Abstract
Background
Latent tuberculosis infection (LTBI) has been associated with increased cardiovascular risk. We investigated the activation and pro-inflammatory profile of monocytes in individuals with LTBI and their association with coronary artery disease (CAD).
Methods
Individuals 40–70 years old in Lima, Peru, underwent QuantiFERON-TB testing to define LTBI, completed a coronary computed tomography angiography to evaluate CAD, and provided blood for monocyte profiling using flow cytometry. Cells were stimulated with lipopolysaccharide to assess interleukin-6 (IL-6) and tumor necrosis factor (TNF)–α responses.
Results
The clinical characteristics of the LTBI (n = 28) and non-LTBI (n = 41) groups were similar. All monocyte subsets from LTBI individuals exhibited higher mean fluorescence intensity (MFI) of CX3CR1 and CD36 compared with non-LTBI individuals. LTBI individuals had an increased proportion of nonclassical monocytes expressing IL-6 (44.9 vs 26.9; P = .014), TNF-α (62.3 vs 35.1; P = .014), and TNF-α+IL-6+ (43.2 vs 36.6; P = .042). Among LTBI individuals, CAD was associated with lower CX3CR1 MFI on classical monocytes and lower CD36 MFI across all monocyte subsets. In multivariable analyses, lower CD36 MFI on total monocytes (b = −0.17; P = .002) and all subsets remained independently associated with CAD in LTBI.
Conclusions
Individuals with LTBI have distinct monocyte alterations suggestive of an exacerbated inflammatory response and tissue migration. Whether these alterations contribute to cardiovascular disease pathogenesis warrants further investigation.
Keywords: cardiovascular disease, coronary artery disease, inflammation, latent tuberculosis, monocytes
About a quarter of the world population has been infected with Mycobacterium tuberculosis (Mtb) [1]. Most of these individuals will remain asymptomatic through their lifetime, carrying a condition known as latent tuberculosis infection (LTBI) [2]. In contrast to the traditional view of LTBI as a state of mycobacterial dormancy, LTBI is now recognized as a continuous spectrum of host–pathogen interactions where replicating and metabolically quiescent mycobacterial populations coexist and are constrained by variable host immune responses within each granuloma [3–5]. Most persons with LTBI will achieve long-term Mtb control without antimicrobial treatment, as a result of their immune system's ability to contain mycobacterial replication [3, 6, 7]. However, the cost of keeping the infection in check may lead to persistently or intermittently enhanced systemic immune activation, which, in turn, could contribute to the progression and/or development of inflammation-driven chronic diseases. Chronic infections such as HIV are capable of driving alterations of inflammatory responses, as demonstrated by the presence of inflammatory soluble proteins and activation markers on monocytes [8–10], which have been associated with atherosclerotic disease [10–12]. Similarly, chronic inflammatory conditions such as rheumatoid arthritis and systemic lupus erythematosus are associated with pro-inflammatory alterations of monocytes [13–15]. Nevertheless, these immune alterations have not been comprehensively assessed in LTBI.
LTBI has been associated with increased ocurrence of cardiovascular disease (CVD). We demonstrated that LTBI was associated with 2-fold increased odds of having an acute myocardial infarction, after adjusting for traditional CVD risk factors [16]. More recently, LTBI has been associated with subclinical obstructive coronary atherosclerosis, symptomatic coronary artery disease (CAD) [17, 18], and increased risk of developing hypertension and diabetes mellitus [19, 20].
Monocytes have an important role in CVD as they are recruited into atherosclerotic plaque and can be a source of pro-inflammatory stimuli [21]. Three major monocyte subsets are recognized based on CD14 and CD16 expression: classical, intermediate, and nonclassical monocytes. Alterations in the proportions of these subsets, as well as variations in the expression of activation receptors, have been associated with increased cardiovascular risk [12, 21]. In prior studies, LTBI was associated with increased proportion of total monocytes [22], and increased HLA-DR expression in the setting of HIV coinfection [23]. However, a comprehensive assessment of monocyte phenotypes and functional responses down to the subset level has not been conducted in LTBI. Of particular interest, the chemokine receptor CX3CR1 is highly expressed in CD16+ monocytes and promotes pro-atherogenic endothelial changes, as well as monocyte recruitment into tissues [24]. Another important monocyte marker is the scavenger receptor CD36, which uptakes oxidized lipids, thus promoting intracellular lipid accumulation and triggering pro-inflammatory responses [25]. In this study, we investigated the activation and pro-inflammatory profile of circulating monocytes in individuals with LTBI. We hypothesized that LTBI is associated with a pro-inflammatory monocyte profile. We also explored whether such a pro-inflammatory profile would be associated with CAD in LTBI.
METHODS
Participant Recruitment
We conducted a cross-sectional study of individuals with and without LTBI between March 2018 and October 2019 in Lima, Peru, as previously described [26]. TB disease is prevalent in Peru, with an incidence rate of 119 TB cases per 100 000 inhabitants for 2019. The overall prevalence of LTBI in Lima is ∼50%–60% [16]. Briefly, individuals 40–70 years of age were identified from markets and outpatient medicine clinics in downtown Lima. Participants were enrolled at the Hospital Nacional Dos de Mayo Clinical Research Unit in Lima, Peru. We excluded individuals with chronic kidney disease stage ≥3, history of acute myocardial infarction, ischemic stroke, active cancer, immunosuppression, dyslipidemia therapy, pregnancy, breastfeeding, or prior LTBI treatment. Participants underwent a coronary computed tomography angiography (CCTA) to examine coronary atherosclerosis and provided blood for QuantiFERON TB Plus (QFT) testing to define LTBI (QFT positive) and non-LTBI (QFT negative) groups. HIV status was assessed by history and confirmed via commercial rapid testing at study entry. Participants also provided blood for plasma and peripheral blood mononuclear cell (PBMC) isolation. For this project, we included LTBI and non-LTBI individuals with available, stored plasma and PBMC samples.
