Abstract
There is currently insufficient knowledge of gestational age dependent medicine disposition in neonates. Accordingly, the use of off‐label medication, i.e., use of medicines outside its approved marketing authorization, is high in the neonatal departments. By using data from the Danish National Pharmaceutical Hospital Purchase Database, we identified the most commonly occurring medications and calculated the on/off‐label ratios for premature and term neonates. Data was extracted on ATC level 5 and based on defined daily doses as per WHO. Data covered the 4 high‐level NICUs and 10 of 13 of the intermediate/standard level Danish neonatal departments. Of the identified medication, 87% and 70% did not have approved marketing authorization for use in premature and full‐term neonates, respectively. Furthermore, one‐fifth of the top 100 medicines did not have a (Danish) marketing license. Overall, off‐label medication was widespread covering virtually all ATC groups and no ATC group had an off‐label level lower than 50% (range 50%–100%). Finally, in 21% of medications, additives from 8 different chemical groups with potential deleterious effects for neonates were identified. In conclusion, off‐label medication in the Danish neonatal departments is widespread. The pharmaceutical industry is unlikely to solve this problem, and we may for a very long time be occasionally forced to use off‐label medication. Practical solution must therefore come from multidisciplinary clinical and academic collaboration. Use of formulation list as guidance for prescriptions and NICU‐friendly galenic formulations may mitigate the problem temporarily while waiting for definitive studies.
Keywords: European Paediatric Regulation, infant, newborn, NICU, off‐label medicines, pharmacology
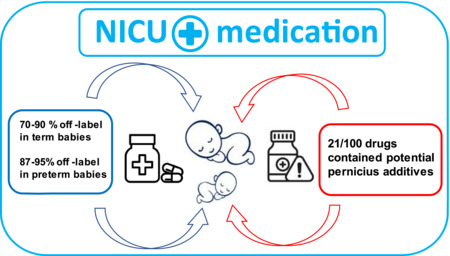
Off‐label medications are massively present in the NICUs. As some even contain potential pernicious additives, this puts term and particularely preterm babies at risk of increased risk of adverse or insufficient drug effects. Gade et al. Massive presence of off‐label medicines in Danish neonatal departments. Pharmacol. Res. Perspect 2022.
Abbreviations
- ATC
anatomical therapeutic chemical classification
- DDD
defined daily doses
- EMA
European Medicines Agency
- MA
Marketing Authorisation
- NICU
neonatal intensive care unit
- PIP
Pediatric Investigational Plan
- SmPC
Summary of Product Characteristics
1. INTRODUCTION
Pharmaceutical treatment in the neonatal department is challenging and off‐label medicine use, i.e., use of a medicine outside its regulatory approved marketing authorization, is high. 1 , 2 , 3 , 4 , 5 It is well known that infants in the neonatal intensive care unit (NICU) are exposed to a large number of medicines. This was once again demonstrated in a recent international review of studies of drug utilization in the NICUs showing a high mean number of medicines per infant, with several studies reporting >30 medicines per infant. 6 As increasingly more premature and even more complex neonatal illnesses are being treated, we are likely to see these challenges intensified in the coming years. 7
Generally, there is insufficient scientific knowledge of gestational age dependent drug disposition, 8 which potentially puts the neonates at risk of receiving suboptimal drug doses with a subsequent increased risk of adverse or insufficient drug effects. And almost adding insult to injury, a Delphi survey 9 revealed that, although the use of ‘off‐label’ medicines in neonates is widespread in Europe, there exists no common scientific and regulatory approach to this phenomenon. Therefore, a shared clinical meaningful and legally well‐defined definition of the off‐label use concept is currently missing.
To reduce off‐label drug use in pediatric patients, the Pediatric Regulation was implemented by the European Union in 2007 and has prompted further pediatrics drug research. 10 , 11 , 12 , 13 , 14 , 15 , 16 Yet, for ethical and practical reasons, neonates and particularly the preterm neonates are extremely rarely included in traditional drug development programs, 13 , 17 , 18 and pharmaceutical companies largely refrain from proactively investing in the neonatal sector, as reported by the European Medicines Agency (EMA) among others. 12 , 19 , 20 , 21 This seems to be an indisputable fact as several international studies have cemented a massive off‐label medicine use in the European NICUs of around 90%, even more than a decade after the Pediatric Regulation came into force. 19 , 22
The aim of this study was to estimate the off‐label medication use in all neonatal departments in Denmark using data from the Danish National Pharmaceutical Hospital Purchase Database (ApoBi) and to evaluate the effects of the qualities of The Summary of Product Characteristics (SmPC).
