Abstract
Microwave imaging technology is a useful method often applied in medical diagnosis and can be used by the food industry to ensure food safety and quality. For fruit, ripeness is the primary characteristic which determines quality for the consumer. This paper proposes a novel microwave imaging system to determine the ripeness of watermelon as a proof of concept. The design employs a circular array with 10 Coplanar Vivaldi antennas offering wide bandwidth, high gain, and high efficiency. S-parameters between antennas are collected quickly via automated channel switching for fast image generation. Eight different watermelon samples of varying ripeness, type, dimensions, and origin are scanned and imaged. Comparisons with sample cross-sections show distinct differences in image characteristics based on watermelon maturity. Sugar concentration of unripe and ripe watermelon is also measured and plotted for further validation of the imaging technique.
Keywords: Food quality, Microwave imaging, Vivaldi antenna, Watermelon, Maturity, Ripeness
Graphical abstract
Highlights
-
•
Accurately determining watermelon maturity is important to reduce profit loss.
-
•
Microwave imaging exhibits distinct advantages over other imaging technologies.
-
•
A circular antenna array with automated rapid accurate microwave imaging.
-
•
Dielectric contrast within images distinguishes between ripe and unripe watermelon.
Abbreviations
- MWI
Microwave Imaging
- CV
Coplanar Vivaldi
- VSWR
Voltage Standing Wave Ratio
- VNA
Vector Network Analyzer
- MERIT
Microwave Radar-based Imaging Toolbox
- DAS
Delay and Sum
- USB
Universal Serial Bus
- GPIB
General Purpose Interface Bus
- SP12T
Single Pole Twelve Throw
- SPDT
Single Pole Double Throw
- USDA
United States Department of Agriculture
1. Introduction
Watermelon is a seasonal fruit, and accurately determining ripeness has long since been an interest to farmers and consumers alike. According to the United States Department of Agriculture (USDA) Economic Research Service, over 100,000 acres of watermelons were grown in the US in 2020, producing 38 million pounds (Agricultural Marketing Resource Center, 2021). Despite this large yield, various factors contribute to undesirable post-harvest loss which can negatively impact profits. For example, some watermelons are harvested prematurely or even after their optimal harvest date, meaning they end up being sold at a discounted price at the market. Most producers and marketers experienced loss of up to 40%, and some greater than 60% (Adepoju and Ologan, 2020). These profit losses demonstrate the need for a method of rapid, reliable scanning and imaging to accurately determine watermelon maturity.
Determining the ripeness of watermelon can be challenging. Farmers often determine ripeness based on time to maturity, field spot color, plant indicators such as the curly tendril on the main vine turning brown, or the leaf growing closest to the melon turning yellow (Dekker, 2022). Sometimes, consumers determine ripeness based on thumping the fruit to hear the noise, examining the rind's glossiness and texture, or even puncturing the rind with a fingernail to determine its firmness (Dekker, 2022). Though these methods may help eliminate completely unripe melons, they are often inaccurate, which may result in avoidable post-harvest loss.
Among various other imaging technologies in the food industry, microwave imaging (MWI) technology is advantageous in multiple aspects. It is contactless, nondestructive, and has both physical and chemical variations. It is fast, sensitive, safe, and offers direct internal characteristics measurement with wide frequency bandwidth(Meng et al., 2018; Ricci et al., 2022; Farina et al., 2018; Alsawaftah et al., 2022). Developing noninvasive imaging technology has been a focus of researchers to quickly and reliably image food to determine its characteristics (Arunkumar et al., 2021). The features of various technologies for food imaging are summarized in Table 1.
Table 1.
Features of various food imaging technologies.
| Feature | Ultrasound Spectroscopy | Near-Infrared Spectroscopy | Computer Vision | Hyperspectral Imaging | Electric Impedance | Microwave Imaging |
|---|---|---|---|---|---|---|
| Nondestructive | Yes | Yes | Yes | Yes | No | Yes |
| Contactless | No | Yes | Yes | Yes | No | Yes |
| Complexity | Low | Moderate | Moderate | High | Low | Low |
| Resolution | Low | Low | Low | High | Low | Moderate |
| Scanning Time | Fast | Fast | Moderate | Moderate | Moderate | Fast |
| 2D/3D | Both | Both | 2D | 3D | 2D | Both |
| Cost | Low | Low | Moderate | High | Moderate | Low |
| Size | Moderate | Moderate | Moderate | Moderate | Small | Moderate |
| Safety | Moderate | Low | High | Low | Moderate | High |
Spectroscopy methods exhibit strengths such as being contactless, nondestructive, rapid, accurate, and low-cost (Arrobas et al., 2015; Moustakas and Pitris, 2009; Guermazi et al., 2018; Karaliunas et al., 2019; Bhargava et al., 2021). However, they often provide low spatial resolution. Computer vision is contactless, nondestructive, and can be used on a wide range of foods but fails to measure internal characteristics (Nandhini et al., 2013; Siswantoro and Asmawati, 2016; Pfisterer et al., 2019) if taking natural image as input. Using MWI technology, food can be analyzed based on the variation of its dielectric properties. MWI has proven effective at analyzing a wide array of food products, such as detecting fine contaminants in hazelnut-cocoa cream (Tobon Vasquez, Scapaticci, Turvani, Ricci, Farina, Litman, Casu, Crocco and Vipiana, 2020), or effectively identifying the health status of eggs (Tai et al., 2020). MWI is also helpful to assess the quality of fruit to determine ripeness and distinguishing characteristics (Ghavami et al., 2019; Zidane et al., 2020). Many research efforts have been made on the determination of the internal quality of watermelon based on indices such as sugar content, firmness, and maturity (Sun et al., 2010), including spectroscopy for maturity detection (Jie et al., 2019). However, current state-of-the-art has made limited progress on complete microwave imaging of watermelon to determine its maturity.
