Abstract
In recent clinical assays, our cholera vaccine candidate strain, Vibrio cholerae 638 El Tor Ogawa, was well tolerated and immunogenic in Cuban volunteers. In this work we describe the construction of 638T, a thymidine auxotrophic version of improved environmental biosafety. In so doing, the thyA gene from V. cholerae was cloned, sequenced, mutated in vitro, and used to replace the wild-type allele. Except for its dependence on thymidine for growth in minimal medium, 638T is essentially indistinguishable from 638 in the rate of growth and morphology in complete medium. The two strains showed equivalent phenotypes with regard to motility, expression of the celA marker, colonization capacity in the infant mouse cholera model, and immunogenicity in the adult rabbit cholera model. However, the ability of this new strain to survive environmental starvation was limited with respect to that of 638. Taken together, these results suggest that this live, attenuated, but nonproliferative strain is a new, promising cholera vaccine candidate.
Cholera remains the cause of high rates of morbidity and mortality in poor-sanitation areas in the developing world (20). Vibrio cholerae, the etiologic agent of cholera, is a gram-negative prototrophic bacterium able to persist for long periods of time in the environment and reemerge as a fully virulent pathogen for humans (14, 26).
Live oral cholera vaccines seem the most promising for elicitation of multifactorial and long-lasting immunity after a single dose (32). However, the implicit release of living bacteria into the environment continues to be a cause of concern worldwide. The El Tor Ogawa live cholera vaccine candidate strain 638 was recently demonstrated to be well tolerated and immunogenic in Cuban volunteers (5), as was CVD103HgR in North American volunteers (32). Inactivation of the thyA gene has been proposed as a biological containment tool for microorganisms intended to be released into the environment (23). The thyA gene codes for thymidylate synthase (TS), the enzyme responsible for the catalytic conversion of dUMP into dTTP (21). Bacterial strains bearing deletions within the thyA gene are auxotrophic for thymine or thymidine and are not expected to proliferate in the environment, where free pyrimidines are absent. Previous to this work, undefined mutants of V. cholerae with thymidine requirements had been selected by trimethoprim resistance. For example, CVD102, a spontaneous thyA-defective derivative of CVD101, was poorly excreted by humans and minimally immunogenic (19). Further experiments demonstrated the CVD102 colonization defect to be unrelated to the thyA mutation, and similar thyA mutants of CVD101 colonized well in the infant mouse cholera model (2). The construction of a thyA-defined mutant of V. cholerae has not been reported previous to this work. The present paper describes cloning and nucleotide sequencing of the thyA gene from V. cholerae and the construction of a thyA-defined mutant derived from our vaccine candidate strain 638 (V. cholerae O1, El Tor Ogawa, ΔCTXΦ, hap::celA). The resultant mutant, 638T, was unable to proliferate in thymidine-free minimal medium unless it was complemented by plasmid pVT1 carrying the V. cholerae thyA gene. The environmental survival of 638T was also examined and demonstrated to be diminished with respect to that of 638. Additionally, this mutant was able to colonize in the infant mouse cholera model and was also immunogenic in rabbits, suggesting that although limited in proliferation, the vaccine candidate effectively stimulates the mucosal immune system to induce a serological response.
MATERIALS AND METHODS
Bacterial strains, plasmids, and media.
The bacterial strains and plasmids used in this study are listed in Table 1. All strains were conserved frozen at −70°C in Luria-Bertani (LB) medium containing 20% glycerol. Bacterial strains were routinely propagated at 37°C in LB medium with the adequate supplements. Antibiotics were added at the following concentrations: ampicillin, 100 μg/ml; polymyxin B, 13.2 μg/ml, and trimethoprim, 50 μg/ml. Thymidine was used at 50 or 200 μg/ml when necessary. Some of the genomic sequence data referred to in this paper were obtained through early release from the Institute for Genomic Research (TIGR) website at http://www.tigr.org.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference |
|---|---|---|
| E. coli | ||
| DH5α | supE44 ΔlacU169 (Φ80lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1 | 3 |
| SY327λpir | Δ(lac pro) argE(Am) rif nalA recA56 (λpirR6K), host for suicide vectors | 30 |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2Tc::Mu (λpirR6K), Kmr, host for suicide vectors with transfer functions integrated in the chromosome | 25 |
| V. cholerae | ||
| C7258 | Wild type, O1, El Tor, Ogawa, from Peru 1991 | 6 |
| 81 | C7258 Δ(cep orfU ace zot ctxA ctxB) | 6 |
| 815 | Spontaneous thymidine auxotroph of 81 | 27 |
| 638 | 81 hap::celA | 27 |
| 638T | thyA mutant of 638 | This study |
| Plasmids | ||
| pBR322 | General cloning vector, Ampr Tetr | 8 |
| pUC19 | General cloning vector for sequencing, Ampr | 34 |
| pCVD442 | Suicide vector, Ampr, confers sucrose sensitivity | 15 |
| pVT1 | pBR322 carrying thyA as an EcoRI-HindIII fragment | This study |
| pVT2 | pUC19 carrying the EcoRI-PstI fragment from pVT1 | This study |
| pVT5 | pUC19 carrying the BglII-HindIII fragment from pVT1 | This study |
| pBMT1 | pVT1 with a deletion between BglII and MluI | This study |
| pVT9 | pUC19 with a blunted EcoRI-HindIII fragment from pBMT1 | This study |
| pEST | pCVD442 carrying SacI fragment from pVT9 | This study |
Selection of spontaneous thyA mutant.
Spontaneous thyA mutants were selected by growing V. cholerae 81 in LB broth containing trimethoprim and thymidine at 200 μg/ml, as reported by Miller (24). Several trimethoprim-resistant, thymidine-requiring mutants were selected and tested for phenotypic stability. Mutant 815 was selected for its low reversion frequency of 10−8 in LB. This mutant was further characterized and used as the host for cloning the V. cholerae thyA gene.
