Abstract
Background
We aimed to describe attitudes toward treatment of herpes simplex virus type 2 (HSV-2) meningitis and prioritize future trials.
Methods
This was a self-administered online survey of HSV-2 meningitis treatment among infectious diseases (ID) specialists in France, Sweden, Australia, and Denmark.
Results
A total of 223 ID specialists (45% female) from France (36%), Denmark (24%), Sweden (21%), and Australia (19%) participated in the survey, primarily from university hospitals (64%). The estimated overall response rate was 11% and ranged from 6% (Australia) to 64% (Denmark). Intravenous (IV) acyclovir followed by oral valacyclovir was the favored treatment in 110 of 179 (61%), whereas monotherapy with either IV acyclovir or oral valacyclovir was used by 35 of 179 (20%) and 34 of 179 (19%), respectively. The median total duration was reported to be 7 days (interquartile range, 7–10 days) regardless of antiviral regimen. Immunocompromise influenced decisions on antiviral treatment in 110 of 189 (58%) of respondents, mainly by prolonged total duration of treatment (36/110 [33%]), prolonged IV administration (31/110 [28%]), and mandatory antiviral treatment (25/110 [23%]). Treatment with acyclovir/valacyclovir versus placebo and comparison of acyclovir versus valacyclovir were assigned the highest prioritization scores for future randomized controlled trials on HSV-2 meningitis.
Conclusions
Perceptions of indications for as well as type and duration of antiviral treatment varied substantially among ID specialists.
Keywords: HSV-2, meningitis, management, survey, trial
Herpes simplex virus 2 (HSV-2) is a frequent cause of genital ulcers but may also occasionally present as viral meningitis. HSV-2 meningitis typically occurs in adult females (76%) at a median age of 35 years [1]. Although it is often considered a benign condition, a substantial proportion may suffer from prolonged neurocognitive impairment and up to 11% may not regain usual functional level, including work capacity, within 6 months after discharge [1–4]. In addition, quality of life has been shown to be substantially reduced at 1 year after hospitalization compared with the background population [4].
Valacyclovir is used for treatment of genital herpes and intravenous (IV) acyclovir for herpes simplex virus type 1 (HSV-1) encephalitis [5–7]. However, efficacy for acute HSV-2 meningitis has never been examined in randomized controlled trials (RCTs), which may lead to large variations in treatment practices. Moreover, adjunctive corticosteroids are part of standard care in treatment of bacterial meningitis and their use is currently being explored in a trial of HSV-1 encephalitis (ClinicalTrials.gov identifier: NCT03084783). This anti-inflammatory effect could also prove beneficial in HSV-2 meningitis. Understanding perceptions and attitudes toward treatment of HSV-2 meningitis is crucial for implementation of evidence-based guidelines and for defining key clinical research questions.
This international survey among infectious diseases (ID) specialists in France, Sweden, Australia, and Denmark was conducted to clarify current practices in antiviral (ie, IV acyclovir or valacyclovir) treatment of HSV-2 meningitis and prioritize future clinical trials.
METHODS
The questionnaire was developed de novo for this study as a self-administered online survey on treatment of HSV-2 meningitis using the REDCap electronic research data capture tool [8]. The main author (J. B.) drafted the questionnaire based upon available clinical studies and discussions with the coauthors. Next, it was piloted among ID physicians at Aalborg University Hospital under the supervision of the main author, after which the questionnaire was finalized (Supplementary material). The survey was distributed among ID specialists from 11 November to 2 December 2020, in Denmark and from 2 May to 30 May 2022, in France, Sweden, and Australia.
Baseline parameters obtained in the survey comprised country of residence, age group, sex, years of experience as an ID specialist, hospital characteristics (university or nonuniversity, number of beds), and annual number of aseptic meningitis and HSV-2 meningitis at each site as well as availability of national and local treatment guidelines for HSV-2 meningitis. Next, respondents were questioned about preferred antiviral treatment such as route of administration, dosages, and duration of treatment. A hypothetical case was also presented, and respondents were asked whether they would initiate antiviral treatment and if selected circumstances may influence such decisions. Finally, study participants were asked to prioritize 4 research questions for RCTs on treatment of HSV-2 meningitis from 1 (high priority) to 6 (low priority) and their willingness to include patients in these trials. A complete response was defined as study participants who answered all questions in the survey.