Recent studies analyzing watermelon have mainly focused on determining the complex relative permittivity (Yoiyod, 2017) and sweetness or sugar content (Chawgien and Kiattisin, 2021; Isa et al., 2009) using destructive methods. Thus, the need for rapid, noninvasive microwave imaging is evident. MWI is accomplished by using single or multiple antennas directed toward a target to improve the image resolution. The electromagnetic waves traveling from the antenna radiation aperture are reflected or pass through the target based on its characteristics. The different dielectric properties of the target result in different reflection levels for the transmitted waves.
In this study, a noninvasive MWI system with an automated rapid scan mechanism is developed and demonstrated for determining the ripeness of various watermelons. Here, the words ripeness and maturity are used interchangeably. As a reference for determining watermelon ripeness, the sweetness (sugar distribution) is recorded. Multiple watermelons of varying species, origin, size, shape, and weight are scanned and imaged to determine their maturity.
2. Materials and methods
2.1. Antenna design
As MWI relies on electromagnetic wave propagation through food, the antenna is a critical component of the system. The antenna requirements include high efficiency, wideband, and symmetrical radiation pattern. Among the different types of available antennas, the Vivaldi antenna exhibits several advantages such as wide bandwidth, high gain, high efficiency, and simple fabrication. These benefits make it suitable for accurate microwave imaging. A Coplanar Vivaldi (CV) antenna is designed, simulated, and fabricated for integration in the automated MWI system as shown in Fig. 1.
Fig. 1.
(a) Simulated and (b) fabricated Coplanar Vivaldi antenna.
A total of ten CV antennas are fabricated with Rogers RO3003 substrate. The antenna dimensions are 62 × 55 × 0.76 mm3. Skin effect, the non-uniform distribution of AC current over the surface or skin of a conductor, is directly related to skin depth, the depth below the surface of the conductor at which the current density has fallen by a certain amount (Smith, 2017). Skin depth inversely increases with increased frequency, conductor diameter, and material permeability. Considering the high water content of watermelon, an antenna which supports a lower frequency band is required for adequate wave penetration. Thus, the lower band of the antenna is designed to be 2 GHz. Its bandwidth covers 2–8 GHz, and a high gain of 5–7 dBi is achieved. The simulated and measured radiation patterns at different frequencies are shown in Fig. 2. These exhibit directional radiation with high gain across a wide frequency range for both E− and H-planes. The simulated and measured voltage standing wave ratio (VSWR) is shown in Fig. 3. The proposed antenna exhibits excellent performance with less than 10% reflected power across its bandwidth from 2 to 8 GHz.
Fig. 2.
Coplanar Vivaldi antenna radiation patterns at different frequencies.
Fig. 3.
VSWR of Coplanar Vivaldi antenna.
2.2. System architecture
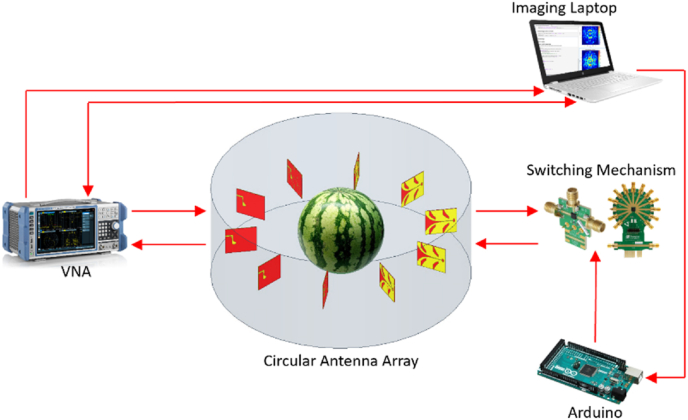
To obtain accurate microwave images of watermelon, the ten CV antennas are arranged in a circular fashion. Each antenna is positioned 36° from one another for full 360-degree scanning. The transmitting power is set to be −18 dBm which is approximately 105 smaller than that of cellular communication or WiFi signals according to the regulation from Federal Communications Commission (FCC), which is safe to human body (FCC, 2020). The radius of the circular scan area is 13.8 cm. To facilitate microwave imaging, an imaging laptop is connected to a vector network analyzer (VNA) using general purpose interface bus (GPIB). The laptop is also connected via universal serial bus (USB) to an Arduino microcontroller board which controls signals of low-loss RF switches for automated data collection. The simplified system architecture is depicted in Fig. 4.
Fig. 4.
Simplified block diagram of automated microwave imaging system.
