Highlights
-
•
Somatic yolk sac tumor differentiation associated with malignant neoplasms is uncommon and associated with poor outcome.
-
•
In the gynecologic tract, somatic yolk sac differentiation most often arises in postmenopausal patients.
-
•
Somatic yolk sac differentiation shares driver mutations with and likely differentiates from the corresponding carcinoma.
-
•
This is the first report of somatic yolk sac differentiation in the gynecologic tract from a non-epithelial malignancy.
1. Introduction
Yolk sac tumor (YST) is a type of malignant germ cell tumor (GCT) that morphologically recapitulates the fetal yolk sac (Rutgers, 1987). YSTs typically present in younger women (frequently younger than 20) as a pure YST or part of a mixed GCT (Rutgers, 1987, Skala, 2020). YST differentiation (YSTd) was first reported in association with an ovarian epithelial carcinoma (ovarian endometrioid carcinoma) by Rutgers et al (Rutgers, 1987). The authors describe an ovarian mass in a 50-year-old woman showing an endometrioid carcinoma (EC) containing foci of YST. Trophoblastic differentiation in somatic neoplasms ranges from hormonal secretion only, hormonal secretion with syncytiotrophoblast-like giant cells, to areas morphologically indistinguishable from a GCT (Rutgers, 1987). The authors proposed the term neometaplasia to describe the process of germ cell differentiation in a carcinoma and hypothesized a somatic/carcinomatous origin for the germ cell components (Rutgers, 1987).
We report two cases of malignant ovarian tumors associated with somatic YSTd. Case 1 is a patient with an ovarian EC with somatic YSTd. Case 2 is a patient with sarcomatous overgrowth (SO) of an ovarian adenosarcoma with focal somatic YSTd. Somatic YSTd arising from an adenosarcoma has not previously been reported.
2. Case 1
2.1. Case presentation:
A 65-year-old nulliparous woman was referred to our center for a large pelvic mass. She complained of abdominal bloating, discomfort, and distention for one year. Contrast-enhanced computed-tomography (CT) scan (Fig. 1) showed a pelvic mass measuring 28.9 × 20.4 × 26.5 cm with solid components and a small amount of ascites. There was a 2.2 cm ill-defined expansile sclerotic lesion in the manubrium and a borderline enlarged left retropectoral lymph node. Serum tumor markers showed: CA125 = 716 U/mL, CA19-9 = 480 U/mL, carcinoembryonic antigen (CEA) = 2.7 ug/mL, and lactate dehydrogenase (LDH) = 249 U/L. Her past medical history was significant for osteoarthritis, high body mass index (BMI) (40 kg/m2), cholecystectomy, and remote measles and mumps infection. She quit smoking more than 30 years ago. Her family history was insignificant for malignancy. Menarche was at the age of 13 and menopause at age 55. She had lifelong regular menstrual cycles and never used oral contraceptive pills (OCP) or hormone replacement therapy (HRT).
Fig. 1.
Preoperative CT scan, sagittal view (A) and coronal view (B), showing a large complex multiloculated mixed cystic/solid mass in the pelvis measuring 28.9 × 20.4 × 26.5 cm. Representative images from the ovarian mass showing the endometrioid carcinoma with somatic yolk sac tumor differentiation at 4x magnification (C), endometrioid carcinoma at 20x magnification (D), and yolk sac tumor at 20x magnification (E).
The patient underwent a midline total abdominal hysterectomy, bilateral salpingo-oophorectomy (TAH BSO), and staging, which included bilateral pelvic lymph node dissection, infracolic omentectomy, appendectomy, small bowel resection, and pelvic washing. There was a 20 cm mass arising from the right ovary adherent to the sigmoid colon. Frozen histological assessment reported an EC. The patient was discharged home post-operative day 4.
2.2. Pathology:
The final pathology showed ovarian EC (FIGO grade 1) with somatic YSTd arising in the right ovary with areas suspicious for surface involvement (Fig. 1). The somatic YSTd comprised 36 % of the tumor and was discrete from the EC areas. Endometriosis was present on both ovaries, uterine serosa, and small bowel serosa. The pelvic fluid cytology and pelvic lymph nodes were negative for malignancy. The mismatch repair (MMR) immunohistochemistry (IHC) profile was normal. On IHC staining, the YST component was positive for Glypican 3 and CDX2 and negative for ER and PAX8. The EC component was positive for ER, p16 (mosaic pattern), and PAX8 and negative for Glypican 3 and CDX2. Both components were negative for WT1 and showed wild-type staining with p53.
