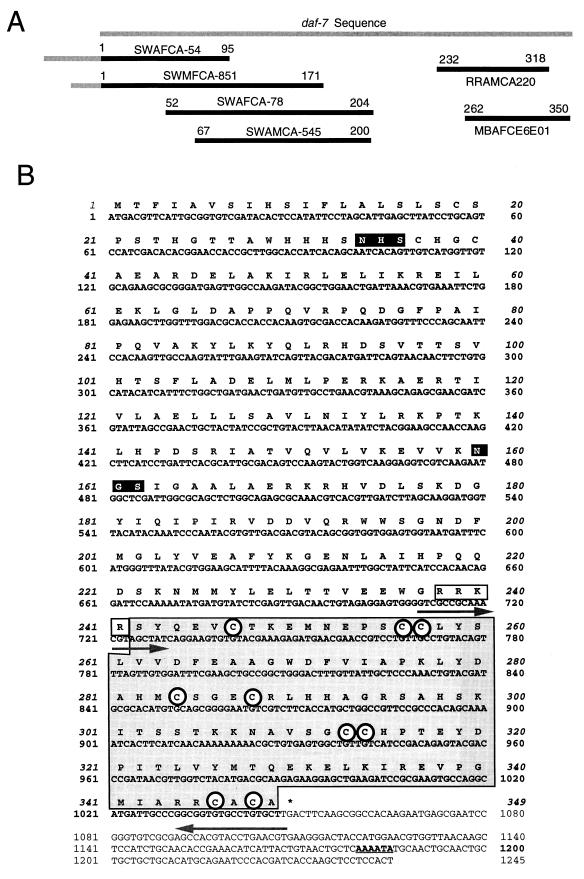
The EST data set generated by the Filarial Genome Project was screened for the presence of TGF-β-like sequences by searching with the DAF-7 protein sequence. Three B. malayi ESTs were identified in this way, and from these one nucleotide sequence was then used to screen the same database, yielding three further clones. From the six ESTs identified, four represented the 5′ end of the cDNA and two the 3′ end (Fig. 1A). The ESTs were present in cDNA libraries from three stages, microfilaria, adult female, and adult male. Full-length cDNA was then isolated by PCR from the microfilarial library by using oligonucleotide primers corresponding to the predicted 5′ and 3′ coding ends of the new gene. This cDNA was named Bm-tgh-2 (B. malayi TGF-β homolog-2). The tgh-2 cDNA consists of 1,266 bp encoding a deduced protein of 349 amino acids (Fig. 1B) with a hydrophobic region at the N terminus, indicating the presence of a signal peptide.
FIG. 1.
(A) Map of six EST sequences with similarity to DAF-7. EST sequences represent single 5′ reads from variably truncated clones. ESTs SWAFCA-54 and SWMFCA-851 include 130 and 68 nucleotides, respectively, of 5′ UTR upstream of the initiation codon (gray bar). The 22-nucleotide nematode spliced leader is not present in either of these regions. EST identification codes are given above the black bars which correspond to sequence data deposited in dbEST. Numbers correspond to amino acid positions in the C. elegans DAF-7 protein. (B) Nucleotide and deduced amino acid sequence of the B. malayi tgh-2 cDNA. The TGF-β homology region, located at the carboxyl end, is shown in a shaded box. The potential proteolytic cleavage site is an open box. The potential glycosylation sites (NHS and NGS) are shown in solid boxes. Nine cysteine residues found in invariant positions in the active domain are circled. The 3′ polyadenylation site is underlined. The sequences used for the RT-PCR primers (709 to 729 and 1030 to 1047) are shown with arrows. The full cDNA sequence has been deposited in GenBank with the accession no. AF104016.
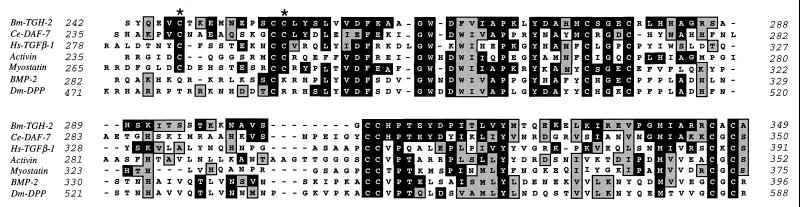
The predicted gene product contains the conserved characteristics of the TGF-β superfamily, namely a large N-terminal preprotein separated from a C-terminal 110- to 140-amino-acid cysteine-rich active domain by a tetrabasic cleavage site (6, 26). TGH-2 shows significant similarity to DAF-7 in both the N-terminal (not shown) and C-terminal (Fig. 2) segments and contains an RRKR motif that is thought to serve as a substrate for proteolytic cleavage. Within the C-terminal active domain of all TGF-β superfamily members are seven invariant cysteine residues, six of which form a rigid, heat-stable “cysteine knot” (13). The C-terminal 108-residue span of TGH-2 contains the seven invariant cysteines, as well as two additional cysteines previously found only in the vertebrate homologs TGF-β, activin, and myostatin and in the C. elegans homolog daf-7 (Fig. 2).