2.3. Automated microwave imaging system
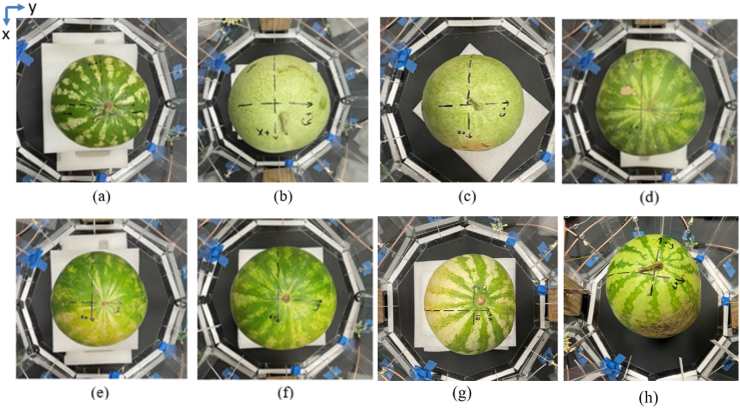
In the food industry, a method of rapid, instant, reliable scanning and imaging of food is critical. To meet this requirement, an automated microwave imaging system is designed and implemented with the antennas, RF switches, coaxial cables, Arduino, and laptop. A total of 10 antennas are placed around a watermelon, allowing a 360-degree angular field of view. Since the microwave imaging is accomplished by collecting the S21 transmission parameters for all channels, both ports 1 and 2 of the VNA are connected to a single pole twelve throw (SP12T) switch. This allows each antenna to either transmit or receive depending on channel selection. To control the switching logic, Arduino code is implemented with physical connections to each switch type. The two SP12T switches are controlled by the Arduino, and four control pins select between the 10 antennas. For each of the ten single pole double throw (SPDT) switches, -5V control voltage is provided to one of their two pins to either transmit or receive. The implemented switches support fast switching up to 10 ns, which can be used in future applications. All measurements were conducted at a consistent room temperature (21 °C) in the laboratory at The University of Alabama. To create an image of the target sample, the sample is first placed within the circular antenna array space. All eight watermelons scanned and imaged within the circular antenna array are shown in Fig. 5.
Fig. 5.
Watermelon samples (a) 1, (b) 2, (c) 3, (d) 4, (e) 5, (f) 6, (g) 7, and (h) 8within the circular antenna array.
After calibrating the VNA, computer code written in the C# language is run on the imaging laptop to save the transmission coefficients (i.e., S21 parameters) between all 45 channels. To generate an image from the measurements, the Microwave Radar-based Imaging Toolbox (MERIT) (O’Loughlin et al., 2019) is used. The algorithm utilizes reflected waves which occur due to the different dielectric materials within the watermelon including water, sugar, and fiber. Two sets of images are used to generate a watermelon image; one is a reference image which is made when no object is involved, while the other is a raw watermelon image. Then, two images are subtracted to remove artifact such as mutual coupling between antennas and reflection due to the surrounding measurement setup fixture. To obtain clear microwave images, delay-and-sum (DAS) beamforming algorithm is used. DAS is simple, robust, and computationally light. The signals are delayed by time (Ti) calculated from the observation location to the antennas. The focus point for imaging is assumed to be p = (x,y,z). The time delay (Tmn) is set as the time delay of the signal from the mth transmitting antenna to the nth receiving antenna. Tmn at the focal point p is expressed as (Kwon et al., 2016)
| (1) |
where v is velocity of the electromagnetic waves. m and n are index of the antennas and vary from 1 to 10, respectively. With 10 antennas, there are 45 channels which define the transmitting and receiving antenna pair. The delay time is calculated for each channel based on the focal point and relative permittivity. The field intensity of a reconstructed image is computed on all points of interest in watermelon. The integration window τ is determined by the input pulse width. In this study, τ is set to be 50% longer than the input pulse width. The intensity (Ip) of the reconstructed image at the given focal point can be written as
| (2) |
where wi is the location-dependent weight calculated during preprocessing, rxi is the received signal, M is the number of channels between transmitting and receiving antennas, and is the time delay ().
Automated data collection takes only 35 s, and image generation within MERIT takes approximately 60 s. Including the time taken to transfer data to the imaging laptop, the time required from data collection to image generation is about 2 min. This rapid scanning and imaging are implemented by the imaging laptop which has an 11th Gen Intel Core i7 processor operating at 2.50 GHz with 32.0 GB of useable RAM. The generated images can detect contrast of internal characteristics of the watermelon, specifically the concentration and distribution of water and sugar. In each image, high permittivity is categorized by strong heat signatures (red), which indicate the region where water concentration is greatest.
To further validate the distinguishing characteristics between ripe and unripe watermelon, the sugar content is measured. Though it varies by species, watermelons contain approximately 92% water and 6% sugar (Hicks-Hamblin, 2022). Despite sugar making up only a small percentage of watermelon, it is a key indicator of ripeness – the sugar content of watermelons continues to increase until fully mature (Zeng et al., 2014). A one-inch-thick slice is cut from the center of the watermelon where the image was taken. The slice is then cut into a 1 cm × 1 cm grid, and the juice from each individual piece is squeezed into a refractometer (VWR International, Radnor, PA, USA) to measure its sugar content in units of Brix. This enables a direct comparison to be made between the dielectric properties of the watermelon pulp and its sweetness depending on maturity level.
3. Results and discussion
A total of eight different watermelons are scanned and imaged using the MWI setup to determine their maturity. Ripeness is then validated based on sugar concentration and distribution. The detailed information including type, dimensions, weight, origin of production, and date and place of purchase is summarized in Table 2. To easily differentiate between ripe and unripe watermelons, the place of purchase is varied. Ripe watermelons are purchased from public grocery stores, whereas unripe watermelons are purchased from local farms. Images for vertical polarization are generated by placing the antennas in the vertical position (as shown in Fig. 4).
