Abstract
Escherichia coli K1 traversal across the blood-brain barrier is an essential step in the pathogenesis of neonatal meningitis. We have previously shown that invasive E. coli promotes the actin rearrangement of brain microvascular endothelial cells (BMEC), which constitute a lining of the blood-brain barrier, for invasion. However, signal transduction mechanisms involved in E. coli invasion are not defined. In this report we show that tyrosine kinases play a major role in E. coli invasion of human BMEC (HBMEC). E. coli induced tyrosine phosphorylation of HBMEC cytoskeletal proteins, focal adhesion kinase (FAK), and paxillin, with a concomitant increase in the association of paxillin with FAK. Overexpression of a dominant interfering form of the FAK C-terminal domain, FRNK (FAK-related nonkinase), significantly inhibited E. coli invasion of HBMEC. Furthermore, we found that FAK kinase activity and the autophosphorylation site (Tyr397) are important in E. coli invasion of HBMEC, whereas the Grb2 binding site (Tyr925) is not required. Immunocytochemical studies demonstrated that FAK is recruited to focal plaques at the site of bacterial entry. Consistent with the invasion results, overexpression of FRNK, a kinase-negative mutant (Arg454 FAK), and a Src binding mutant (Phe397 FAK) inhibited the accumulation of FAK at the bacterial entry site. The overexpression of FAK mutants in HBMEC also blocked the E. coli-induced tyrosine phosphorylation of FAK and its association with paxillin. These observations provide evidence that FAK tyrosine phosphorylation and its recruitment to the cytoskeleton play a key role in E. coli invasion of HBMEC.
Escherichia coli K1 is one of the most common gram-negative bacteria causing meningitis during the neonatal period. The mortality and morbidity associated with E. coli meningitis remain significant, with case fatality rates ranging from 15 to 40% of infected neonates. Incomplete understanding of the pathogenesis and pathophysiology of this disease has contributed to the high morbidity and mortality. For example, most cases of E. coli meningitis develop as a result of hematogenous spread, but the mechanisms by which E. coli traverses across the blood-brain barrier are not clearly understood. Mechanisms of bacterium-host interactions can often be elucidated using in vitro cell culture systems. However, establishment of the appropriate host target cell cultures for these studies is required to address issues relevant to the disease. We have developed an in vitro human brain microvascular endothelial cell (HBMEC) culture with characteristics of the blood-brain barrier, which is a natural target for meningitis (39). Using this HBMEC tissue culture model, we have shown that the process of E. coli invasion into HBMEC is multifactorial, i.e., utilizes S fimbriae for binding and OmpA, IbeA, IbeB, and Yjp for invasion (17, 18, 27, 40, 42). Binding and invasion of E. coli have been verified in the newborn rat model of hematogenous meningitis, and the results are in agreement with our in vitro model (12). However, it is not clear whether the above structures act cooperatively or independently for E. coli invasion of HBMEC. Invasion begins by the bacteria interacting with the HBMEC plasma membrane, which progressively enwraps the bacteria (26). This event, called the zipper mechanism, requires bacterium-induced actin rearrangements in HBMEC for bacterial uptake. The actin that polymerizes underneath the bacteria forms focal plaques containing protein complexes of highly phosphorylated proteins (10, 11). Bacterial entry in this fashion is sensitive to cytochalasin D, an inhibitor of microfilament polymerization and inhibitors of tyrosine kinases (N. V. Prasadarao, J. Zhou, C. A. Wass, M. A. Arditi, and K. S. Kim, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., p. 38, 1997).
There is increasing evidence that focal adhesion kinase (FAK) may play a key role in regulating the dynamic changes of actin reorganization (34). FAK is associated with focal adhesions in all eukaryotic cells, and it plays a critical role in mediating cell adhesion and motility (19). Focal adhesions are discrete regions associated with the ventral cell surface where integrin receptors interact with adhesive extracellular matrix protein of the substratum (4, 34). Inside the cell, the cytoplasmic tail of the integrins directs the assembly of a protein complex which functions to anchor actin filament bundles, thereby providing the physical support necessary for cell spreading and locomotion (22). FAK has emerged as a likely component of the signal transduction pathways underlying integrin-mediated changes in cells, and the role of tyrosine phosphorylation of this protein in these events has been demonstrated (14, 15, 19, 44). FAK, a widely expressed nonreceptor protein tyrosine kinase (PTK), is a 125-kDa protein that contains a central kinase domain flanked by an amino-terminal domain and a carboxy-terminal domain (34). The amino-terminal domain contains an autophosphorylation site (Tyr397), while the carboxy-terminal domain contains a region required for its localization to focal adhesions (FAT [focal adhesion targeting] site) and binding sites for the cytoskeletal proteins paxillin and talin (6, 15, 16, 37). The autophosphorylation site of FAK serves as a binding site for the SH2 domain of Src family of PTKs in vivo (42). Phosphorylation of tyrosine residues in the FAK carboxy-terminal domain by Src kinases at Tyr925 creates a binding site for the SH2 domain of Grb2 (14, 23, 36). Thus, FAK appears to act as a scaffold for organizing a network of signaling and cytoskeletal proteins. Human and rat brain cells express several FAK alternative splice forms that appear to be able to regulate FAK phosphorylation. A C-terminal domain of FAK, designated FRNK (FAK-related nonkinase), is expressed autonomously as a 41- to 43-kDa protein. FRNK can also localize to focal adhesions because of structural integrity of the FAT domain (2, 31). However, overexpression of FRNK inhibits the phosphorylation of focal adhesion proteins and inhibits FAK function (31). Thus, FAK is itself a highly regulated factor that is required for transducing phosphorylation signals for a variety of cellular functions.
In this study, we investigated the role of FAK in E. coli invasion of HBMEC by overexpressing the dominant-negative mutants of FAK. Our results indicate that FAK is critical for E. coli invasion of HBMEC and that Tyr397 and the kinase activity of FAK play an important role in the invasion process. In addition, the recruitment of FAK to the bacterial entry site is also needed for efficient entry of E. coli into HBMEC.
MATERIALS AND METHODS
Bacteria.
E. coli E44 is a spontaneous rifampin-resistant mutant of E. coli K1 strain RS 218 (serotype O18:K1:H7) isolated from the cerebrospinal fluid of a neonate with meningitis and invades HBMEC in a cell culture model. E91 is a noninvasive derivative of E44 with a disrupted ompA gene, which expresses no OmpA (26). HB101 (K-12 capsular polysaccharide), a laboratory strain, is noninvasive in HBMEC. Listeria monocytogenes, which also invades HBMEC, was used as a positive control strain (13). All bacteria were grown overnight in brain heart infusion broth with appropriate antibiotics as necessary. All bacterial media were purchased from Difco Laboratories (Detroit, Mich.).
Materials.