DNA techniques and analysis.
The alkaline lysis method of Birnboim and Doly (7) was used to isolate plasmid DNA from bacterial strains. Transformation of V. cholerae strains with plasmid DNA was achieved by electroporation, and suicide vectors were mobilized from donor strain SM10λpir to receptor V. cholerae strains by conjugation at a donor-to-receptor strain ratio of 2:1. Recombinant plasmids were constructed using standard methods (28) and tested by restriction assays. DNA restriction and modification enzymes were used according to the manufacturer's instructions (Boehringer Mannheim and Amersham). V. cholerae chromosomal DNA was prepared as previously described (3). For Southern blots, DNA was transferred to nitrocellulose filters by the downward alkaline capillary transfer technique (12), and detection was performed by using digoxigenin-labeled probes generated with the DNA labeling and detection kit from Boehringer Mannheim.
Cloning of thyA gene from V. cholerae.
Chromosomal DNA from strain C7258 and plasmid DNA from pBR322 were doubly digested with EcoRI and BamHI, SalI and EcoRI, BamHI and HindIII, and EcoRI and HindIII. The correspondent ligations were set between vibrio DNAs and equally digested plasmid. After the reaction, each ligation was used to transform strain 815 by electroporation, and transformants were selected on LB plates supplemented with ampicillin. Plasmid DNAs purified from clones showing no requirement for thymidine were electroporated into Escherichia coli DH5α for propagation.
Sequencing of thyA gene.
Double-stranded plasmid DNA was sequenced by the method of Sanger et al. (29) using the T7-Sequenase 7-deaza-dGTP sequencing kit V2.0 (Amersham Life Science) according to the manufacturer's instructions. The strategy used for sequencing (Fig. 1A) was based on plasmids pVT2 and pVT5, derivatives of pUC19 containing the overlapping fragments EcoRI-PstI and BglII-HindIII, respectively. In addition to universal primers for pUC, primers I (GTATCACCCAGCAATG), II (GTTGCCCACATCGTAG), III (CATGCTGGGTGATAC), and IV (TGGGTCACTTTGGATG) were also designed for double-stranded sequencing of the insert. The nucleotide sequence was analyzed with software Gene Runner V 3.02. CLUSTAL W (33) and BLAST (1) softwares were used for protein alignment and comparisons.
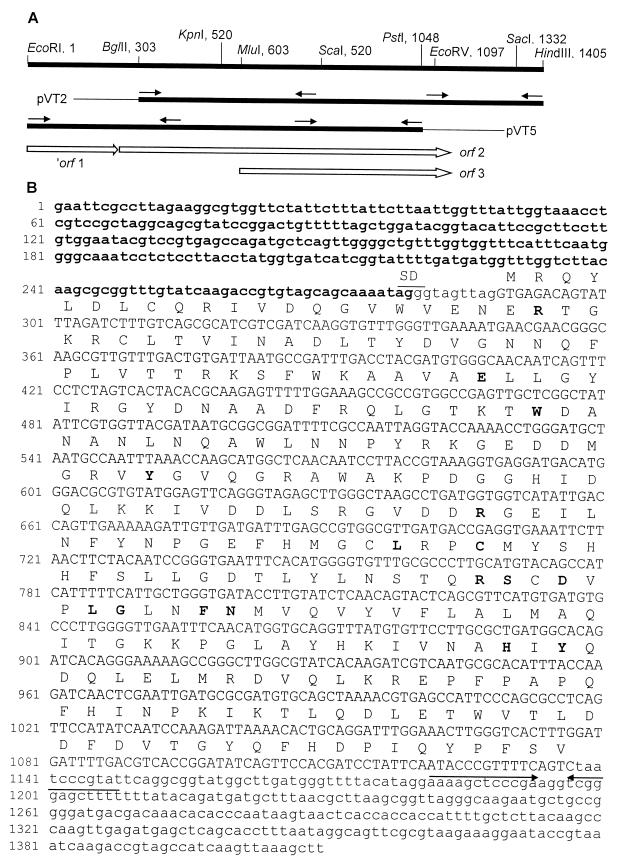
FIG. 1.
Restriction map, sequencing strategy (A), and nucleotide sequence of the EcoRI-HindIII fragment in pVT1 (B). (A) Heavy lines represent V. cholerae DNA. pVT2 and pVT5 are subclones in pUC19; arrows indicate the direction of sequencing from the subclones, thin lines correspond to the pUC19 vector, and the open reading frames of interest are represented by open arrows. (B) Nucleotide sequence corresponding to orf 2 is represented in uppercase and flanking DNA in lowercase letters. The predicted polypeptide encoded in orf 2 is shown above, with residues involved in substrate and cofactor binding in boldface. Putative Shine-Dalgarno (SD) and transcription terminator (→←) sequences are overscored. The partial sequence of orf 1 appears in boldface.
Construction of a V. cholerae strain defective in TS.