Table 2.
Watermelon samples imaged with the automated MWI system.
| Sample | Ripeness | Type | Length (cm) | Diameter (cm) | Weight (kg) | Origin of Production | Date of Purchase | Place of Purchase |
|---|---|---|---|---|---|---|---|---|
| Sample 1 | Unripe (3 weeks) | Crimson Sweet | 23.0 | 20.5 | N/A | Alabama | 06.29.2022 | Local Farm |
| Sample 2 | Unripe (3 weeks) | Charleston Gray | 43.0 | 19.5 | N/A | Alabama | 07.13.2022 | Local Farm |
| Sample 3 | Unripe (1.5 weeks) | Charleston Gray | 34.5 | 20.5 | 7.0 | Alabama | 07.20.2022 | Local Farm |
| Sample 4 | Ripe (7 days) | Red Seedless | 29.5 | 24.0 | N/A | North Carolina | 07.01.2022 | H Mart |
| Sample 5 | Ripe (5 days) | Red Seedless | 24.5 | 21.0 | 5.6 | South Carolina | 07.18.2022 | Publix |
| Sample 6 | Ripe (3 days) | Red Seedless | 26.5 | 22.5 | 7.0 | Indiana | 07.25.2022 | Walmart |
| Sample 7 | Ripe | Crimson Sweet | 21.5 | 20.5 | 4.60 | Alabama | 7.29.2022 | Farmers Market |
| Sample 8 | Unripe | Jubilee | 39.5 | 20.0 | 9.40 | Alabama | 07.28.2022 | Farmers Market |
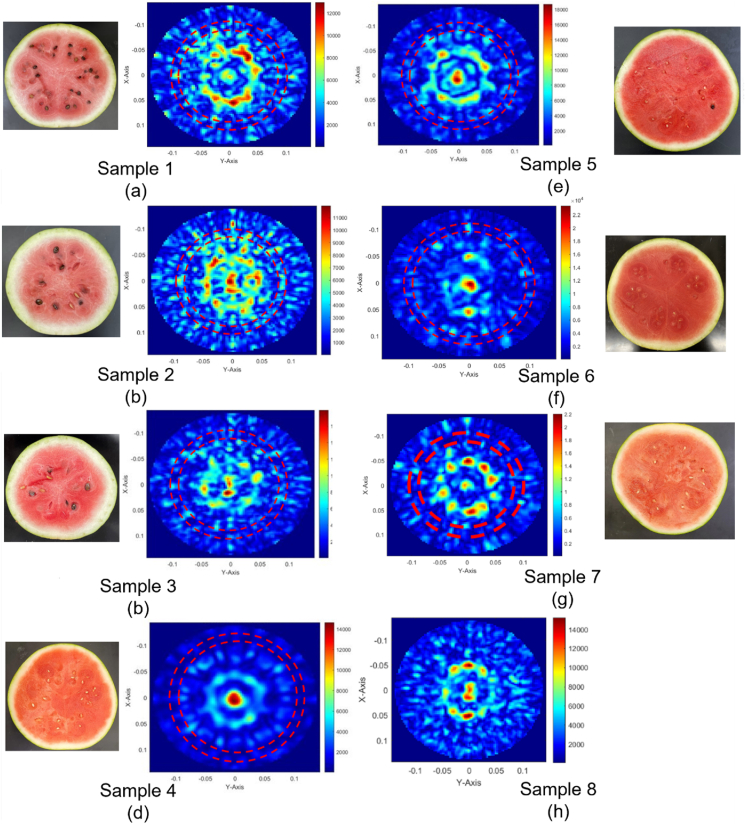
For each of the eight watermelons shown in Fig. 5, scans for all channels are collected and the resulting microwave images are generated. The cross-sections and images of all eight samples are provided in Fig. 6.
Fig. 6.
Camera photograph and microwave images for watermelon samples. (a) Sample 1,(b) Sample 2, (c) Sample 3, (d) Sample 4, (e) Sample 5, (f) Sample 6, (g) Sample 7, (h) Sample 8. Note that sample 8 camera photograph is not able to be taken due to on-going measurement.
3.1. Unripe watermelon
As a watermelon grows, distinct sectors (placentas) begin to enlarge throughout the pulp where nutrients are supplied to. Before the melon reaches maturity, the locule walls dividing these placentas appear white in color and is relatively low in water and sugar concentration (Shiu et al., 2016). Thus, water and sugar are mainly confined to the placentas along the outer circumference of the watermelon flesh, rather than within the heart. The cross-sections and images of unripe Samples 1, 2, and 3 are shown in Fig. 6(a–c).
As seen in its cross-section, Sample 1 exhibits many qualities of an unripe watermelon. The streaks of white locule walls dividing the pulp into sectors are clearly visible, leading to a lack of water and sugar in the center. Analyzing the image, it is important to note that the strongest signatures on the colormap (red) are associated with the highest relative permittivity. In this case, water has the highest permittivity. Thus, from the image, it can be determined that the highest water concentration is located at the center of the nutrient-rich placentas of the melon. These locations of high-water concentration are validated by the two concentric circles placed on the image. The outer circle represents the diameter of the melon, whereas the inner represents the rind/flesh interface. There is not much sugar at the nutrient sectors, nor is there much water in the center. Additionally, there is some scattering and irregularity in the image. The green and yellow signatures throughout the image imply that nutrients in the pulp are not yet evenly distributed. These are distinguishing qualities of an unripe watermelon on an MWI image.