Monoclonal antibodies to FAK and paxillin were purchased from Transduction Laboratories (Lexington, Ky.). Polyclonal anti-FAK antibody, which recognizes the C-terminal end, was from Santa Cruz Biotechnology (Santa Cruz, Calif.). Monoclonal antiphosphotyrosine antibody 4G10 was purchased from Upstate Biotechnology, Inc. (Lake Placid, N.Y.). Hemagglutinin epitope (HA) antibody CA125 and protein A-agarose were from Boehringer Mannheim (Indianapolis, Ind.). Kinase inhibitors genistein, herbimycin A, and PP1 were purchased from Calbiochem (San Diego, Calif.). Horseradish peroxidace-conjugated secondary antibodies were obtained from Bio-Rad Laboratories (Hercules, Calif.), and fluorescein isothiocyanate-conjugated secondary antibodies were purchased from Molecular Probes (San Diego, Calif.). SuperSignal chemiluminescence reagent and the bicinchonic acid protein assay kit were from Pierce Chemical Co. (Rockford, Ill.). [γ-32P]ATP (3,000 Ci/mmol) was obtained from New England Nuclear (Boston, Mass.), and Lipofectamine was from Gibco-BRL (Bethesda, Md.). All other chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.). Mammalian expression vectors for FAK proteins were described previously (33). Both wild-type and mutant FAK proteins were cloned in pCDNA3 and confer G418 resistance to transfected cells. FRNK was cloned into pCDNA3.1, which confers hygromycin resistance. All FAK proteins had a triple HA tag at the C-terminal end to differentiate between endogenous FAK and ectopically expressed FAK.
Endothelial cell culture and transfections.
HBMEC were isolated and cultured as described earlier (39). HBMEC cultures were routinely grown in RPMI 1640 containing 10% heat-inactivated fetal bovine serum, 10% Nu-Serum, 2 mM glutamine, 1 mM pyruvate, penicillin (100 U/ml), streptomycin (100 μg/ml), essential amino acids, and vitamins. HBMEC were transfected with mammalian expression vectors using Lipofectamine. Briefly, DNA-Lipofectamine complex in RPMI 1640 was added to 50% confluent HBMEC monolayers. After 6 h of incubation at 37°C, the cells were washed with RPMI 1640 and complete medium was added. Three days following the transfection, HBMEC were transferred to medium containing the antibiotic G418 (400 μg/ml) or hygromycin B (100 μg/ml) for 2 to 3 weeks, and the antibiotic-resistant colonies were pooled or separated into individual clones for further analysis. Green fluorescence protein (GFP) cloned in pCDNA3 or pCDNA3.1 was used to determine the transfection efficiency by counting fluorescent and nonfluorescent cells in a fluorescence microscope.
E. coli invasion assays.
E. coli invasion of HBMEC was tested as described earlier (25, 27). Briefly, confluent endothelial cell monolayers in 24-well plates were incubated with 107 E. coli (multiplicity of infection of 100) in experimental medium (M199–HamF-12 [1:1] containing 5% heat-inactivated fetal bovine serum, 2 mM glutamine, and 1 mM pyruvate) for 1.5 h at 37°C. The monolayers were then washed with RPMI 1640 and further incubated with experimental medium containing gentamicin (100 μg/ml) for 1 h to kill extracellular bacteria. The monolayers were washed again and lysed in 0.5% Triton X-100. The released intracellular bacteria were enumerated by plating on sheep blood agar plates. In duplicate experiments, the number of total cell-associated bacteria was determined as described above except that the gentamicin step was omitted. For inhibition studies, various kinase inhibitors at different concentrations were incubated with HBMEC in experimental medium for 1 h before addition of the bacteria. Effects of the inhibitors on HBMEC were assessed by the trypan blue exclusion method, and effects on bacterial viability were tested by colony plating (27).
Preparation of cell lysates and immunoprecipitation.
For stimulation with bacteria, confluent monolayers of HBMEC grown on collagen-coated dishes (60-mm diameter) were washed with RPMI 1640, and E. coli suspended in experimental medium was added. After stimulation for the times indicated at 37°C, the monolayers were washed with cold phosphate-buffered saline (PBS) containing 1 mM sodium orthovanadate and lysed in modified radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris [pH 7.4], 150 mM NaCl, 2 mM EGTA, 1% Triton X-100, 0.5% deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM sodium orthovanadate, 50 mM NaF, 10 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride, 10 μg each of leupeptin and aprotinin per ml) at 4°C. The cell lysates were centrifuged at 16,000 × g for 20 min at 4°C. Supernatants were collected, and the protein content in each sample was determined by the bicinchoninic acid method (Pierce). For immunoprecipitation, 300 to 500 μg of protein was incubated with appropriate antibody overnight at 4°C and then for an additional hour with protein A-agarose. The immune complexes were washed four times with RIPA buffer without deoxycholate and SDS. The immune complexes were subjected to Western blotting or an in vitro kinase assay.
Western blotting.
Proteins in immune complexes or total cell lysates were resolved by polyacrylamide gel electrophoresis (PAGE) on SDS–10% polyacrylamide gels and transferred to polyvinylidene difluoride membranes. The blots were blocked with TBST (25 mM Tris [pH 7.4], 150 mM NaCl, 0.1% Tween 20) containing 3% bovine serum albumin for 2 h at room temperature and incubated with appropriate primary antibody overnight at 4°C or for 2 h at room temperature. The blots were then washed with TBST and incubated with horseradish peroxidase-conjugated secondary antibody (1:10,000 dilution) for 1 h at room temperature. Subsequently, the blots were washed with TBST for 1 h (four times for 15 min each) and developed with SuperSignal chemiluminescence reagent (Pierce). The blots were exposed to X-ray film to visualize the proteins.
In vitro kinase assays.
FAK immune complexes were washed twice with kinase assay buffer (20 mM HEPES [pH 7.4], 150 mM NaCl, 10% glycerol, 10 mM MgCl2, 10 mM MnCl2). The kinase reaction was performed by incubating protein A-agarose containing the immune complexes in 30 μl of kinase assay buffer containing 2.5 μl of [γ-32P]ATP (3,000 Ci/mmol, 10 μCi/μl) for 15 min at 37°C. The reaction was terminated by the addition of Laemmli sample buffer and boiling for 5 min. Protein A-agarose was removed by centrifugation for 5 min, and the supernatant was loaded onto SDS–10% polyacrylamide gels. Following SDS-PAGE, gels were fixed in 10% methanol–10% acetic acid in water, dried, and exposed to X-ray film to locate 32P-labeled FAK proteins. The FAK bands were excised and counted to determine radioactivity.
Immunofluorescence staining.
HBMEC were grown in eight-well chamber slides coated with collagen and stimulated with E. coli as described above. The monolayers were then washed with PBS and fixed with 2% paraformaldehyde for 15 min at room temperature. Subsequently, the monolayers were permeabilized with 1% Triton X-100 in PBS containing 5% normal goat serum for 30 min at room temperature and further incubated with primary antibody in PBS-normal goat serum NGS for 1 h at room temperature. The cells were then washed with PBS and incubated with secondary antibody conjugated to tetramethyl rhodamine isothiocyanate (TRITC) for 30 min at room temperature. The cells were washed again, the chambers were removed, and the slides were mounted in 50% glycerol. Cells were viewed with a Zeiss Axioscope laser scanning confocal microscope.