A defined mutant deficient in TS biosynthesis was constructed by allelic replacement of the thyA gene in the chromosome of strain 638 with a mutated allele from plasmid pEST. The stepwise construction was done as follows. pBMT1 was constructed by deletion of the 300-bp BglII-MluI fragment from the thyA gene in plasmid pVT1 (Fig. 1); consequently, pBMT1 did not complement the 815 thyA defect. The mutated thyA gene was excised as an EcoRI-HindIII fragment from pBMT1, blunted, gel purified, and cloned into SmaI-digested pUC19 in the same direction as the β-galactosidase gene to obtain pVT9. A SacI fragment from pVT9 containing the mutated thyA gene was cloned into pCVD442 to obtain pEST. E. coli SM10λpir was used to mobilize pEST into V. cholerae by conjugation. The replication of pEST is restricted to lysogens of λpir expressing protein π. Since 638 does not support autonomous replication of pEST, bacterial transformants after mobilization of pEST arose by homologous recombination of the plasmid into the bacterial chromosome to form ampicillin-resistant cointegrates. Ten exconjugants growing on LB-ampicillin-polymyxin plates were screened for the presence of the correct cointegrate by Southern blotting with a V. cholerae thyA-specific probe generated from the EcoRI-HindIII insert of pVT1. One of seven clones that contained the expected genotype was allowed to segregate overnight in LB broth supplemented with thymidine, and appropriate dilutions were plated in LB agar supplemented with thymidine at 200 μg/ml and 15% sucrose to counterselect merodiploid vibrios. Sucrose-resistant, ampicillin-sensitive colonies were analyzed by Southern blotting and PCR for the structure of the thyA locus. Bacteria in which the mutated allele replaced the wild-type thyA exhibited dependence on thymidine for growth in minimal medium. One such mutant was designated 638T and further characterized in vitro and in vivo.
Thymidine requirement, characteristics of growth, and morphological examination of 638T.
The thymidine requirement of V. cholerae 638T was examined by culturing in LB supplemented with thymidine at 200 μg/ml, washing the cells three times in phosphate-buffered saline (PBS), and plating of appropriate dilutions on parallel plates of thymidine-free and thymidine-supplemented M9 minimal medium. Parallel cultures in M9 broth were also set. For growth characterization, wild-type or thyA mutant V. cholerae was cultured overnight in LB broth or LB-thymidine (200 μg/ml). After overnight growth, vibrios were harvested, washed three times in PBS, and inoculated at a density of 107 cells per ml in 70 ml of LB or LB containing thymidine at 50 or 200 μg/ml. The optical density and CFU were recorded every 15 min until the stationary phase of growth and later at 24 h of incubation at 37°C and 200 rpm. Appropriate samples were taken at each interval for electron microscopy examinations. At least 10 fields were microscopically examined for morphological characterization of harvested bacteria, and a characteristic field was photographed.
Environmental survival assays.
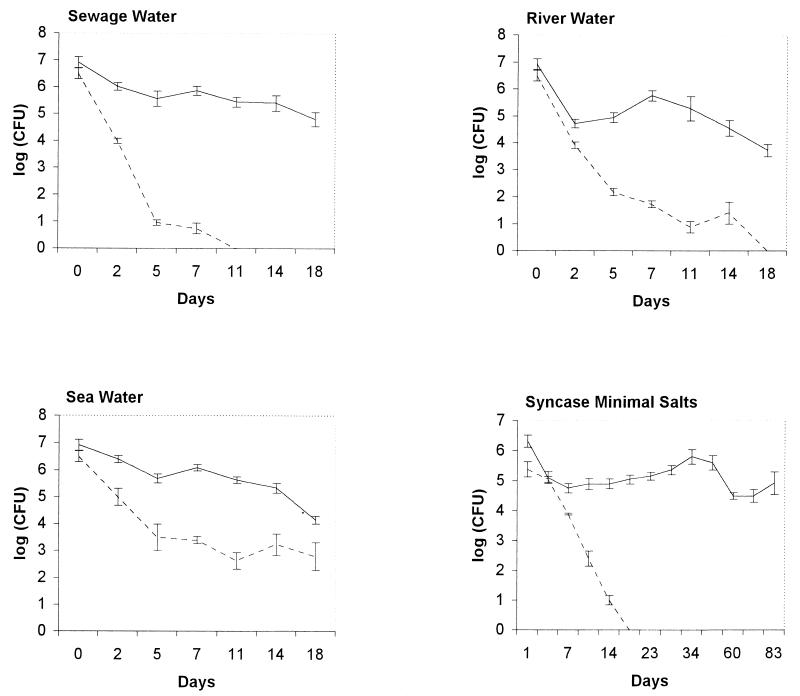
Previous to environmental survival assays, the thyA mutant 638T or 638T transformed with pVT1 (638/pVT1) was cultured in LB with adequate supplements to mid-log phase. Cells were harvested, washed twice in saline, and adjusted to about 108 cells/ml; 100 μl of each suspension was inoculated into 10 ml of either syncase minimal salts (Na2HPO4 and K2HPO4 at 0.5% and NH4Cl at 0.118%), sewage water, river water, or seawater. Water from each source was used both untreated and autoclaved. Triplicate experiments for each strain evaluated were settled for the appropriate time at room temperature. CFU were recorded at appropriate times for at least 20 days by plating 10-μl droplets of 10-fold serial dilutions of bacterial suspensions. The day 0 counts are reflected in Fig. 3.
FIG. 3.
Environmental survival of 638T (dashed lines) compared to 638T complemented with pVT1 as modeled in vitro under laboratory conditions in sewage water, river water, and seawater and in syncase minimal salts. Each value represents the average for three samples in a single experiment. The standard deviations are represented by error bars.
Animal models.
The colonizing potential of the mutant 638T was evaluated in the suckling mouse cholera model as previously reported (9). BALB/c mice (3 to 5 days old) were inoculated intragastrically with 106 CFU of 638, 638T, or 638T transformed with pVT1. Two independent groups of five mice were used for each strain tested. Bacterial strains were cultured in LB broth with appropriate supplements to an optical density of 1.0 at 600 nm, washed twice with PBS, and adjusted to about 2 × 107 CFU per ml. The exact amount in the inoculum (50 μl) was determined by triplicate plating of appropriately diluted bacteria. After 16 h, mice were sacrificed, and the small intestines were removed, washed three times in PBS, and homogenized individually in 5 ml of the same buffer. Viable-cell counts were determined by serial dilution and plating of 10-μl droplets in triplicate on LB plates containing adequate supplements.