The survey was sent by e-mail to the heads of Danish and Swedish ID departments or members of the Danish Study Group of Infections of the Brain for subsequent distribution among ID specialists at their hospital [9]. Each head of department in Denmark and Sweden was asked to provide the total number of ID specialists working at participating hospitals. In France, the survey was sent by email to the approximately 700 members of the French Society of Infectious Diseases (Société de Pathologie Infectieuse de Langue Française) [10]. In Australia and New Zealand, the survey was distributed by the ID mail list “Ozbug,” which also comprises 700 contacts [11]. An email reminder was sent 2 weeks before closure in all participating countries.
Statistical Analyses
Binary variables are presented as n/N to account for missing values and percentages. Continuous variables are described as medians with interquartile ranges (IQRs). Response rates were estimated by division of respondents by the total number of ID specialists employed at each included hospital in Denmark and Sweden. For the French and Australian distribution networks, response rates were defined as number of respondents divided by total number of ID physicians (specialists and residents) in the research networks (approximately 700 in each country). A sensitivity analysis restricted to participants with complete responses was also performed. This was a descriptive survey of HSV-2 meningitis treatment, and a sample size calculation was not meaningful for this type of study. Stata MP version 16 (StataCorp, College Station, Texas) was used for all statistical analyses.
Ethical Considerations and Funding
Participation in the survey was voluntary and completely anonymous. There were no financial or other incentives for physicians to complete the questionnaire and no funding was obtained for the conduct of this study. Approval from an ethical committee was not required for this type of study in the participating countries.
Patient Consent Statement
No patients were involved in this study and the survey conforms to standards applied in Denmark.
RESULTS
During the study period, 223 ID specialists (45% female) responded to the survey, of whom 78 (36%) were from France, 52 (24%) from Denmark, 47 (21%) from Sweden, and 42 (19%) from Australia (Table 1). The corresponding response rates were 78 of 700 (11%) in France, 52 of 81 (64%) in Denmark, 47 of 469 (10%) in Sweden, and 42 of 700 (6%) in Australia. Most respondents worked at university hospitals (69%) and had between 5 and 14 years of experience as specialists (41%). The annual number of HSV-2 meningitis cases managed at each site was reported to be ≤4 in 91 of 215 (42%), 5–9 in 53 of 215 (25%), 10–19 in 17 of 215 (8%), and ≥20 in 6 of 215 (3%) and was unknown in 48 of 215 (22%). National guidelines for treatment of HSV-2 meningitis existed in Denmark and Sweden, whereas local hospital guidelines were present according to 50 of 163 (23%) physicians. Complete responses were provided by 183 of 223 (82%) study participants.
Table 1.
Characteristics of 223 Infectious Diseases Specialists From France, Denmark, Sweden, and Australia Participating in a Survey on Management of Herpes Simplex Virus Type 2 Meningitis
| Characteristic | no./No. | (%) |
|---|---|---|
| Overall | 223 | (100) |
| Female sex | 98/218 | (45) |
| Country | ||
| France | 78/219 | (36) |
| Denmark | 52/219 | (24) |
| Sweden | 47/219 | (21) |
| Australia | 42/219 | (19) |
| Age, y | ||
| ≤34 | 16/218 | (7) |
| 35–44 | 94/218 | (43) |
| 45–54 | 63/218 | (29) |
| 55–64 | 39/218 | (18) |
| ≥65 | 6/218 | (3) |
| Specialist experience, y | ||
| ≤4 | 62/216 | (29) |
| 5–14 | 88/216 | (41) |
| 15–24 | 37/216 | (17) |
| ≥25 | 29/216 | (13) |
| Hospital | ||
| University | 139/218 | (64) |
| Nonuniversity | 79/218 | (36) |
| No. of beds | ||
| 0–200 | 11/217 | (5) |
| 201–500 | 72/217 | (33) |
| ≥501 | 125/217 | (58) |
| Don’t know | 9/217 | (4) |
| No. of patients with aseptic meningitis treated per year | ||
| ≤4 | 21/215 | (10) |
| 5–9 | 23/215 | (11) |
| 10–19 | 39/215 | (18) |
| ≥20 | 77/215 | (36) |
| Don’t know | 55/215 | (26) |
| No. of patients with HSV-2 meningitis treated per year | ||
| ≤4 | 91/215 | (42) |
| 5–9 | 53/215 | (25) |
| 10–19 | 17/215 | (8) |
| ≥20 | 6/215 | (3) |
| Don’t know | 48/215 | (22) |
| Complete response | 183/223 | (82) |
Abbreviation: HSV-2, herpes simplex virus type 2.
