Abstract
Mycoplasma penetrans is a newly isolated Mollicute from the urine of patients infected with human immunodeficiency virus that demonstrates the capacity to adhere to and invade human cells. A previous report, based on assays with mouse red blood cells (RBCs), indicated that M. penetrans lacked hemolytic activity. In our studies, we incubated different isolates of M. penetrans with various RBC species and observed hemolytic zones surrounding individual mycoplasma colonies. All M. penetrans strains displayed hemolysis after 2 to 3 days of incubation. Hemolytic activity diffused from single colonies, eventually causing complete lysis. Hemolysis was most pronounced with sheep RBCs, followed by horse, chicken, and human cells. Furthermore, hemolytic activity was demonstrable in both intact mycoplasma cell preparations and spent culture supernatant. However, unlike intact mycoplasmas, the hemolytic activity in the supernatant was dependent on the reducing agent, cysteine. In addition to hemolysis, a brown precipitate was closely associated with mycoplasma colonies, suggesting oxidation of hemoglobin. Absorption spectra indicated that hemoglobin was oxidized to methemoglobin, and the addition of catalase demonstrated H2O2-mediated hemoxidation. Other experiments suggested that hemoxidation enhanced total hemolysis, providing the first evidence of both hemolytic and hemoxidative activities in M. penetrans.
Mycoplasmas, members of the cell wall-less Mollicutes and considered among the smallest self-replicating cells, have been detected in a wide range of hosts, including humans, other vertebrates, invertebrates, and plants (2, 19, 20, 27). In previous studies of pathogenic mycoplasmas, hemadsorption and cytadherence activities were closely associated with virulence potential (2, 30). For example, hydrogen peroxide-mediated and membrane-associated hemolytic activities have been found in numerous Mycoplasma species (15, 24, 30), and analysis of mycoplasma genomic sequences revealed the presence of a hemolysin-like gene (VXpSPT7_orf424) in Mycoplasma pneumoniae (13) and a homologous gene (MG146) in Mycoplasma genitalium (10). It is well known that bacterial hemolysins lyse erythrocytes (RBCs) and a variety of other cell types, including mast cells, neutrophils, and polymorphonuclear cells, which enables hemolytic microorganisms to directly damage host tissues as well as induce inflammatory responses (5). However, a new species, Mycoplasma penetrans, which was isolated from the urine of patients infected with human immunodeficiency virus (22), was shown to actively invade mammalian cells in culture (21) and cause cytopathology in experimentally infected chicken embryos (12) but lack hemolytic activity (24). In this report we describe hemolytic activity in all isolates of M. penetrans and show that H2O2-mediated hemoxidative activity contributes to total mycoplasma-mediated hemolysis.
MATERIALS AND METHODS
Bacterial strains and medium.
M. penetrans cells (GTU-54 and isolates from France and Texas) (11) were obtained from J. Tully (National Institute of Allergy and Infectious Diseases, Bethesda, Md.). These strains had been isolated from AIDS patients and passaged less than 10 times in vitro. Upon receipt, each strain was grown in SP-4 broth (21) and frozen at −70°C. New cultures were initiated from frozen stocks.
Culture media.
For the detection of hemolytic activity in culture supernatant fluids, it was necessary to remove phenol red from the SP-4 medium because the dye interfered with spectrophotometric assays. Therefore, in specific experiments, M. penetrans was grown in SP-M medium, which is a modification of SP-4 that contains 20% (instead of 17%) heat-inactivated serum and 5% (instead of 3.5%) yeast extract and lacks CMRL medium (Gibco-BRL, Grand Island, N.Y.) and phenol red. Mycoplasma cultures were incubated statically in 100 ml of SP-M or SP-4 broth in glass bottles at 37°C for 48 h, harvested by centrifugation at 12,000 × g for 15 min, washed three times with phosphate-buffered saline (PBS) and used immediately or stored as cell pellets at −70°C.
Hemolytic activity on plates.