FIG. 2.
Alignment of the C-terminal amino acid sequences of TGH-2 and five other representatives of the TGF-β superfamily. Residues that are identical to TGH-2 in any of the other five proteins are shown in solid boxes; similarities to TGH-2 in any of the other proteins are outlined and shaded. Gaps introduced to optimize the alignment are represented by dashes. Two of the conserved cysteines found only in TGF-β, activin, and DAF-7 are shown with asterisks. Numbers at the start and finish of each line correspond to amino acid numbers in each respective sequence. Accession numbers for the sequences shown are as follows: C. elegans DAF-7, U72883; human TGF-β1, P01137; human activin, X82540; human myostatin, AF019627; human BMP-2, P12643; and D. melanogaster DPP, P07713.
TGH-2 is a member of the TGF-β subfamily.
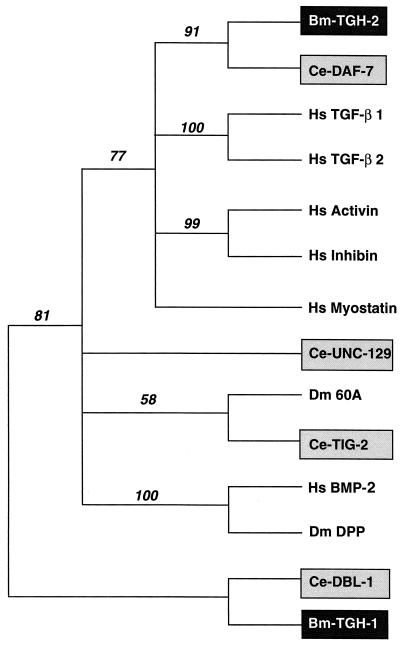
Comparison of the C-terminal ligand domain of TGH-2 with the entire GenBank protein database showed that its closest relatives are the other nine-cysteine proteins C. elegans DAF-7, human TGF-β2, and human growth/differentiation factor-8 or myostatin (respectively 41, 37, and 32% identical with Bm-TGH-2). Activin, BMP-2 and Dm-DPP show lower degrees of identity (respectively, 26, 27, and 24%) with Bm-TGH-2. In general, the N-terminal preprotein domains of TGF-βs are highly divergent, but the predicted TGH-2 precursor shows 25% identity over 240 residues with the predicted DAF-7 precursor. To investigate further the relationship between active domains of members of the TGF-β superfamily, their sequences were analyzed by the PAUP program. A presentation of this tree (Fig. 3) groups TGH-2 with the DAF-7/TGF-β/activin/myostatin subfamily and shows that DAF-7 from C. elegans is the closest relative. In contrast, the previously described TGH-1 groups with the DPP/BMP subfamily.
FIG. 3.
Phylogenetic representation of the sequence relationship between active domain of members of the TGF-β superfamily. Numbers represent bootstrap values. The accession numbers are as follows: human TGF-β1, P01137; human TGF-β2, P08112; C. elegans DAF-7, U72883; human activin, X82540; human BMP-2, P12643; D. melanogaster DPP, P07713; human inhibin, X72498; human myostatin, AF019627; C. elegans DBL-1, AF004395; B. malayi TGH-1, AF010495; D. melanogaster 60A, P27091; C. elegans UNC-129, AF029887; C. elegans TIG-2 (cosmid F39G3), AF016424.1.
Expression of tgh-2 during the life cycle.
A full-length tgh-2 cDNA was isolated from the microfilarial library, and identical sequences were found to be present in the five ESTs obtained from the adult female and the adult male libraries. Among the >18,000 B. malayi ESTs now deposited in the NCBI dbEST, a total of 11 clones have been identified, of which 3 are from microfilaria, 6 are from adult female, and 5 are from adult male cDNA libraries.