CCTA and CAD Assessments
Participants underwent a CCTA using a 160-slice Toshiba Aquilion Prime CT scanner at Hospital Nacional Dos de Mayo, following guidelines from the Society of Cardiac Computed Tomography [27]. Stored CCTA DICOM images were interpreted by an expert CCTA reader blinded to the participants’ demographic and clinical characteristics including LTBI status. CAD was defined as the presence of plaque in any of the coronary segments.
Plasma and PBMC Isolation
Fresh blood was processed the same day of collection for isolation of plasma and PBMCs at the Impacta Peru Clinical Trials Unit in Lima, Peru. PBMCs were separated using Ficoll-Hypaque gradients following the cross-network HIV/AIDS Network Coordination (HANC) protocol [28]. PBMCs were maintained in liquid nitrogen for long-term cryopreservation. Plasma samples were stored at −80°C. Plasma and PBMCs were then transferred to the University of Cincinnati on dry ice and liquid nitrogen, respectively. PBMCs remained in liquid nitrogen and plasma at −80°C until they were thawed for further analyses. Viability of cells was confirmed using Trypan blue at the time of thawing from all processed samples.
Multiplex Assays for Inflammation and Immune Activation Markers in Plasma
Plasma levels of high-sensitivity C-reactive protein (hs-CRP), tumor necrosis factor–α (TNF-α), interferon-gamma-induced protein 10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), soluble CD163 (sCD163), soluble CD14 (sCD14), and soluble tissue factor (sTF) were measured using 2 customized Luminex assays (Luminex; R&D Systems). Interleukin-6 (IL-6) levels were determined using an enzyme-linked immunosorbent assay (R&D Systems). All individual samples were tested in duplicate on the same plate, and the average was analyzed to minimize variability.
Immunophenotyping of Monocytes at Rest and After In Vitro Stimulation
Cryopreserved PBMCs were thawed and rested in RPMI 1640 containing 10% heat-inactivated FBS and 10 mM EDTA. A total of 1.5 × 106 cells were incubated for 20 minutes at room temperature with Zombie UV (Biolegend), followed by Fc receptor blockage. PBMCs were then incubated for 30 minutes at room temperature with optimized doses and combinations of mouse antihuman antibodies (Supplementary Table 1).
We followed a similar protocol for in vitro stimulation of PBMCs. Briefly, after thawing, 1.5 × 106 cells were stimulated in 48-well flat-bottom plates (Costar, Corning, NY, USA) with 1 μg/mL of E. coli lipopolysaccharide 055:B5 (LPS; Invivogen) and incubated for 6 hours at 37°C in 5% CO2 in the presence of 10 μg/mL of Brefeldin A. After incubation, the PBMCs were harvested and washed with 2 mL of 1X PBS (Biolegend). Cells were then incubated for 20 minutes at room temperature with Zombie UV (Biolegend), followed by Fc receptor blockage. Optimized doses of mouse antihuman antibodies, as previously mentioned, were added to PBMCs for 30 minutes at room temperature. Then, the cells were fixed and permeabilized with the Foxp3/Transcription Factor Staining (Thermo Fisher). Subsequently, standardized doses of anti-IL-6 Alexafluor 700 (Thermo Fisher) and anti-TNF-α Brilliant Violet 650 (Mab11, Biolegend) were added and incubated for 30 minutes at 4°C. We could not consistently measure IL-1β under the described experimental conditions, and therefore this cytokine was not included in the final panel.
After antibody staining, the cells were washed with 1X permeabilization buffer (Thermo Fisher) and acquired on an LSR Fortessa cytometer (BD) using FACS Diva, version 6.0. At least 50 000 CD14+ events were acquired per sample. Flow cytometry data were analyzed using FlowJo software, version 10.8.1 (Tree Star, Inc., Ashland, OR, USA). Fluorescence minus one (FMO) controls were included to define positive thresholds. All samples were analyzed alongside aliquots of cryopreserved PBMCs from the same blood bank donor collected at the Hoxworth Blood Center in Cincinnati, Ohio. These reference PBMCs were used to check for batch effect and normalize data using the R package CytoNorm and FlowJo [29]. A validated gating strategy was used to identify total monocytes and their subsets based on CD14 and CD16 expression and their respective activation markers (Supplementary Figure 1A, B).
Statistical Analyses
Study data were collected and managed using REDCap [30]. We used 2-sample t tests or Mann-Whitney U tests for comparisons of numeric and flow cytometry data between the LTBI and non-LTBI groups. Categorical variables were compared using the chi-square test or Fisher exact test. Pearson's or Spearman's correlation coefficients were used to evaluate the correlation between 2 continuous variables. In multivariable linear regression models that assessed the relationship between flow cytometry variables of interest and CAD, we included 10-year pooled cohort equation (PCE) atherosclerotic CVD (ASCVD) risk as a covariate to adjust for traditional ASCVD risk factors [31]. P values <.05 were considered statistically significant. All P values were 2-tailed. We used Stata, version 12 (College Station, TX, USA), R, version 4.1.0 (R Foundation), and Prism, version 9.2.0 (GraphPad Software, San Diego, CA, USA), for data analyses and generation of graphics.
Patient Consent
The human participants protocol was reviewed and approved by the University of Cincinnati Institutional Review Board (IRB), the Ethical Committee of Hospital Nacional Dos de Mayo, and the Office of Public Health Strategic Interventions at the Ministry of Health in Peru. All participants provided written consent before being involved in any research procedures.
RESULTS
A total of 41 LTBI (60%) and 28 non-LTBI (40%) participants had stored plasma available for soluble marker analyses. Of these participants, 36 LTBI (62%) and 22 non-LTBI (38%) had stored PBMCs available for complete flow cytometry analyses. Overall, there were no significant differences in demographic characteristics of LTBI and non-LTBI participants, except for a higher body mass index observed in the LTBI group (Table 1). All study participants were HIV negative.
Table 1.