2.3. Outcome:
Tumor board review recommended six cycles of Carboplatin AUC 5 and Paclitaxel 175 mg/m2 every-three weeks. Chemotherapy was started nine weeks after the surgery. The treatment was delayed for one week after cycle four due to neutropenia. A follow-up CT scan showed no evidence of residual disease. The left retropectoral lymph node had decreased in size and the bony lesion remained unchanged. Serum tumor markers reverted to normal after chemotherapy. The patient was in remission during her last follow-up nine months after chemotherapy treatment.
3. Case 2
3.1. Case presentation:
A 75-year-old woman (G3P2A1) was referred to our center for a large pelvic mass and ascites. Fifteen years previously, she had an abdominal TAH BSO and staging for postmenopausal bleeding, thickened endometrial lining, and a large complex adnexal cyst with normal serum tumor markers. The previous pathology was reported as an ovarian serous cystadenofibroma and endometrial hyperplasia without atypia, hence, she was discharged from gynecologic oncology clinic.
Menarche commenced at age 11 and menopause at age 58 with regular menstrual periods. She used an OCP for two years and had never used HRT. She quit smoking more than 55 years ago. Her mother was diagnosed with breast cancer at age 65 and her grandmother had pancreatic cancer at age 80.
A large pelvic mass with ascites was present on CT scan (Fig. 2). Core biopsy showed a high-grade malignant tumor, unable to further classify. Her serum CA125 was elevated (589 U/L), whereas serum CEA, CA 19–9, and LDH were normal. She underwent debulking surgery and was found to have two large masses in the pelvis with ascites. Both masses were resected without residual disease. Her postoperative period was unremarkable.
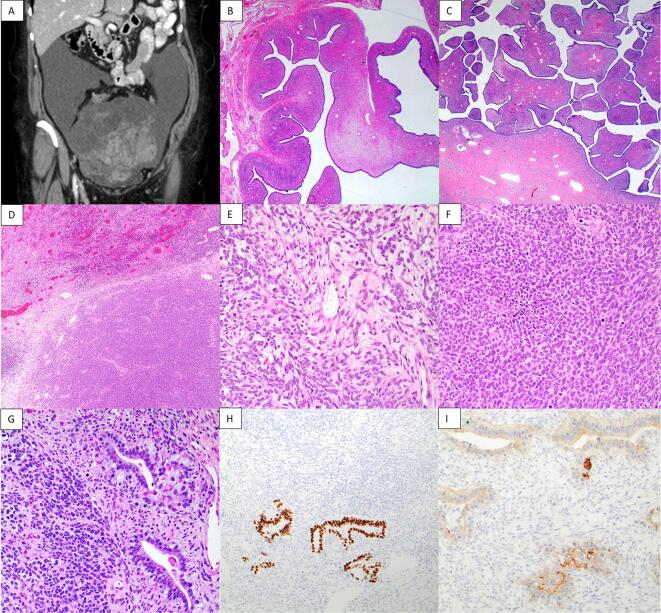
Fig. 2.
Preoperative CT scan, coronal view, showing a large pelvic mass measuring 20.1 × 16.7 cm (A). Representative images from the previously resected ovarian tumor at 2x magnification (B) and recurrent tumor showing the adenosarcoma at 2x magnification (C). Representative images from the sarcomatous overgrowth at 4x magnification (D) and 20x magnification (E, F). Sarcomatous overgrowth with somatic yolk sac tumor differentiation 20x magnification (G), CDX2 immunohistochemistry 10x magnification (H), Glypican 3 immunohistochemistry 20x magnification (I).
3.2. Pathology:
The first mass was received in two fragments (23 cm and 17 cm in largest dimension) and the second mass measured 18 cm is largest dimension. The first mass was an adenosarcoma and the second mass was an adenosarcoma with SO and focal somatic YSTd (Fig. 2). The area of somatic YSTd comprised less than 5 % of the overall tumor volume and was enveloped within the SO. The SO was homologous, monomorphic, and mitotically active with areas of necrosis. IHC showed the SO was positive for WT1, PR (focal), CyclinD1 (focal), and CD117 (focal) and negative for cytokeratin AE1/3, PAX8, CD10, ER, Desmin, Caldesmon, and Myogenin. ARID1B, INI1, BRG1, MSH6, and PMS2 were intact (normal). Glypican 3 and CDX2 highlighted the small focus of somatic YSTd (Fig. 2). p53 was wild type in both components. Custom panel based RNA fusion analysis that included nearly all previously reported genetic fusions in ovarian and uterine tumors was negative for genetic fusions.