RESULTS
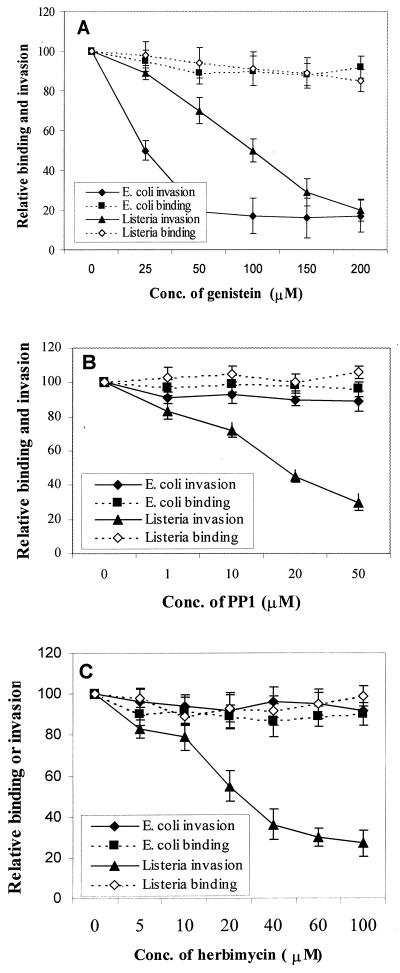
Genistein blocks E. coli invasion of HBMEC.
We have previously shown that the invasion of E. coli induces actin rearrangement in HBMEC, which requires activation of specific signal transduction pathways in host cells (26). Tyrosine phosphorylation of several host cell proteins by various tyrosine kinases plays a significant role in relaying signals for the reorganization of actin filaments. Thus, to examine the involvement of tyrosine kinases in these events, E. coli invasion assays were performed by pretreating HBMEC with various tyrosine kinase inhibitors. L. monocytogenes, which also invades HBMEC, was used as a comparison to assess the effects of these inhibitors on HBMEC. The general PTK inhibitor genistein blocked the E. coli invasion of HBMEC quite effectively in a dose-dependent manner with an 50% inhibitory concentration of 25 μM (10,565 ± 1,000 CFU without inhibitor versus 1,565 ± 430 CFU with 50 μM genistein per well, P < 0.002) (Fig. 1A). The invasion of L. monocytogenes was also inhibited in a dose-dependent manner, but the inhibition was achieved with a 50% inhibitory concentration of 100 μM (3.2 × 105 ± 0.4 × 105 CFU without inhibitor versus 7.5 × 104 ± 0.8 × 104 CFU with 200 μM genistein per well, P < 0.005). To rule out the possibility that the observed inhibition was due to inefficient association of either E. coli or L. monocytogenes to genistein-treated HBMEC, the number of total HBMEC-associated bacteria was determined as described in Materials and Methods. Genistein treatment did not show any effect on the total cell-associated bacteria compared to untreated cells (Fig. 1A). Neither herbimycin A, an inhibitor of Src family member kinases, nor PP1, a Src kinase-specific inhibitor, blocked the invasion of E. coli (Fig. 1B and C). However, treating HBMEC with these inhibitors significantly decreased the invasion of L. monocytogenes. These inhibitors showed no effect on the total number of cell-associated E. coli and L. monocytogenes. Also, none of the inhibitors showed any effect on bacterial viability under the experimental conditions used. These results suggest that tyrosine kinases other than Src kinases may be involved in E. coli invasion of HBMEC. Moreover, these data suggest that the observed inhibition of E. coli invasion of HBMEC was not due to nonspecific shutdown of other cellular functions since Listeria could still invade HBMEC.
FIG. 1.
Effects of PTK inhibitors genistein, PP1, and herbimycin A on E. coli and Listeria binding and invasion of HBMEC. Confluent monolayers of HBMEC were treated with the indicated concentrations of tyrosine kinase inhibitors for 1 h before addition of the bacteria, and invasion assays were carried out. The level of total cell-associated bacteria (binding) was determined as described in Materials and Methods. Values are means ± standard deviations of results from at least three separate experiments carried out in triplicate. The results are expressed as relative binding or invasion, taking the value for the no-inhibitor control as 100%.
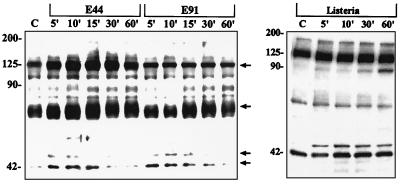
E. coli induces tyrosine phosphorylation of HBMEC proteins.
Having demonstrated the requirement of PTKs in E. coli invasion of HBMEC, we examined the tyrosine phosphorylation pattern of HBMEC proteins following stimulation with E. coli. Confluent HBMEC monolayers were incubated with invasive E. coli (strain E44), L. monocytogenes, or the noninvasive E. coli strain E91 for various periods of time, and the cell lysates were prepared. Proteins in the cell lysates were separated by SDS-PAGE, transferred to a polyvinylidene difluoride membrane, and immunoblotted with antiphosphotyrosine antibody 4G10. Cell lysates prepared from HBMEC infected with HB101 were used as a negative control. As shown in Fig. 2, the invasive E. coli strain E44 rapidly (by 5 min) stimulated tyrosine phosphorylation of several HBMEC proteins with apparent molecular masses of 125, 65, and 44/42 kDa. Tyrosine phosphorylation of the 125- and 65- kDa proteins was predominant in these cell lysates. Phosphorylation of these proteins peaked between 10 to 15 min and declined thereafter. The noninvasive E. coli strain E91 failed to stimulate tyrosine phosphorylation of these proteins between 5 and 10 min, although there was some increased phosphorylation of 65-kDa protein between 15 and 30 min. However, strain HB101 showed a phosphorylation pattern similar to that of E91 (data not shown), suggesting that other structures may have contributed to this increase. The increases in tyrosine phosphorylation levels of 125- and 65-kDa proteins in E44-treated HBMEC were fourfold greater than those of E91-treated HBMEC, as estimated by densitometric evaluation. The phosphorylation pattern of 44/42-kDa proteins did not show significant differences in cells treated with either E44 or E91. HBMEC infected with L. monocytogenes also showed slightly increased phosphorylation of a 125-kDa protein and 44/42-kDa proteins compared to uninfected cells (Fig. 2). The increase in the phosphorylation of 125-kDa protein following infection with Listeria was not as dramatic as that of HBMEC infected with E44. HBMEC treated with Listeria did not show any increased tyrosine phosphorylation of 65-kDa protein compared to untreated cells. These results showed that E44 invasion is associated with rapid and specific tyrosine phosphorylation of 125- and 65-kDa proteins.
FIG. 2.
Invasive E. coli induces increased tyrosine phosphorylation of 125- and 65-kDa proteins of HBMEC. Confluent monolayers of HBMEC were infected with either invasive E. coli (E44), noninvasive E. coli (E91), or Listeria for indicated periods of time (minutes). The cell lysates were prepared, separated by SDS-PAGE, and immunoblotted with antiphosphotyrosine antibody 4G10 as described in Materials and Methods. Arrows indicate the protein bands with increased density in E44-treated HBMEC compared to cells treated with E91 or Listeria. Sizes are indicated in kilodaltons. C, control.