Respondents were asked about treatment decisions on a hypothetical case of a 30-year-old healthy man hospitalized with a clinical presentation of meningitis (ie, headache, neck stiffness, and a temperature of 39.1°C), normal conscious state, C-reactive protein level of 42 mg/L, and a cerebrospinal fluid (CSF) leukocyte count of 400 × 106/L with 80% lymphocytes (Table 2). Empiric acyclovir would be administered by 108 of 223 (48%) respondents, antibiotics for bacterial meningitis by 98 of 223 (44%), adjunctive dexamethasone by 50 of 223 (22%), and no treatment by 56 of 223 (25%). Over the next 2 days, HSV-2 was confirmed by positive polymerase chain reaction of the CSF and symptoms had regressed to a more moderate level. In this scenario, 90 of 188 (48%) would switch to valacyclovir, 47 of 188 (25%) would continue IV acyclovir for a few more days before switching to valacyclovir, 37 of 188 (20%) would stop antiviral treatment, and 14 of 188 (7%) would continue IV acyclovir throughout treatment.
Table 2.
Hypothetical Case Presentation and Associated Treatment Decisions
| Case Presentation | ||
| It's after midnight and a 30-year-old previously healthy male is admitted with headache, photophobia, neck stiffness, and a normal conscious state. Vitals show a temperature of 39.1°C and are otherwise within normal ranges. Blood C-reactive protein is 42 mg/L and B-leukocytes are 12 × 109/L. Lumbar puncture shows a CSF white blood cell count of 400 × 106/L with 80% lymphocytes. | ||
| Would you treat this patient empirically with any of these options?a | no./No. | (%) |
| Noneb | 56/223 | (25) |
| Acyclovir | 108/223 | (48) |
| Dexamethasone | 50/223 | (22) |
| Antibiotics for bacterial meningitis | 98/223 | (44) |
| Other | 10/223c | (4) |
| Case Presentation—Continued | ||
| The meningitis patient was started on empiric IV acyclovir by the admitting doctors. Two days later, the symptoms have regressed considerably, although he still has nausea, slight photophobia, intermittent spikes of fever, and requires analgetics for his headache. PCR of the CSF is positive for HSV-2. | ||
| How would you manage this patient? | no./No. | (%) |
| Stop antiviral treatmentb | 37/188 | (20) |
| Continue IV acyclovir throughout treatment | 14/188 | (7) |
| Continue IV acyclovir for now (switch to oral valacyclovir after further improvement) | 47/188 | (25) |
| Switch to oral valacyclovir | 90/188 | (48) |
Abbreviations: CSF, cerebrospinal fluid; HSV-2, herpes simplex virus type 2; IV, intravenous; PCR, polymerase chain reaction.
Several choices possible.
Automatically excludes other answers.
Other treatments included analgetics (n = 3) and valacyclovir (n = 2).
Others required further information before treatment decisions could be made (eg, CSF microscopy, glucose, and protein levels, suspicion of neuroborreliosis or not, and Hoen score).