2. MATERIALS AND METHODS
2.1. Study setting
Denmark has approximately 5.9 million inhabitants and 61–63.000 births per year. 23 The overall prematurity rate is 6%–7%. 24 For this study, all Danish unique neonatal departments were identified through information acquired from the Danish Pediatric Society and cross‐referenced with the Danish National Hospital Pharmacy Sales Database (ApoBI/Targit). All neonatal departments or subunits were contacted by phone or e‐mail to verify their pharmaceutical purchase policy and debtor information. Neonatal departments that were administratively registered as a subdivision of another department and therefore had shared pharmaceutical purchase systems, were excluded. Thereby only data from departments or subunits with a dedicated debtor account for neonatal use was included in the study.
2.2. Data collection
Data on all pharmaceutical products purchased in Danish neonatal departments from 1 April 2020 to 31 March 2021 was extracted from the Danish National Hospital Pharmacy Sales Database (ApoBI/Targit). April 1st represents the official date on which the supply list is published in accordance with the national procurement agreements. Data was extracted on anatomical therapeutic chemical classification (ATC) level 1–5 and based on defined daily doses (DDD) as per WHO. 25
2.3. Data analysis
2.3.1. Data cleaning
Data cleaning took place in three steps.
Each pharmaceutical product was evaluated for clinical relevance on ATC‐Level 1 by three senior neonatologists (MDs), independently. Obvious non‐medications, e.g., disinfectants, bandages, and diet supplements were excluded. In case of divergent evaluations, consensus was achieved by a round table discussion with participation of 2 senior clinical pharmacologists (MDs).
A list of 100+ medicines were constructed based on the quantity of purchased products measured in DDDs. In case more than one product was purchased within the same generic substance and ATC group, the product with the highest quantity measured in DDDs was selected for further analysis. For example, purchase of solutions in the strengths of 1 mg/ml and 5 mg/ml were both registered for the generic substance midazolam (ATC Code N05CD08) in the quantities of 503 DDDs and 2.513 DDDs, respectively. Based on quantity, the midazolam solution for injection/infusion in the strength of 5 mg/ml was selected for further analysis.
The procedure was repeated, where 3 neonatologists independently reviewed the selected medicines, now corresponding to ATC‐level 5. In this step obvious non‐neonatal medications, e.g., 500 mg paracetamol and 400 mg ibuprofen tablets purchased for co‐hospitalized mothers were excluded.
Eventually each medicine on the final list of 100 products was reviewed and analyzed for off‐label and off‐license use in addition to content of potential pernicious additives by a clinical pharmacologist. See the final “Top 100” list in the supplementary, Table S1.
The Summary of Product Characteristics (SmPC) for each pharmaceutical product, officially approved by either the EMA or the Danish Medicines Agency, was obtained. The SmPCs were consulted on the 15th of May 2022 at the latest. The on/off‐label classification was then registered for each drug for the pre‐specified age groups: (i) prematurely born children (i.e., neonates born before 37 completed weeks of gestation), (ii) term born children (i.e., neonates born after 37 completed weeks of gestation), but within the neonatal period (i.e., within the first 28 days after birth).
Due to the hierarchical structure of a SmPC, the lower age limit specified in the SmPC section 4.1 (indication(s)) or 4.2 (posology) was noted for each pharmaceutical product. In addition, it was noted if age specified neonatal data were presented in SmPC section 5.2 (pharmacodynamic and pharmacokinetic properties) in case there was no authorized pediatric indication in accordance with the EMA SmPC guideline. 26
2.3.2. Potential pernicious additives
The SmPC for each individual medicine was reviewed for potential pernicious additives according to the European Commission guideline on “Excipients in the labelling and package leaflet of medicinal product for human use”. 27
2.3.3. Licensing status
Licensed medicinal products were defined as products with a Danish marketing authorization and a valid SmPC as per EMA or the Danish Health Authorities. Pharmaceutical products available for compassionate use or manufactured by extemporaneous preparation did not have a valid SmPC and were classified as unlicensed. 9
2.3.4. Off‐label use
Definitions of off‐label drug use were adapted from the official EMA off‐label definition provided in the European Glossary, which reads as follows “the use of a medicine for an unapproved indication or in an unapproved age group, dose, or route of administration”. 28 Since purchasing data was used and not individualized reel life data, only off‐label use based on age groups could be evaluated in the present study. According to the EMA, age limits must be stated for the first time in the summary of product characteristics in section 4.1, which refers to the indication(s) the medicine is approved for. 29
By a random sample test, we found a tendency for the population of neonates rarely to be specified in 4.1. We therefore chose to extend the on‐label classification to include section 4.2, which refers to Posology. The Danish Patient Safety Authority and The Danish Medicine Agency were contacted and consulted in this question to achieve consensus on the on/off‐label definition in the population of neonates. However, no clear distinction between on/off‐label classification in the neonatal population could be achieved from the Danish health authorities in relation to the framework of this study.