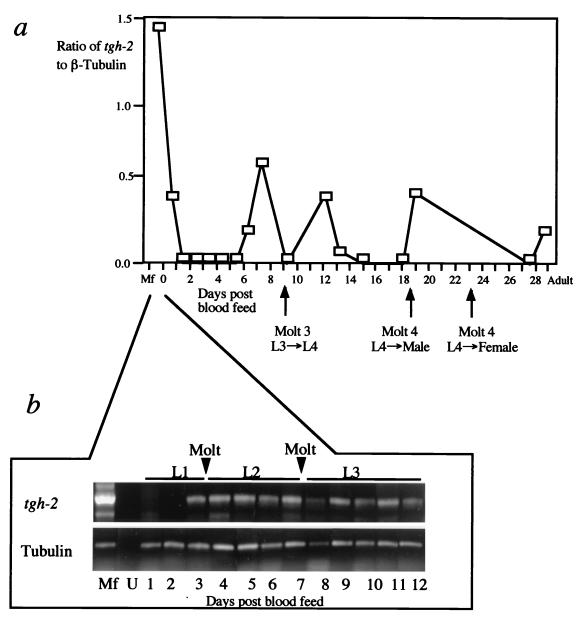
In order to determine further the expression pattern of tgh-2, first-strand cDNA was synthesized from the closely related species B. pahangi, taken at 1- to 2-day intervals over the first 4 weeks postinfection of the jird, and from parasites taken at 1-day intervals (for 12 days) after mosquitoes were blood fed. These first-strand cDNAs were amplified with primers specific for tgh-2. To provide a relative quantification of expression levels, each reaction mixture of samples from the mammalian stages contained control primers to simultaneously amplify β-tubulin transcripts (22), as this gene shows similar abundance at each stage of the life cycle. The abundance of the test transcript was then expressed as the ratio of the amount of its amplified product to that of the control transcript. First-strand cDNAs from mosquito stages were amplified with both tgh-2 and β-tubulin primers, and the PCR products were analyzed on an agarose gel.
Four separate peaks of mRNA abundance of the tgh-2 transcript were detected in the mammalian stages (Fig. 4A). The first peak corresponds to the microfilarial stage, the second peak appears just before the first molt in the host (day 9), the third peak appears before the second molt of the males in the host (day 18), and the fourth peak appears just before the second molt of the females in the host (day 23). In the mosquito stages, the tgh-2 transcript started to be visible 72 h after a blood meal with microfilariae (Fig. 4B).
FIG. 4.
Expression pattern of the tgh-2 transcript. (a) Expression data from semiquantitative RT-PCR for the tgh-2 gene performed on a time course of RNA samples taken from B. pahangi microfilariae, infective L3, at 1- to 2-day intervals over the first 4 weeks postinfection, and adults. The life cycle stage and timing of the molt relating to this time course are indicated on the x axis. The y axis represents the ratio of detected test mRNA over control β-tubulin mRNA, determined as previously described (19). (b) First-strand cDNAs from microfilariae, uninfected mosquitoes (U), and infected mosquitoes taken every day for 12 days after blood feeding were used as templates in PCRs using the tgh-2-specific primer pair indicated in Fig. 1B. The positive control for β-tubulin used primers described in Materials and Methods. The time points associated with each molt are indicated.
Secretion of TGH-2 by adult parasites in culture.
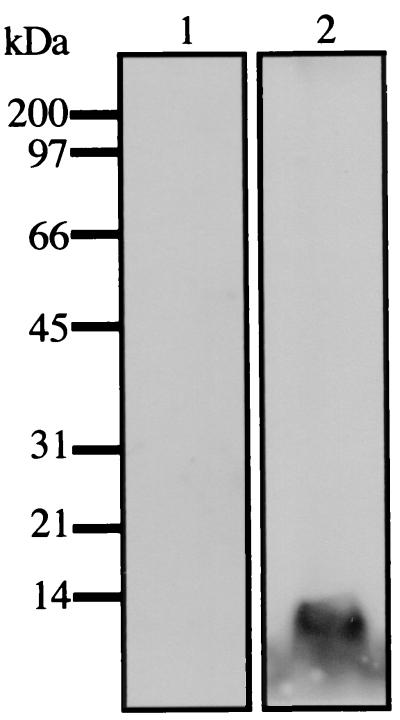
Polyclonal antibodies raised against recombinant TGH-2 were used to identify proteins secreted by adult worms in culture by Western blotting. Antisera to TGH-2 reacted against a 12-kDa protein released from adult parasites cultured in serum-free medium. This corresponds to the expected molecular mass of the TGH-2 active domain. No reactivity was observed on Western blot analysis of ES products probed with normal mouse serum (Fig. 5).
FIG. 5.
Secretion of TGH-2 by parasites in culture. Shown is a Western blotting of adult parasite ES products probed with normal mouse serum (lane 1) or mouse anti-recombinant TGH-2 antibodies (lane 2) followed by ECL detection and autoradiography. The positions of the molecular mass markers (in kilodaltons) are shown on the left.
Expression of the TGH-2 polypeptide in insect cells.