The cross-section of Sample 2 exhibits slightly different qualities since it is a longer, narrower Charleston Gray melon. Despite this, the characteristics of an unripe watermelon remain. The fibrous white locule walls separate the inner flesh into placenta sectors where the nutrients are supplied to the center of the melon. Water content is still limited in the center, though slightly higher in the image due to the smaller diameter of the melon. The same signatures in the image are present near the locations of the nutrient sectors, indicating high water concentration and relatively low sugar concentration. Again, the green and yellow signatures throughout the image remain.
Sample 3 is another Charleston Gray watermelon, only one week riper than the previous sample. This is evident from the cross-section based on the slightly darker, redder pulp. Characteristics of an unripe melon can still be seen, such as the white locule walls in the center and sectors along the outer ring of the pulp with higher nutrient concentration. Comparing this to the image, the heart still lacks high water concentration as the signature is irregular. The placenta sectors can be seen around it, and there are still some scattered green and yellow signatures throughout. This sample is clearly riper than Sample 2 but still exhibits qualities of an unripe melon.
For further validation of unripe watermelon characteristics (i.e., sugar concentration), the sweetness of the sample was measured. From Fig. 6 (b), it is expected that this sample will have lower sugar concentration than a fully mature watermelon. The interpolated data for the sugar content of Sample 3 is shown in Fig. 7.
Fig. 7.
Sample 3 (a) measurement process and (b) interpolated sugar concentration map.
3.2. Completely ripe watermelon
As a watermelon develops, the placentas enlarge to take the whole volume within the locules. Therefore, when imaging ripe watermelons, it is expected that the water and sugar concentration and distribution is more even throughout the watermelon flesh. As such, both flesh color and water/sugar concentration should be uniform throughout the melon. The cross-sections and images of the ripe Samples 4 and6 are shown in Fig. 6 (d and f).
As shown in the cross-section of Sample 4, the fibrous locule walls has mostly disappeared, apart from the Y-shaped lines which meet in the center. This is an indicator that the nutrient-rich placentas have fully developed. Also, the flesh has taken on a deeper, pinkish-red hue. Despite this, remnants of unripe placentas can be seen. There are still darker red spots around the outside of the pulp, as well as in the center. Judging from the image, the highest water concentration is now at the center of the melon. The strong signatures seen surrounding the center near the placenta sectors have now decreased, suggesting that the water has moved closer to the center and that the sugar is more evenly distributed. Furthermore, the scattered green and yellow signatures seen in the unripe images have mostly disappeared. This image uniformity is a common characteristic of ripe watermelon.
For further validation of ripe watermelon characteristics, the sweetness of the sample is measured. From Fig. 6 (f), it is expected that this sample will have higher sugar concentration compared to unripe watermelon. The interpolated data for the sugar content of Sample 5 is shown in Fig. 8. Compared to Sample 3 in Fig. 7, this melon exhibits higher sweetness based on increased sugar concentration throughout the sample. Near the center, higher Brix values are present, and moderate sweetness values spread out farther and more evenly in the pulp than in the unripe Sample 3.
Fig. 8.
Sample 5 (a) measurement process and (b) interpolated sugar concentration map.
Reviewing the cross-section and image of Sample 6, the melon is ripe. Similar qualities such as the uniform flesh color and general absence of the white fibrous locule walls are clear indicators of watermelon maturity. Comparing once again to the image, the strongest signature is in the center where water concentration is highest. The minimal signatures surrounding the heart signify the remnants of possible unripe sectors in the melon which have now been mostly distributed throughout the flesh. The green and yellow signatures scattered throughout the unripe sample images have also all but disappeared.
3.3. Barely ripe watermelon
Barely ripe watermelons shows characteristics between a completely ripe and unripe watermelon. Water concentrations begin to transition to the edge of the heart of the watermelon. Water begins to concentrate in the center of the watermelon. Scattered patterns found in the unripe watermelons located in the placenta region begin to diminish.
Examining the cross-section of Sample 5, 7 and 8 show usual qualities of a ripe watermelon are present. The fibrous locule walls have mostly disappeared, and the flesh is dark-pink or red in color. Like Sample 4, the remnants of possible unripe sections can still be seen in the darker red areas around the outside of the pulp. Comparing the image to the cut watermelon, the signatures are not as uniform as the previous sample. However, the center signature is still dark red and circular in shape, meaning that the highest water concentration is in the center. The signatures of the placenta sectors have reduced, though not as much as Sample 4. This is partially due to the smaller diameter of the melon. As for the outer portions of the image, the scattered green and yellow signatures are mostly absent, allowing the conclusion to be made that this watermelon sample is ripe.
To summarize the image characteristics across samples, the differentiating features among watermelons discussed previously are provided in Table 3. For a more quantitative representation of the images, a few key statistics which differentiate between unripe and ripe watermelons are provided in Table 4. These values are calculated by separating a watermelon image into a three-by-three boxes. Each pixel displayed in Fig. 6 represents a relative permittivity contrast in a given location. The center box is then isolated from watermelon, mean and standard deviation is taken from these samples.