Demographic Characteristics of the Study Cohorta
| No LTBI (n = 28) |
LTBI (n = 41) |
P Value | |
|---|---|---|---|
| Age, y | 51 (44–65) | 53 (47–59) | .769 |
| Male sex | 15 (54) | 19 (46) | .555 |
| Body mass index | 26.3 (24–27.7) | 27.2 (25.9–29.1) | .023 |
| Hypertension | 4 (14) | 7 (17) | .756 |
| Diabetes mellitus | 4 (14) | 11 (27) | .215 |
| Dyslipidemia | 9 (32) | 11 (27) | .633 |
| Current tobacco use | 1 (4) | 4 (10) | .331 |
| Hemoglobin A1c, % | 5.6 (5.4–5.9) | 5.7 (5.5–6.8) | .162 |
| Total cholesterol | 189 (166–210) | 199 (177–222) | .183 |
| HDL cholesterol | 46 (40–53) | 42 (37–47) | .135 |
| LDL cholesterol | 111 (93–131) | 114 (102–134) | .505 |
| Coronary artery disease | 9 (32) | 15 (37) | .704 |
| ASCVD PCE score | 4.6 (1–8.2) | 4.7 (1.6–9.5) | .844 |
| Identified from outpatient clinic | 6 (21) | 13 (32) | .348 |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; HDL, high-density lipoprotein; IQR, interquartile range; LTBI, latent tuberculosis infection; LDL, low-density lipoprotein; PCE, pooled cohort equation.
Median (IQR) for continuous variables and No. (%) for categorical variables.
Plasma Markers of Systemic Inflammation and Monocyte Activation Were Similar in LTBI and Non-LTBI Individuals
We previously reported that LTBI individuals have higher IFN-γ values in the nil (unstimulated) QFT tube as compared with non-LTBI individuals [32], which was also noted within our study population (Supplementary Table 2). However, we found no significant differences between the non-LTBI and LTBI groups in plasma levels of IL-6, TNF-α, IP-10, MCP-1, sCD14, sCD163, and sTF (Supplementary Table 2).
LTBI Individuals Exhibited Alterations in Expression of CD36, CX3CR1, and HLA-DR on Their Circulating Monocytes
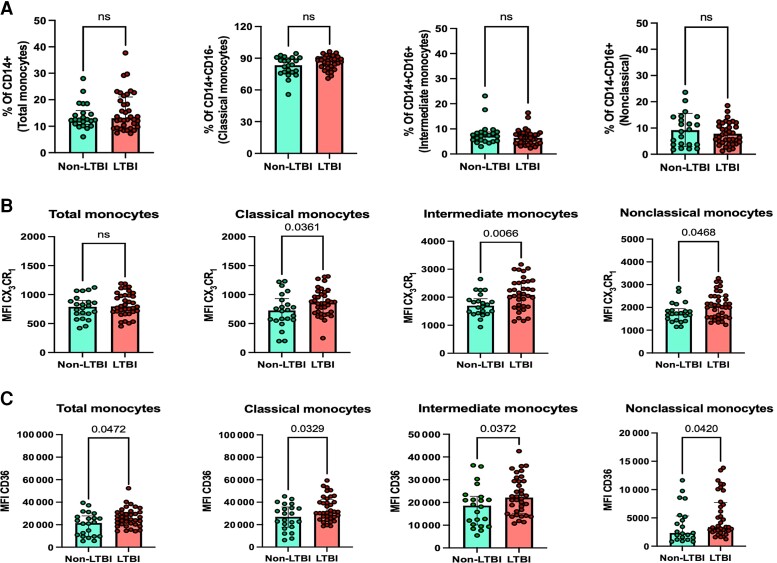
There were no significant differences in percentage of CD14+ total monocytes (LTBI: 13 vs non-LTBI: 12.4; P = .795), CD14+ CD16− classical (87.8 vs 83.4; P = .058), CD14+ CD16+ intermediate (6.5 vs 7.3; P = .239), or CD14dim CD16+ nonclassical monocyte subsets (7.02 vs 9.1; P = .315) between the non-LTBI and LTBI groups (Figure 1A).
Figure 1.
Immunophenotyping of monocytes in LTBI. A, Percentage of total monocytes, classical monocytes, intermediate monocytes, and nonclassical monocytes in non-LTBI and LTBI individuals. MFI of (B) CX3XR1 and (C) CD36 on total monocytes, classical monocytes, intermediate monocytes, and nonclassical monocytes in the non-LTBI and LTBI groups. P values are from the Mann-Whitney test. P values <.05 are considered significant. Abbreviations: LTBI, latent tuberculosis infection; MRI, mean fluorescence intensity; ns, not statistically significant.
Individuals with LTBI exhibited an increased percentage of classical monocytes expressing the chemokine receptor CX3CR1 (Supplementary Figure 2A). LTBI individuals also had an increased MFI of CX3CR1 across all monocyte subsets (Figure 1B), including classical monocytes (860 vs 725; P = .041), intermediate monocytes (2084 vs 1699; P = .006), and nonclassical monocytes (2053 vs 1724; P = .031).
The MFI of CD36 receptor was higher on total monocytes (28 826 vs 23 245; P = .047), classical monocytes (29 612 vs 21 826; P = .033), intermediate monocytes (30 746 vs 26 991; P = .043), and nonclassical monocytes (4080 vs 2826; P = .042) from LTBI individuals compared with non-LTBI individuals (Figure 1C). No differences were observed on the percentage of total monocytes and monocyte subsets expressing CD36 (Supplementary Figure 2A).
Another marker that was altered in the LTBI group was HLA-DR. LTBI individuals exhibited a lower percentage of total monocytes positive for HLA-DR compared with non-LTBI individuals (Supplementary Figure 2A), but no differences were found in the MFI of HLA-DR (Supplementary Figure 2B).
Additionally, we found that LTBI participants had a higher percentage of CD80 on nonclassical monocytes (Supplementary Figure 2A), but this difference was not observed across other monocyte subsets, nor did we find differences in MFI of CD80 between the LTBI and non-LTBI groups. Finally, we found no differences in percentage or MFI of CD86, CCR2, and CD163 receptors on monocytes by LTBI status (Supplementary Figure 2A, B).