Antiviral treatment was always used for patients with HSV-2 meningitis by 88 of 223 (39%) of study participants, whereas 22 of 223 (10%) answered that they never used antivirals for this condition (Table 3). Important indications for antiviral treatment included immunocompromising conditions (84/223 [38%]), severe symptoms (76/223 [34%]), concurrent HSV-2 ulcers (46/223 [21%]), and previous diagnosis of HSV-2 meningitis (44/223 [20%]). Among the different types of antiviral treatments, IV acyclovir followed by valacyclovir was the most favored regimen by 110 of 179 (61%). Others primarily used monotherapy with either IV acyclovir (35/179 [20%]) or valacyclovir (34/179 [19%]). The median total duration was reported to be 7 days (IQR, 7–10 days), regardless of antiviral regimen. Preferred dosages were 10 mg/kg 3 times a day (TID) in 132 of 145 (91%) for IV acyclovir and 1000 mg TID for valacyclovir in 122 of 144 (85%). Adjunctive dexamethasone was used by 2 of 189 (1%). A total of 110 of 189 (58%) respondents reported to treat immunocompromised patients differently, mainly by prolonged total duration of treatment by 36 of 110 (33%), prolonged IV treatment by 31 of 110 (28%), and mandatory treatment with antivirals by 25 of 110 (23%). Long-term prophylaxis for recurrent HSV-2 meningitis was used by 95 of 189 (50%).
Table 3.
Indications and Preferred Antiviral Treatment of Herpes Simplex Virus Type Meningitis Among Respondents
| Indication | no./No. | (%) |
|---|---|---|
| What do you consider as indications for acyclovir or valacyclovir treatment of patients with confirmed HSV-2 meningitis?a | ||
| I never or only rarely treat these patients with antiviralsb | 22/223 | (10) |
| Severe symptoms | 76/223 | (34) |
| No improvement in symptoms after 48 h of diagnosis | 27/223 | (12) |
| Immunocompromising conditions | 84/223 | (38) |
| Previous diagnosis of HSV-2 meningitis | 44/223 | (20) |
| Concurrent HSV-2 ulcers (genital or oral) | 46/223 | (21) |
| Patient indication (ie, patient requests antiviral treatment) | 11/223 | (5) |
| I always treat these patients with antiviralsb | 88/223 | (39) |
| Favored antiviral regimen | ||
| IV acyclovir followed by valacyclovir | 110/179 | (61) |
| Monotherapy with IV acyclovir | 35/179 | (20) |
| Monotherapy with valacyclovir | 34/179 | (19) |
| Duration of treatment (n = 174), d, median (IQR) | 7 | (7–10) |
| Preferred IV acyclovir dosages | ||
| 5 mg/kg 3 times daily | 8/145 | (6) |
| 10 mg/kg 3 times daily | 132/145 | (91) |
| 15 mg/kg 3 times daily | 5/145 | (3) |
| Preferred valacyclovir dosages | ||
| 1000 mg 3 times daily | 122/144 | (85) |
| 1000 mg 4 times daily | 3/144 | (2) |
| 2000 mg 3 times daily | 12/144 | (8) |
| 2000 mg 4 times daily | 2/144 | (1) |
| Other | 5/144 | (3) |
| Adjunctive dexamethasone | 2/189 | (1) |
| Treat immunocompromised patients differently | 110/189c | (58) |
Abbreviations: HSV-2, herpes simplex virus type 2; IQR, interquartile range; IV, intravenous.
Several choices possible.
Automatically excludes other answers.
Prolonged total duration of treatment (n = 36), prolonged IV acyclovir (n = 31), and always treat with antivirals (n = 25).