3.3. Outcome:
Tumor board review recommended dual platinum-based chemotherapy. She declined further treatment and follow-up.
4. Discussion
Since the initial report by Rutgers et al, multiple case reports and series describing germ cell differentiation associated with carcinomas have been published. Skala et al described eight YSTs (six arising in the ovary and two in the endometrium) in patients over the age of 35; six cases were associated with an epithelial component (Skala, 2020). The YST components harbored similar mutations to their respective epithelial carcinomas: PTEN, PIK3CA, FGFR2, CTNNB1 in those associated with ECs and TP53 and PIK3CA in those associated with high-grade carcinomas, supporting the theory that YSTd is somatically derived from the carcinoma component (Skala, 2020). Of note, isochromosome 12p (i(12p)), a genetic abnormality associated with malignant GCTs arising in younger patients, was found in three cases associated with high-grade carcinomas (in addition to TP53 and PIK3CA mutations). In these cases, i(12p) was postulated to be secondary to chromosomal instability and aneuploidy; most cases had a poor prognosis (Skala, 2020). Acosta et al tested nine tumors of the gynecologic tract (six ovarian and three uterine) with epithelial and germ cell/trophoblastic components (seven with YSTd, one with choriocarcinoma differentiation, and one with epithelioid trophoblastic tumor differentiation) and demonstrated shared mutations between the epithelial and germ cell/trophoblast components (Acosta, 2020). Similar to Skala et al, the shared mutations were driver mutations typically described for the respective epithelial components (Acosta, 2020). In contrast to Skala et al, none of the YST components showed i(12p), however, four of the cases showed aneuploidy of chromosome 12 (Acosta, 2020). All of the tumors tested showed high copy number variations (CNVs), suggesting that germ cell/trophoblastic differentiation may reflect genomic instability (Acosta, 2020). The largest case series described 18 cases of YST in women over 40; seventeen cases were ovarian primaries and one was a uterine primary (McNamee, 2016). A total of 31 cases of ovarian carcinoma with somatic YSTd including outcomes have been reported to date (McNamee, 2016, Abe, 2008, Lopez, 2003, Wang, 2018, Ahn, 2020, McCarthy, 2016, Roth, 2011, Nogales, 1996, Hodgson, 2020, Roma and Pryzybycin, 2014). Twelve cases of pure ovarian YST (10/12 patients ≥ 50 years old) have also been reported (McNamee, 2016, Wang, 2018, Roth, 2011, Roma and Pryzybycin, 2014). Somatic YSTd occurs with variable epithelial components. 28 % (12/43) occurred with EC (one had EC, immature teratoma, and carcinoid), 28 % (12/43) as pure somatic YST (two cases had concurrent endometriosis), 21 % (9/43) with high-grade serous carcinoma (HGSC) (one with serous tubal intraepithelial carcinoma), 9 % (4/43) with clear cell carcinoma (CC) (one borderline clear cell adenofibroma), 5 % (2/43) with low-grade serous carcinoma (LGSC), 5 % (2/43) with large cell neuroendocrine carcinoma (LCNEC), and 2 % (1/43) with carcinosarcoma (CS). In one case, the epithelial carcinoma was reported as an adenocarcinoma, not further specified (Wang, 2018). Table 1 lists previously reported cases with clinical follow-up (36 cases) grouped according to the epithelial component. In cases of ovarian EC with somatic YSTd, 43 % (3/7) of stage I cases died of disease and 100 % (4/4) cases > stage I died of disease. In cases of pure ovarian somatic YST in older patients (youngest age 40), 25 % (1/4) of stage I patients died of disease and 60 % (3/5) cases > stage I died of disease. In cases where the epithelial component was non-endometrioid, only 20 % (3/15) were stage I and 60 % (9/15) patients had either recurred and were alive with disease or had died of disease.
Table 1.