For functional characterization of the 349-amino acid TGH-2 polypeptide, the entire protein-coding region, including a His tag at the C-terminal end, was expressed in Sf21 insect cells under the control of the polyhedrin promoter. As shown by SDS-PAGE analysis of 35S-labeled cells, TGH-2 protein was secreted to the medium by insect cells as a 38.5-kDa polypeptide. The 40.6-kDa protein in TGH-2-infected insect cell extracts corresponds to the polypeptide with a signal sequence still bound due to the inability of insect cells to process properly the high amount of TGH-2 protein expressed. This reconciles with the expected molecular mass of the TGH-2 polypeptide (39.4 kDa) with a 1.2-kDa peptide tag. Time course experiments revealed that the expression levels of the recombinant protein reached a maximum 48 h after infection (data not shown).
TGH-2 shows low level of binding to mammalian receptors.
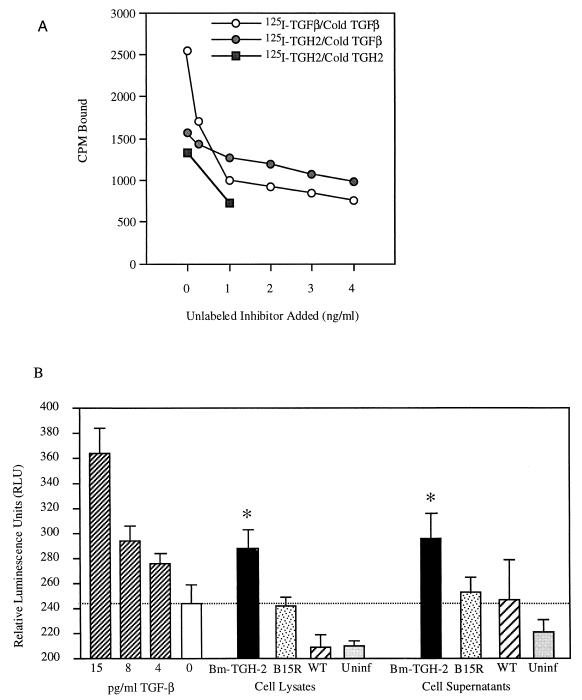
To measure whether Bm-TGH-2 binds directly to mammalian TGF-β receptors, we assayed the binding of iodine-labeled recombinant protein to mink lung cells. As shown in Fig. 6, baculovirus-expressed B. malayi TGH-2 (Bm-TGH-2) does bind to these cells, and binding can be inhibited by nanogram per-milliliter-concentrations of homologous ligand or mammalian TGF-β administered at concentrations of nanograms per milliliter. In four separate experiments, maximal inhibition of TGH-2 binding by TGF-β averaged 29% (range, 16 to 38%).
FIG. 6.
TGH-2 binding to mammalian cells. 125I-labeled TGF-β and TGH-2 were incubated with mink lung cells and washed. Incubations were performed in the presence of up to 4 ng of inhibitor/ml as indicated; 1 ng of inhibitor/ml represents a 33-fold molar excess over 125I-labeled ligand, and all assays were performed in the presence of 2 mg of BSA/ml. Shown are binding of TGF-β inhibited by various concentrations of unlabeled TGF-β (○), binding of TGH-2 inhibited by unlabeled TGF-β (●), and binding of TGH-2 inhibited by unlabeled TGH-2 (■). CPM, counts per minute. (B) Bioactivity of TGH-2 produced by baculovirus-infected insect cells. SF21 cells were infected with TGH-2 virus, B15R virus, or wild-type virus added at a multiplicity of infection of 20 PFU/cell. At 48 h postinfection, cells and supernatants were collected and assayed for their ability to induce PAI-1 expression by using the MLEC luciferase assay (1). ∗, responses to TGH-2 are significantly higher than those of medium alone (P values of <0.025 for both cell lysate and supernatant samples) and significantly higher than responses to the control recombinant B15R (P value of <0.025 for cell lysate, P value of <0.05 for supernatant) using Student's t test for unpaired samples. This experiment was performed three times, with positive stimulation by TGH-2 on each occasion. WT, wild type.
In order to further show that TGH-2 has a biological effect similar to that of TGF-β, we measured the responsiveness of an MLEC line which had been transfected with a luciferase reporter construct linked to the promoter for a gene up-regulated by TGF-β, plasminogen activator inhibitor-1 (1). Modified MLECs cultured in the presence of cell lysates and cell supernatants from insect cells infected with the wild-type virus and B15R virus (a 40- to 44-kDa vaccinia virus secretory glycoprotein that functions as a soluble interleukin-1 receptor [2]) did not generate significant levels of luciferase activity. However, modified MLECs cultured with either cell lysate or cell supernatant from insect cells infected with the TGH-2 virus both showed significant increases in luciferase activity. The relative luminescence generated by recombinant Bm-TGH-2 was similar to the signal observed by stimulation with 8 pg of recombinant human TGF-β/ml.