In addition, we classified off‐label use as lack of neonatal data in general or if contraindicated (SmPC section 4.3) in the newborn population in the SmPC, as per definition adapted from consensus approaches in previously internationally published studies on the subject. 9 , 30 We accepted any mention of prematurity, regardless of specific gestational age, as on‐label for premature children. Subclassification in degrees of prematurity, e.g., a gestational age above/below 28 weeks.
For pharmaceutical products available for compassionate use or manufactured by extemporaneous preparation no (valid) SmPCs are available and hence no specific lower age limit for approval could be defined.
2.4. Statistical analysis
Standard descriptive statistics were used as applicable. Data were analyzed using R software (R Core Team R 2022).
2.5. Ethical considerations
Ethics approval was not required because the study used data from administrative registers and was therefore exempt from obtaining individual informed consent as per national regulations for register‐based studies in Denmark.
2.6. Nomenclature of targets and ligands
Key Protein targets and ligands in this article are hyperlinked to the corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to Pharmacology (Harding et al., 2018), and are permanently archived in the Concise Guide to Pharmacology 2019/2020 (Alexander et al., 2019). 31
3. RESULTS
Data from all of Denmark's 4 high‐level NICUs and 10 of 13 of the intermediate/standard level neonatal departments was included in the present study. These departments cover approximately 60.000 of the annual 63.000 births in 2021, 23 and approximately 6000 neonatal hospitalization of which approximately 3.600 are premature. 24
Three intermediate/standard level neonatal departments were excluded as they did not have a unique dedicated debtor‐account making it impossible to distinguish between the neonatal department and the associated pediatric department. Of the 100 most purchased medicines in Danish neonatal departments, we found that 70% did not have an approved marketing authorization (off‐label) for use in the population of neonates, i.e., infants younger than 28 days, born at term. For the population of premature infants, we found that the amount of off‐label classified medicines increased to 87%. For details, see Table 1.
TABLE 1.
Off‐label/licensed drugs in Danish neonatal departments
| Classification | Prematures a | Neonates b |
|---|---|---|
| Off‐label c | 87% | 70% |
| Off‐licensed | 19% | 19% |
| Off‐label as per EMA d | 95% | 90% |
Note: ATC, Anatomical Therapeutic Chemical (ATC) classification system.
Prematures: any gestational age <37 weeks.
Neonates: newborns at term and up to 28 days after birth.
As per inclusive definition of on/off‐label.
When using the EMA requirement of age limits being specified in section 4.1 of the SmPC (See text for details).
In a sub‐analysis, when applying the EMA regulatory criteria of requiring the specification of the age limits to be stated in SmPC section 4.1, the off‐label classification rate rose to 90% in neonates and 95% in premature neonates (See Table 1). Furthermore, 16/100 (16%) of the top 100 medicines had an approval for children, i.e., children or pediatric population stated in SmPC section 4.1. or 4.2, without any age limit specified. Four percent of the medicines had no age specifications at all. Five percent of the medicines had a weight limit, but no specific age limit specified. For full data on classification on individual medicines and their additives, see (Table S1).
The identified top 100 medicines covering 12 of the 14 ATC level one groups in the Anatomical Therapeutic Chemical (ATC) Classification System proposed by the World Health Organization Collaborating Centre for Drug Statistics Methodology (WHOCC). 32 The exemptions were the ATC groups L (Antineoplastic and immunomodulating agents) and P (Antiparasitic products, insecticides, and repellents). Among the individual ATC groups, we found the level of off‐label classified medicines to range between 50% and 100%. For details, see Table 2. Notably, in the largest ATC groups J (Anti‐infectives for systemic use), 7 of 15 (47%) medicines had an approved marketing authorization for use in the population of neonates, while in the equally sized group A (Alimentary tract and metabolism), only 4 of 15 (30%) of medicines could be classified as on‐label. In 6 of 12 (50%) of the individual ATC groups, the medicines were classified as 100% off‐label for use in premature, i.e., ATC group D, G, H, M, S, and V. For details, see Table 3.
TABLE 2.
Off‐label medications in Danish neonatal departments per ATC group
| ATC group* | Prematures a | Neonates b |
|---|---|---|
| A: Alimentary tract and metabolism (n = 15) | 93% | 73% |
| B: Blood and blood forming organs (n = 12) | 67% | 58% |
| C: Cardiovascular system (n = 16) | 88% | 81% |
| D: Dermatologicals (n = 4) | 100% | 100% |
| G: Genito‐urinary system and sex hormones (n = 1) | 100% | 100% |
| H: Systemic hormonal preparations (n = 7) | 100% | 67% |
| J: Antiinfectives for systemic use (n = 15) | 87% | 53% |
| M: Musculo‐skeletal system (n = 3) | 100% | 67% |
| N: Nervous system (n = 14) | 86% | 71% |
| R: Respiratory system (n = 6) | 67% | 67% |
| S: Sensory organs (n = 6) | 100% | 86% |
| V: Various (n = 2) | 100% | 50% |
Note: ATC, Anatomical Therapeutic Chemical (ATC) classification system.