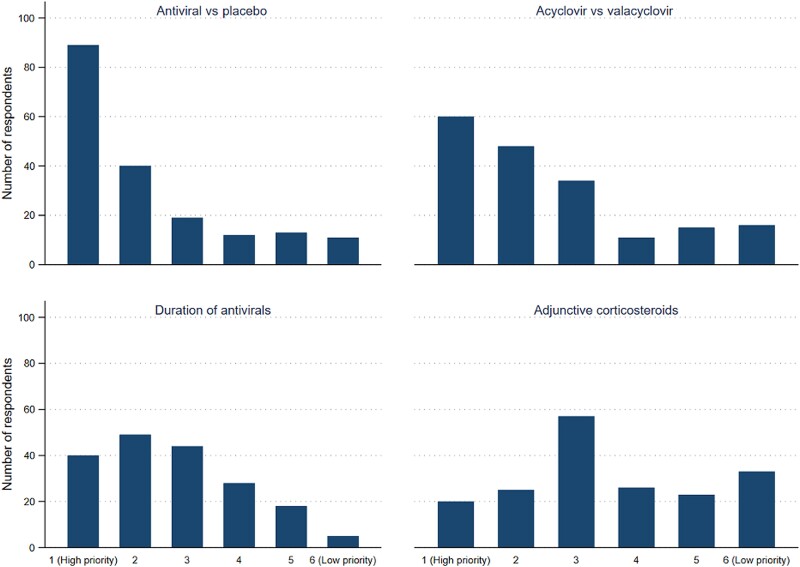
A high prioritization score was assigned to future RCTs on IV acyclovir or valacyclovir versus placebo for HSV-2 meningitis and IV acyclovir versus valacyclovir (Figure 1). In contrast, clinical trials on duration of antiviral treatment and adjunctive corticosteroid therapy were considered less crucial for management currently. Overall, willingness to include patients in RCTs on HSV-2 meningitis treatment was high and ranged from 70% for adjunctive corticosteroids to 91% for studies on duration of antiviral therapy (Table 4).
Figure 1.
Prioritization scores for future randomized clinical trials on treatment of acute herpes simplex virus type 2 meningitis.
Table 4.
Willingness to Enroll Patients in Future Randomized Clinical Trials on Treatment of Acute Herpes Simplex Virus Type 2 Meningitis
| Randomized Controlled Trial | Willingness to Include Patients in Trial | |
|---|---|---|
| no./No. | (%) | |
| Acyclovir or valacyclovir vs placebo | 143/183 | (78) |
| Acyclovir vs valacyclovir | 159/183 | (87) |
| Duration of therapy | 167/183 | (91) |
| Adjunctive dexamethasone | 128/183 | (70) |
Sensitivity analyses restricted to complete respondents did not substantially change the results of the survey (data not shown).
DISCUSSION
This survey showed that a large proportion of respondents were inclined to start empirical IV acyclovir for viral meningitis and switch to valacyclovir after a few days if HSV-2 was detected in the CSF for a total treatment duration of 7 days. Furthermore, the results of the survey indicated that many physicians considered immunocompromising conditions, severe symptoms, concurrent genital ulcers, and recurrent meningitis as important indications for antiviral treatment in confirmed HSV-2 meningitis. RCTs examining antiviral versus placebo or IV acyclovir versus valacyclovir received the highest priority scores.
We have not been able to identify previous surveys of treatment practices for HSV-2 meningitis. In this study, 48% of ID physicians were likely to use empirical IV acyclovir in a clinically stable patient with presumed viral meningitis (ie, without signs of encephalitis), whereas 10% indicated that they never treat HSV-2 meningitis with antivirals. Consistently, previous observational studies of patients with HSV-2 meningitis showed that almost all hospitalized patients in Denmark (96%) and other countries (60%–100%) were treated with antivirals despite the lack of evidence of clinical efficiency in RCTs [1, 4, 12–14]. Moreover, Danish guidelines recommend withholding acyclovir/valacyclovir until HSV-2 or varicella zoster virus have been confirmed as the causative pathogen in patients with viral meningitis [15]. On the other hand, a South Korean study observed no neurological sequelae among 53 patients with only symptomatic treatment of HSV-2 meningitis [16].