DISCUSSION
Host defenses against pathogens rely on a network of cytokines for activation and coordination of the immune response. It is not surprising, therefore, that infectious organisms encode cytokine-like molecules and other products which interfere with cytokine-mediated communication (3, 43). Most known examples come from viruses, which are likely to have evolved most of their cytokine mimics by horizontal gene capture from their hosts. Such a process would be less common among eukaryotic parasites, and for metazoan helminths in particular it seems more likely that convergent evolution between members of ancient gene families may give rise to functional cytokine homologs in parasites. The TGF-β superfamily emerged early in the evolution of metazoa, and helminths would have had the opportunity to adapt these molecules for the purposes of subversion of host responses.
We therefore investigated homologs of TGF-β in B. malayi, a prominent tissue-dwelling filarial nematode. We have isolated and characterized two related genes. One, designated tgh-1, belongs to the differentiation-inducing subfamily of DPP and BMP and is expressed at a relatively low level during growth points of the parasite (19). The second which we describe here is more abundant, more closely related to DAF-7 and to human TGF-β itself, is secreted by the parasite in culture, shows some binding for host TGF-β receptors, and seems to have a similar biological effect as human TGF-β on the MLECs. Perhaps the most striking feature of this new gene is that it is maximally expressed in the blood stage microfilarial parasites, which are exposed to the full force of the host immune system and are at the same time in a state of arrested development. Moreover, the appearance of microfilariae during natural infection is most closely associated with suppression of the host immune response. These considerations suggest that TGH-2 may have an immunomodulatory function, rather than inducing growth and differentiation. Thus, on the basis of sequence similarities, expression patterns, and receptor binding, TGH-2 is a candidate for an immune evasion molecule. Further studies are now necessary to test this supposition.
TGH-2 is one of a growing set of helminth homologs of host immune system molecules. For example, B. malayi homologs of macrophage migration inhibitory factor have recently been cloned (40), while migration inhibition factor-like biological activity has been demonstrated in a range of nematode species (42) and a gamma interferon-like protein reported in the nematode Trichuris muris (21). It is also likely that parasites will express cytokine-binding proteins and receptors. Thus, receptors for TGF-β-like ligands have been cloned from B. malayi (20) and S. mansoni (14), while the existence of tumor necrosis factor alpha receptors in S. mansoni is implied by the dependence of egg-laying on the presence of this cytokine (5). As parasite genome projects expand apace (9), it is likely that many more cytokine mimics will be discovered to play major roles in the successful immune evasion by helminth organisms.
ACKNOWLEDGMENTS
We thank the Leverhulme Trust and the Medical Research Council for support.
We thank Steve Williams of the Filarial Genome Project for provision of B. malayi cDNA libraries. We thank Don Riddle (Columbia, Mo.) for sharing the DAF-7 sequence prior to publication and for general advice and Mark Blaxter (ICAPB, Edinburgh, United Kingdom) for advice on phylogenetic analysis. We thank D. B. Rifkind (New York University Medical Center) and Antonio Alcami (University of Cambridge) for their kind gifts of a transfected MLEC line and B15R-containing baculovirus, respectively.