The immunizing potential of 638T was evaluated in the adult rabbit cholera model. The inoculum was prepared as for the infant mouse colonization assay. Six New Zealand rabbits, weighing 1 to 1.2 kg, were fasted overnight, intraduodenally inoculated (17) with a single dose of about 5 × 108 CFU, and caged separately. Blood samples were taken at days 0, 7, 14, 21, and 27, stored at −70°C, and later examined for the presence of vibriocidal and antilipopolysaccharide (LPS) immunoglobulin G (IgG) antibodies in the serum.
Immune response to vaccine strains.
Sera collected on days 0, 7, 14, 21, and 28 were analyzed for vibriocidal antibody titers in a microassay (4) as previously reported by Benítez et al. (5). Titers of IgG raised by the vaccine strain were evaluated with the anti-LPS IgG enzyme-linked immunosorbent assay described in reference 5.
Statistical analysis.
Statistical analysis of experimental data was performed with software Epi Info 6, version 6.04a, July 1996 (Centers for Disease Control and Prevention, Atlanta, Ga.). Means were compared by the paired t test or by the unpaired t test (P < 0.05), as required.
The data from the environmental survival assays were analyzed with software Statistica v4.3 (Statsoft Inc., 1993). Data were presented in a multiple analysis of variance and analyzed by means of the F test. Individual comparisons were performed following the algorithm of Duncan.
Nucleotide sequence accession number.
The nucleotide sequence of the V. cholerae thyA gene reported here was deposited in the EMBL database and given accession number Y17135.
RESULTS
Cloning, sequencing, and analysis of the V. cholerae TS gene.
The thyA gene was cloned from V. cholerae as described in Materials and Methods. The smallest plasmid able to complement 815 was pVT1, which contained a 1.4-kb EcoRI-HindIII insert. This fragment was excised, labeled, and used to probe a Southern blot containing V. cholerae DNA restricted with different enzymes. It was shown to recognize a single 1.4-kb EcoRI-HindIII fragment in the genome of C7258. All plasmids able to complement the thymidine requirement in 815 were shown to contain inserts homologous to the probe by Southern blotting.
A restriction map of the EcoRI-HindIII insert in pVT1 was done with the restriction enzymes represented in Fig. 1A. The internal BglII-HindIII and EcoRI-PstI overlapping segments within this fragment were cloned into pUC19 to obtain pVT2 and pVT5, respectively. In contrast to pVT1, pVT2 and pVT5 were unable to complement V. cholerae 815, suggesting that BglII and PstI restriction sites mapped within the thyA gene, as was demonstrated later.
Clones pVT1, pVT2, and pVT5 were used as templates for DNA sequencing according to the strategy depicted in Fig. 1A. The nucleotide sequence of the EcoRI-HindIII fragment is shown in Fig. 1B. It revealed two complete open reading frames: orf 2, which is 849 nucleotides long and extends from nucleotides 289 to 1137, and orf 3, which is 540 nucleotides long and extends from nucleotides 598 to 1137. orf 2 could code for a 283-amino-acid chain, and orf 3 could code for a 180-residue peptide. A putative Shine-Dalgarno sequence (AGG) was detected preceding the start codon of orf 2 (GTG289), and a probable rho-independent terminator was identified as a dyad symmetry region 42 bp downstream from its TAA1138 stop codon. Since plasmid pVT5 contains the entire orf 3 and is unable to complement the thyA defect in 815, we concluded that orf 2 codes for the V. cholerae TS. This conclusion is further supported by the fact that the molecular weight and amino acid sequence of the orf 2-encoded protein are similar to the average molecular weight and amino acidic sequence of TSs from different sources.
A partial orf was found upstream of the V. cholerae thyA gene running in the same direction of transcription (′orf 1 in Fig. 1). Theoretical translation of this partial orf produces a 92-amino-acid sequence with high percent identity to the C-terminal end of LGT from Salmonella enterica serovar Typhimurium (63%), E. coli (60%) and Haemophilus influenzae (55.5%). In E. coli, LGT is an essential protein exhibiting phosphatidylglycerol:prolipoprotein diacylglycerol transferase activity (16). On the basis of these comparisons, it seems likely that the identified orf corresponds to the Igt gene of V. cholerae.
Properties of vaccine strain.
The genetic manipulation performed in 638 to obtain 638T generated a 300-bp deletion in its thyA coding sequence, which prevents the synthesis of an active TS. Consequently, 638T is impaired in the ability to produce dTTP, an essential precursor of DNA biosynthesis. Like 638, 638T lacks the CTXΦ prophage sequences and preserves a single RS1 copy in the chromosome, is defective in expression of the hemagglutinin/protease (HA/P), and does not assemble the mannose-sensitive hemagglutinin to the bacterial surface. The defect in HA/P expression is due to the insertion of the endoglucanase A marker (celA) from Clostridium thermocellum into this locus, which serves to differentiate the vaccine strain from wild-type vibrios in vitro (27). Aside from the genetic manipulation in its thyA gene, 638T was indistinguishable in motility, biochemical tests, serotyping, and growth rate from its parental strain 638 in complete medium (LB supplemented with thymidine at 200 μg/ml). However, in minimal medium, the mutation introduced in thyA generated a nutritional requirement for thymidine, which was eliminated by transformation with plasmid pVT1, containing thyA as a 1.4-kb EcoRI-HindIII fragment. In thymidine-limiting conditions, 638T acquired a filamentous appearance in which the cell length was proportional to the thymidine limitation (Fig. 2A).
FIG. 2.