The case scenario presented in the survey was intended to capture some of the challenges in management of patients with viral meningitis in clinical practice. Symptoms and signs such as headache, photophobia, neck stiffness, and a normal conscious state at admission in a 30-year-old patient with 400 × 106 leukocytes/L (80% lymphocytes) in the CSF were indeed suggestive of viral meningitis. However, HSV-2 meningitis is more often diagnosed in females (76%) and the median level of C-reactive protein has been reported to be 3 mg/L (IQR 1–6 mg/L) in a nationwide prospective cohort study of 205 patients [1]. Different treatment strategies may therefore be justified depending on the overall interpretation of the clinical presentation by the physician on duty and given the lack of evidence on management of patients with viral meningitis.
Primary treatment or transition to valacyclovir seems to be common practice based on the current survey and observational studies [1, 12–14, 17]. This is supported by CSF measurements of valacyclovir in 10 patients with multiple sclerosis, although information on inhibitory concentrations from in vitro models do not directly translate into clinical efficiency [18, 19]. Respondents in the current survey suggested a median treatment duration of 7 days, which was only slightly shorter than the 10 days observed in 2 recent prospective cohorts from the United Kingdom from 2011 to 2014 and Denmark from 2015 to 2020 [1, 4]. Of note, a recommendation of 7 days of antiviral treatment for confirmed HSV-2 meningitis was published in Danish national guidelines in 2018 [15]. Other studies on mostly immunocompromised patients reported treatment durations of 14–21 days [1, 4, 13, 14, 20].
ID physicians consider immunocompromising conditions, severity of symptoms, genital ulcers, and previous HSV-2 meningitis as major determinants for antiviral treatment of HSV-2 meningitis. Even though these criteria seem reasonable, there are few data to support them. A Danish population-based prospective study observed an increased proportion with unfavorable outcome at 6 months after discharge in immunocompromised patients (24%) compared with patients without immunocompromise (9%) [1]. Yet, these differences were not significant in adjusted analyses. A retrospective single-center study examined the role of antiviral therapy in 42 patients with HSV-2 meningitis among whom 15 (36%) were immunocompromised (10 with human immunodeficiency virus [HIV], 3 with diabetes mellitus, and 2 on high-dose corticosteroids) [13]. They observed no neurological sequelae regardless of antiviral treatment in immunocompetent patients. On the other hand, 3 immunocompromised patients received supportive care only and all had neurological sequelae including chronic headache (n = 2) and paresthesia of the hands and discoordination (n = 1). For comparison, 2/12 treated with antivirals had persistent paresthesia of the hands (n = 1) and left arm pain (n = 1). Another single-center retrospective study on 13 immunocompromised patients (HIV and/or hematological malignancies) found that 2 patients with delayed antiviral treatment >6 hours developed encephalitis [20]. Acyclovir has been shown to shorten duration of genital HSV-2 mucocutaneous lesions by 1–2 days [5]. The previously mentioned recent large Danish prospective study found that 83 of 174 (47%) patients with HSV-2 meningitis had a history of genital ulcers, but active lesions were present during hospitalization in only 15 of 205 (8%) [1]. Patients with recurrent HSV-2 meningitis may have a milder course of illness and improved outcome for each recurrence [1]. Yet, such data remain difficult to interpret since diagnosis is often more straightforward in case of recurrences and antiviral treatment may be initiated at an earlier stage of disease along with improved self-management and care. Finally, long-term prophylaxis was used by 95 of 189 (50%) even though a previous placebo-controlled RCT did not find a substantial effect on recurrence with prophylactic valacyclovir through 1 year and an increased risk of HSV-2 meningitis after treatment cessation [21]. Reasons for use of long-term prophylaxis were not obtained in the current survey and should be explored further in future studies.
Respondents suggested a high prioritization for studies on antiviral treatment versus placebo along with willingness to include patients for such a trial. Although other research questions were not assigned as high prioritization scores, physicians were still willing to include patients in those trials supporting their clinical relevance and lack of safety concern. Responses in the current survey could inform design of clinical trials for HSV-2 meningitis. However, the feasibility of trials on HSV-2 meningitis may be challenging due to the low incidence of this infection.