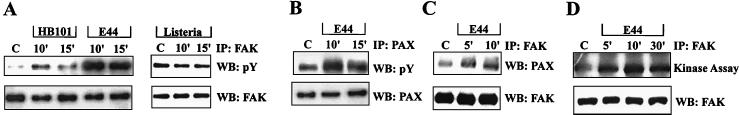
Activation of FAK in HBMEC stimulated with E. coli.
FAK has been shown to play a significant role in the dynamic regulation of actin structures. Since the size of the 125-kDa protein induced by E. coli appears to be within the range for FAK, we examined cell lysates prepared from HBMEC stimulated with E44 by immunoprecipitation with an anti-FAK monoclonal antibody followed by immunoblotting with an antiphosphotyrosine antibody. As shown in Fig. 3A, tyrosine phosphorylation of FAK is increased following stimulation with E. coli, with maximum phosphorylation at 10 min. A duplicate blot of these immunoprecipitates was immunoblotted with anti-FAK antibody to confirm the presence of equal amounts of FAK protein. Strain HB101 activated FAK very weakly at 10 min after infection of HBMEC, to a level about fourfold less than that induced by E44. Noninvasive strain E91 showed FAK phosphorylation levels similar to those of HB101 (data not shown). In contrast, HBMEC infected with Listeria did not show any increase in the phosphorylation of FAK.
FIG. 3.
E. coli invasion of HBMEC stimulates the tyrosine phosphorylation and kinase activity FAK in HBMEC and the phosphorylation of paxillin and its association with FAK. Cell lysates obtained after treatment of HBMEC with either E. coli E44 or HB101 or Listeria for different periods of time (minutes) were immunoprecipitated (IP) with either anti-FAK antibody (A and C) or antipaxillin antibody (B) and subjected to Western blotting (IB) with antiphosphotyrosine antibody (pY; A and B), anti-FAK antibody (FAK; A and C), or antipaxillin antibody (PAX; B and C). (D) Cell lysates were immunoprecipitated with anti-FAK antibody and subjected to in vitro kinase assay as described in Materials and Methods. The immune complexes were also subjected to immunoblotting with anti-FAK antibody. C, control.
The FAT domain of FAK contains binding sites for cytoskeletal proteins such as paxillin and talin. Activation of FAK has been shown to increase tyrosine phosphorylation of its associated protein paxillin, which has a molecular mass of 65 kDa. Since E. coli also showed increased phosphorylation of a 65-kDa protein, we examined whether FAK activation results in increased paxillin tyrosine phosphorylation. Cell lysates from HBMEC stimulated with invasive E. coli strain E44 were immunoprecipitated with an antipaxillin antibody, and the protein complexes were analyzed by Western blotting with an antiphosphotyrosine antibody (Fig. 3B). Tyrosine phosphorylation of paxillin was also increased significantly by 10 min compared to untreated control cells. Immunoblotting with an antipaxillin antibody showed equal amounts of paxillin in these immunoprecipitates. The cells treated with noninvasive bacteria (E91 and HB101) exhibited phosphorylation of paxillin similar to that of control cells (data not shown). Since the tyrosine phosphorylation of paxillin was increased with invasive E. coli, we examined whether the association of paxillin with FAK was also enhanced. As shown in Fig. 3C, immunoblotting of FAK immunoprecipitates with antipaxillin antibody showed a maximum association of paxillin with FAK by 10 min after infection of HBMEC with E. coli. This increased association of paxillin correlates with the increased phosphorylation of paxillin as shown above.
We further verified whether FAK kinase activity was increased during the invasion of E. coli into HBMEC by an in vitro kinase assay. As shown in Fig. 3D, the kinase activity of FAK increased threefold (2,500 ± 300 cpm for HBMEC infected with E44 versus 650 ± 450 cpm for untreated control cells) following stimulation with invasive E. coli by 10 min postinfection. One caveat for this result was that the tyrosine kinase activity observed in FAK immunoprecipitates could also be due to Src kinase that coimmunoprecipitated with FAK. Therefore, we performed the in vitro kinase assay in the presence of the Src-specific inhibitor PP1. However, PP1 failed to inhibit the tyrosine kinase activity observed in FAK immunoprecipitates (the pattern was similar to that of Fig. 3D), indicating that the observed kinase activity was not due to Src. Taken together, these results suggest that E. coli invasion of HBMEC results in increased tyrosine phosphorylation and kinase activity of FAK. In addition, the phosphorylation of paxillin and its association with FAK were also increased. These results demonstrated the involvement of FAK and the associated protein paxillin in E. coli invasion of HBMEC.
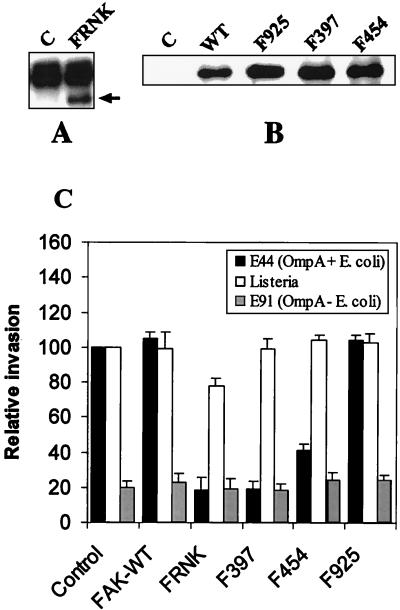
Overexpression of FRNK inhibits E. coli invasion.
To further determine the role of FAK in E. coli invasion, HBMEC were transfected with mammalian expression vectors containing HA-tagged wild-type FAK (FAK-WT) and FRNK; the resulting cell lines were designated FAK-WT/HBMEC and FRNK/HBMEC. FRNK is a mutant lacking the amino-terminal end, including the kinase domain of FAK, and is expressed in vivo as a result of regulation of an alternative promoter residing within an intron of the FAK gene. FRNK has been shown to negatively regulate the function of FAK (21, 31). FAK-WT transfectants were selected using G418, and FRNK transfectants were selected using hygromycin B. The cell lysates of pooled antibiotic-resistant cells were immunoprecipitated with anti-HA tag antibody, and the immunoprecipitates were subjected to immunoblotting with anti-FAK antibody, which recognizes both FAK and FRNK. As shown in Fig. 4A and B, HA-tagged FAK and FRNK could be detected in cells transfected with the appropriate expression vector but not in cells transfected with a GFP-expressing vector. The protein band present in both control and FRNK-transfected HBMEC (Fig. 4A) was due to immunoglobulin G present in immunoprecipitates. HBMEC transfectants expressing FAK or FRNK were used in E. coli invasion assays as described in Materials and Methods. HBMEC transfected with a GFP expression vector (G418 or hygromycin resistant) were used as controls. Overexpression of FAK-WT and GFP resulted in E. coli invasion similar to that of nontransfected HBMEC (10,965 ± 985 CFU for GFP/HBMEC versus 10,180 ± 1,200 CFU for HBMEC) (Fig. 4C). In contrast, FRNK overexpression significantly (>80%) inhibited the E. coli invasion of HBMEC. Noninvasive E. coli strain E91 invasion was not affected by the overexpression of these proteins. Listeria invasion of FAK-WT/HBMEC was similar to that of GFP/HBMEC; however, Listeria invasion of FRNK/HBMEC was reduced by only 20%. The level of total cell-associated bacteria, for both E. coli and Listeria, was not significantly different from that of nontransfected HBMEC. These results indicate that invasion of HBMEC by E. coli is dependent on FAK. Also, E. coli invades HBMEC by a different mechanism that does Listeria.