REFERENCES
- 1.Abe M, Harpel J G, Metz C N, Nunes I, Loskutoff D J, Rifkin D B. An assay for transforming growth factor-β using cells transfected with a plasminogen activator inhibitor-1 promoter-luciferase construct. Anal Biochem. 1994;216:276–284. doi: 10.1006/abio.1994.1042. [DOI] [PubMed] [Google Scholar]
- 2.Alcami A, Smith G L. A soluble receptor for interleukin-1β encoded by vaccinia virus: a novel mechanism of virus modulation of the host response to infection. Cell. 1992;71:153–167. doi: 10.1016/0092-8674(92)90274-g. [DOI] [PubMed] [Google Scholar]
- 3.Alcami A, Smith G L. Cytokine receptors encoded by poxviruses: a lesson in cytokine biology. Immunol Today. 1995;16:474–478. doi: 10.1016/0167-5699(95)80030-1. [DOI] [PubMed] [Google Scholar]
- 4.Allen J E, Maizels R M. Immunology of human helminth infection. Int Arch Allergy Appl Immunol. 1996;109:3–10. doi: 10.1159/000237225. [DOI] [PubMed] [Google Scholar]
- 5.Amiri P, Locksley R M, Parslow T G, Sadick M, Rector E, Ritter D, McKerrow J H. Tumour necrosis factor α restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 6.Barr P J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991;66:1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- 7.Barral A, Barral-Netto M, Yong E C, Brownell C E, Twardzik D R, Reed S G. Transforming growth factor β as a virulence mechanism for Leishmania braziliensis. Proc Natl Acad Sci USA. 1993;90:3442–3446. doi: 10.1073/pnas.90.8.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barral-Netto M, Barral A, Brownell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-β in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 9.Blaxter M L, Aslett M, Guiliano D, Daub J The Filarial Genome Project. Parasitic helminth genomics. Parasitology. 1999;118:S39–S51. doi: 10.1017/s0031182099004060. [DOI] [PubMed] [Google Scholar]
- 10.Bootcov M R, Bauskin A R, Valenzuela S M, Moore A G, Bansal M, He X Y, Zhang H P, Donnellan M, Mahler S, Pryor K, Walsh B J, Nicholson R C, Fairlie W D, Por S B, Robbins J M, Breit S N. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-β superfamily. Proc Natl Acad Sci USA. 1997;94:11514–11519. doi: 10.1073/pnas.94.21.11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cate R L, Mattaliano R J, Hession C, Tizard R, Farber N M, Cheung A, Ninfa E G, Frey A Z, Gash D J, Chow E P, Fisher R A, Bertonis J M, Torres G, Wallner B P, Ramachandran K L, Ragin R C, Manganaro T F, MacLaughlin D T, Donahoe P K. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 12.Colavita A, Krishna S, Zheng H, Padgett R W, Culotti J G. Pioneer axon guidance by UNC-129, a C. elegans TGF-β. Science. 1998;281:706–709. doi: 10.1126/science.281.5377.706. [DOI] [PubMed] [Google Scholar]
- 13.Daopin S, Piez K A, Ogawa Y, Davies D R. Crystal structure of transforming growth factor-β2: an unusual fold for the superfamily. Science. 1992;257:369–373. doi: 10.1126/science.1631557. [DOI] [PubMed] [Google Scholar]
- 14.Davies S J, Shoemaker C B, Pearce E J. A divergent member of the transforming growth factor β receptor family from Schistosoma mansoni is expressed on the parasite surface membrane. J Biol Chem. 1998;273:11234–11240. doi: 10.1074/jbc.273.18.11234. [DOI] [PubMed] [Google Scholar]
- 15.Estevez M, Attisano L, Wrana J L, Albert P S, Massagué J, Riddle D L. The daf-4 gene encodes a bone morphogenetic protein receptor controlling C. elegans dauer larva development. Nature. 1993;365:644–649. doi: 10.1038/365644a0. [DOI] [PubMed] [Google Scholar]
- 16.Ferguson E L, Anderson K V. decapentaplegic acts as a morphogen to organize dorsal-ventral pattern in the Drosophila embryo. Cell. 1992;71:451–461. doi: 10.1016/0092-8674(92)90514-d. [DOI] [PubMed] [Google Scholar]
- 17.Gazzinelli R T, Oswald I P, Hieny S, James S L, Sher A. The microbicidal activity of interferon-γ-treated macrophages against Trypanosoma cruzi involves l-arginine-dependent, nitrogen oxide-mediated mechanism inhibitable by interleukin-10 and transforming growth factor-β. Eur J Immunol. 1992;22:2501–2506. doi: 10.1002/eji.1830221006. [DOI] [PubMed] [Google Scholar]
- 18.Georgi L L, Albert P S, Riddle D L. daf-1, a C. elegans gene controlling dauer larva development, encodes a novel receptor protein kinase. Cell. 1990;61:635–645. doi: 10.1016/0092-8674(90)90475-t. [DOI] [PubMed] [Google Scholar]