Monocytes From LTBI Individuals Showed Enhanced Pro-inflammatory Responses Upon LPS Stimulation
Following LPS stimulation, LTBI individuals exhibited a lower MFI of HLA-DR on classical monocytes (6802 vs 8842; P = .049) and intermediate monocytes (9735 vs 12 483; P = .049) compared with non-LTBI individuals (Supplementary Figure 3B). No differences were observed in percentage or density of CX3CR1, CCR2, CD80, CD86, and CD163 receptors by LTBI status (Supplementary Figure 3A, B).
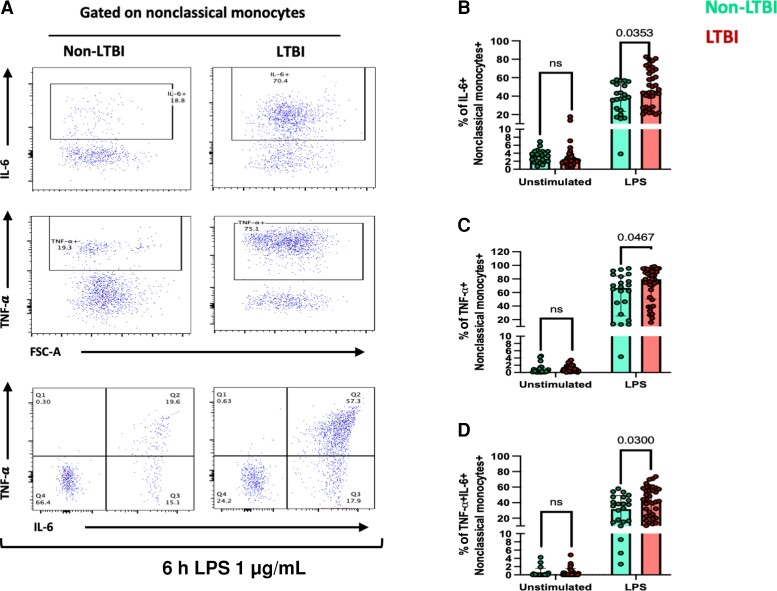
To determine monocyte expression of pro-inflammatory cytokines, the gating strategy shown in Figure 2A was used. Individuals with LTBI exhibited a higher percentage of nonclassical monocytes expressing IL-6 (68.6 vs 54; P = .035) (Figure 2B), TNF-α (79.5 vs 66.7; P = .047) (Figure 2C), and both IL-6 and TNF-α (43.2 vs 36.6; P = .042) (Figure 2D) compared with non-LTBI individuals. There were no differences in the percentage of IL-6+ and TNF-α+ total monocytes, classical, and intermediate monocyte subsets (Supplementary Figure 3C).
Figure 2.
Alteration in the proinflammatory response of LPS-stimulated monocytes from LTBI individuals. Peripheral blood mononuclear cells were stimulated with LPS 1 μg/mL for 6 hours. Unstimulated control was included. A, Gating strategy for detection of nonclassical monocytes (CD14dimCD16+) positive for IL-6, TNF-α, and IL-6+ TNF-α+. Percentage of nonclassical monocytes positive for (B) IL-6, (C) TNF-α+, and (D) IL-6+ TNF-α+ from the non-LTBI (green color) and LTBI (red color) groups. P values are from the Mann-Whitney test. P values <.05 are considered significant. Abbreviations: IL-6, interleukin-6; LTBI, latent tuberculosis infection; LPS, lipopolysaccharide; ns, not statistically significant; TNF, tumor necrosis factor.
To examine whether alterations in expression of CX3CR1 and CD36 on LTBI monocytes influenced the enhanced pro-inflammatory cytokine responses in the nonclassical monocyte compartment, we conducted a correlation analysis between baseline MFI of CX3CR1 and CD36 with post-LPS cytokine expression. The MFI of CX3CR1 at baseline positively correlated with IL-6+ nonclassical monocytes in the LTBI group (r = 0.438; P = .008) (Supplementary Figure 4A), which was also observed in the non-LTBI group (r = 0.464; P = .029). There were no significant associations between MFI of CX3CR1 and percentage of TNF-α+ or IL-6+ TNF-α+ nonclassical monocytes (Supplementary Figure 4A). No significant correlations were found between MFI of CD36 and IL-6 or TNF-α expression in LTBI vs non-LTBI participants (Supplementary Figure 4B).
Among LTBI individuals, the percentage of total monocytes of HLA-DR+ negatively correlated with the percentage of IL-6+ (r = −0.335; P = .046) and IL-6+ TNF-α+ total monocytes (r = −0.421; P = .011) (Supplementary Figure 4C). No significant correlation was found between HLA-DR and TNF-α+ total monocytes (Supplementary Figure 4C).
CX3CR1 and CD36 Expression Were Associated With CAD in LTBI Individuals
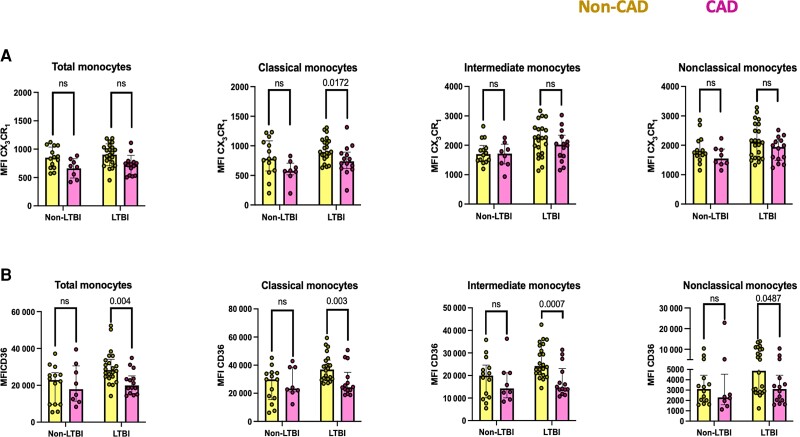
We then explored if monocyte parameters found to be altered in LTBI individuals were associated with CAD. Baseline MFI CX3CR1 on classical monocytes was significantly lower in LTBI individuals with CAD compared with LTBI individuals without CAD (Figure 3A). Similar but nonsignificant trends were observed in total, intermediate, and nonclassical monocytes (Figure 3A). The baseline MFI of CD36 on total monocytes and all monocyte subsets was lower in LTBI individuals with CAD as compared with those without CAD (Figure 3B). Baseline and post-LPS HLA-DR monocyte MFIs were not associated with CAD (Supplementary Table 3). No associations between CAD status and MFIs of CX3CR1, CD36, or HLA-DR were observed in non-LTBI controls. No associations were found between percentage of classical monocytes + CX3CR1 + baseline and post-LPS IL-6 and TNF-α expression with CAD status in individuals with or without LTBI (Supplementary Table 3).