Table 3.
Differentiating features of watermelon images.
| Watermelon Maturity | Center | Inner Circumference | Outer Circumference |
|---|---|---|---|
| Unripe | Irregular, scattered | Stronger, prevalent | Scattered, green and yellow |
| Ripe | Uniform, strong | Minimal, weaker | Uniform, minimal |
Table 4.
Quantitative statistics of watermelon images.
| Watermelon Maturity | Sample | Mean | Standard Deviation |
|---|---|---|---|
| Completely | 4 | 0.0150 | 0.00731 |
| Ripe | 6 | 0.0159 | 0.00784 |
| Barely Ripe | 5 | 0.0169 | 0.00940 |
| 7 | 0.0177 | 0.00981 | |
| 8 | 0.0174 | 0.00949 | |
| Unripe | 3 | 0.0184 | 0.01077 |
| 2 | 0.0185 | 0.01111 | |
| 1 | 0.0187 | 0.01180 |
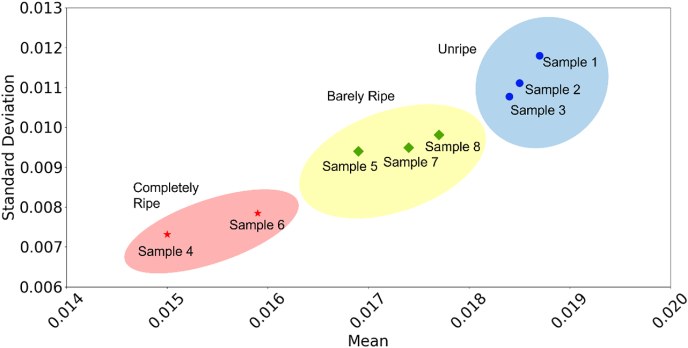
As shown in Table 4, the qualitative differentiating features extracted from the unripe and ripe watermelon images are of sound statistical basis. Comparing the samples, both the mean and standard deviation are consistently higher for the unripe watermelons. The higher mean can be attributed to the higher water concentration in the placenta sectors along the inner circumference of the flesh, as opposed to in the center. As for standard deviation, unripe watermelon images have higher values due to the starker color (and thus permittivity) contrast. Unripe watermelon images have greater scattering and irregularity which corresponds to a higher standard deviation. Shown in Fig. 9 is a chart of mean permeability on a scale of completely ripe to unripe. In the center, there is a middle ground where values are in scale. These can be defined as barely ripe, this contains samples 5, 8, and 7. The samples in this categories have a progression of high intensity of water in the center, and still contain scattering throughout the watermelon. This is unnoticeable for human consumption, but the watermelons have not fully developed the characteristics of a ripe watermelon.
Fig. 9.
Watermelon maturity for eight watermelon samples. X and y axis are the mean and standard deviation of the normalized microwave images.
4. Further remarks
The proposed MWI system offers several advantages. It can switch bands based on different target characteristics and contains broadband antennas and switches. It is cost-effective relative to other imaging technologies such as X-ray, millimeter wave, or hyperspectral imaging, requires no chamber for scanning, and is capable of being deployed on inline food production lines in food factories.
The watermelon samples scanned and imaged in this project vary in maturity, type, dimensions, weight, and origin. Although it is important to image varied samples to obtain an accurate representation of different watermelons, a set of eight samples have its limitations. Image characteristics can vary from type to type when comparing them at different stages of maturity due to water and sugar contents (Ito and Sugiyama, 2000). The diameters of the imaged watermelon vary from 19.5 cm to 24 cm. Future research would include a variety of different sizes of watermelon samples to classify each watermelon type at progressive stages of maturity and to validate the proposed microwave imaging system. Even then, it is crucial to note that the same watermelon species from different origins could result in slight image differences. At the time of this study, the sample 8 has not been cut to evaluate the level of deterioration over time.
The MWI system with a circular antenna array is well-suited for imaging watermelon samples of different types. If the different sized fruits are to be imaged, the antenna array will need to be adjusted according to the size of the fruit. However, future work looks to expand the array by adding more antenna elements. This will not only increase the resolution of the image but should also improve its symmetry (Lagovsky et al., 2016). The proposed CV antennas can also be improved with higher gain for deeper penetration of the watermelon, leading to higher contrast in reconstructed image (Abushakra et al., 2022). The images for watermelon samples in this project were generated with vertical antenna polarization, though minimal scans were taken using horizontal polarization. These images resulted in distinctly different, yet possibly still useful data. Creating antennas with dual polarization would allow more data to be garnered from each sample image to better determine the watermelon maturity (Abou-Khousa et al., 2018).
5. Conclusions
The proposed MWI system with automatic channel switching capability generated images to effectively distinguish between watermelon samples of various maturity stages. A novel Coplanar Vivaldi antenna was designed and fabricated to offer wide bandwidth, high gain, and high efficiency for adequate penetration and determination of internal characteristics of watermelon. The circular antenna array for the MWI system was well-suited for watermelon samples, and scan data and microwave images were saved and generated quickly. Comparing the cross-sections of the watermelon samples to their respective images allowed for accurate maturity determination as validated by the sugar concentration measurements.
Funding
This work was supported by the USDA and Alabama Department of Agriculture and Industries [Award number: AM200100XXXXG028] and partially supported by USDA (2022-67021-36866).