This survey has limitations. Generalizability may be limited due to the relatively low response rates among ID specialists in France, Sweden, and Australia although the absolute number of respondents was high. We consider the denominator for the response rates to be accurate as information on number of ID specialists was provided directly by heads of departments of participating hospitals in Sweden and Denmark as well as consistency with other estimates of ID physicians in France, and members of the Ozbug email list in Australia and New Zealand [10, 11, 22]. Reasons for declining to participate in the survey may include limited experience with treatment as HSV-2 meningitis is a rare disease, low priority of responding to surveys in busy clinical practices, and burnout among ID physicians during the coronavirus disease 2019 pandemic [23, 24]. It is also conceivable that a more personal introduction of the survey than invitation by email may increase response rates. Another limitation is that HSV-2 meningitis may sometimes be managed by other medical specialties (eg, neurologists, general internal medicine) and the survey may not be representative of attitudes and perceptions toward treatment in such settings. Missing values were addressed by providing proportions of actual responses for each question in the survey and incomplete responses were accounted for by sensitivity analyses of complete responses only.
In conclusion, perceptions and attitudes toward management of HSV-2 meningitis differs significantly between physicians. RCTs on antiviral treatment versus placebo or IV acyclovir versus valacyclovir are of high priority.
Supplementary Material
Contributor Information
Jacob Bodilsen, Department of Infectious Diseases, Aalborg University Hospital, Aalborg, Denmark; European Society of Clinical Microbiology and Infectious Diseases Study Group for Infectious Diseases of the Brain, Basel, Switzerland; Department of Clinical Medicine, Aalborg University Hospital, Aalborg, Denmark.
Pierre Tattevin, European Society of Clinical Microbiology and Infectious Diseases Study Group for Infectious Diseases of the Brain, Basel, Switzerland; Department of Infectious Diseases and Intensive Care Unit, Pontchaillou University Hospital, Rennes, France; Réseau National de Recherche Clinique en Infectiologie, Paris, France.
Steven Y C Tong, Victorian Infectious Diseases Service, The Royal Melbourne Hospital at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia; Department of Infectious Diseases, The University of Melbourne at the Peter Doherty Institute for Infection and Immunity, Melbourne, Australia.
Pontus Naucler, Department of Infectious Diseases, Karolinska University Hospital, Stockholm, Sweden; Division of Infectious Diseases, Department of Medicine Solna, Karolinska Institutet, Stockholm, Sweden.
Henrik Nielsen, Department of Infectious Diseases, Aalborg University Hospital, Aalborg, Denmark; European Society of Clinical Microbiology and Infectious Diseases Study Group for Infectious Diseases of the Brain, Basel, Switzerland; Department of Clinical Medicine, Aalborg University Hospital, Aalborg, Denmark.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. This study is a collaborative study within the European Study Group for Infectious Diseases of the Brain.
Author contributions. All authors contributed to design, analysis, and interpretation of data. J. B. wrote the first draft of the report.
Financial support. No financial support was obtained for this study.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jakobsen A, Skov MT, Larsen L, et al. Herpes simplex virus 2 meningitis in adults: a prospective, nationwide, population-based cohort study. Clin Infect Dis 2022; 75:753–60. [DOI] [PubMed] [Google Scholar]
- 2. Schmidt H, Heimann B, Djukic M, et al. Neuropsychological sequelae of bacterial and viral meningitis. Brain 2006; 129:333–45. [DOI] [PubMed] [Google Scholar]
- 3. Damsgaard J, Hjerrild S, Andersen H, Leutscher PDC. Long-term neuropsychiatric consequences of aseptic meningitis in adult patients. Infect Dis 2015; 47:357–63. [DOI] [PubMed] [Google Scholar]
- 4. McGill F, Griffiths MJ, Bonnett LJ, et al. Incidence, aetiology, and sequelae of viral meningitis in UK adults: a multicentre prospective observational cohort study. Lancet Infect Dis 2018; 18:992–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gupta R, Warren T, Wald A. Genital herpes. Lancet 2007; 370:2127–37. [DOI] [PubMed] [Google Scholar]
- 6. Sköldenberg B, Alestig K, Burman L, et al. Aciclovir versus vidarabine in herpes simplex encephalitis. Lancet 1984; 324:707–11. [DOI] [PubMed] [Google Scholar]
- 7. Whitley RJ, Alford CA, Hirsch MS, et al. Vidarabine versus acyclovir therapy in herpes simplex encephalitis. N Engl J Med 1986; 314:144–9. [DOI] [PubMed] [Google Scholar]
- 8. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodilsen J, Larsen L, Brandt CT, et al. Existing data sources for clinical epidemiology: the Danish Study Group of Infections of the Brain Database (DASGIB). Clin Epidemiol 2021; 13:921–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [. Accessed 15 June 2022.]. http://www.infectiologie.com French Society of Infectious Diseases, Société de Pathologie Infectieuse de Langue Française.