- 19.Gomez-Escobar N, Lewis E, Maizels R M. A novel member of the transforming growth factor-β (TGF-β) superfamily from the filarial nematodes Brugia malayi and B. pahangi. Exp Parasitol. 1998;88:200–209. doi: 10.1006/expr.1998.4248. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Escobar N, van den Biggelaar A, Maizels R M. A member of the TGF-β receptor gene family in the parasitic nematode Brugia. Gene. 1997;199:101–109. doi: 10.1016/s0378-1119(97)00353-3. [DOI] [PubMed] [Google Scholar]
- 21.Grencis R K, Entwistle G M. Production of an interferon-gamma homologue by an intestinal nematode: functionally significant or interesting artefact. Parasitology. 1997;115:S101–S105. doi: 10.1017/s0031182097002114. [DOI] [PubMed] [Google Scholar]
- 22.Guénette S, Prichard R K, Matlashewski G. Identification of a novel Brugia pahangi β-tubulin gene (β2) and a 22-nucleotide spliced leader sequence on β1-tubulin mRNA. Mol Biochem Parasitol. 1992;50:275–284. doi: 10.1016/0166-6851(92)90225-9. [DOI] [PubMed] [Google Scholar]
- 23.Hirsch C S, Hussain R, Toossi Z, Dawood G, Shahid F, Ellner J J. Cross-modulation by transforming growth factor β in human tuberculosis: suppression of antigen-driven blastogenesis and interferon γ production. Proc Natl Acad Sci USA. 1996;93:3193–3198. doi: 10.1073/pnas.93.8.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch C S, Yoneda T, Averill L, Ellner J J, Toossi Z. Enhancement of intracellular growth of Mycobacterium tuberculosis in human monocytes by transforming growth factor-β1. J Infect Dis. 1994;170:1229–1237. doi: 10.1093/infdis/170.5.1229. [DOI] [PubMed] [Google Scholar]
- 25.Hunter C A, Bermudez L, Beernink H, Waegell W, Remington J S. Transforming growth factor-β inhibits interleukin-12-induced proliferation of interferon-γ by natural killer cells: a role for transforming growth factor-β in the regulation of T cell-independent resistance to Toxoplasma gondii. Eur J Immunol. 1995;25:994–1000. doi: 10.1002/eji.1830250420. [DOI] [PubMed] [Google Scholar]
- 26.Kingsley D M. The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- 27.Lecuit T, Brook W J, Ng M, Calleja M, Sun H, Cohen S M. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature. 1996;381:387–393. doi: 10.1038/381387a0. [DOI] [PubMed] [Google Scholar]
- 28.Letterio J J, Roberts A B. Regulation of immune responses by TGF-β. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Hunter C A, Farrell J P. Anti-TGF-β treatment promotes rapid healing of Leishmania major infection in mice by enhancing in vivo nitric oxide production. J Immunol. 1999;162:974–979. [PubMed] [Google Scholar]
- 30.Ling N, Ying S Y, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature. 1986;321:779–782. doi: 10.1038/321779a0. [DOI] [PubMed] [Google Scholar]
- 31.Maizels R M, Bundy D A P, Selkirk M E, Smith D F, Anderson R M. Immunological modulation and evasion by helminth parasites in human populations. Nature. 1993;365:797–805. doi: 10.1038/365797a0. [DOI] [PubMed] [Google Scholar]
- 32.Markwell M A K, Fox C F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3α,6α-diphenylglycoluril. Biochemistry. 1978;17:4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- 33.Massagué J. Identification of receptors for type-β transforming growth factor. Methods Enzymol. 1987;146:174–195. doi: 10.1016/s0076-6879(87)46020-5. [DOI] [PubMed] [Google Scholar]
- 34.McPherron A C, Lawler A M, Lee S-J. Regulation of skeletal muscle mass by a new TGF-β superfamily member. Nature. 1997;387:83–90. doi: 10.1038/387083a0. [DOI] [PubMed] [Google Scholar]
- 35.Morita K, Chow K L, Ueno N. Regulation of body length and male tail ray pattern formation of Caenorhabditis elegans by a member of TGF-β family. Development. 1999;126:1337–1347. doi: 10.1242/dev.126.6.1337. [DOI] [PubMed] [Google Scholar]
- 36.Nakane A, Asano M, Sasaki S, Nishikawa S, Miura T, Kohanawa M, Minagawa T. Transforming growth factor β is protective in host resistance against Listeria monocytogenes in mice. Infect Immun. 1996;64:3901–3904. doi: 10.1128/iai.64.9.3901-3904.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nellen D, Burke R, Struhl G, Basler K. Direct and long-range action of a DPP morphogen gradient. Cell. 1996;85:357–368. doi: 10.1016/s0092-8674(00)81114-9. [DOI] [PubMed] [Google Scholar]
- 38.Omer F H, Riley E M. Transforming growth factor β production is inversely correlated with severity of murine malaria infection. J Exp Med. 1998;188:39–48. doi: 10.1084/jem.188.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oswald I P, Gazzinelli R T, Sher A, James S L. IL-10 synergizes with IL-4 and transforming growth factor-β to inhibit macrophage cytotoxic activity. J Immunol. 1992;148:3578–3582. [PubMed] [Google Scholar]