CRediT authorship contribution statement
Joe Garvin: Methodology, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Writing – review & editing, Visualization. Feras Abushakra: Methodology, Software, Investigation, Resources, Writing – original draft, Writing – review & editing, Visualization. Zachary Choffin: Methodology, Validation, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization. Bayley Shiver: Methodology, Software, Validation, Investigation, Data curation. Yu Gan: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Lingyan Kong: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition. Nathan Jeong: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Resources, Data curation, Writing – original draft, Writing – review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Nathan Jeong reports financial support was provided by USDA and Alabama Department of Agriculture. Yu Gan reports financial support was provided by US Department of Agriculture.
Acknowledgments
The authors acknowledge Larry Turner of New Lexington Farms in New Lexington, AL for providing Alabama-grown Crimson Sweet and Charleston Gray watermelons, as well as Sara Ghulamani and Shiori Johnson for assisting in sugar content measurements of watermelon. Special thanks to the previous UA Capstone senior design team of John Kramer, Andrew Ridenour, and Darian Wilson for assisting in development of preliminary hardware and software.
Data availability
The data that has been used is confidential.
References
- Abou-Khousa M.A., Rahman M.S.U., Xingyu X. Dual-polarized microwave imaging probe. IEEE Sensor. J. 2018;19(5):1767–1776. [Google Scholar]
- Abushakra F., Jeong N., Awasthi A., Kolpuke S., Simpson C., Reyhanigalangashi O., Elluru D., Bhandari A., Taylor D., Gogineni S. Ultra-wideband coplanar Vivaldi antenna array with dielectric patch antenna for grating lobes suppression. IEEE Access. 2022;10:54410–54420. [Google Scholar]
- Adepoju A., Ologan O. Post-harvest loss along the watermelon value chain in the tropics. Int. J. Veg. Sci. 2020;27:1–11. doi: 10.1080/19315260.2020.1848961. [DOI] [Google Scholar]
- Agricultural Marketing Resource Center . 2021. Watermelon.https://www.agmrc.org/commodities-products/vegetables/watermelon Retrieved from. Accessed. [Google Scholar]
- Alsawaftah N., El-Abed S., Dhou S., Zakaria A. Microwave imaging for early breast cancer detection: current state, challenges, and future directions. Journal of Imaging. 2022;8:123. doi: 10.3390/jimaging8050123. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrobas B.G., Ciaccheri L., Mencaglia A.A., Rodriguez-Pulido F.J., Stinco C., González-Miret M.L., Heredia F.J., Mignani A.G. vols. 1–4. 2015. Raman spectroscopy for analyzing anthocyanins of lyophilized blueberries. (Proceedings of the 2015 IEEE SENSORS). [DOI] [Google Scholar]
- Arunkumar M., Rajendran A., Gunasri S., Kowsalya M., Krithika C. Non-destructive fruit maturity detection methodology - a review. Mater. Today Proc. 2021 doi: 10.1016/j.matpr.2020.12.1094. 2021. [DOI] [Google Scholar]
- Bhargava N., Mor R.S., Kumar K., Sharanagat V.S. Advances in application of ultrasound in food processing: a review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105293. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawgien K., Kiattisin S. Machine learning techniques for classifying the sweetness of watermelon using acoustic signal and image processing. Comput. Electron. Agric. 2021;181 doi: 10.1016/j.compag.2020.105938. 2021. [DOI] [Google Scholar]
- Dekker S., Gardener's Path How to pick a ripe watermelon. 2022. https://gardenerspath.com/plants/fruit/harvest-watermelon/ Retrieved from. Accessed.
- Farina L., Vasquez J.A.T., Scapaticci R., Rivero J., Crocco L., Litman A., Vipiana F. Proceedings of the 2018 18th Mediterranean Microwave Symposium. MMS); 2018. In-line monitoring of food contamination via microwave imaging; pp. 382–383. [DOI] [Google Scholar]
- FCC . 2020. Wireless Devices and Health Concerns.https://www.fcc.gov/consumers/guides/wireless-devices-and-healthconcerns#:~:text=For%20exposure%20to%20RF%20energy,the%20relevant%20RF%20exposure%20limit [Google Scholar]
- Hicks-Hamblin K., Gardener's Path . 2022. The Taste of Summer: How to Plant and Grow Watermelons.https://gardenerspath.com/plants/fruit/grow-watermelons/ Retrieved from. Accessed. [Google Scholar]
- Ghavami N., Sotiriou I., Kosmas P. Proceedings of the 2019 13th European Conference on Antennas and Propagation. EuCAP); 2019. Experimental investigation of microwave imaging as means to assess fruit quality. 1–5. [Google Scholar]
- Guermazi M., Fendri A., Kanoun O., Derbel N. Potential of impedance spectroscopy for real-time assessing of food quality. IEEE Instrum. Meas. Mag. 2018;21:44–48. doi: 10.1109/MIM.2018.8573593. 2018. [DOI] [Google Scholar]
- Isa M.M., Ibrahim N., Shamsudin R., Marhaban M.H. Proceedings of the 2009 IEEE Student Conference on Research and Development (SCOReD) 2009. Sugar content in watermelon juice based on dielectric properties at 10.45GHz. 529–532. [DOI] [Google Scholar]