Prematures: any gestational age < 37 completed weeks;
Neonates: newborns at term and up to 28 days after birth (See text for details).
12 of the 14 ATC groups were represented.
TABLE 3.
Potentially pernicious additives in “top 100” medicines in Danish neonatal wards
| Additive | No of drugs and routes of administration | Potential deleterious effect(s) for neonates using the relevant routes of administration* |
|---|---|---|
| Benzalkonium chloride | 3 a | Has caused eye irritation and dryness in adults. No data are available in neonates |
| Benzoic acid | 2 b | Absorption through the immature skin of neonates is significant. May cause bilirubinemia and jaundice in neonates (up to 4 weeks old) due to displacement of bilirubin from albumin |
| Benzyl alcohol | 2 c | Intravenous administration of benzyl alcohol has been associated with serious adverse events and death in neonates (“Gasping syndrome”). The minimum amount of benzyl alcohol at which toxicity may occur is not known |
| Cetyl or cetostearyl alcohols | 3 d | May cause local skin reactions |
| Ethanol | 3 e | Co‐administration with medicines containing, e.g., propylene glycol or ethanol may lead to accumulation of ethanol and induce systemic toxicity |
| Parahydroxybenzoates and their esters | 10 f | May cause allergic reactions (possibly delayed) |
| Polysorbate 80 | 2 g | Doses >80 mg/kg/day of polysorbate has caused severe (fatal) hepatoxicity in neonates |
| Propylene glycol | 2 h | Co‐administration with any substrate for alcohol dehydrogenase may induce serious adverse events in neonates, e.g., metabolic acidosis and hyperosmolarity |
| In total | In total, 21/100 drugs (21%) contained one or more of 8 different groups of potentially pernicious additives. Only the potential deleterious effect(s) for the relevant route of administration are given in this table (See text and below) | |
Topical (ophthalmic) administration.
Topical (skin) administration.
Oral and parental administration.
Topical (skin) and oral administration.
Oral and parental administration.
Rectal, oral, and parental administration.
Oral administration.
Oral administration.
As per EMA (ref https://www.ema.europa.eu/en/annex‐european‐commission‐guideline‐excipients‐labelling‐package‐leaflet‐medicinal‐products‐human; https://www.ema.europa.eu/en/documents/scientific‐guideline/draft‐information‐package‐leaflet‐regarding‐polysorbates‐used‐excipients‐medicinal‐products‐human_en.pdf).
When assessing the SmPCs for each of the individual medicines included on the top 100 list (Table S1 in supplementary), we found that 21% of the medicines contained various additives that have either a known or suspected detrimental effect on newborns. A total of 8 different such groups of additives were identified. Several of these additives have been associated with severe and even fatal adverse events. See Table 3 for details.
We found their latest update of the SmPCs to be of various time intervals ranging from 12 days to 4325 days, which is almost 12 years (median 565 days, IQR [325;1203]). Despite 75% of SmPCs were updated within the recent 3.3 years, only 42% of the SmPCs contained specific information about newborn/premature children in section 4.1, with 58% of the SmPCs containing this information in section 4.2. However, when scrutinizing the SmPCs of the 70% off‐label medicines, we did find additional information on neonates of various clinical relevance in section 5.1 or 5.2 in 29% (20 out of 70 medicines), yet this information was not enough to render them on‐label for use in a neonatal/premature population.
4. DISCUSSION
Authorization of pharmaceutical products for specific uses and groups of patients is intended to ensure safety, efficacy, and overall quality. 21 Yet, the results of the present study based on nationwide pharmaceutical hospital purchase data demonstrate a massive presence of off‐label medicines in the Danish neonatal departments.
4.1. Off label use in Danish NICUs
The included neonatal departments represent almost all neonatal care in Denmark. We found that as little as one‐tenth and one‐third of the most commonly used medicines in Danish neonatal departments had an approved marketing authorization for use in premature and full‐term neonates, respectively. Furthermore one‐fifth of the top 100 medicines did not have a (Danish) marketing license at all. In general, we found widespread off‐label medicine use covering virtually all ATC groups, as we found that no ATC group had a lower off‐label level than 50%. In half of the ATC groups, all identified medicines were classified as off‐label for use in preterm neonates, which should be seen in the light of the additional finding of 1 in 5 medicines containing additives that have either a known or suspected adverse effect on neonates.