Exponentially growing M. penetrans cells in SP-M broth were diluted in fresh medium to a cell density of 2 × 103 mycoplasmas per ml, and samples (0.1 ml) were plated on SP-M agar. After 1 week of incubation, the plates were examined for mycoplasma colonies. To detect hemolysis, we overlaid individual plates with a sterile mixture of 0.75% agar in PBS (pH 7.4 and kept molten at 42°C) plus PBS-buffered RBCs at a final concentration of 2%. After 2 to 3 days, the plates were examined for hemolysis. Sheep RBCs were received from BioWhittaker, Walkersville, Md., and horse and chicken RBCs were received from Pel Freez, Rogers, Ark. Human blood was collected from a healthy donor. Since aged RBCs tended to provide variable results, RBCs were used immediately or stored at 4°C and used within 1 week of receipt.
Sample preparation for hemolytic activity.
M. penetrans cultures grown in SP-M broth were centrifuged at 10,000 × g for 20 min at 4°C. Cells and culture supernatants were collected aseptically at various growth stages and stored at −70°C until analyzed. To measure hemolytic activity in sonicated cell fractions, we resuspended M. penetrans cells in PBS and then subjected them to ultrasonic disruption with a Braun sonicator (three 20-s periods at 40 W with 45-s intervals on ice between periods). Unbroken cells were removed by centrifugation at 10,000 × g for 15 min. PBS served as a control. Protein concentrations of mycoplasma preparations were determined using the bicinchoninic acid protein assay kit (Pierce, Rockford, Ill.).
Spectrophotometric assessment of hemolysis and hemoxidation.
Hemolytic activity was determined as described by Chu and Holt (7) with the following modifications. M. penetrans cells and sonicated cellular fractions at 50 μg of mycoplasma protein/100 μl, or 100 μl of culture-grown supernatants, were preincubated with cysteine (see Table 2 for cysteine concentrations) and then added to sheep RBCs (final concentration, 1% RBCs) in PBS. Heat-inactivated samples (100°C for 10 min) of each test preparation were used as baseline controls since heat inactivation abolished hemolytic and hemoxidative activities. Test samples were placed in a rotatory shaker (100 rpm) at 37°C for 3 h. To detect RBC lysis, we added 0.1 ml of each test mixture to 0.9 ml of PBS and subjected them to centrifugation at 1,500 × g for 10 min and measurement of released hemoglobin by determination of the optical density at 405 nm in a Shimadzu UV-160 spectrophotometer. The highest dilution that demonstrated 50% hemolysis was considered 1 hemolytic unit. All reactions were performed in triplicate. To measure hemoxidation, defined as the oxidation of hemoglobin to methemoglobin, we added 0.4 ml of the same test preparations used for hemolytic activity to 1.6 ml of distilled water to lyse RBCs. Released methemoglobin was determined by measuring supernatant absorbance at 630 nm (6). Hydrogen peroxide (30%, wt/wt) and catalase were purchased from Sigma Chemical Co., St. Louis, Mo.
TABLE 2.
Effect of cysteine on M. penetrans hemolytic activity
| Cysteine concn (mM) | Hemolytic activitya |
|---|---|
| 0 | 2.98 ± 0.09 |
| 0.5 | 3.09 ± 0.18 |
| 1.0 | 3.09 ± 0.18 |
| 3.0 | 16.47 ± 0.85 |
| 6.0 | 34.03 ± 1.04 |
| 12.0 | 9.01 ± 1.80 |
Assayed using intact mycoplasma cells. Hemolytic activity was assayed as described in Materials and Methods. Values represent the means ± standard deviations of three independent determinations at each cysteine concentration.