- Ito H., Sugiyama J. vol. 579. 2000. Nondestructive harvest time decision of melons by a portable firmness tester; pp. 367–371. (II Balkan Symposium on Vegetables and Potatoes). October. [Google Scholar]
- Jie D., Zhou W., Wei X. Nondestructive detection of maturity of watermelon by spectral characteristic using NIR diffuse transmittance technique. Sci. Hortic. 2019;257 doi: 10.1016/j.scienta.2019.108718. 2019. [DOI] [Google Scholar]
- Karaliunas M., Dapšys I., Urbanowicz A., Vektaris G., Vektariene A., Bražinskiene D., Asadauskas S., Kasalynas I., Valušis G. 2019. Inspection of oils, caffeine containing foods and consumable plant leaves by time-domain THz spectroscopy. (Proceedings of the 2019 44th International Conference on Infrared, Millimeter, and Terahertz Waves (IRMMW-THz)). 1–2. [DOI] [Google Scholar]
- Lagovsky B., Samokhin A., Shestopalov Y. 2016. Increasing effective angular resolution of measuring systems based on antenna arrays; pp. 432–434. (2016 URSI International Symposium on Electromagnetic Theory (EMTS)). [Google Scholar]
- Meng Z., Wu Z., Gray J. Microwave sensor technologies for food evaluation and analysis: methods, challenges and solutions. Trans. Inst. Meas. Control. 2018;40:3433–3448. doi: 10.1177/0142331217721968. [DOI] [Google Scholar]
- Moustakas C., Pitris C. vols. 1–3. 2009. Raman spectroscopy for determining nutritional facts. (Proceedings of the 2009 9th International Conference on Information Technology and Applications in Biomedicine). [DOI] [Google Scholar]
- Nandhini P., Jaya J., George J. Proceedings of the 2013 International Conference on Current Trends in Engineering and Technology. ICCTET); 2013. Computer vision system for food quality evaluation — a review. 85–87. [DOI] [Google Scholar]
- O’Loughlin D., Elahi M.A., E P.e.a. 2019. Microwave Radar-Based Imaging Toolbox: Efficient Reconstruction Software.https://github.com/EMFMed/MERIT [Google Scholar]
- Pfisterer K.J., Amelard R., Syrnyk B., Wong A. Proceedings Of the 2019 IEEE/CVF Conference On Computer Vision And Pattern Recognition Workshops (CVPRW), 490–492. 2019. Towards computer vision powered color-nutrient assessment of puréed food. [DOI] [Google Scholar]
- Ricci M., Crocco L., Vipiana F. Proceedings Of the 2022 16th European Conference On Antennas And Propagation (EuCAP), 1–3. 2022. A microwave imaging device for detecting contaminants in water-based food products. [DOI] [Google Scholar]
- Shiu J.W., Slaughter D.C., Boyden L.E., Barrett D.M. Correlation of descriptive analysis and instrumental puncture testing of watermelon cultivars. J. Food Sci. 2016;81(6):S1506–S1514. doi: 10.1111/1750-3841.13316. [DOI] [PubMed] [Google Scholar]
- Siswantoro J., Asmawati E. Proceedings Of the 2016 2nd International Conference On Science In Information Technology (ICSITech), 74–78. 2016. A new framework for measuring volume of axisymmetric food products using computer vision system based on cubic spline interpolation. [DOI] [Google Scholar]
- Smith J., Circuit Globe . 2017. Skin Effect.https://circuitglobe.com/skin-effect.html Retrieved from. Accessed. [Google Scholar]
- Sun T., Huang K., Xu H., Ying Y. Research advances in nondestructive determination of internal quality in watermelon/melon: a review. J. Food Eng. 2010;100:569–577. doi: 10.1016/j.jfoodeng.2010.05.019. 2010. [DOI] [Google Scholar]
- Tai T.C., Wu H.W., Hung C.Y., Wang Y.H. Food security sensing system using a waveguide antenna microwave imaging through an example of an egg. Sensors. 2020:20. doi: 10.3390/s20030699. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobon Vasquez J.A., Scapaticci R., Turvani G., Ricci M., Farina L., Litman A., Casu M.R., Crocco L., Vipiana F. Noninvasive inline food inspection via microwave imaging technology: an application example in the food industry. IEEE Antenn. Propag. Mag. 2020;62:18–32. doi: 10.1109/MAP.2020.3012898. 2020. [DOI] [Google Scholar]
- Yoiyod P. Proceedings Of the 2017 International Symposium On Antennas And Propagation (ISAP), 1–2. 2017. Complex relative permittivity analysis for monitoring watermelon. [DOI] [Google Scholar]
- Zeng W., Huang X., Müller-Arisona S., et al. Classifying watermelon ripeness by analysing acoustic signals using mobile devices. Personal Ubiquitous Comput. 2014;18:1753–1762. doi: 10.1007/s00779-013-0706-7. [DOI] [Google Scholar]
- Zidane F., Lanteri J., Marot J., Brochier L., Joachimowicz N., Roussel H., Migliaccio C. Nondestructive control of fruit quality via millimeter waves and classification techniques: investigations in the automated health monitoring of fruits. IEEE Antenn. Propag. Mag. 2020;62:43–54. doi: 10.1109/MAP.2020.3003222. 2020. [DOI] [Google Scholar]
- Kwon S., Lee S. Recent Advances in Microwave Imaging for Breast Cancer Detection. Int. J. Biomed. Imag. 2016;2016 doi: 10.1155/2016/5054912. Article ID 5054912, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.