Summary of previously reported cases of ovarian carcinoma with somatic yolk sac tumor differentiation with outcomes.
| Reference | Patient age | Histotype | Stage | Treatment | Outcome |
|---|---|---|---|---|---|
| Roth (Roth, 2011) | 48 | EC, CC and YST | IA | Chemo NOS × 5 cycles | NED at 24 months |
| Nogales (Nogales, 1996) | 71 | EC and YST | IA | Chemo NOS × 6 cycles | NED at 12 months |
| Nogales (Nogales, 1996) | 64 | EC and YST | IA | Chemo NOS × 3 cycles | Recurrence at 8 months, DOD at 14 months |
| Abe (Abe, 2008) | 52 | EC and YST | IC | BEP × 3 cycles, CT × 3 cycles | NED at 20 months |
| Rutgers (Rutgers, 1987) | 50 | EC and YST | IC | VDC × 5 cycles | Recurrence at 7 months, DOD |
| Lopez (Lopez, 2003) | 51 | EC and YST | IC | CEB × 4 cycles | Recurrence at 10 weeks, DOD at 10 months |
| Hodgson (Hodgson, 2020) | 27 | EC and YST | IC | BEP × 3 cycles | NED at 15 months |
| Nogales (Nogales, 1996) | 31 | EC and YST | III | Chemo NOS × 6 cycles | Recurrence at 1 month, DOD at 8 months |
| Nogales (Nogales, 1996) | 71 | EC and YST | III | Chemo NOS × 1 cycle | DOD at 3 months |
| Nogales (Nogales, 1996) | 40 | EC and YST | IV | Chemo NOS × 3 cycles | DOD at 5 months |
| McNamee (McNamee, 2016) | 63 | EC and YST | IVB | n/a | DOD at 10 months |
| McNamee (McNamee, 2016) | 50 | YST and EM | IC | n/a | NED at 22 months |
| McNamee (McNamee, 2016) | 60 | YST | IC | n/a | NED at 22 months |
| Roth (Roth, 2011) | 60 | YST | IC | CT | NED at 14 months |
| Wang (Wang, 2018) | 55 | YST | IC | BEP × 6 cycles | DOD at 30.8 months |
| Wang (Wang, 2018) | 60 | YST | IIC | BEP × 4 cycles | NED at 40.6 months |
| Wang (Wang, 2018) | 55 | YST | IIC | BEP × 5 cycles | DOD at 18.5 months |
| McNamee (McNamee, 2016) | 40 | YST | IIIB | n/a | DOD at 27 months |
| Roma (Roma and Pryzybycin, 2014) | 70 | YST and EM | IIIC | Chemo NOS × 6 cycles | Recurrence at 7 months, AWD |
| McNamee (McNamee, 2016) | 42 | YST | IVB | n/a | DOD at 8 months |
| Wang (Wang, 2018) | 50 | YST | n/a | DDP × 3 cycles, FP × 1 cycle | DOD at 8.5 months |
| McCarthy (McCarthy, 2016) | 62 | HGSC and YST | IC3 | Chemo NOS | NED |
| Wang (Wang, 2018) | 77 | HGSC and YST | IIIC | NACT × 3 cycles, CT a 1 cycle | NED at 7 months |
| McNamee (McNamee, 2016) | 68 | HGSC and YST | IIIC | n/a | NED at 1 month |
| Roma (Roma and Pryzybycin, 2014) | 61 | HGSC and YST | IIIC | Chemo NOS × 6 cycles | Recurrence at 7 months, AWD |
| McNamee (McNamee, 2016) | 56 | HGSC and YST | IIIC | n/a | DOD at 4 months |
| McNamee (McNamee, 2016) | 62 | HGSC and YST | IIIC | n/a | DOD at 20 months |
| Nogales (Nogales, 1996) | 73 | CS and YST | III | None | AWD at 2 months |
| Wang (Wang, 2018) | 61 | LGSC and YST | IIIB | BEP × 2 cycles, PEV × 1 cycle, CT × 1 cycle | Recurrence at 8 months, AWD at 23 months |
| Roth (Roth, 2011) | 67 | LGSC and YST | IIIC | None | Died of post-op complications |
| McNamee (McNamee, 2016) | 79 | BCCAF and YST | IA | n/a | NED at 21 months |
| Wang (Wang, 2018) | 58 | CC and YST | IC | BEP × 3 cycles | NED as 12 months |
| Roth (Roth, 2011) | 49 | CC and YST | IIIA | Chemo NOS | Recurrence at 3 months, DOD at 15 months |
| McNamee (McNamee, 2016) | 48 | CC and YST | IIIC | n/a | DOD at 12 months |
| McNamee (McNamee, 2016) | 59 | LCNEC and YST | IIB | n/a | DOD at 21 months |
| Ahn (Ahn, 2020) | 82 | LCNEC and YST | IIIC | None (patient choice) | Recurrence at 9 months |
EC: Endometrioid carcinoma.
CC: Clear cell carcinoma.
YST: Yolk sac tumor.
HGSC: High grade serous carcinoma.