RESULTS AND DISCUSSION
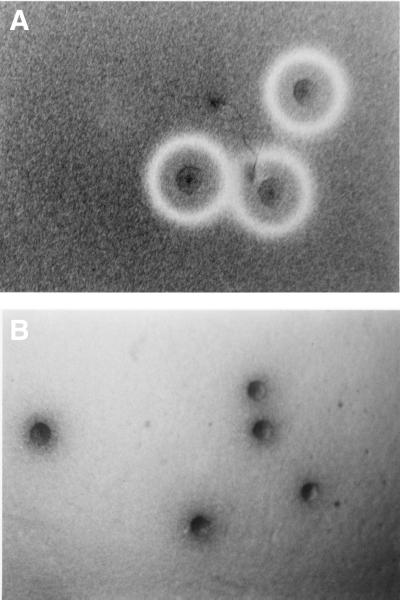
M. penetrans GTU-54 colonies are visible microscopically on SP-M agar plates on day 3 of incubation at 37°C, and by day 7 they can be observed with the unaided eye. At this stage, the colonies were overlaid with a mixture of sheep RBCs in molten agar. Within 2 h of incubation, an area of greening or incomplete hemolysis was evident at the leading edges of the mycoplasma colonies. Clear zones of beta-hemolysis were apparent around the colonies after 48 h (Fig. 1A). Within 7 to 10 days, hemolytic zones began to coalesce, and eventually hemolysis of the entire plate occurred (Fig. 1B). The observed hemolytic activity is consistent with the release and diffusion into the medium of a soluble hemolysin from mycoplasma cells. In addition, a brown precipitate formed only around individual colonies (Fig. 1B). Since the hemolytic activity of M. penetrans was reported to be negative in an earlier communication (24), different M. penetrans isolates were analyzed. All strains exhibited similar hemolytic zones.
FIG. 1.
Hemolytic and hemoxidative activities of M. penetrans. Colonies of M. penetrans strain GTU-54 on SP-M plates were overlaid with a mixture of 1% agar plus 2% sheep blood and incubated in air-CO2 at 37°C. The colonies were photographed at 9 (A) and 15 (B) days. Hemolytic zones are visible in panel A, and the precipitate is readily observed in panel B.
The hemolytic activity of M. penetrans on sheep RBCs was compared to that on other RBC species. Hemolytic activity on horse RBCs showed a smaller zone of hemolysis after a 48-h incubation, and even smaller zones were observed with chicken RBCs. In similar experiments using human blood, pinpoint zones of hemolysis were detected (Table 1). The different hemolytic intensities among RBC species may explain the previously reported negative results since RBCs from different animal species vary in sensitivity to bacterial hemolysins, including Leptospira interrogans (25), Staphylococcus aureus (4), certain Vibrio species (14, 18, 35, 36), and Borrelia burgdorferi (34). Concerning M. penetrans hemolytic activity, the molecular basis of the difference in RBC sensitivity is unknown. Mycoplasma penetrans hemolysin(s) may bind to specific RBC receptors, act on distinct molecules in RBC membranes, or bind to target molecules that are more abundant in sheep RBCs.
TABLE 1.
Hemolytic activity of M. penetrans colonies using different RBC species
| RBC | Hemolytic zonea |
|---|---|
| Chicken | ++ |
| Horse | +++ |
| Human | + |
| Sheep | ++++ |
Symbols indicate size and intensity (+, pinpoint; ++++, complete).
To test for cold-induced hemolytic activation, we incubated M. penetrans-RBC plates at 37°C until incomplete zones of hemolysis appeared (24 h) and then transferred selected plates to 4°C and incubated them overnight. The zones of hemolysis were not increased; however, the opaque hemolytic zones seen at 37°C cleared during cold induction, indicating an enhancement of hemolysis during the hot-cold shift (data not shown). Hemolytic activity induced by hot-cold incubation has been reported for the hemolysins of Leptospira (3, 25, 29), the beta-hemolysins of Borrelia (34) and Staphylococcus (28), and the alpha-toxin of Clostridium (32). In these bacteria, the hemolysins are classified as phospholipase A and/or phospholipase C. Therefore, the observed cold enhancement of hemolytic activity of M. penetrans suggests a similar type of hemolysin. It has been reported that M. penetrans possesses a membrane-associated phopholipase C (26). However, the involvement of other M. penetrans enzymes, such as proteases or additional phospholipases, in hemolysis cannot be eliminated.