The findings in the present study are in concordance with international studies reporting similar percentages of off‐label use. 1 , 2 , 3 , 5 , 33 , 34 A meta‐analysis from 2015 35 revealed that 71%–100% of the neonates received at least one off‐label/off‐licensed drug during their stay in the NICU, and that premature neonates were more likely to receive off‐label medication as compared to term born neonates. Overall, the off‐label/off‐license medication use in neonates was found to be in the range of 28.4%–91.8%. It should be noticed that off‐label use can be recorded in different ways and in addition the off‐label classification may vary from study to study, making direct comparison between our result between our results and the existing literature difficult. 19
4.2. Off label classification
Importantly, there is no uniform classification on off‐label medication in Europe, making research in this topic extremely complicated. In the present study, we found that depending on the intended enforcement by the health authorities regarding age‐specific approval requirements specified in section 4.1 or 4.2 of the SmPC, the percentage of neonatal off‐label use ranged between 70%–90%. Here the neonatal population appears to be more frequently included in SmPC section 4.2 rather than in 4.1. This despite the Delphi panel back in 2008 stressed the importance of detailed information in the SmPC section 4.1 in relation to defining off‐label use. Concordantly, the EMA SmPC guideline states that “a specific requirement for the indication section is the necessity to state the age limits of the indicated population in that this attribution of an age group in the indication statement allows the differentiation between medicinal products with or without paediatric indication”. 29 , 36 Thus, strictly following this guidance, the presence of off‐label medicines in the NICUs in Denmark is as high as 90% and 95% for term born neonates and preterm neonates, respectively (Table 1).
This illustrates that not only are off‐label medicines extensively used in neonatology but also that the definition hereof is difficult, particularly for non‐regulatory knowledgeable healthcare professionals. Despite the effort of the EMA to standardize the pediatric information in a SmPC guideline, the age limits are still not uniformly reported. This may be due to delayed procedures for harmonizing the SmPCs, but we nevertheless found most SmPCs updated within the recent 3 years. Consequently, the clinical prescription as well as the legal framework under statutory obligations becomes complicated for the neonatologists to interpret.
4.3. Potentially pernicious additives
Another problem in the wake of prescribing off‐label medicines, is the risk of administering potentially harmful additives to the newborn. Neonates and in particular preterm neonates are subjects for a not insignificant toxicity risk when exposed to some additives, e.g., benzyl alcohol, propylene glycol and polysorbate, see Table 3. Nevertheless, a widespread presence of these in the most used medicines in the Danish NICUs was demonstrated. The manufacturer is obliged to specify potentially problematic additives in the SmPC, 37 but it is ultimately up to the neonatologist to interpret the risk. Severe toxicity syndromes may occur due to the physiological immature clearance pathways in neonates. 14 , 38 , 39 In addition, the exact content of additives is rarely stated in the SmPC, just as threshold values are unknown for most additives. 27 , 40 To make matters worse, there is great variation in the use of additives from manufacturer to manufacturer, and even within medicines products with origin from the same manufacturer, e.g. the medicine product Solu‐Medrol where the solvents for the strengths 500 mg and 1 mg contain benzyl alcohol but not in the strengths 40 mg and 125 mg. 41 It even appears that medicines developed and approved for use in the pediatric population may contain problematic additives, e.g., the oral solution Pinex junior®, which contains both benzyl alcohol and propylene glycol. Demonstrating the fact that the neonatal population is often overlooked, also in the regulatory system.
Overall neonatal pharmacotherapy is practice based and clinical neonatologists are often left with insufficient information, making decision on adequate drug doses very difficult. Our findings, and that of others, indicate that the high quality and safety standards applied to adult drug use assessment have not been applied to children, leading to extensive off‐label and even off‐science drug use in neonatology.
4.4. Strengths and limitations
This study draws strength from being a nationwide register‐based study. As all purchased medicines are registered in the ApoBi database, we have virtually full coverage of all hospitalized neonates, and we believe the omission of 3 minor intermediate/standard level neonatal departments is unlikely to significantly skew the results. However, we do not have individual patient data, e.g., born at term or prematurely, thus the number of off‐label medicines administered to preterm and/or term born could not be registered. In addition, not all purchased medicines are necessarily administered to a patient and there may be significant waste.
Furthermore, we dichotomously divided the neonates into preterm/term children, yet our finding of 13% of medications being on‐label for preterm infants does not necessarily mean that these medicines are on‐label for all degrees of prematurity as detailed in the appendix. Finally, as doses and administration routes were not examined in detail in this study, we may underestimate the off‐label use of an otherwise classified on‐label drug, for instance given in doses that exceeds the dose specified in SmPC section 4.2 or medicines that are manipulated or administered by other routes, e.g., intravenous drug formulation administered orally in a gastrointestinal probe which is common practice in neonatology.