- 40.Pastrana D V, Raghavan N, FitzGerald P, Eisinger S W, Metz C, Bucala R, Schleimer R P, Bickel C, Scott A L. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun. 1998;66:5955–5963. doi: 10.1128/iai.66.12.5955-5963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson G I, Padgett R W. TGF β-related pathways. Roles in Caenorhabditis elegans development. Trends Genet. 2000;16:27–33. doi: 10.1016/s0168-9525(99)01916-2. [DOI] [PubMed] [Google Scholar]
- 42.Pennock J L, Behnke J M, Bickle Q D, Devaney E, Grencis R K, Isaac R E, Joshua G W, Selkirk M E, Zhang Y, Meyer D J. Rapid purification and characterization of L-dopachrome-methyl ester tautomerase (macrophage-migration-inhibitory factor) from Trichinella spiralis, Trichuris muris and Brugia pahangi. Biochem J. 1998;335:495–498. doi: 10.1042/bj3350495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ploegh H L. Viral strategies of immune evasion. Science. 1998;280:248–253. doi: 10.1126/science.280.5361.248. [DOI] [PubMed] [Google Scholar]
- 44.Reddi A H. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- 45.Ren P, Lim C-S, Johnsen R J, Albert P S, Pilgrim D, Riddle D L. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 46.Rodrigues V, Jr, Santana da Silva J, Campos-Neto A. Transforming growth factor beta and immunosuppression in experimental visceral leishmaniasis. Infect Immun. 1998;66:1233–1236. doi: 10.1128/iai.66.3.1233-1236.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruvkun G, Hobert O. The taxonomy of developmental control in Caenorhabditis elegans. Science. 1998;282:2033–2041. doi: 10.1126/science.282.5396.2033. [DOI] [PubMed] [Google Scholar]
- 48.Sher A, Gazzinelli R T, Oswald I P, Clerici M, Kullberg M, Pearce E J, Berzofsky J A, Mosmann T R, Lames S L, Morse III H C, Shearer G M. Role of T-cell derived cytokines in the downregulation of immune responses in parasitic and retroviral infection. Immunol Rev. 1992;127:183–204. doi: 10.1111/j.1600-065x.1992.tb01414.x. [DOI] [PubMed] [Google Scholar]
- 49.Sporn M B, Roberts A B, Wakefield L M, Assoian R K. Transforming growth factor-β: biological function and chemical structure. Science. 1986;233:532–534. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- 50.Stavnezer J. Regulation of antibody production and class switching by TGF-β. J Immunol. 1995;155:1647–1651. [PubMed] [Google Scholar]
- 51.Suzuki Y, Yandell M D, Roy P J, Krishna S, Savage-Dunn C, Ross R M, Padgett R W, Wood W B. A BMP homolog acts as a dose-dependent regulator of body size and male tail patterning in Caenorhabditis elegans. Development. 1999;126:241–250. doi: 10.1242/dev.126.2.241. [DOI] [PubMed] [Google Scholar]
- 52.Swofford D L. PAUP: Phylogenetic Analysis Using Parsimony. Champaign, Ill: Illinois Natural History Survey; 1991. [Google Scholar]
- 53.Thomsen G, Woolf T, Whitman M, Sokol S, Vaughan J, Vale W, Melton D A. Activins are expressed early in Xenopus embryogenesis and can induce axial mesoderm and anterior structures. Cell. 1990;63:485–493. doi: 10.1016/0092-8674(90)90445-k. [DOI] [PubMed] [Google Scholar]
- 54.Tsutsui N, Kamiyama T. Transforming growth factor β-induced failure of resistance to infection with blood-stage Plasmodium chabaudi in mice. Infect Immun. 1999;67:2306–2311. doi: 10.1128/iai.67.5.2306-2311.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J. Purification and characterisation of an FSH releasing protein from porcine ovarian follicular fluid. Nature. 1986;321:776–779. doi: 10.1038/321776a0. [DOI] [PubMed] [Google Scholar]
- 56.Wahl S M. Transforming growth factor β: the good, the bad, and the ugly. J Exp Med. 1994;180:1587–1590. doi: 10.1084/jem.180.5.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams M E, Caspar P, Oswald I, Sharma H K, Pankewycz O, Sher A, James S L. Vaccination routes that fail to elicit protective immunity against Schistosoma mansoni induce the production of TGF-β, which down-regulates macrophage antiparasitic activity. J Immunol. 1995;154:4693–4700. [PubMed] [Google Scholar]
- 58.Williams S A. Deep within the filarial genome: an update on progress in the Filarial Genome Project. Parasitol Today. 1999;15:219–224. doi: 10.1016/s0169-4758(99)01454-4. [DOI] [PubMed] [Google Scholar]
- 59.Wilson M E, Young B M, Davidson B L, Mente K A, McGowan S E. The importance of TGF-β in murine visceral leishmaniasis. J Immunol. 1998;161:6148–6155. [PubMed] [Google Scholar]