Growth data for 638T in LB supplemented with different concentrations of thymidine and for 638 in LB. (A) Length of V. cholerae cells at different times of growth, as measured by electron microscopy. (B) Growth curve for 638 in LB and for 638T in LB supplemented with thymidine at 0 and 50 μg/ml. Each curve represents three replicas in three independent experiments. MGT, mean generation time.
Characteristics of growth and morphological observation.
The mean generation time of 638T growing in LB supplemented with thymidine at 200 μg/ml was equivalent to that of 638 growing in LB (Fig. 2A), and cells from both strains were also morphologically indistinguishable. When thymidine supplements were dropped to 50 μg/ml, the mean generation time remained unchanged, but in contrast to 638, single cells of 638T were found to elongate (Fig. 2A). When 638T complemented with pVT1 grew in unsupplemented LB, the elongated phenotype did not occur and the strain behaved like 638 (result not shown).
Similar growth curves were obtained for 638 in LB and 638T in LB supplemented with 50 or 200 μg of thymidine per ml (Fig. 2B). In contrast, in LB broth without thymidine, 638T grew at a lower rate and stopped growing at 1.0 to 2.0 units of absorbance at 600 nm. From this time on, the cell count began to decay, presumably due to thymineless death of bacteria, and at 24 h the CFU were as low as 103 per ml. In this condition of growth, practically 100% of the cholera bacilli showed the elongated phenotype.
Environmental survival.
The survival of V. cholerae 638T and 638T/pVT1 was evaluated in water from three different sources: sewage, river, and sea. Survival in each source was determined with untreated and autoclaved water. In untreated water, no viable cells of 638T or 638T/pVT1 were detected at day 5 (not shown). Therefore, we could not make a reasonable comparison between the two strains in untreated samples. A more convenient analysis could be performed in autoclaved water from different sources. The results are presented in Fig. 3. In the more immediate human environment of sewage water, the counts of 638T fell to undetectable levels at day 11, while counts of 638T/pVT1 remained at 105 CFU/ml until day 18. The number of CFU for 638T was significantly lower than for 638T/pVT1 after the second day of evaluation (P < 0.01).
In the less immediate human environment of river water, the number of CFU per milliliter dropped to undetectable levels at day 18 of the assay for 638T, while 638T/pVT1 remained at 104. Statistically significant differences were observed between 638T and 638T/pVT1 from the fifth day of evaluation (P < 0.01).
In seawater, although statistically significant differences were established between 638T and 638T/pVT1 from day 2 through day 18, both strains had detectable CFU during the 20 days for which survival was analyzed.
Under laboratory conditions in syncase minimal salts, the counts of 638T dropped to undetectable levels at day 18, while those of 638T/pVT1 remained between 104 and 106 for the same interval. The counts of 638T were significantly lower than the counts for the complemented strain after day 7 of the assay (P < 0.01).
Under the same conditions, the results found for parental strain 638 (not shown) were similar to the results found for 638T/pVT1.
Colonization properties and immune response.
As indicated in Table 2, 638T was able to colonize the small bowel of the infant mice as well as strain 638 did. Under the optical microscope, a typical filamentous or elongated phenotype was observed for 638T vibrios colonizing the intestine of mice (data not shown).
TABLE 2.
Preclinical findings for 638T, 638T transformed with pVT1, and 638 in mice and rabbits
| Strain | Colonizationa (no. of vibrios)
|
Immune responseb
|
||||
|---|---|---|---|---|---|---|
| Vibriocidal antibody GMT (range) on day:
|
Anti- LPS IgG GMT (range) on day:
|
|||||
| Inoculated | Recovereda | 14 | 28 | 14 | 28 | |
| 638 | 5.9 × 106 | 1.5 × 106 | 800 (320–2,500) | 500 (320–1,200) | 150 (100–300) | 316 (200–400) |
| 638T | 1.0 × 106 | 3.0 × 105 | 485 (80–2,560) | 368 (80–1,280) | 355 (100–1,600) | 848 (100–3,200) |
| 638T/pVT1 | 3.6 × 106 | 1 × 106 | 640 (160–1,280) | 320 (160–640) | 640 (100–3,200) | 640 (100–3,200) |
Each value represents the average for at least eight mice from two independent experiments.
Each value represents the average for at least six rabbits. The day 0 titers were under 1:20 for vibriocidal antibodies and under 1:25 for anti-LPS IgG.
In two competition experiments involving 13 mice, equivalent CFU of 638T and 638 were recovered from homogenates of the small bowel of mice intragastrically coinoculated with 106 CFU of both strains in a 1:1 ratio (not shown). We also found that 638T transformed with pVT1 colonized in the infant mouse cholera model as well as 638T, with the peculiarity that the counts of vibrios recovered from the intestines of mice were very similar in LB supplemented with thymidine and in LB supplemented with ampicillin, indicating no loss of the plasmid during intestinal colonization (not shown).
It was also observed that rabbits intraduodenally inoculated with a single dose of 638T seroconverted at day 7 with high geometric mean titers (GMT) (mean GMT [range] = 211 [40 to 1,280]) of vibriocidal antibodies which peaked at day 14 and remained high up to day 28 (Table 2). Consistent with these results, high titers of anti-LPS IgG were induced by 638T at day 21 in all immunized rabbits (GMT = 479 [180 to 1,600]), with the peak observed at day 28 (GMT = 848 [100 to 3,200]). Among six rabbits, three seroconverted at day 7 and the other three seroconverted at day 21. Similar results were obtained with 638 and 638T/pVT1 (Table 2). No statistically significant differences could be detected in the titers of antibodies elicited by 638T and 638T/pVT1 or 638.