YST: Yolk sac tumor.
CS: Carcinosarcoma.
LGSC: Low grade serous carcinoma.
BCCAF: Borderline clear cell adenofibroma.
CC: Clear cell carcinoma.
LCNEC: Large cell neuroendocrine carcinoma.
EM: Endometriosis.
Chemo NOS: chemotherapy with no further details provided.
BEP: Bleomycin, etoposide, platinum.
CT: Carboplatin and paclitaxel.
VDC: Vincristine, dacrinomycin, cyclophosphamide.
CEB: Cisplatin, etoposide, bleomycin.
DDP: Cisplatin, adriomycin, 5-fluorouracil.
FP: 5-fluorouracil and cisplatin.
NACT: Neoadjuvant chemotherapy.
PEV: Cisplatin, epirubicin, vinorelbine.
NED: No evidence of disease.
DOD: Dead of disease.
AWD: Alive with disease.
The cases reported are associated with poor outcomes, even in patients with stage I disease, all of whom received adjuvant chemotherapy. In contrast, classic GCTs arising in young patients typically have a good prognosis and excellent response to chemotherapy (Acosta, 2020). Diagnostic nomenclature should highlight that germ cell/trophoblastic differentiation associated with a carcinoma has a poor prognosis compared to classic GCTs (Acosta, 2020). It is uncertain if germ cell/trophoblastic differentiation is an independent predictor of poor prognosis (Acosta, 2020). Serum AFP levels are elevated in the majority of cases and can be helpful to monitor treatment response and disease recurrence (Wang, 2018). Somatic YSTd has been reported in tumors arising at other sites including the bladder (Collins, 2022), vulva (Kolin, 2022), cervix (Liu, 2022) and colorectum (Takashi, 2020) and is also reported to have poor outcomes.
To the best of our knowledge, case two is the first reported case of adenosarcoma with SO showing somatic YSTd. It is unclear whether the YSTd is clonally related to the SO. El Hallani et al described cases of mixed mullerian adenosarcoma and EC in which the EC is clonally related to the sarcoma (El Hallani, 2021). It is thus plausible that adenosarcoma may give rise to the component of YSTd observed here either directly or indirectly through an unsampled EC component.
Malignant tumors associated with somatic YST differentiation are uncommon. They are understudied and the optimal treatment (germ cell chemotherapy protocol versus chemotherapy directed to the somatic component) is uncertain. Based on studies showing the molecular alterations in the GCT components match the common driver mutations in the corresponding epithelial component, most authors suggest treatment regimens directed towards the epithelial component. To the best of our knowledge, this is the first report of somatic GCT differentiation in a non-epithelial malignancy in the gynecologic tract. Pathologists and clinicians should be aware of these rare cases, which arise predominantly in post-menopausal patients and show aggressive behavior compared to GCTs arising in younger patients.
5. Author contributions:
Dr. A Bassi: First author, helped write the paper (clinical case presentations), edit the paper and provided CT images for Fig. 1, Fig. 2. Obtained consent from both patients for the case report.
Dr. G Nelson: Assisted Dr. Bassi with obtaining consent and edited the paper.
Dr. CH Lee: Gynecologic and sarcoma pathologist who was consulted on the adenosarcoma case and edited the paper.
Dr. T Ogilvie: Gynecologic and breast pathologist reporting part of the pathology from case 2 (adenosarcoma with sarcomatous overgrowth and somatic yolk sac tumor differentiation).
Dr. A Cota: Pathologist reporting the pathology of case 1 (ovarian endometrioid carcinoma with yolk sac tumor differentiation).
Dr. S Lee: Gynecologic pathologist reporting the final pathology for case 2, tumor board review pathologist for case 1, selected the two cases for the case report and helped write and edit the paper.