Soluble M. penetrans hemolytic activity was monitored spectrophotometrically. Preliminary experiments using culture supernatants consistently yielded negative results, which conflicted with the observed diffusible hemolysis on plates. Possible oxidation of the hemolysin(s) may have occurred during the manipulation of the soluble fractions. To prevent or reverse oxidative damage to the hemolysin(s), we added the reducing agent cysteine to the culture supernatant for 30 min prior to performing hemolytic assays. Under these conditions, hemolytic activity was readily demonstrable (see Fig. 3). In agar plates, hemolysis occurred without added cysteine, possibly due to the partial anaerobic environment that accompanies the overlay agar procedure. To further characterize hemolytic activity in M. penetrans cells, we incubated intact mycoplasmas with sheep RBCs. As expected, and in contrast to culture supernatant and sonicated cell preparations, intact cells exhibited hemolytic activity (2.98 ± 0.09 U/ml) in the absence of added cysteine. However, to maximize hemolytic activity in intact cells, we added various cysteine concentrations to test samples. Preincubation of intact mycoplasmas with 6 mM cysteine for 30 min enhanced hemolytic activity more than 10-fold (Table 2). Increased cysteine concentrations caused a precipitation due to the reaction of cysteine with released hemoglobin (Hb). Also, the observed hemolytic activation by cysteine suggests that a sulfhydryl group(s) may be essential for hemolytic activity, as described with other oxygen-labile hemolysins including streptolysin O (16), pneumolysin (33), perfringolysin O (31), and listeriolysin O (23). On the basis of our observations with cysteine activation, the M. penetrans hemolysin may also contain a cysteine residue that must be present in a reduced state for lytic activity.
FIG. 3.
Hemolytic and hemoxidative activities of M. penetrans. Aliquots (100 μl) of intact and sonicated cell preparations, which were preincubated with 6 mM cysteine for 30 min, were incubated with 1% sheep RBCs for 3 h at 37°C and photographed. RBC lysis and brown precipitate are readily observed in the treated samples and absent in the PBS-RBC control.
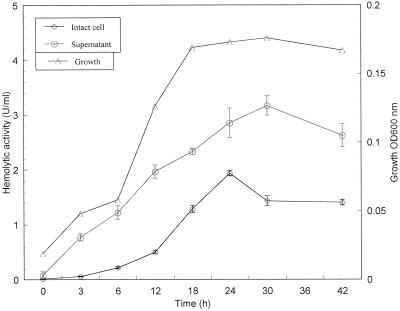
We detected differences in the hemolytic activity of intact cell preparations at various stages of the mycoplasma growth phase (Fig. 2). For example, activity was low during the initial hours of growth and reached maximal levels at later stages (19.28 ± 0.51 at 24 h) followed by decreasing values. Like intact cells, spent culture supernatant exhibited less hemolytic activity during early mycoplasma growth. However, maximal levels were observed only at stationary phase (31.63 ± 1.76 at 30 h [Fig. 2]). Furthermore, differences were detected in cell-associated and extracellular hemolytic activities, indicating that nearly two-thirds of the total hemolytic activity was associated with the culture supernatant (Fig. 2).
FIG. 2.
Relationship between M. penetrans GTU-54 growth and hemolytic activities. Mycoplasmas were grown in SP-M medium at 37°C, and the growth stage and extent of hemolytic activity associated with intact cells were compared. Results are the mean and standard deviation of three independent determinations at each time point.
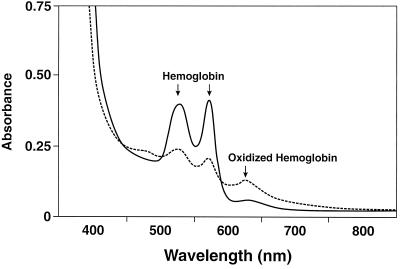
It was interesting that intact or sonicated M. penetrans cell preparations caused a brown precipitate to form during a 3-h incubation with sheep RBCs (Fig. 3). A similar precipitate was observed when spent supernatant was incubated with RBCs (data not shown). Chu et al. reported similar observations with Treponema denticola (8). To determine whether the brown precipitate was due to Hb oxidation, we incubated sheep RBCs with spent supernatant for 3 h, pelleted and lysed the RBCs in distilled water, and analyzed the supernatant for Hb content. Heat-inactivated spent media served as negative controls. We used multiwavelength rapid-scanning spectrophotometry to measure the interconversion of spectrally distinct Hb derivatives. Spectral scans (400 to 800 nm) were recorded at fixed intervals over specified periods (Fig. 4). Comparisons of the Hb absorption spectra of control versus treated samples show a distinct shift (interconversion) from 540 and 575 nm to 630 nm, indicating the oxidation of Hb to methemoglobin (metHb). Treatment of sheep RBCs with sonicated or intact cells also gave similar results (data not shown).