FIG. 4.
Overexpression of FRNK, Phe397 FAK, and Arg454 FAK inhibited the E. coli invasion of HBMEC. Cell lysates of HBMEC that had been transfected with plasmids containing GFP (Control or C) or HA-tagged FRNK (A) and FAK-WT, Phe925, FAK, Phe327 FAK, or Arg454 FAK (B) were immunoprecipitated with anti-HA tag antibody. The immune complexes were subjected to immunoblotting with anti-FAK antibody. The arrow indicates the FRNK protein (A). The protein above the FRNK band was due to immunoglobulin G present in the immune complexes. (C) HBMEC transfected with FAK mutants were used for invasion assays as described in Materials and Methods. Values are means ± standard deviations of results from at least five separate experiments carried out in triplicate, and invasion is expressed as a percentage of the value for GFP-expressing cells (control). P < 0.01 for FRNK, Phe397 FAK, and Arg454 FAK for E44 compared to control cells by unpaired two-tailed t test.
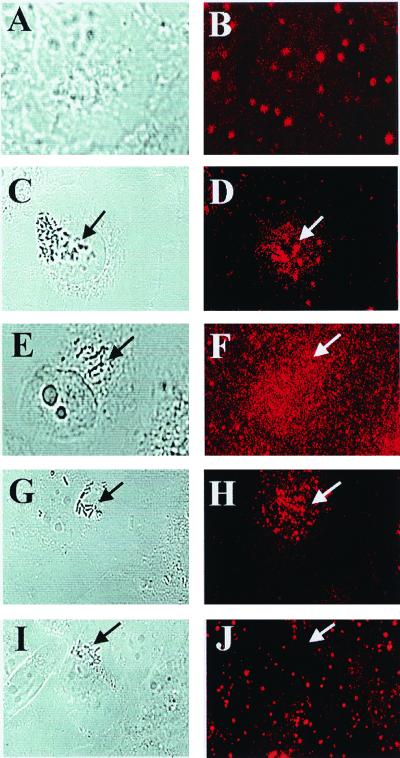
Since E. coli invasion is associated with actin condensation at the site of bacterial entry (26), FAK may colocalize with the accumulated actin, and this colocalization may be important for uptake of the bacteria by HBMEC. It has been shown in cell spreading experiments that overexpressed FRNK competes with endogenous FAK to recruit to focal adhesions, thus displacing the FAK from focal adhesions (31). To verify whether this could be a mechanism of FRNK inhibition of E. coli invasion into HBMEC, FRNK-overexpressing transfectants were subjected to immunocytochemistry using polyclonal anti-FAK antibody (which also recognizes FRNK) followed by TRITC-conjugated secondary antibody and visualized by confocal fluorescence microscopy. Nontransfected HBMEC without bacteria showed a punctate staining at the apical side of the cell (Fig. 5A and B), whereas HBMEC infected with strain E44 showed accumulation of FAK under groups of bacteria (Fig. 5C and D). Our previous experiments also showed accumulation of actin under groups of bacteria similar to FAK accumulation in HBMEC (26). In contrast, FRNK/HBMEC (Fig. 5E and F) showed very diffuse staining underneath the bacteria, indicating that overexpression of FRNK displaced and/or blocked the recruitment of endogenous FAK to the sites of bacterial entry.
FIG. 5.
Overexpression of mutant forms of FAK inhibits the accumulation of native FAK at bacterial entry sites. HBMEC, uninfected and nontransfected, were used as controls (A and B) to show the normal pattern. HBMEC either nontransfected (C and D) or transfected with plasmids containing FRNK (E and F), Phe397 FAK (G and H), and Arg454 FAK (I and J) were treated with E44 for 15 min, fixed, and stained with anti-FAK antibody as described in Materials and Methods. Confocal laser microscopy was used to visualize the bacteria by transmitted light optics (A, C, E, G, and I) and FAK by TRITC-conjugated secondary antibody to anti-FAK antibody (B, D, F, H, and J). Arrows indicate locations of bacteria or accumulation of FAK.
Kinase-negative and autophosphorylation mutants of FAK also inhibit E. coli invasion.
Upon activation by extracellular signals, FAK is autophosphorylated at Tyr397, to which Src kinase binds, and in turn phosphorylates additional tyrosine residues on FAK, including Tyr925. To determine the roles of these tyrosine residues in E. coli invasion of HBMEC, we examined the effects of overexpressing FAK mutants that were defective in autophosphorylation (Phe397 FAK), kinase activity (Arg454 FAK), and Grb2 binding (Phe925 FAK). Invasion of E. coli was reduced in cells expressing Phe397 FAK by >80%, in which Tyr397 was mutated to Phe, compared to control cells (Fig. 4C). However, HBMEC expressing Arg454 FAK (in which lysine 454 was mutated to arginine) showed approximately 50% reduction in the invasion of E. coli. In contrast, overexpression of Phe925 FAK, in which Tyr925 was mutated to Phe, showed E. coli invasion similar to that observed with GFP/HBMEC. To rule out the possibility that the presence of insufficient quantities of Phe925 FAK caused no inhibition of E. coli invasion, lysates from the transfected cells were immunoprecipitated with anti HA tag antibody and immunoblotted with anti-FAK antibody. As shown in Fig. 4B, FAK mutants expressed significant quantities of mutated FAK proteins compared to GFP-expressing control cells. L. monocytogenes, on the other hand, showed no significant differences in the invasion of Phe397 FAK-, Arg454 FAK-, and Phe925 FAK-transfected HBMEC (Fig. 4). The invasion frequency of noninvasive E. coli did not change in any of these transfectants. These results demonstrated that Lys454 (for FAK kinase activity) and the autophosphorylation site (Tyr397) of FAK are required for E. coli invasion, whereas the Grb2 binding site Tyr925 may not be critical in this process.
In addition, localization of FAK at the site of bacterial entry was examined by immunocytochemistry using the Phe397 FAK, Arg454 FAK, and Phe925 FAK transfectants. Both Phe397 FAK- and Arg454 FAK-overexpressing HBMEC showed no association of FAK at the bacterial entry site (Fig. 5G to J). In contrast, Phe925 FAK-expressing cells showed FAK association with bacteria similar to that observed for nontransfected HBMEC (as seen in Fig. 5C and D). These results suggest that overexpression of dominant interfering mutant FAK proteins inhibits E. coli invasion of HBMEC probably by blocking the formation of FAK complex necessary for the reorganization of actin at the site of bacterial entry.
Overexpression of Phe397 FAK, Arg454 FAK, and FRNK inhibits the tyrosine phosphorylation of FAK.