4.5. Future directions
Off‐label use of medication will constantly play an important role in neonatology as long as there is limited authorized treatment options. The European Pediatric Regulation made a Pediatric Investigational Plan (PIP) mandatory back in 2007, nontheless Toma et al 19 recently described that even though the number of PIPs including neonates and infants has actually increased over the last decade, the number of medicines approved for preterm and term newborn remains extremely low. Thus, one can only hope that more PIPs will lead to more available medicines with an official approved neonatal indication in a future perspective. Similarly, many SmPCs do not specify any age, and in the future regulatory authorities should strive towards ensuring explicitly specified age groups on both approved and not‐approved populations. Cynical speaking, it seems unlikely that pharmaceutical companies will feel obligated to solve this problem under the current regulatory measures. 18 , 19 , 42 Practical solution must therefore come from a multidisciplinary regulatory, clinical and academic collaboration.
Optimized use of already available knowledge can already today help increase safety and efficacy in the NICU, as off‐label does not necessarily equal off‐science. The development of support tools for neonatologists based on available science from both the pharmaceutical industry and academia as well expert knowledge is therefore important to ensure safe medication of the newborn. 40 , 43 Consequently, formulary/recommendation lists for neonates have been developed in some European countries, e.g., the Netherlands 44 and Denmark. 45 Such measures could advantageously be extended to more countries and pharmacological communities and is strongly encouraged by the WHO as are Drug and Therapeutics committees (DTCs), which has been shown to be very effective in safeguarding and promoting efficient and rational use of medicines in hospitals. 46 It is important that these kinds of initiatives can be implemented quickly and without the need for complex and time‐consuming clinical studies.
Finally, optimized pharmacy‐developed galenic formulations may prove a solution the medications currently containing potentially pernicious additives.
5. CONCLUSION
We demonstrated a very high presence of off‐label medicine in the Danish neonatal intensive care units. Despite the EMA's efforts, there is still an unacceptable shortage of medicines with an approved marketing authorization for use in the neonatal population. Harmonization of the SmPCs to include accurate information on age limits should begin immediately, just as the wider pharmacological scientific community should work together to address safe medication of the newborn. In the meantime, optimized use of already available knowledge through formulary/recommendation lists for neonates governed by Drug and Therapeutics Committees together with optimized pharmacy‐developed galenic formulations represent a rational solution.
AUTHOR CONTRIBUTIONS
Christina Gade Associate Professor, MD, PhD, conceptualized and designed the study, drafted the initial manuscript, designed the data collection instrument, and approved the final manuscript as submitted. Ulrik Lausten‐Thomsen MD, PhD: conceptualized and designed the study, drafted the initial manuscript, designed the data collection instrument, and approved the final manuscript as submitted. Stine Trolle MSc: conceptualized and designed the study, designed the data collection instrument, and approved the final manuscript as submitted. Jon Trærup Andersen Professor, MD, PhD, Mette‐Louise Mørk MD, Thorkild Jakobsen MD, Peter Fruergaard Andersen MD, and Anna Elisabeth Lewis MD conceptualized and designed the study, designed the data collection instrument, reviewed, and revised the manuscript, and approved the final manuscript as submitted.
7. FUNDING INFORMATION
This study was a collaboration between the Copenhagen University Hospitals Righospitalet and Bispebjerg and Frederiksberg together with the Hospital Pharmacy of the Capital Region of Denmark, The study recieved no external funding.
8. ETHICS STATEMENT
Ethics approval was not required as the study used data from administrative registers and was therefore exempt from obtaining individual informed consent as per national regulations for register‐based studies in Denmark as per Danish law.
DISCLOSURE
None.
Supporting information
Table S1.
6. ACKNOWLEDGEMENTS
The authors wish to thank the local Hospital Pharmacies and Neonatal Departments and subunit for providing info on pharmaceutical purchase policies.
Gade C, Trolle S, Mørk M‐L, et al. Massive presence of off‐label medicines in Danish neonatal departments: A nationwide survey using national hospital purchase data. Pharmacol Res Perspect. 2023;11:e01037. doi: 10.1002/prp2.1037
DATA AVAILABILITY STATEMENT
Data available on request from the authors.