FIG. 4.
Spectral changes induced in sheep RBC Hb by M. penetrans. Sheep RBCs were treated with either 100 μl of spent supernatant (test sample, –––) or 100 μl of heat-inactivated spent supernatant (control, ——) from late-log-phase cultures of M. penetrans. After incubation for 3 h, RBCs were lysed with water. Increases at 630 nm and decreases at 540 and 575 nm (arrows) indicate the conversion of Hb to metHb. Each value represents three independent determinations.
Since the formation of metHb enhances hemolysis (9), we monitored the effect of exogenous H2O2 on sheep RBC lysis. We observed no direct lysis of RBCs by H2O2 (20 to 200 nM/ml). However, the color of RBCs changed from red to brown, and the absorption spectra of H2O2-treated RBCs exhibited an absorbance peak at 630 nm, which was similar to the spectrum observed in the M. penetrans-treated samples (Fig. 4). This suggested that H2O2 produced by mycoplasmas or related mycoplasma molecules mediated the oxidation of Hb to metHb. However, these results did not resolve the possible relationships between M. penetrans hemolytic and hemoxidative activities and the cumulative effects of H2O2 on hemolysis. For example, Barnard and Stinson (1) reported that H2O2 mimics alpha-hemolysin, and Kellogg and Fridovich (17) observed protection by catalase against both H2O2-mediated Hb oxidation and RBC lysis. To further elucidate the role of H2O2 on M. penetrans-mediated hemolysis and hemoxidation, we added catalase to mycoplasma samples (intact cells, sonicated cell fractions, and culture-grown supernatants) prior to assaying for hemolytic activity. When 1 hemolytic unit of spent supernatant was incubated with 100 U of catalase for 10 min at 37°C, 29% ± 1% of the activity was inactivated. Using intact mycoplasma cells as the source of hemolytic activity, we observed that catalase inactivated 1 unit of hemolytic activity by 24% ± 2%. Thus, the presence of exogenous catalase reduced but did not abolish hemolytic activity, reinforcing the distinct nature of M. penetrans hemolytic activity. However, no black precipitation was observed in catalase-treated test samples, and spectrophotometric analysis indicated the absence of the metHb peak. Thus, it appears that hemoxidation is directly linked to mycoplasma-generated H2O2. Further studies concerning the nature and pathological impact of hemolytic and hemoxidative activities of M. penetrans on mammalian cells should clarify their roles in virulence and disease pathogenesis.
ACKNOWLEDGMENTS
We are grateful to L. Chu and S. C. Holt for helpful experimental advice.
This work was supported by grant AI 41010 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Barnard J P, Stinson M W. The alpha-hemolysin of Streptococcus gordonii is hydrogen peroxide. Infect Immun. 1996;64:3853–3857. doi: 10.1128/iai.64.9.3853-3857.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baseman J B, Reddy S P, Dallo S F. Interplay between mycoplasma surface proteins, airway cells, and the protean manifestations of mycoplasma-mediated human infections. Am J Respir Crit Care Med. 1996;154:S137–S144. doi: 10.1164/ajrccm/154.4_Pt_2.S137. [DOI] [PubMed] [Google Scholar]
- 3.Bauer D C, Eames L N, Sleight D, Ferguson L C. The significance of leptospiral hemolysin in the pathogenesis of Leptospira pomona infections. J Infect Dis. 1961;108:229–236. doi: 10.1093/infdis/108.2.229. [DOI] [PubMed] [Google Scholar]
- 4.Bernheimer A W. Staphylococcal alpha toxin. Ann N Y Acad Sci. 1965;128:112–123. doi: 10.1111/j.1749-6632.1965.tb11633.x. [DOI] [PubMed] [Google Scholar]
- 5.Braun V, Focareta T. Pore-forming bacterial protein hemolysins (cytolysins) Crit Rev Microbiol. 1991;18:115–158. doi: 10.3109/10408419109113511. [DOI] [PubMed] [Google Scholar]