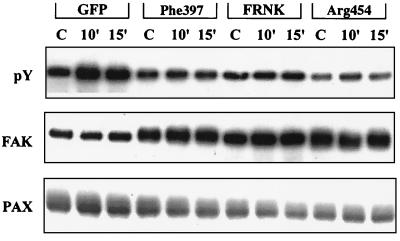
To assess whether the overexpression of FAK mutants inhibits the tyrosine phosphorylation of native FAK, we examined E. coli-stimulated tyrosine phosphorylation of FAK in HBMEC transfected with the FAK mutants. Control HBMEC and HBMEC expressing FRNK, Phe397 FAK, and Arg454 FAK were stimulated with E. coli for the indicated periods of time, and cell lysates were immunoprecipitated with anti-FAK antibody. FAK immune complexes were immunoblotted with an antiphosphotyrosine antibody. A duplicate blot was immunoblotted with anti-FAK antibody to verify the presence of equal quantities of FAK protein in these cell lysates. As shown in Fig. 6, transfected HBMEC cell lysates exhibited increased concentrations of FAK protein compared to GFP-expressing HBMEC; however, the tyrosine phosphorylation of FAK was greatly reduced in cells transfected with FRNK, Phe397 FAK, and Arg454 FAK even after stimulation with E. coli. Thus, tyrosine phosphorylation patterns of FAK protein in these transfectants followed the same pattern as changes in invasion of E. coli in these cells. The immunoprecipitates were also immunoblotted with antipaxillin antibody, which showed that the association of FAK with paxillin was also reduced in cells expressing FRNK, Phe397 FAK, and Arg454 FAK. Therefore, the inability of E. coli to invade HBMEC transfected with FAK mutants may be due to the inhibition of FAK tyrosine phosphorylation and its association with paxillin, which is necessary for the formation of focal plaques.
FIG. 6.
Invasive E. coli-induced tyrosine phosphorylation of FAK and its association with paxillin was blocked by overexpression of mutant forms of FAK. Confluent HBMEC transfected with GFP, Phe397 FAK, FRNK, and Arg454 FAK were infected with E44 for different periods of time (minutes); total cell lysates were prepared and immunoprecipitated with anti-FAK antibody. The immune complexes were separated by SDS-PAGE and subjected to immunoblotting with antiphosphotyrosine antibody (pY), anti-FAK antibody (FAK), or antipaxillin antibody (PAX). C, control.
DISCUSSION
Our previous results showed that cytochalasin D and latrunculin A, inhibitors of actin polymerization, completely blocked OmpA+ E. coli invasion of HBMEC, suggesting the requirement of intact actin cytoskeleton (26). In addition, E. coli entry of HBMEC has been shown to be associated with actin condensation, indicating that invasive E. coli transduces signals for cytoskeletal reorganization required for the formation of focal plaques. Our present data for assays using genistein revealed that tyrosine phosphorylation-mediated signal transduction processes play a significant role in E. coli invasion of HBMEC. The concentration of genistein required for blocking E. coli invasion of HBMEC was three to four times less than that required for Listeria; thus, it is possible that a low-level inhibition of tyrosine kinases may be sufficient to block E. coli invasion. The observed effect of genistein on E. coli invasion was not due to the lack of E. coli adherence to HBMEC, as genistein-treated and untreated HBMEC showed no significant differences in total cell-associated bacteria. However, herbimycin A or PP1-treated HBMEC were still capable of internalizing E. coli but not L. monocytogenes, indicating that Src kinases are needed for Listeria invasion and not for E. coli invasion. Based on these results, we investigated which tyrosine kinases are required for E. coli invasion of HBMEC.
FAK is implicated in pathways that regulate cytoskeletal organization and cell motility and may possibly be involved in cell growth control as well (30, 34). Phosphorylation of different tyrosine residues on FAK enhances its ability to interact with a number of other signaling molecules via their SH2 domains, including Src kinase family members, phosphatidylinositol 3-kinase (PI-3 kinase), and Grb2 (3, 5, 9, 29, 34). In addition, the C-terminal domain serves as a docking site for a number of cytoskeletal and signaling molecules, including paxillin and talin (16, 34). During these interactions, signaling and structural proteins in the focal complex are also tyrosine phosphorylated. As expected, the tyrosine phosphorylation of FAK was significantly and consistently increased in HBMEC infected with invasive E. coli within a short time. In contrast, noninvasive E. coli (strains E91 and HB101) did not stimulate an increase in tyrosine phosphorylation of FAK, indicating that the invasive property of E. coli is necessary for inducing the signals for FAK phosphorylation. These results are in agreement with the data obtained for cytoskeletal rearrangements in which the HBMEC actin accumulation was associated with invasive E. coli but not with noninvasive E. coli (26). Because E. coli interacts with several HBMEC receptors (24, 25), it remains to be determined which of these receptors induces FAK phosphorylation.
Tyrosine phosphorylation of FAK is critical for its function. The C-terminal domain of FAK functions in subcellular localization and directing the transmission of downstream signaling, i.e., by binding and recruiting substrates (37). In this study, E. coli-induced activation of FAK also increased its association with paxillin, as well as tyrosine phosphorylation of paxillin. Furthermore, overexpression FRNK in HBMEC inhibited E. coli invasion, suggesting that FRNK acts as an inhibitor of FAK activation. Due to the presence of an intact FAT domain, FRNK can target to focal adhesions but may not be sufficient to regulate the catalytic activity and/or tyrosine phosphorylation. However, targeting of FRNK to focal adhesions in general is sufficient to block FAK function in integrin-dependent cell migration (37). Thus, overexpression of FRNK might displace the recruitment of FAK to the focal plaques (31), which subsequently may inhibit the formation of the proper FAK signaling complex required for cytoskeletal rearrangement that mediates E. coli invasion. FRNK overexpression may also promote FAK dephosphorylation at Tyr397, which could inhibit its association with other signaling molecules, thereby preventing the cytoskeletal reorganization. Alternatively, FRNK may sequester critical FAK-associated proteins. Therefore, it appears that the major determinant in E. coli invasion of HBMEC is the activation of FAK to target to focal plaques to recruit other signaling and structural proteins for the reorganization of actin filaments.
The autophosphorylation site of FAK (Tyr397) is required for the association with the SH2 domain of Src family kinases and/or of the p85 subunit of PI-3 kinase. The inability of PP1 to block E. coli invasion suggests that the catalytic activity of Src may not be involved in invasion. Noninvolvement of Src kinases has been revealed recently in studies of signal transduction mechanisms of platelet growth-derived factor receptor in mouse embryos harboring functional null mutations of the ubiquitously expressed Src family kinases Src, Yes, and Fyn (20). These investigators showed that Src family kinases are largely dispensable for receptor-induced signaling, whereas they are absolutely required to mediate specific functions regulated by extracellular matrix proteins (28). Src family protein tyrosine kinases binding to FAK at Tyr397 after integrin stimulation is required for subsequent Src-mediated phosphorylation of Tyr925 on FAK (35). However, the inability to block E. coli invasion by overexpression of Grb2 binding mutant (Phe925 FAK) also point out that Src kinase activity may not be essential for invasion. Alternatively, loss of the Grb2 binding site on FAK in E. coli invasion may be compensated for by other phosphotyrosines on FAK. The role of PI-3 kinase in E. coli is currently under investigation. Thus, it is possible that FAK itself promotes the tyrosine phosphorylation of paxillin that binds to its C terminus. This possibility is in contrast to the scenario that occurs in cell spreading, where the Src bound to FAK also phosphorylates paxillin (32).