Financial support:
Financial support was received from the Tom Baker Cancer Center Gynecological Oncology to cover the publishing fee.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Abe A., et al. A case of ovarian endometrioid adenocarcinoma with a yolk sac tumor component. International Journal of Gynecol Cancer. 2008;18:168–192. doi: 10.1111/j.1525-1438.2007.00952.x. [DOI] [PubMed] [Google Scholar]
- Acosta A.M., et al. Malignant tumors of the uterus and ovaries with Mullerian and germ cell or trophoblastic components have a somatic origin and are characterized by genomic instability. Histopathology. 2020;77:788–797. doi: 10.1111/his.14188. [DOI] [PubMed] [Google Scholar]
- Ahn H., et al. Ovarian yolk sac tumor with epithelial tumor component in a postmenopausal woman – case report and literature review. Int J Clin Exp Pathol. 2020;13(9):2401–2406. [PMC free article] [PubMed] [Google Scholar]
- Collins K., et al. Somatic-type yolk sac tumor arising as a predominant component of bladder urothelial carcinoma. Int. J. Surg. Pathol. 2022;30(2):207–213. doi: 10.1177/10668969211030688. [DOI] [PubMed] [Google Scholar]
- El Hallani S., et al. Mixed endometrioid adenocarcinoma and Mullerian adenosarcoma of the uterus and ovary: clinicopathologic characterization with emphasis on its distinction from carcinosarcoma. Am J Surg Pathol. 2021;45(3):374–383. doi: 10.1097/PAS.0000000000001643. [DOI] [PubMed] [Google Scholar]
- Hodgson A., et al. Somatically derived yolk sac tumor of the ovary in a young woman. International Journal of Gynecological Pathology. 2020;40:296–300. doi: 10.1097/PGP.0000000000000673. [DOI] [PubMed] [Google Scholar]
- Kolin D.L., et al. Vulvar yolk sac tumors are somatically derived SMARCB1 (INI1)-deficient neoplasms. Am. J. Surg. Pathol. 2022;46:169–178. doi: 10.1097/PAS.0000000000001777. [DOI] [PubMed] [Google Scholar]
- Liu X.L., et al. Yolk sac tumor originating from cervical adenocarcinoma: a case predominated by enteroblastic differentiation. International Journal of Gynecological Pathology. 2022;00:1–5. doi: 10.1097/PGP.0000000000000891. [DOI] [PubMed] [Google Scholar]
- Lopez J.M., et al. Ovarian yolk sac tumor associated with endometrioid carcinoma and mucinous cystadenoma of the ovary. Ann. Diagn. Pathol. 2003;7:300–305. doi: 10.1016/s1092-9134(03)00081-9. [DOI] [PubMed] [Google Scholar]
- McCarthy W.A., et al. Ovarian yolk sac tumor with high-grade serous carcinoma in a 62-year-old woman. Int. J. Surg. Pathol. 2016;24(4):360–365. doi: 10.1177/1066896915626796. [DOI] [PubMed] [Google Scholar]
- McNamee T., et al. Yolk sac tumors of the female genital tract in older adults derive commonly from somatic epithelial neoplasms: somatically derived yolk sac tumors. Histopathology. 2016;69:739–751. doi: 10.1111/his.13021. [DOI] [PubMed] [Google Scholar]
- Nogales F.F., et al. Ovarian endometrioid tumors with yolk sac tumor component, an unusual form of ovarian neoplasm: analysis of six cases. Am. J. Surg. Pathol. 1996;20(9):1056–1066. doi: 10.1097/00000478-199609000-00003. [DOI] [PubMed] [Google Scholar]
- Roma A., Pryzybycin C.G. Yolk sac tumor in postmenopausal patients: pure or associated with adenocarcinoma, a rare phenomenon. International Journal of Gynecological Pathology. 2014;33:477–482. doi: 10.1097/PGP.0000000000000078. [DOI] [PubMed] [Google Scholar]
- Roth L.M., et al. Ovarian yolk sac tumors in older women arising from epithelial ovarian tumors or with no detectable epithelial component. International Journal of Gynecological Pathology. 2011;30:442–451. doi: 10.1097/PGP.0b013e3182164386. [DOI] [PubMed] [Google Scholar]
- Rutgers J.L., et al. Ovarian yolk sac tumor arising from an endometrioid carcinoma. Hum. Pathol. 1987;18(12) doi: 10.1016/s0046-8177(87)80418-5. [DOI] [PubMed] [Google Scholar]
- Skala S.L., et al. Molecular characterization of uterine and ovarian tumors with mixed epithelial and germ cell features confirms frequent somatic derivation. Mod. Pathol. 2020;33:1989–2000. doi: 10.1038/s41379-020-0548-6. [DOI] [PubMed] [Google Scholar]
- Takashi M., et al. Colorectal adenocarcinoma with enteroblastic differentiation: a clinicopathologic study of five cases. Histopathology. 2020;76:325–332. doi: 10.1111/his.13973. [DOI] [PubMed] [Google Scholar]
- Wang Y., et al. Ovarian yolk sac tumor in postmenopausal females. A case series and a literature review. Medicine. 2018;97(33):e11838. doi: 10.1097/MD.0000000000011838. [DOI] [PMC free article] [PubMed] [Google Scholar]