DISCUSSION
During this work, a DNA fragment containing the thyA gene from V. cholerae was cloned, sequenced, and demonstrated to contain two complete open reading frames, as represented in Fig. 1A. Bacterial TSs are highly conserved proteins, especially in regions involved in dUMP and folate binding (21). The predicted amino acid sequence encoded in orf 2 is shown above the corresponding coding sequence in Fig. 1B. Comparison of this sequence with those of proteins deposited in the Swiss-Prot database showed 78.4% identity with H. influenzae TS, 31.8% with Neisseria gonorrhoeae TS, and 31.4% with E. coli TS enzyme. According to the crystallographic data available on the stereochemical mechanism and structure of E. coli TS, the most important residues involved in substrate and cofactor binding have been established (21). Previous comparisons of TS amino acid sequences from different sources marked Arg-21, -126, -127, and -166; Glu-58; Trp-80; Tyr94 and -209; Leu-143 and -172; Cys-146; Ser-167; Asp-169; Gly-173; Phe-176; Asn-177; and His-207 as conserved residues implicated in substrate binding and catalytic function of the enzyme. For numbering, the TS of E. coli was taken as the reference. Except for Arg-127, V. cholerae TS is shown to contain all of the above residues in equivalent positions, as represented in Fig. 1B. These facts, together with the requirement for thymidine observed in several orf 2 mutants of V. cholerae constructed in our laboratory (results not shown), as well as the complementing ability of plasmids containing the complete orf 2, permit us conclude that orf 2 corresponds to the thyA structural gene of V. cholerae. In E. coli, Igt and thyA form an operon, and TS levels are regulated by transcription from the Igt promoter and by translational coupling due to the overlap of Igt and the ribosome-binding site of thyA (16). This overlap is also present in V. cholerae (Fig. 1B), and consequently, regulation by translational coupling seems plausible. The analysis of this region in the chromosome of 638 permitted us to design a procedure to mutate thyA, leaving intact the essential Igt gene, and obtain a viable mutant of V. cholerae by allelic replacement.
Biological containment is a desired feature of live cholera vaccines. Thymine or thymidine auxotrophy has been proposed as an environmental biosafety feature for environmentally released vaccines, since free pyrimidines are scarce in natural ecosystems (23). One important goal in developing the thyA mutant 638T was enhancement of the environmental safety of V. cholerae strain 638. We therefore evaluated the survival of 638T in minimal salts and in autoclaved and untreated water from different environmental sources in comparison to that of 638T transformed with pVT1. From our analysis, the following conclusions were derived. (i) Environmental survival in a culturable state of strain 638T is limited with respect to the control strain 638T complemented with pVT1 in autoclaved water from all sources tested. (ii) The survival ability of 638T is more limited in sewage and river water than in seawater. (iii) A sort of heat-sensitive factor(s), presumably biotic, exists in all environmental sources of water that limits the survival of V. cholerae in a culturable state and hampered comparisons in untreated water.
Previous reports demonstrated that LB-grown wild-type vibrios achieved the viable but nonculturable state at 20 days of incubation in a carbon source-free minimal medium (14). During our studies, the control strain survived at high counts for more than 20 days in autoclaved water from different sources. While this is the case for the control strain 638T/pVT1, the 638T counts became undetectable after 11 and 18 days in sewage and river water, respectively. These results point to a significant contribution of the wild-type thyA gene to the survival of V. cholerae and reinforce our proposal to use 638T as a cholera vaccine prototype with enhanced environmental safety features. The greater persistence of 638T in seawater than in the rest of the waters tested remains unexplained, but it is highly encouraging that in the more immediate human environment, 638T has diminished survival with respect to the control strain. This is even more important since it was recently demonstrated that the hemagglutinin protease of V. cholerae is capable of proteolytic inactivation of CTXΦ (18). As in 638T, the HA/P coding gene is inactivated; this strain seems more vulnerable to infection by CTXΦ. However, in addition to the immunity conferred by the remnant RS1 in 638T to reacquisition of this phage, the thyA mutation makes superfluous this and most of the other means of acquisition of genetic information.
Although 638T displayed limited survival in the conditions tested, it was not at the expense of its colonization properties. Conclusions drawn from previous experiments on the relevance of thyA for colonization of the small bowel were debatable. V. cholerae CVD102, a spontaneous thymine-dependent auxotroph of CVD101 (ctxA ctxB+ live oral cholera vaccine candidate), was studied in volunteers. This strain showed diminished immunogenicity and colonization potential with respect to CVD101, which suggested that thymine auxotrophy was overattenuating (19). Later studies in the infant mouse cholera model showed that the reduced colonizing potential of this strain could not be compensated for with a functional thyA gene (2). Additionally, CVD102 showed reduced synthesis of toxin-coregulated pili during in vitro growth, suggesting that this second mutation was responsible for the overattenuation observed (2). Mutant 815 was constructed in our laboratory by growth in trimethoprim and thymidine using the same procedures as for CVD102. This strain was also defective for colonization of the small bowel of mice compared to its parental strain 81 (27), suggesting that the thymidine requirement in V. cholerae was associated with a colonization defect.
Since CVD102 and 815 were both spontaneous mutants selected by trimethoprim resistance, it was interesting to explore if thyA mutations had pleiotropic effects over genes essential for colonization or if the colonization defect was due to the procedure used for mutant selection. Mutant 638T is a genetically defined thyA mutant generated by allelic replacement. This mutant, although unable to grow in thymidine-free minimal medium, was able to colonize the small bowel of mice to the same extent as its parental strain 638 and evoked a strong immune response in rabbits after intraduodenal inoculation. We conclude that an intact thyA gene is not essential for colonization of the small bowel of mice or rabbits and that the mutation in thyA is not necessarily associated with a mutant phenotype in any other factor essential for colonization. It seems possible that the procedure used to select spontaneous thyA mutants with trimethoprim favors the selection of parallel mutations that interfere with colonization. Note that 815 was selected on the basis of being able to grow in LB supplemented with thymidine but not in LB alone, but 638T is able to grow in unsupplemented LB to a density of approximately 1 to 2 absorbance units at 600 nm (Fig. 2).