Figure 3.
Immune alteration in monocytes from LTBI and their association with CAD. Baseline MFI of (A) CX3CR1 and (B) CD36 on total monocytes, classical monocytes, intermedial monocytes, and nonclassical monocytes in non-CAD (yellow color) and CAD (yellow color) individuals with non-LTBI or LTBI. P values are from the Mann-Whitney test. P values <.05 are considered significant. Abbreviations: CAD, coronary artery disease; LTBI, latent tuberculosis infection; MFI, mean fluorescence intensity; ns, not statistically significant.
In multivariable analysis, lower density of CD36 on total monocytes and all monocyte subsets of LTBI individuals was independently associated with presence of CAD (Table 2). CX3CR1, HLA-DR, and pro-inflammatory cytokine expression were not associated with CAD after adjusting for ASCVD PCE scores.
Table 2.
Results of Linear Regression Models Assessing the Relationship Between CAD and Log10-Transformed CD36 Density on Monocytes in LTBI Individuals
| Unadjusted b Coefficient (95% CI) | P Value | Adjustedab Coefficient (95% CI) | P Value | |
|---|---|---|---|---|
| CD36 MFI on total monocytes | −0.14 (−0.23 to −0.05) | .003 | −0.17 (−0.28 to −0.07) | .002 |
| CD36 MFI on classical monocytes | −0.14 (−0.22 to −0.05) | .002 | −0.18 (−0.27 to 0.08) | .001 |
| CD36 MFI on intermediate monocytes | −0.18 (−0.28 to −0.09) | <.001 | −0.24 (−0.35 to −0.13) | <.001 |
| CD36 MFI on nonclassical monocytes | −0.22 (−0.41 to −0.02) | .032 | −0.27 (−0.51 to −0.04) | .023 |
Abbreviations: ACC/AHA ASCVD, American College of Cardiology/American Heart Association atherosclerotic cardiovascular disease; CAD, coronary artery disease; LTBI, latent tuberculosis infection; MFI, mean fluoresce intensity.
Adjusted by ACC/AHA ASCVD pooled cohort equation risk scores.
DISCUSSION
We found that monocytes from individuals with LTBI exhibited distinct alterations in expression of surface marker receptors and pro-inflammatory cytokine responses. Specifically, individuals with LTBI had higher MFIs of CD36 lipid scavenger receptor and CX3CR1 chemokine receptor, lower percentages of HLA-DR, and increased proportions of nonclassical monocytes producing IL-6 and TNF-α post–LPS stimulation. Among individuals with LTBI, CAD was associated with lower MFI of CD36 across all monocyte subsets. No influence of LTBI on peripheral blood soluble markers of immune activation was found, indicating that LTBI-driven immune alterations are not sufficiently exacerbated for being reflected at a systemic level but are noticeable at the cellular levels.
CX3CR1 is a chemokine receptor important in monocyte adhesion and migration to tissues. We found increased expression of CX3CR1 on monocytes from individuals with LTBI, similar to what has been described in other inflammatory conditions, including HIV infection [33]. CX3CR1 is also important in atherosclerotic plaque development, as it plays an essential role in recruitment of CD16+ monocytes into the vascular tissue [24, 34]. Importantly, we found that the increased CX3CR1 expression among LTBI individuals was more pronounced on nonclassical monocytes, which is the monocyte subset with the overall highest expression for this receptor as they patrol the vasculature for endothelial injury [8]. A possible explanation for the decreased expression of CX3CR1 on circulating monocytes of LTBI individuals with CAD could be that monocytes with higher CX3CR1 densities may have migrated to tissues already, leaving monocytes with lower CX3CR1 in the circulation [8], but addressing this hypothesis would require tissue samples, which were not available in this study. A similar negative correlation between CAD and CX3CR1 density was reported in a cohort of persons with HIV in Uganda [35].
CD36 is a lipid scavenger receptor that allows uptake of modified lipids into cells, particularly oxidized-LDL [36, 37]. Mtb infection has been shown to increase CD36 receptor expression in a TLR-2 manner, inducing intracellular lipid accumulation in monocyte/macrophages [38–40]. Intracellular lipid accumulation induces foamy cell formation, a hallmark component of TB granulomas, but also atherosclerotic plaque lesions [41]. Although we found that LTBI was associated with increased CD36 expression as compared with non-LTBI, we also observed lower CD36 expression across all monocyte subsets among LTBI individuals with CAD. Both upregulation and downregulation of CD36 have been implicated in atherogenesis, and thus a tight protective window of CD36 expression has been postulated [25]. Activation of CD36 is downregulated by corticosteroids, HDL, and TLR signals including LPS, indicating that CD36 is downregulated during inflammation [42, 43]. Therefore, it is possible that the lower CD36 expression in LTBI individuals with CAD could result from a negative feedback mechanism to counterbalance ongoing inflammation. Alternatively, it is possible that monocytes with higher CD36 densities may have migrated out of the circulation, similar to what may have happened with high CX3CR1-expressing monocytes.