Integrins act as receptors for many pathogenic bacteria for efficient entry of host eukaryotic cells (7, 8, 43). FAK has been shown to be involved in invasin-mediated uptake of Yersinia pseudotuberculosis, which requires integrins as the receptor molecules in chicken embryo fibroblasts (1). These studies showed that overexpression of a kinase-defective FAK did not inhibit the invasin-mediated uptake in a manner similar to the mechanisms described for cell migration (38). In addition, Yersinia invasion requires Src kinase activity, as observed by inhibition of invasion by overexpression of Src kinase-negative mutant. In contrast, as shown here, E. coli invasion of HBMEC requires the kinase activity of FAK, which was increased twofold in response to E. coli interaction. However, Src kinase activity may not be necessary for E. coli invasion of HBMEC, suggesting that the invasion mechanism may not be similar to that of integrin-mediated uptake. We have previously shown that GlcNAcβ1,4-GlcNAc epitopes present on HBMEC glycoproteins interact with OmpA for E. coli invasion of HBMEC (25). Consistent with these studies, GlcNAcβ1,4-GlcNAc oligomers blocked the actin condensation associated with invasive E. coli (25). The inability of RGD peptides to block these cytoskeletal changes suggests that receptors other than integrins may be involved E. coli invasion of HBMEC. Furthermore, we have identified a 95-kDa OmpA binding molecule on HBMEC (unpublished results). Our preliminary data also showed that the purified receptor protein blocked the OmpA+ E. coli-induced tyrosine phosphorylation of HBMEC proteins, suggesting that the 95-kDa protein may play a role in transducing the signals for cytoskeletal reorganization in E. coli invasion of HBMEC. Alternatively, E. coli OmpA interacts with the 95-kDa receptor on HBMEC, which may trigger the synthesis of new virulence factors. These factors may be secreted into the medium, which would then be taken up by HBMEC to activate FAK. In this scenario, FAK can directly interact with phospholipase C-γ to catalyzes the hydrolysis of phosphatidylinositol 4,5-bisphosphate to generate the second messenger diacylglycerol, which activates protein kinase C for subsequent actin rearrangement (45). Studies are in progress to explore these possibilities.
In summary, while the molecular control of actin rearrangement which drives HBMEC for the uptake of E. coli is not known, we have demonstrated that the autophosphorylation and kinase activity of FAK are essential for this process. Furthermore, FAK's association with paxillin and its localization to focal plaques are linked to efficient entry of E. coli into HBMEC. Since E. coli invasion of HBMEC is integrin independent, we envision a novel mechanism involved in the interaction of E. coli OmpA with the corresponding HBMEC receptor, which subsequently regulates actin condensation. Future studies will be directed toward elucidating the interactions of the OmpA receptor and HBMEC cellular proteins and how they function in actin rearrangement for the uptake of E. coli by HBMEC.
ACKNOWLEDGMENTS
We appreciate the excellent technical assistance of Ernesto Barron. We also thank Scott Filler and Barbara Driscoll for critical reading of the manuscript.
This work was supported by NIH grants R29 AI40567 (N.V.P.), RO1 NS26310 and HL61951 (K.S.K.), and R29 CA75240 (D.D.S.). The Doheny Eye Institute confocal microscope core facility at the University of Southern California School of Medicine is funded by NEI/NIH core grant EY03040.
REFERENCES
- 1.Alrutz M A, Isberg R R. Involvement of focal adhesion kinase in invasin-mediated uptake. Proc Natl Acad Sci USA. 1998;95:13658–13663. doi: 10.1073/pnas.95.23.13658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andre E, Becker-Andre M. Expression of an N-terminally truncated form of human focal adhesion kinase in brain. Biochem Biophys Res Commun. 1993;190:140–147. doi: 10.1006/bbrc.1993.1022. [DOI] [PubMed] [Google Scholar]
- 3.Bachelot C, Rameh L, Parsons T, Cantley L C. Association of phosphatidylinositol 3-kinase, via the SH2 domains of p85, with focal adhesion kinase in polyoma middle t-transformed fibroblasts. Biochim Biophys Acta. 1996;1311:45–52. doi: 10.1016/0167-4889(95)00176-x. [DOI] [PubMed] [Google Scholar]
- 4.Cary L A, Guan J L. Focal adhesion kinase in integrin-mediated signaling. Front Biosci. 1999;4:D102–D113. doi: 10.2741/cary. [DOI] [PubMed] [Google Scholar]
- 5.Chen H C, Appeddu P A, Isoda H, Guan J L. Phosphorylation of tyrosine 397 in focal adhesion kinase is required for binding phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:26329–26334. doi: 10.1074/jbc.271.42.26329. [DOI] [PubMed] [Google Scholar]
- 6.Chen H C, Appeddu P A, Parsons J T, Hildebrand J D, Schaller M D, Guan J L. Interaction of focal adhesion kinase with cytoskeletal protein talin. J Biol Chem. 1995;270:16995–16999. doi: 10.1074/jbc.270.28.16995. [DOI] [PubMed] [Google Scholar]
- 7.Clark M A, Hirst B H, Jepson M A. M-cell surface β1 integrin expression and invasin-mediated targeting of Yersinia pseudotuberculosis to mouse Peyer's patch M cells. Infect Immun. 1998;66:1237–1243. doi: 10.1128/iai.66.3.1237-1243.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dehio M, Gomez-Duarte O G, Dehio C, Meyer T F. Vitronectin-dependent invasion of epithelial cells by Neisseria gonorrhoeae involves alpha(v) integrin receptors. FEBS Lett. 1998;424:84–88. doi: 10.1016/s0014-5793(98)00144-6. [DOI] [PubMed] [Google Scholar]
- 9.Eide B L, Turck C W, Escobedo J A. Identification of Tyr-397 as the primary site of tyrosine phosphorylation and pp60src association in the focal adhesion kinase, pp125FAK. Mol Cell Biol. 1995;15:2819–2827. doi: 10.1128/mcb.15.5.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 11.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garner A M, Kim K S. The effects of Escherichia coli S-fimbriae and outer membrane protein A on rat pial arterioles. Pediatr Res. 1996;39:604–608. doi: 10.1203/00006450-199604000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Greiffenberg L, Goebel W, Kim K S, Weiglein I, Bubert A, Engelbrecht F, Stins M, Kuhn M. Interaction of Listeria monocytogenes with human brain microvascular endothelial cells: In1B-dependent invasion, long-term intracellular growth, and spread from macrophages to endothelial cells. Infect Immun. 1998;66:5260–5267. doi: 10.1128/iai.66.11.5260-5267.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan J L. Role of focal adhesion kinase in integrin signaling. Int J Biochem Cell Biol. 1997;29:1085–1096. doi: 10.1016/s1357-2725(97)00051-4. [DOI] [PubMed] [Google Scholar]
- 15.Hanks S K, Polte T R. Signaling through focal adhesion kinase. Bioessays. 1997;19:137–145. doi: 10.1002/bies.950190208. [DOI] [PubMed] [Google Scholar]