- 6.Chu L, Kennell W, Holt S C. Characterization of hemolysis and hemoxidation activities by Treponema denticola. Microb Pathog. 1994;16:183–195. doi: 10.1006/mpat.1994.1019. [DOI] [PubMed] [Google Scholar]
- 7.Chu L, Holt S C. Purification and characterization of a 45 kDa hemolysin from Treponema denticola ATCC 35404. Microb Pathog. 1994;16:197–212. doi: 10.1006/mpat.1994.1020. [DOI] [PubMed] [Google Scholar]
- 8.Chu L, Ebersole J L, Kurzban G P, Holt S C. Cystalysin, a 46-kilodalton cysteine desulfhydrase from Treponema denticola, with hemolytic and hemoxidative activities. Infect Immun. 1997;65:3231–3238. doi: 10.1128/iai.65.8.3231-3238.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Falcioni G C, Coderoni S, Tedeschi G G, Brunori M, Rotilio G. Red cell lysis induced by microorganisms as a case of superoxide- and hydrogen peroxide-dependent hemolysis mediated by oxyhemoglobin. Biochim Biophy Acta. 1981;678:437–441. doi: 10.1016/0304-4165(81)90125-2. [DOI] [PubMed] [Google Scholar]
- 10.Fraser C M, Gocayne J D, White O, Adams M D, Clayton R A, Fleischmann R D, Bult C J, Kerlavage A R, Sutton G, Kelley J M, Fritchman J L, Weidman J F, Small K V, Sandusky M, Fuhrmann J, Nguyen D, Utterback T R, Saudek D M, Phillips C A, Merrick J M, Tomb J-F, Dougherty B A, Bott K F, Hu P-C, Lucier T S. The minimal gene complement of Mycoplasma genitalium. Science. 1995;270:397–403. doi: 10.1126/science.270.5235.397. [DOI] [PubMed] [Google Scholar]
- 11.Giron J A, Lange M, Baseman J B. Adherence, fibronectin binding, and induction of cytoskeleton reorganization in cultured human cells by Mycoplasma penetrans. Infect Immun. 1996;64:197–208. doi: 10.1128/iai.64.1.197-208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayes M M, Li B J, Wear D J, Lo S C. Pathogenicity of Mycoplasma fermentans and Mycoplasma penetrans in experimentally infected chicken embryos. Infect Immun. 1996;64:3419–3424. doi: 10.1128/iai.64.8.3419-3424.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Himmelreich R, Hilbert H, Plagens H, Pirkl E, Li B C, Herrmann R. Complete sequence analysis of the genome of the bacterium Mycoplasma pneumoniae. Nucleic Acids Res. 1996;24:4420–4449. doi: 10.1093/nar/24.22.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Honda T, Finkelstein R A. Purification and characterization of a hemolysin produced by Vibrio cholerae biotype El Tor: another toxic substance produced by cholera vibrios. Infect Immun. 1979;26:1020–1027. doi: 10.1128/iai.26.3.1020-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jarvill-Taylor K J, Minion C F. The effect of thiol-active compounds and sterols on the membrane-associated hemolysin of Mycoplasma pulomonis. FEMS Microbiol Lett. 1995;128:213–218. doi: 10.1111/j.1574-6968.1995.tb07525.x. [DOI] [PubMed] [Google Scholar]
- 16.Kehoe M A, Miller L, Walker J A, Boulnois G J. Nucleotide sequence of streptolysin O (SLO) gene: structural homologies between SLO and other membrane damaging, thiol-activated toxins. Infect Immun. 1987;55:3228–3232. doi: 10.1128/iai.55.12.3228-3232.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellogg E W, III, Fridovich I. Liposome oxidation and erythrocyte lysis by enzymically generated superoxide and hydrogen peroxide. J Biol Chem. 1977;252:6721–6728. [PubMed] [Google Scholar]
- 18.Kothary M H, Kreger A S. Purification and characterization of an extracellular cytolysin produced by Vibrio damsela. Infect Immun. 1985;49:25–31. doi: 10.1128/iai.49.1.25-31.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause D C, Taylor-Robinson D. Mycoplasma which infect humans. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 417–444. [Google Scholar]