Seroconversion in rabbits intraduodenally immunized with live cholera vaccine candidates has provided us with an estimate of their immunogenic potential for humans (17). In this work, we demonstrate that a single intraduodenal dose of 638T suffices to stimulate the mucosal immune system of rabbits to produce a systemic response of antibacterial antibodies similar to that generated by 638 or 638T transformed with pVT1. There are three explanations for the ability of 638T to colonize the bowel of mice and rabbits. (i) The pool of thymidine within V. cholerae is enough to support a few rounds of replication. (ii) The intestinal lumen contains enough thymidine or thymine to support survival of vibrios in both animal models. (iii) V. cholerae has evolved an adequate mechanism to recover thymidine from or more efficiently use thymidine in the intestinal milieu. Although the first explanation seems the most likely, further experimentation is required for an answer to this question. The second explanation seems unlikely since bacterial cells of 638T directly isolated from the intestine of infant mice exhibit the filamentous shape adopted in thymidine-limiting conditions in vitro (not shown). Hypothesis i or iii will be confirmed or discarded after testing this strain in human volunteers.
Other auxotrophies have been shown to be nonattenuating for the colonizing capacity of V. cholerae in infant mice, including auxotrophies to several amino acids, in particular to uracil and arginine (11). For these mutants, exogenous uracil acts as the immediate precursor of UMP, which is used in turn for the biosynthesis of all pyrimidine nucleotides (e.g., UTP, CTP, dCTP, and dTTP). As it has also been found that thymine or thymidine auxotrophy does not attenuate the colonizing potential of V. cholerae, it seems that pyrimidine auxotrophs of this bacterium are unimpaired in their ability to colonize the small bowel of mice. In contrast, purine auxotrophs have been shown to be defective in intestinal colonization of infant mice (11). It would be interesting to know whether this behavior is inherent to V. cholerae or is dependent on the animal model.
The marker of auxotrophy introduced into 638 to obtain 638T provides the advantage of an easier purity control for vaccine lots. As V. cholerae is predominantly prototrophic, contaminants in vaccine preparations can be detected by growth in syncase minimal salts containing sucrose at 0.5 %, a concentration at which the auxotrophic vaccine strain does not grow at all.
As seen for E. coli and Shigella flexneri, thyA mutants of V. cholerae elongated when grown in thymidine-limiting conditions (10, 13). As the cell is unable to synthesize thymidine, it is affected in DNA synthesis but not in other functions. Cellular elongation is thought to be due to an arrest of cellular septum formation awaiting the completion of genome duplication; meanwhile, the cell continues growing as if it were preparing for cellular division. This characteristic is important for the induction of a multifactorial immune response, since it is well known that bacterial cells during adherence and colonization of the human gut upregulate the expression of several colonization factors specifically induced in vivo.
The only undesired property detected so far in this vaccine strain is the resistance to trimethoprim inherent to the thyA mutation. However, the thyA mutation is recessive, and the antibiotic resistance is only manifested in the presence of thymidine or thymine. These two features make superfluous the possible horizontal transfer of this marker, and since this antibiotic is not used for the treatment of cholera, its presence is not expected to limit volunteer studies.
Finally, the introduction of a stable and defined thymidine auxotrophy in V. cholerae provides us with a useful tool for the expression of foreign genes in the vaccine strain from a balanced lethal system that would ensure the production of desired gene products during fermentation or colonization of the human intestine. In vivo maintenance of this plasmid was demonstrated in 638T by recovering equal CFU from the intestines of mice orally inoculated with 638T transformed with pVT1 in both LB-thymidine and LB-ampicillin.
ACKNOWLEDGMENTS
We thank Arlenis Moreno for excellent technical assistance in the preservation and maintenance of bacterial strains. We are also indebted to Odelsa Ancheta for the electron microscopy analysis of our V. cholerae strains.
We also appreciate the support given by members of the Division for Environmental Studies at Centro Nacional de Investigaciones Cientíificas with the analysis of water from the different sources employed in this study, especially Mario Cruz and Caridad Ramos. Finally, we acknowledge Angela Pérez for reviewing the manuscript.