Recent studies indicate that nonclassical monocytes are involved in atherosclerosis development [44]. We reported that M. bovis BCG induces a relative expansion of nonclassical monocytes, which correlates with the extent of aortic plaque development in atherosclerotic mice [45]. Although we found that the proportion of nonclassical monocytes was similar in individuals with and without LTBI, we demonstrated that nonclassical monocytes from LTBI individuals had a higher expression of pro-inflammatory cytokines IL-6 and TNF-α upon LPS challenge. The nonclassical monocytes patrol the endothelium both at steady state and during inflammation and play an important role in homeostasis of vascular tissue [46]. However, this subset of monocytes is associated with atherogenesis when immune dysregulation is present [47].
We have found that persons with HIV with LTBI coinfection had an increased density of HLA-DR on monocytes [23]. In contrast, here we report that LPS-induced expression of HLA-DR was diminished in HIV-uninfected individuals with LTBI compared with non-LTBI. These data suggest that LTBI induces monocyte alterations through different mechanisms depending on the host HIV status. Decreased HLA-DR expression has been described on monocytes of patients with conditions where immune responses and antigen presentation are abnormally regulated such as systemic lupus erythematosus or pulmonary TB disease [48–50].
People with active TB have an increased risk of cardiovascular disease events [51]. Studies indicate that this risk remains elevated among TB survivors [52]. Monocytes from patients with active TB show increased cytokine activity upon stimulation with PPD antigens [53]. Additionally, TB patients have an augmented proportion of CD16+ monocytes, which are prone to TNF-α production and cell death [54]. Markers of monocyte activation are high at TB diagnosis and remain elevated throughout the course of TB treatment [55, 56]. Our findings suggest that pro-inflammatory monocytes may be found through different stages of the TB spectrum, including LTBI. However, monocyte alterations seem more subtle in LTBI and may not be equally present within the entire LTBI continuum.
Our study had limitations. Our sample size was limited to detecting small differences on monocyte markers between study groups. Nevertheless, to our knowledge, our work represents the largest study to date to profile monocyte subsets and their pro-inflammatory responses in LTBI. Furthermore, we were able to find distinct alterations in total and subset monocytes. LPS may induce downregulation of CD16, which could pose a challenge to identify CD16+ monocyte subsets post-LPS. However, our experiments showed that the overall expression of both CD14 and CD16 after LPS stimulation was adequate for analyses. Furthermore, we obtained very similar results using alternative gating strategies [57]. The significant differences in functional responses and MFI of CX3CR1 and CD36 between the LTBI and non-LTBI groups were modest. The biological and clinical relevance of these findings needs future study. We analyzed CAD as a binary variable (presence or absence of plaque) and at a single time point, which limits causal associations. Future longitudinal studies that analyze monocyte activation and quantify plaque progression among individuals with LTBI might be informative of more gradual changes and interactions between inflammation and ASCVD risk over time. We included participants over 40 years of age, and thus CAD risk factors were prevalent in our study population, which may not be fully representative of individuals with TB infection in endemic areas but comprises a population at risk for inflammation-driven chronic diseases.
In conclusion, individuals with LTBI exhibit distinct pro-inflammatory alterations of their monocytes, including increased CX3CR1 and CD36 expression as well as production of pro-inflammatory cytokines. These findings support the capacity of Mtb infection to alter the integrity of immune responses. Whether these monocyte alterations contribute to cardiovascular disease pathogenesis warrants further investigation.
Supplementary Material
Acknowledgments
We thank all the study participants.
Financial support . This work was supported in part by the National Center for Advancing Translational Sciences (grant numbers KL2 TR001426 and R03TR004097 to M.A.H.), the National Institute of Allergy and Infectious Diseases (grant number UM1AI069501 to C.J.F. and M.A.H.), and the National Heart, Lung, and Blood Institute (grant number 1R01HL156779 to M.A.H.) at the National Institutes of Health.
Disclaimer. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the institutions with which the authors are affiliated. The funding source had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Contributor Information
Manuel G Feria, Division of Infectious Diseases, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Cecilia Chang, Asociacion Civil Impacta Salud y Educacion, Lima, Peru.
Eduardo Ticona, Hospital Nacional Dos de Mayo, Lima, Peru; Universidad Nacional Mayor de San Marcos, Lima, Peru.
Anissa Moussa, Division of Infectious Diseases, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Bin Zhang, Division of Biostatistics, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA; Department of Pediatrics, University of Cincinnati, Cincinnati, Ohio, USA.
Isabel Ballena, Hospital Nacional Dos de Mayo, Lima, Peru.
Ruben Azañero, Hospital Nacional Dos de Mayo, Lima, Peru.
Cesar Ticona, Hospital Nacional Dos de Mayo, Lima, Peru.
Carlo N De Cecco, Division of Cardiothoracic Imaging, Department of Radiology and Imaging Sciences, Emory University, Atlanta, Georgia, USA.
Carl J Fichtenbaum, Division of Infectious Diseases, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Robert E O’Donnell, Division of Cardiovascular Health and Disease, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA.
Alberto La Rosa, Asociacion Civil Impacta Salud y Educacion, Lima, Peru.
Jorge Sanchez, Asociacion Civil Impacta Salud y Educacion, Lima, Peru; Centro de Investigaciones Tecnologicas, Biomedicas y Medioambientales, Callao, Peru.
Sandra Andorf, Division of Biostatistics, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA; Department of Pediatrics, University of Cincinnati, Cincinnati, Ohio, USA; Divisions of Biomedical Informatics and of Allergy and Immunology, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA.
Laura Atehortua, Division of Immunobiology, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA; Graduate Program in Immunology, Cincinnati Children's Hospital Medical Center and University of Cincinnati, Cincinnati, Ohio, USA.
Jonathan D Katz, Department of Pediatrics, University of Cincinnati, Cincinnati, Ohio, USA; Division of Immunobiology, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA; Graduate Program in Immunology, Cincinnati Children's Hospital Medical Center and University of Cincinnati, Cincinnati, Ohio, USA.