- 20.Lee I-M, Davis R E. Mycoplasmas which infect plants and insects. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 379–390. [Google Scholar]
- 21.Lo S C, Hayes M M, Kotani H, Pierce P F, Wear D J, Newton P B, 3d, Tully J G, Shih J W. Adhesion onto and invasion into mammalian cells by Mycoplasma penetrans: a newly isolated mycoplasma from patients with AIDS. Mod Pathol. 1993;6:276–280. [PubMed] [Google Scholar]
- 22.Lo S C, Hayes M M, Tully J G, Wang R Y, Kotani H, Pierce P F, Rose D L, Shih J W. Mycoplasma penetrans sp. nov., from the urogenital tract of patients with AIDS. Int J Syst Bacteriol. 1992;42:357–364. doi: 10.1099/00207713-42-3-357. [DOI] [PubMed] [Google Scholar]
- 23.Menguad J, Vicente M-F, Chenevert J, Pereira J M, Geoffroy C, Gicquel-Sanzey B, Baquero F, Perez-Diaz J-C, Cossart P. Expression in Escherichia coli and sequence analysis of the listeriolysin O determinant of Listeria monocytogenes. Infect Immun. 1988;56:766–772. doi: 10.1128/iai.56.4.766-772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Minion C F, Jarvill-Taylor K. Membrane-associated hemolysin activities in mycoplasmas. FEMS Microbiol Lett. 1994;116:101–106. doi: 10.1111/j.1574-6968.1994.tb06682.x. [DOI] [PubMed] [Google Scholar]
- 25.Russell C M. A “hemolysin” associated with leptospirae. J Immunol. 1956;77:405–409. [PubMed] [Google Scholar]
- 26.Shibata K, Sasaki T, Watanabe T. AIDS-associated mycoplasmas possess phospholipases C in the membrane. Infect Immun. 1995;63:4174–4177. doi: 10.1128/iai.63.10.4174-4177.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simecka J W, Davis J K, Dvidson M K, Ross S E, Stadtlander C T K-H, Cassell G H. Mycoplasma diseases of animals. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 391–416. [Google Scholar]
- 28.Smith M L, Price S A. Staphylococcus β haemolysin. J Pathol Bacteriol. 1938;47:361–377. [Google Scholar]
- 29.Stamm L V, Charon N W. Plate assay for detection of Leptospira interrogans serovar pomona hemolysin. J Clin Microbiol. 1979;10:590–592. doi: 10.1128/jcm.10.4.590-592.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tryon V V, Baseman J B. Pathogenic determinants and mechanisms. In: Maniloff J, McElhaney R N, Finch L R, Baseman J B, editors. Mycoplasmas: molecular biology and pathogenesis. Washington, D.C.: American Society for Microbiology; 1992. pp. 457–472. [Google Scholar]
- 31.Tweten R K. Nucleotide sequence of the gene for perfringolysin O (theta-toxin) from Clostridium perfringens: significant homology with the genes for streptolysin O and pneumolysin. Infect Immun. 1988;56:3235–3240. doi: 10.1128/iai.56.12.3235-3240.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Heyningen W H. The biochemistry of the gas gangrene toxins. 2. Partial purification of the toxins of Cl. welchii, type A. Separation of α and θ toxins. Biochem J. 1941;35:1257–1269. doi: 10.1042/bj0351257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walker J A, Allen R L, Falmagne P, Johnson M K, Boulnois G J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987;55:1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams L W, Austin F E. Hemolytic activity of Borrelia burgdorferi. Infect Immun. 1992;60:3224–3230. doi: 10.1128/iai.60.8.3224-3230.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamanaka H, Shimatani S, Tanaka M, Katsu T, Ono B, Shinoda S. Susceptibility of erythrocytes from several animal species to Vibrio vulnificus hemolysin. FEMS Microbiol Lett. 1989;52:251–255. doi: 10.1016/0378-1097(89)90206-1. [DOI] [PubMed] [Google Scholar]
- 36.Zen-Yoji H, Hitokoto H, Morozumi S, Le Clair R A. Purification and characterization of a hemolysin produced by Vibrio parahaemolyticus. J Infect Dis. 1971;123:665–667. doi: 10.1093/infdis/123.6.665. [DOI] [PubMed] [Google Scholar]