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Attridge S R. Thymine auxotrophy as an attenuating marker in Vibrio cholerae. Microb Pathog. 1995;19:11–18. doi: 10.1006/mpat.1995.0040. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1995. [Google Scholar]
- 4.Benenson A, Saad S, Mosley A. Serological studies in cholera. 2. The vibriocidal antibody response of cholera patients determined by a microtechnique. Bull W H O. 1968;38:277–285. [PMC free article] [PubMed] [Google Scholar]
- 5.Benítez J A, García L, Silva A, García H, Fando R, Cedré B, Pérez A, Campos J, Rodríguez B L, Pérez J L, Valmaseda T, Pérez O, Pérez A, Ramírez M, Ledón T, Jydi M D, Lastre M, Bravo L, Sierra G. Preliminary assessment of the safety and immunogenicity of a new CTXφ-negative, hemagglutinin/protease-defective El Tor candidate cholera vaccine strain. Infect Immun. 1999;67:539–545. doi: 10.1128/iai.67.2.539-545.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benítez J A, Silva A, Rodríguez B L, Fando R, Campos J, Robert A, García H, García L, Pérez J L, Oliva R, Torres C A, Ledón T. Genetic manipulation of Vibrio cholerae for vaccine development: construction of live attenuated El Tor candidate vaccine strains. Arch Med Res. 1996;27:275–283. [PubMed] [Google Scholar]
- 7.Birnboim H C, Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979;7:1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bolivar F, Rodriguez R L, Greene P J, Betlach M C, Heyneker H L, Boyer H W, Crosa J H, Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2:95–113. [PubMed] [Google Scholar]
- 9.Cedré B, García L, García H, Fariñas M, Talavera A, Infante J F. Intestinal colonization of the infant mouse model by attenuated and virulent Vibrio cholerae strains. Arch Med Res. 1998;29:231–234. [PubMed] [Google Scholar]
- 10.Cersini A, Salvia A M, Bernardini M L. Intracellular multiplication and virulence of Shigella flexneri auxotrophic mutants. Infect Immun. 1998;66:549–557. doi: 10.1128/iai.66.2.549-557.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiang S L, Mekalanos J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 12.Chomczynski P. One-hour downward alkaline capillary transfer for blotting of DNA and RNA. Anal Biochem. 1992;201:134–139. doi: 10.1016/0003-2697(92)90185-a. [DOI] [PubMed] [Google Scholar]
- 13.Churchward G, Bremer H, Young R. Transcription in bacteria at different DNA concentrations. J Bacteriol. 1982;150:572–581. doi: 10.1128/jb.150.2.572-581.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colwell R R, Tamplin M L, Brayton P R, Gauzens A L, Tall B D, Herrington D, Levine M M, Hall S, Huq A, Sack D A. Environmental aspects of Vibrio cholerae in transmission of cholera. In: Sack R B, Zinnaka Y, editors. Advances in research on cholera and related diarrheas. 7th ed. Tokyo, Japan: KTK Scientific Publishers; 1990. pp. 327–343. [Google Scholar]
- 15.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gan K, Sankaran K, Williams M G, Aldea M, Rudd K E, Kushner S R, Wu H C. The umpA gene of Escherichia coli encodes phosphatidylglycerol:prolipoprotein diacylglyceryl transferase (Igt) and regulates thymidylate synthase levels through translational coupling. J Bacteriol. 1995;117:1879–1882. doi: 10.1128/jb.177.7.1879-1882.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García L, Oliva R, Cedré B, Valmaseda T, García H, Talavera A, Pérez J L, Sierra G. Intraduodenal inoculation of adult rabbits for evaluating the immunogenicity of genetically attenuated Vibrio cholerae strains. Lab Anim Sci. 1998;48:538–541. [PubMed] [Google Scholar]
- 18.Kimsey H H, Waldor M K. Vibrio cholerae hemagglutinin/protease inactivates CTXΦ. Infect Immun. 1998;66:4025–4029. doi: 10.1128/iai.66.9.4025-4029.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine M M, Kaper J B, Herrington D, Losonsky G, Morris J G, Clements M L, Black R E, Tall B, Hall R. Volunteer studies of deletion mutants of Vibrio cholerae O1 prepared by recombinant techniques. Infect Immun. 1988;56:161–167. doi: 10.1128/iai.56.1.161-167.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin W, Fullner K J, Clayton R, Sexton J A, Rogers M B, Calia K E, Calderwood S B, Fraser C, Mekalanos J J. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci USA. 1999;96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maley F, Maley G F. A tale of two enzymes, deoxycytidylate deaminase and thymidylate synthase. Prog Nucleic Acid Res Mol Biol. 1990;39:49–80. doi: 10.1016/s0079-6603(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 22.Matthews D A, Appelt K, Oatley S J, Xuong N H. Crystal structure of Escherichia coli thymidylate synthase containing bound 5-fluoro-2′-deoxyuridylate and 10-propargyl-5,8-dideazafolate. J Mol Biol. 1990;214:923–936. doi: 10.1016/0022-2836(90)90346-N. [DOI] [PubMed] [Google Scholar]
- 23.Mekalanos J, Goldberg I, Miller V, Pearson G, Swartz D, Taylor R, Harford N, Gathoye A M, Simoen E, Boon B, de Wilde M. Genetic construction of cholera vaccine prototypes. Vaccines. Cold Spring Harbor; 1985. [Google Scholar]
- 24.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. pp. 218–220. [Google Scholar]
- 25.Miller V L, Mekalanos J J. Synthesis of cholera toxin is positively regulated at the transcriptional level by toxR. Proc Natl Acad Sci USA. 1984;81:3471–3475. doi: 10.1073/pnas.81.11.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravel J, Knight I T, Monahan C E, Hill R T, Colwell R. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology. 1995;141:377–383. doi: 10.1099/13500872-141-2-377. [DOI] [PubMed] [Google Scholar]
- 27.Robert A, Silva A, Benítez J A, Rodríguez B L, Fando R, Campos J, Sengupta D K, Boesman-Finkelstein M, Finkelstein R A. Tagging a Vibrio cholerae El Tor candidate vaccine strain by disruption of its hemagglutinin/protease gene using a novel reporter enzyme: Clostridium thermocellum endoglucanase A. Vaccine. 1996;14:1517–1522. doi: 10.1016/s0264-410x(96)00105-3. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simon R, Priefer U, Pühler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:781–791. [Google Scholar]
- 31.Svennerholm A M, Sack D A, Holmgren J, Bardhan P K. Intestinal antibody response after immunization with cholera B subunit. Lancet. 1982;i:305–308. doi: 10.1016/s0140-6736(82)91568-9. [DOI] [PubMed] [Google Scholar]
- 32.Taket C O, Kotloff K L, Losonsky G, Nataro J P, Michalski J, Kaper J B, Levine M M. Onset and duration of protective immunity in challenged volunteers after vaccination with live oral cholera vaccine CVD103-HgR. J Infect Dis. 1992;170:1518–1523. doi: 10.1093/infdis/166.4.837. [DOI] [PubMed] [Google Scholar]
- 33.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]