REFERENCES
- 1. Cuzzolin L, Agostino R. Off‐label and unlicensed drug treatments in neonatal intensive care units: an Italian multicentre study. Eur J Clin Pharmacol. 2016;72(1):117‐123. [DOI] [PubMed] [Google Scholar]
- 2. Geißler C, Schulze C, Botzenhardt S, Rascher W, Neubert A. Drug utilisation and off‐label use on a German neonatal intensive care unit: a retrospective cohort study and 10‐year comparison. Pharmacy. 2020;8(3):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Flint RB, van Beek F, Andriessen P, et al. Large differences in neonatal drug use between NICUs are common practice: time for consensus? Br J Clin Pharmacol. 2018;84(6):1313‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arocas Casañ V, Cabezuelo Escribano B, Garrido‐Corro B, de la Cruz MP, Blázquez Álvarez Ma J, de la Rubia Nieto MA. Off‐label and unlicensed drug use in a Spanish neonatal intensive care unit. Farm Hosp. 2017;41(3):371‐381. [DOI] [PubMed] [Google Scholar]
- 5. Stark A, Smith P, Hornik C, et al. Medication use in the neonatal intensive care unit and changes from 2010‐2018. J Pediatr. 2021;2:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Al‐Turkait A, Szatkowski L, Choonara I, Ojha S. Review of drug utilization studies in neonatal units: a global perspective. Int J Environ Res Public Health. 2020;17(16):E5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smits A, Annaert P, Cavallaro G, et al. Current knowledge, challenges and innovations in developmental pharmacology: a combined conect4children expert group and European Society for Developmental, perinatal and paediatric pharmacology white paper. Br J Clin Pharmacol. 2022;88(12):4965‐4984. Available from: 10.1111/bcp.14958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mørk ML, Andersen JT, Lausten‐Thomsen U, Gade C. The blind spot of pharmacology: a scoping review of drug metabolism in prematurely born children. Front Pharmacol. 2022;13:828010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Neubert A, Wong ICK, Bonifazi A, et al. Defining off‐label and unlicensed use of medicines for children: results of a Delphi survey. Pharmacol Res. 2008;58(5–6):316‐322. [DOI] [PubMed] [Google Scholar]
- 10. European Medicines Agency—Paediatric medicine ‐ Paediatric Regulation. 2013. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/regulation/document_listing/document_listing_000068.jsp
- 11. Ceci A, Giannuzzi V, Bonifazi D, Felisi M, Bonifazi F, Ruggieri L. Clinical Trials in Paediatrics — Regulatory and Methodological Aspects. 2015.
- 12. Wimmer S, Rascher W, McCarthy S, Neubert A. The EU paediatric regulation: still a large discrepancy between therapeutic needs and approved Paediatric investigation plans. Pediatr Drugs. 2014;16(5):397‐406. [DOI] [PubMed] [Google Scholar]
- 13. Allegaert K. Better medicines for neonates: improving medicine development, testing, and prescribing. Early Hum Dev. 2017;114:22‐25. [DOI] [PubMed] [Google Scholar]
- 14. O'Hara K, Wright IMR, Schneider JJ, Jones AL, Martin JH. Pharmacokinetics in neonatal prescribing: evidence base, paradigms and the future. Br J Clin Pharmacol. 2015;80(6):1281‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Frattarelli DA, Galinkin JL, Green TP, et al. Off‐label use of drugs in children. Pediatrics. 2014;133(3):563‐567. [DOI] [PubMed] [Google Scholar]
- 16. Conroy S, Choonara I, Impicciatore P, et al. Survey of unlicensed and off label drug use in paediatric wards in European countries. BMJ. 2000;320(7227):79‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van den Anker J, Allegaert K. Considerations for drug dosing in premature infants. J Clin Pharmacol. 2021;61(S1):S141‐S151. [DOI] [PubMed] [Google Scholar]
- 18. O'Hara K, Martin JH, Schneider JJ. Barriers and challenges in performing pharmacokinetic studies to inform dosing in the neonatal population. Pharmacy. 2020;8(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Toma M, Felisi M, Bonifazi D, et al. Paediatric medicines in Europe: the Paediatric regulation—is it time for reform? Front Med. 2021;2(8):593281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. the‐european‐union‐pediatric‐legislation‐impact‐on‐pharmaceutical‐research‐in‐pediatric‐populations.pdf. 2017. Available from: http://www.openaccessjournals.com/articles/the‐european‐union‐pediatric‐legislation‐impact‐on‐pharmaceutical‐research‐in‐pediatric‐populations.pdf
- 21. Better medicines for children.pdf. 2022. Available from: https://www.ema.europa.eu/en/documents/leaflet/better‐medicines‐children_en.pdf