Claire A Chougnet, Department of Pediatrics, University of Cincinnati, Cincinnati, Ohio, USA; Division of Immunobiology, Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio, USA; Graduate Program in Immunology, Cincinnati Children's Hospital Medical Center and University of Cincinnati, Cincinnati, Ohio, USA.
George S Deepe, Jr., Division of Infectious Diseases, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA Graduate Program in Immunology, Cincinnati Children's Hospital Medical Center and University of Cincinnati, Cincinnati, Ohio, USA.
Moises A Huaman, Division of Infectious Diseases, Department of Internal Medicine, University of Cincinnati, Cincinnati, Ohio, USA; Graduate Program in Immunology, Cincinnati Children's Hospital Medical Center and University of Cincinnati, Cincinnati, Ohio, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. Houben RM, Dodd PJ. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 2016; 13:e1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah M, Dorman SE. Latent tuberculosis infection. N Engl J Med 2021; 385:2271–80. [DOI] [PubMed] [Google Scholar]
- 3. Barry CE 3rd, Boshoff HI, Dartois V, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol 2009; 7:845–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lin PL, Maiello P, Gideon HP, et al. PET CT identifies reactivation risk in cynomolgus macaques with latent M. tuberculosis. PLoS Pathog 2016; 12:e1005739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dutta NK, Karakousis PC. Latent tuberculosis infection: myths, models, and molecular mechanisms. Microbiol Mol Biol Rev 2014; 78:343–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gideon HP, Phuah J, Myers AJ, et al. Variability in tuberculosis granuloma T cell responses exists, but a balance of pro- and anti-inflammatory cytokines is associated with sterilization. PLoS Pathog 2015; 11:e1004603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gill WP, Harik NS, Whiddon MR, Liao RP, Mittler JE, Sherman DR. A replication clock for Mycobacterium tuberculosis. Nat Med 2009; 15:211–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McCausland MR, Juchnowski SM, Zidar DA, et al. Altered monocyte phenotype in HIV-1 infection tends to normalize with integrase-inhibitor-based antiretroviral therapy. PLoS One 2015; 10:e0139474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hunt PW. HIV and inflammation: mechanisms and consequences. Curr HIV/AIDS Rep 2012; 9:139–47. [DOI] [PubMed] [Google Scholar]
- 10. Nou E, Lo J, Grinspoon SK. Inflammation, immune activation, and cardiovascular disease in HIV. AIDS 2016; 30:1495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hoffmann U, Lu MT, Foldyna B, et al. Assessment of coronary artery disease with computed tomography angiography and inflammatory and immune activation biomarkers among adults with HIV eligible for primary cardiovascular prevention. JAMA Netw Open 2021; 4:e2114923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Funderburg NT, Zidar DA, Shive C, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood 2012; 120:4599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Lee PY, Reeves WH. Monocyte and macrophage abnormalities in systemic lupus erythematosus. Arch Immunol Ther Exp (Warsz) 2010; 58:355–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hirose S, Lin Q, Ohtsuji M, Nishimura H, Verbeek JS. Monocyte subsets involved in the development of systemic lupus erythematosus and rheumatoid arthritis. Int Immunol 2019; 31:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smiljanovic B, Radzikowska A, Kuca-Warnawin E, et al. Monocyte alterations in rheumatoid arthritis are dominated by preterm release from bone marrow and prominent triggering in the joint. Ann Rheum Dis 2018; 77:300–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Huaman MA, Ticona E, Miranda G, et al. The relationship between latent tuberculosis infection and acute myocardial infarction. Clin Infect Dis 2018; 66:886–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoufi EAA. Association between latent tuberculosis and ischemic heart disease: a hospital-based cross-sectional study from Saudi Arabia. Pan Afr Med J 2021; 38:362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alsayed Hasanain AF, El-Maghraby KM, Zayed AA H, Nafee AM A, Abdel-Aal SM, Bakkar SM. Latent tuberculosis infection among patients with coronary artery stenosis: a case-control study. Int J Mycobacteriol 2018; 7:143–7. [DOI] [PubMed] [Google Scholar]
- 19. Mandieka E, Saleh D, Chokshi AK, Rivera AS, Feinstein MJ. Latent tuberculosis infection and elevated incidence of hypertension. J Am Heart Assoc 2020; 9:e019144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Magee MJ, Khakharia A, Gandhi NR, et al. Increased risk of incident diabetes among individuals with latent tuberculosis infection. Diabetes Care 2022; 45:880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Williams H, Mack CD, Li SCH, Fletcher JP, Medbury HJ. Nature versus number: monocytes in cardiovascular disease. Int J Mol Sci 2021; 22:9119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang PH, Wu MF, Hsu CY, et al. The dynamic change of immune checkpoints and CD14+ monocytes in latent tuberculosis infection. Biomedicines 2021; 9:1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huaman MA, Juchnowski SM, Zidar DA, et al. Monocyte activation in persons living with HIV and tuberculosis coinfection. AIDS 2021; 35:447–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roy-Chowdhury E, Brauns N, Helmke A, et al. Human CD16+monocytes promote a pro-atherosclerotic endothelial cell phenotype via CX3CR1-CX3CL1 interaction. Cardiovasc Res 2021; 117:1510–22. [DOI] [PubMed] [Google Scholar]
- 25. Zhao L, Varghese Z, Moorhead JF, Chen Y, Ruan XZ. CD36 and lipid metabolism in the evolution of atherosclerosis. Br Med Bull 2018; 126:101–12. [DOI] [PubMed] [Google Scholar]
- 26. Huaman MA, De Cecco CN, Bittencourt MS, et al. Latent tuberculosis infection and subclinical coronary atherosclerosis in Peru and Uganda. Clin Infect Dis 2021; 73:e3384–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abbara S, Blanke P, Maroules CD, et al. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: a report of the society of cardiovascular computed tomography guidelines committee: endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr 2016; 10:435–49. [DOI] [PubMed] [Google Scholar]
- 28. HIV/AIDS Network Coordination . Cross-network PBMC processing standard operating procedure. HANC-LAB-P00001v6.0. https://www.hanc.info/resources/sops-guidelines-resources/laboratory/cross-network-pbmc-processing-sop.html. Accessed September 1, 2022.