- 16.Hildebrand J D, Schaller M D, Parsons J T. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1995;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang S H, Wass C A, Prasadarao N V, Stins M F, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S H, Chen Y H, Fu Q, Stins M, Wang Y, Wass C, Kim K S. Identification and characterization of an Escherichia coli invasion gene locus, ibeB, required for penetration of brain microvascular endothelial cells. Infect Immun. 1999;67:2103–2109. doi: 10.1128/iai.67.5.2103-2109.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ilic D, Damsky C H, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110:401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- 20.Klinghoffer R A, Sachsenmaier C, Cooper J A, Soriano P. Src family kinases are required for integrin but not PDGFR signal transduction. EMBO J. 1999;18:2459–2471. doi: 10.1093/emboj/18.9.2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nolan K, Lacoste J, Parsons J T. Regulated expression of focal adhesion kinase-related nonkinase, the autonomously expressed C-terminal domain of focal adhesion kinase. Mol Cell Biol. 1999;19:6120–6129. doi: 10.1128/mcb.19.9.6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owen J D, Ruest P J, Fry D W, Hanks S K. Induced focal adhesion kinase (FAK) expression in FAK-null cells enhances cell spreading and migration requiring both. Mol Cell Biol. 1999;19:4806–4818. doi: 10.1128/mcb.19.7.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons J T, Parsons S J. Src family protein tyrosine kinases: cooperating with growth factor and adhesion signaling pathways. Curr Opin Cell Biol. 1997;9:187–192. doi: 10.1016/s0955-0674(97)80062-2. [DOI] [PubMed] [Google Scholar]
- 24.Prasadarao N V, Wass C A, Huang S H, Kim K S. Identification and characterization of a novel Ibe10 binding protein that contributes to Escherichia coli invasion of brain microvascular endothelial cells. Infect Immun. 1999;67:1131–1138. doi: 10.1128/iai.67.3.1131-1138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasadarao N V, Wass C A, Kim K S. Endothelial cell GlcNAc β1-4GlcNAc epitopes for outer membrane protein A enhance traversal of Escherichia coli across the blood-brain barrier. Infect Immun. 1996;64:154–160. doi: 10.1128/iai.64.1.154-160.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prasadarao N V, Wass C A, Stins M F, Shimada H, Kim K S. Outer membrane protein A-promoted actin condensation of brain microvascular endothelial cells is required for Escherichia coli invasion. Infect Immun. 1999;67:5775–5783. doi: 10.1128/iai.67.11.5775-5783.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rankin S, Rozengurt E. Platelet-derived growth factor modulation of focal adhesion kinase (p125FAK) and paxillin tyrosine phosphorylation in Swiss 3T3 cells. Bell-shaped dose response and cross-talk with bombesin. J Biol Chem. 1994;269:704–710. [PubMed] [Google Scholar]
- 29.Reiske H R, Kao S C, Cary L A, Guan J L, Lai J F, Chen H C. Requirement of phosphatidylinositol 3-kinase in focal adhesion kinase-promoted cell migration. J Biol Chem. 1999;274:12361–12366. doi: 10.1074/jbc.274.18.12361. [DOI] [PubMed] [Google Scholar]
- 30.Richardson A, Parsons J T. Signal transduction through integrins: a central role for focal adhesion kinase? Bioessays. 1995;17:229–236. doi: 10.1002/bies.950170309. [DOI] [PubMed] [Google Scholar]
- 31.Schaller M D, Borgman C A, Parsons J T. Autonomous expression of a noncatalytic domain of the focal adhesion-associated protein tyrosine kinase pp125FAK. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaller M D, Hildebrand J D, Shannon J D, Fox J W, Vines R R, Parsons J T. Autophosphorylation of the focal adhesion kinase, pp125FAK, directs SH2-dependent binding of pp60src. Mol Cell Biol. 1994;14:1680–1688. doi: 10.1128/mcb.14.3.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schlaepfer D D, Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J Biol Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- 34.Schlaepfer D D, Hauck C R, Sieg D J. Signaling through focal adhesion kinase. Prog Biophys Mol Biol. 1999;71:435–478. doi: 10.1016/s0079-6107(98)00052-2. [DOI] [PubMed] [Google Scholar]
- 35.Schlaepfer D D, Hunter T. Evidence for in vivo phosphorylation of the Grb2 SH2-domain binding site on focal adhesion kinase by Src-family protein-tyrosine kinases. Mol Cell Biol. 1996;16:5623–5633. doi: 10.1128/mcb.16.10.5623. . (Erratum, 16:7182–7184.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schlaepfer D D, Jones K C, Hunter T. Multiple Grb2-mediated integrin-stimulated signaling pathways to ERK2/mitogen-activated protein kinase: summation of both c-Src- and focal adhesion kinase-initiated tyrosine phosphorylation events. Mol Cell Biol. 1998;18:2571–2585. doi: 10.1128/mcb.18.5.2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen Y, Schaller M D. Focal adhesion targeting: the critical determinant of FAK regulation and substrate phosphorylation. Mol Biol Cell. 1999;10:2507–2518. doi: 10.1091/mbc.10.8.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sieg D J, Hauck C R, Schlaepfer D D. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112:2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- 39.Stins M F, Prasadarao N V, Wass C, Kim K S. Escherichia coli binding to and invasion of brain microvascular endothelial cells derived from humans and rats of different ages. Infect Immun. 1999;67:5522–5525. doi: 10.1128/iai.67.10.5522-5525.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stins M F, Prasadarao N V, Ibric L, Wass C A, Luckett P, Kim K S. Binding characteristics of S fimbriated Escherichia coli to isolated brain microvascular endothelial cells. Am J Pathol. 1994;145:1228–1236. [PMC free article] [PubMed] [Google Scholar]
- 41.Thomas J W, Ellis B, Boerner R J, Knight W B, White G C, Schaller M D. SH2- and SH3-mediated interactions between focal adhesion kinase and Src. J Biol Chem. 1998;273:577–583. doi: 10.1074/jbc.273.1.577. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, Huang S H, Wass C A, Stins M F, Kim K S. The gene locus yijP contributes to Escherichia coli K1 invasion of brain microvascular endothelial cells. Infect Immun. 1999;67:4751–4756. doi: 10.1128/iai.67.9.4751-4756.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watarai M, Funato S, Sasakawa C. Interaction of Ipa proteins of Shigella flexneri with alpha5beta1 integrin promotes entry of the bacteria into mammalian cells. J Exp Med. 1996;183:991–999. doi: 10.1084/jem.183.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zachary I. Focal adhesion kinase. Int J Biochem Cell Biol. 1997;29:929–934. doi: 10.1016/s1357-2725(97)00008-3. [DOI] [PubMed] [Google Scholar]
- 45.Zhang X, Chattopadhyay A, Ji Q S, Owen J D, Ruest P J, Carpenter G, Hanks S K. Focal adhesion kinase promotes phospholipase C-γ1 activity. Proc Natl Acad Sci USA. 1999;96:9021–9026. doi: 10.1073/pnas.96.16.9021. [DOI] [PMC free article] [PubMed] [Google Scholar]