- 11. Watson DAR. Ozbug: an email mailing list for physicians that works. Intern Med J 2003; 33:532–4. [DOI] [PubMed] [Google Scholar]
- 12. Miller S, Mateen FJ, Aksamit AJ. Herpes simplex virus 2 meningitis: a retrospective cohort study. J Neurovirol 2013; 19:166–71. [DOI] [PubMed] [Google Scholar]
- 13. Noska A, Kyrillos R, Hansen G, Hirigoyen D, Williams DN. The role of antiviral therapy in immunocompromised patients with herpes simplex virus meningitis. Clin Infect Dis 2015; 60:237–42. [DOI] [PubMed] [Google Scholar]
- 14. Landry ML, Greenwold J, Vikram HR. Herpes simplex type-2 meningitis: presentation and lack of standardized therapy. Am J Med 2009; 122:688–91. [DOI] [PubMed] [Google Scholar]
- 15. Danish Society of Infectious Diseases. Guidelines for management of viral meningitis in adults. 2018. http://www.infmed.dk/guidelines. Accessed 15 June 2022.
- 16. Moon SM, Kim T, Lee EM, Kang JK, Lee S-A, Choi S-H. Comparison of clinical manifestations, outcomes and cerebrospinal fluid findings between herpes simplex type 1 and type 2 central nervous system infections in adults. J Med Virol 2014; 86:1766–71. [DOI] [PubMed] [Google Scholar]
- 17. Omland LH, Vestergaard BF, Wandall JH. Herpes simplex virus type 2 infections of the central nervous system: a retrospective study of 49 patients. Scand J Infect Dis 2008; 40:59–62. [DOI] [PubMed] [Google Scholar]
- 18. Lycke J, Malmestrom C, Stahle L. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob Agents Chemother 2003; 47:2438–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bodilsen J, Nielsen H, Whitley RJ. Valaciclovir therapy for herpes encephalitis: caution advised. J Antimicrob Chemother 2019; 74:1467–8. [DOI] [PubMed] [Google Scholar]
- 20. Momméja-Marin H, Lafaurie M, Scieux C, Galicier L, Oksenhendler E, Molina J-M. Herpes simplex virus type 2 as a cause of severe meningitis in immunocompromised adults. Clin Infect Dis 2003; 37:1527–33. [DOI] [PubMed] [Google Scholar]
- 21. Aurelius E, Franzen-Röhl E, Glimåker M, et al. Long-term valacyclovir suppressive treatment after herpes simplex virus type 2 meningitis: a double-blind, randomized controlled trial. Clin Infect Dis 2012; 54:1304–13. [DOI] [PubMed] [Google Scholar]
- 22. Brockhoff RA, Hicks SR, Salmanton-García J, et al. Training in infectious diseases across Europe in 2021—a survey on training delivery, content and assessment. Clin Microbiol Infec 2021; 27:1693.e1–8. [DOI] [PubMed] [Google Scholar]
- 23. Melnikow J, Padovani A, Miller M. Frontline physician burnout during the COVID-19 pandemic: national survey findings. BMC Health Serv Res 2022; 22:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shanafelt TD, West CP, Dyrbye LN, et al. Changes in burnout and satisfaction with work-life integration in physicians over the first 2 years of the COVID-19 pandemic. Mayo Clin Proc 2022; 97:2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.