- 29. Van Gassen S, Gaudilliere B, Angst MS, Saeys Y, Aghaeepour N. Cytonorm: a normalization algorithm for cytometry data. Cytometry A 2020; 97:268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Harris PA, Taylor R, Minor BL, et al. The REDCap Consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goff DC Jr, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S49–73. [DOI] [PubMed] [Google Scholar]
- 32. Huaman MA, Henson D, Rondan PL, et al. Latent tuberculosis infection is associated with increased unstimulated levels of interferon-gamma in Lima, Peru. PLoS One 2018; 13:e0202191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guo N, Chen Y, Su B, et al. Alterations of CCR2 and CX3CR1 on three monocyte subsets during HIV-1/Treponema pallidum coinfection. Front Med (Lausanne) 2020; 7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Combadiere C, Potteaux S, Rodero M, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation 2008; 117: 1649–57. [DOI] [PubMed] [Google Scholar]
- 35. Longenecker CT, Bogorodskaya M, Margevicius S, et al. Sex modifies the association between HIV and coronary artery disease among older adults in Uganda. J Int AIDS Soc 2022; 25:e25868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Podrez EA, Febbraio M, Sheibani N, et al. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J Clin Invest 2000; 105:1095–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kunjathoor VV, Febbraio M, Podrez EA, et al. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J Biol Chem 2002; 277:49982–8. [DOI] [PubMed] [Google Scholar]
- 38. Lovewell RR, Sassetti CM, VanderVen BC. Chewing the fat: lipid metabolism and homeostasis during M. tuberculosis infection. Curr Opin Microbiol 2016; 29:30–6. [DOI] [PubMed] [Google Scholar]
- 39. Rajaram MV, Brooks MN, Morris JD, Torrelles JB, Azad AK, Schlesinger LS. Mycobacterium tuberculosis activates human macrophage peroxisome proliferator-activated receptor gamma linking mannose receptor recognition to regulation of immune responses. J Immunol 2010; 185:929–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mahajan S, Dkhar HK, Chandra V, et al. Mycobacterium tuberculosis modulates macrophage lipid-sensing nuclear receptors PPARgamma and TR4 for survival. J Immunol 2012; 188:5593–603. [DOI] [PubMed] [Google Scholar]
- 41. Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol 2010; 7:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zamora C, Cantó E, Nieto JC, Angels Ortiz M, Juarez C, Vidal S. Functional consequences of CD36 downregulation by TLR signals. Cytokine 2012; 60:257–65. [DOI] [PubMed] [Google Scholar]
- 43. Febbraio M, Hajjar DP, Silverstein RL. CD36: a class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J Clin Invest 2001; 108:785–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Clemente C, Rius C, Alonso-Herranz L, et al. MT4-MMP deficiency increases patrolling monocyte recruitment to early lesions and accelerates atherosclerosis. Nat Commun 2018; 9:910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Huaman MA, Qualls JE, Jose S, et al. Mycobacterium bovis bacille-calmette-guerin infection aggravates atherosclerosis. Front Immunol 2020; 11:607957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Auffray C, Fogg D, Garfa M, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 2007;317:666–70. [DOI] [PubMed] [Google Scholar]
- 47. Thomas G, Tacke R, Hedrick CC, Hanna RN. Nonclassical patrolling monocyte function in the vasculature. Arterioscler Thromb Vasc Biol 2015; 35:1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sánchez MD, García Y, Montes C, et al. Functional and phenotypic changes in monocytes from patients with tuberculosis are reversed with treatment. Microbes Infect 2006; 8:2492–500. [DOI] [PubMed] [Google Scholar]
- 49. Shirakawa F, Yamashita U, Suzuki H. Decrease in HLA-DR-positive monocytes in patients with systemic lupus erythematosus (SLE). J Immunol 1985; 134:3560–2. [PubMed] [Google Scholar]
- 50. Palojarvi A, Petaja J, Siitonen S, Janer C, Andersson S. Low monocyte HLA-DR expression as an indicator of immunodepression in very low birth weight infants. Pediatr Res 2013;73:469–75. [DOI] [PubMed] [Google Scholar]
- 51. Huaman MA, Kryscio RJ, Fichtenbaum CJ, et al. Tuberculosis and risk of acute myocardial infarction: a propensity score-matched analysis. Epidemiol Infect 2017; 145:1363–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Basham CA, Smith SJ, Romanowski K, Johnston JC. Cardiovascular morbidity and mortality among persons diagnosed with tuberculosis: a systematic review and meta-analysis. PLoS One 2020; 15:e0235821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pollara G, Turner CT, Rosenheim J, et al. Exaggerated IL-17A activity in human in vivo recall responses discriminates active tuberculosis from latent infection and cured disease. Sci Transl Med 2021; 13:eabg7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Castaño D, García LF, Rojas M. Increased frequency and cell death of CD16+monocytes with Mycobacterium tuberculosis infection. Tuberculosis (Edinb) 2011; 91:348–60. [DOI] [PubMed] [Google Scholar]
- 55. Lawn SD, Labeta MO, Arias M, Acheampong JW, Griffin GE. Elevated serum concentrations of soluble CD14 in HIV- and HIV+ patients with tuberculosis in Africa: prolonged elevation during anti-tuberculosis treatment. Clin Exp Immunol 2000; 120:483–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Feruglio SL, Trøseid M, Damås JK, Kvale D, Dyrhol-Riise AM. Soluble markers of the Toll-like receptor 4 pathway differentiate between active and latent tuberculosis and are associated with treatment responses. PLoS One 2013; 8:e69896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Belge KU, Dayyani F, Horelt A, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol 2002; 168:3536–42. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.