- 22. Guidi B, Parziale A, Nocco L, et al. Regulating pediatric off‐label uses of medicines in the EU and USA: challenges and potential solutions: comparative regulation framework of off label prescriptions in pediatrics: a review. Int J Clin Pharmacol. 2022;44(1):264‐269. [DOI] [PubMed] [Google Scholar]
- 23. Fødsler . 2022. Available from: https://www.dst.dk/da/Statistik/emner/borgere/befolkning/foedsler
- 24. 102373_dkn_aarsrapport_2020_offentlig.pdf. 2022. Available from: https://www.sundhed.dk/content/cms/73/102373_dkn_aarsrapport_2020_offentlig.pdf
- 25. Defined Daily Dose (DDD). 2022. Available from: https://www.who.int/tools/atc‐ddd‐toolkit/about‐ddd
- 26. wording‐therapeutic‐indication‐guide‐assessors‐centralised‐applications_en.pdf. 2022. Available from: https://www.ema.europa.eu/en/documents/regulatory‐procedural‐guideline/wording‐therapeutic‐indication‐guide‐assessors‐centralised‐applications_en.pdf
- 27. EMA . Annex to the European Commission guideline on ‘excipients in labelling and package leaflet of medicinal products for human use’ European Medicines Agency. 2018. Available from: https://www.ema.europa.eu/en/annex‐european‐commission‐guideline‐excipients‐labelling‐package‐leaflet‐medicinal‐products‐human
- 28. EMA . Off‐label use. European Medicines Agency 2022. Available from: https://www.ema.europa.eu/en/glossary/label‐use
- 29. London, 20 November 2008 ‐ smpc_guideline_rev2_en.pdf. 2016. Available from: http://ec.europa.eu/health/files/eudralex/vol‐2/c/smpc_guideline_rev2_en.pdf
- 30. Kimland E, Odlind V. Off‐label drug use in pediatric patients. Clin Pharmacol Ther. 2012;91(5):796‐801. [DOI] [PubMed] [Google Scholar]
- 31. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucleic Acids Res. 2018;46(D1):D1091‐D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. WHOCC ‐ Home. 2022. Available from: https://www.whocc.no/
- 33. Schweigertova J, Durisova A, Dolnikova D, et al. Off‐label and unlicensed use of medicinal products in the neonatal setting in the Slovak Republic. Pediatr Int. 2016;58(2):126‐131. [DOI] [PubMed] [Google Scholar]
- 34. Alonso A, Avila‐Alvarez A, Eiriz M, et al. Use of off‐label drugs in neonatal intensive care. An Pediatría Engl Ed. 2019;91(4):237‐243. [DOI] [PubMed] [Google Scholar]
- 35. Krzyżaniak N, Pawłowska I, Bajorek B. Review of drug utilization patterns in NICUs worldwide. J Clin Pharm Ther. 2016;41(6):612‐620. [DOI] [PubMed] [Google Scholar]
- 36. Sickmüller DB. Betreuer und 1. Referent: Dr. Birka Lehmann Zweiter Referent: 103.
- 37. annex‐european‐commission‐guideline‐excipients‐labelling‐package‐leaflet‐medicinal‐products‐human_en.pdf. 2022. Available from: https://www.ema.europa.eu/en/documents/scientific‐guideline/annex‐european‐commission‐guideline‐excipients‐labelling‐package‐leaflet‐medicinal‐products‐human_en.pdf
- 38. The Gasping Syndrome and Benzyl Alcohol Poisoning|NEJM. 2022. Available from: 10.1056/NEJM198211253072206 [DOI] [PubMed]
- 39. Allegaert K, Turner MA, van den Anker JN. Neonatal formulations and additives. Neonatal Pharmacol Nutr Update. 2015;18:41‐57. [Google Scholar]
- 40. van der Zanden TM, de Wildt SN, Liem Y, Offringa M, de Hoog M. Developing a paediatric drug formulary for The Netherlands. Arch Dis Child. 2017;102(4):357‐361. [DOI] [PubMed] [Google Scholar]
- 41. Solu‐Medrol, pulver og solvens til injektionsvæske, opløsning 40 mg, 125 mg, 500 mg, 1 g.doc. Available from: https://view.officeapps.live.com/op/view.aspx?src=http%3A%2F%2Fspcweb.dkma.dk%2FSPCREPL%2FHuman%2FS%2FSolu‐Medrol%2C%2520pulver%2520og%2520solvens%2520til%2520injektionsv%25c3%25a6ske%2C%2520opl%25c3%25b8sning%252040%2520mg%2C%2520125%2520mg%2C%2520500%2520mg%2C%25201%2520g.doc&wdOrigin=BROWSELINK
- 42. Korppi M, Lepola P, Vettenranta K, Pakkala S, Hoppu K. Limited impact of EU Paediatric regulation on Finnish clinical trials highlights need for Nordic collaboration. Acta Paediatr. 2013;102(11):1035‐1040. [DOI] [PubMed] [Google Scholar]
- 43. Promoting safety of medicines for children. 2022. Available from: https://www.who.int/publications‐detail‐redirect/9789241563437
- 44. Kinderformularium (Dutch Pediatric Formulary) | Health E‐Solutions. 2022. Available from: https://healthesolutions.nl/portfolio/kinderformularium‐dutch‐pediatric‐formulary/
- 45. Maj ‐ Rekommandationsliste for Neonatale (GA 28 dage).pdf. 2022. Available from: https://www.regionh.dk/til‐fagfolk/Sundhed/Sundhedsfaglige‐raad‐og‐komiteer/Documents/Rekommandationsliste%20for%20Neonatale%202022.pdf
- 46. WHO_EDM_PAR_2004.1.pdf. 2022. Available from: https://apps.who.int/iris/bitstream/handle/10665/68553/WHO_EDM_PAR_2004.1.pdf?sequence=1&isAllowed=y
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Data Availability Statement
Data available on request from the authors.


