Abstract
Background:
No data on the permanent and curative effect of bisphosphonate treatment in patients with complex regional pain syndrome type-1 (CRPS-1) are currently available. The aim of this pre-specified, open-label, observational study was to evaluate the long-term efficacy and safety of neridronate treatment.
Design:
A pre-specified, open-label, extension study.
Methods:
Patients treated with intramuscular (IM) placebo in the double-blind phase of the study were assigned to 100 mg intravenous (IV) neridronate treatment administered 4 times over 10 days. These patients, together with those previously treated with 400 mg IM neridronate, were followed for 1 year. Efficacy was assessed using a visual analogue scale (VAS) pain score. Changes in clinical signs and symptoms, quality of life (QoL) using the Short Form Health Survey (SF-36), and the McGill Pain Questionnaire were also assessed.
Results:
Benefits on pain, clinical and functional measures were maintained and further improved over 12 months in most patients treated with neridronate administered either IM or IV. In IM-treated patients, the percentage of those defined as responders (VAS score reduction ≥ 50%) progressively increased up to day 360 to 32 of 35 patients (91.4%). Among the 27 patients referred to as responders at the end of the double-blind phase, 26 reported the same result at day 360 (96.3%). In IV-treated patients, a responder rate of 88% (22 out 25) was found at day 360 (p = 0.66 between groups). Consistent improvements were also observed for all clinical signs and functional questionnaire. No drug-related adverse events were reported during the study.
Conclusion:
In patients with acute CRPS-1, the benefit in pain, clinical, and functional measures observed a few weeks after neridronate treatment administered either IM or IV is maintained and further improved over 12 months. Parenteral neridronate induces permanent disease remission preventing chronic pain and motor dysfunction.
Trial registration:
EU Clinical Trials Register (EudraCT Number): 2014-001156-28
Keywords: algodystrophy, bisphosphonates, complex regional pain syndrome type 1, neridronate, open-label clinical trial
Introduction
Complex regional pain syndrome (CRPS) is a severely painful and disabling disease for which a multitude of therapeutic interventions have been proposed. Unfortunately, most of them remain empirical, without reliable proof of efficacy.
In the past two decades, only bisphosphonates (BPs) gained credibility by employing different BPs in a variety of treatment regimens. The results of six randomized controlled studies (RCT)1–6 and three meta-analyses,7–9 all showed benefit in controlling pain and other clinical manifestations of the disease, mostly when the disease was treated at an early stage. The conclusions of the most recent published meta-analysis9 recommend parenteral bisphosphonates as the first-line therapy in controlling pain and other clinical manifestations, ultimately providing a better efficacy profile compared with other off-label pharmacological approaches.
In recent years, more convincing evidence has become available on the use of parenteral neridronate. Since 2014, this drug is registered and marketed in Italy for the treatment of CRPS. To date, the only therapeutic schedule that is recognized to be able to confer benefit is the intravenous (IV) administration of 100 mg given 4 times over 10 days. To explore if the same total amount of drug retains the same efficacy when administered intramuscularly (IM), in 2021 we published a study demonstrating short-term overlapping results between IV and IM neridronate.6 Although these results are encouraging, all studies on BPs treatment of CRPS published to date have been designed with a short follow-up, the longest ones not exceeding 3 months after the end of treatment.3,4 Some studies reported positive results confirmed over time2,5 but were achieved only on small and selected subgroups of patients, outside of the original study design. To date, it remains an unexplored topic if BPs represent a treatment able to afford a definitive recovery, namely having a curative effect, or whether they must be considered only for palliative care, conferring only a temporary benefit.
As planned by study design (EudraCT Number: 2014-001156), an open-label extension trial was started at the end of the double-blind, placebo-controlled arm investigating neridronate IM. To allow that patients assigned to the placebo group would receive active treatment, those who agreed to participate in the long-term follow-up study were treated with neridronate IV according to the registered therapeutic course with recognized efficacy in the treatment of CRPS. Both patients treated with IM neridronate in the double-blind arm of the study and patients treated with IV neridronate were followed for 1 year to evaluate the long-term efficacy, the possible occurrence of adverse events (AEs) and to explore possible differences between the two routes of administration. As well as assessing the pain course over time, we also assessed the effects of IV and IM neridronate on secondary markers of efficacy including local signs of inflammation, pain at passive motion, allodynia, hyperalgesia, range of motion, and quality of life (QoL).
Here, we report the long-term results in response to parenteral neridronate given both IM and IV.
Methods
Patients
Data on patient recruitment have been previously reported.6 Briefly, all patients included in the study were diagnosed according to the International Association for Study of Pain (IASP) diagnostic criteria for CRPS10 (‘Budapest criteria’ for research purpose).11 Only patients with a disease duration no longer than 4 months and with a bone scan showing increased uptake in the late phase at the disease site were recruited.12 Further inclusion criteria were patients aged ⩾ 18 years; a spontaneous pain intensity in the affected limb of ⩾ 50 mm on a visual analogue scale (VAS) ranging from 0 (no pain) to 100 mm (maximum pain); no major nerve damage suggestive for CRPS-2; prior treatment with BPs and the presence of renal disease.
Nerve blockers and other sympathectomy procedures, spinal cord stimulation, peripheral nerve stimulation, ketamine infusions, acupuncture, electromagnetic field treatment, radiofrequency ablation, mirror therapy, and other biofeedback interventions were prohibited for the full duration of the study. Investigators discouraged patients from taking any medication during the study due to insufficient CRPS pain relief, such as analgesic treatments, including non-steroidal anti-inflammatory agents, calcitonin, corticosteroids, anticonvulsants, antidepressants, and opioids. Except for the aforementioned list of non-permitted drugs, participants were allowed to use any concomitant medication necessary for the treatment of pre-existing concomitant pathologies or for intercurrent diseases. This study was conducted under the provisions of the Declaration of Helsinki, and in accordance with the International Conference on Harmonization Consolidated Guideline on Good Clinical Practice. The study was approved by Milano B Ethics committee (28 October 2014). All patients provided written informed consent to participate in the study.
Study design
At the end of the double-blind phase of the study (30 days from the start of IM treatment), the blind code was broken and patients treated with placebo during the double-blind phase of the study could be treated with IV neridronate at a dose of four 100 mg infusions each diluted in a 500 ml saline isotonic solution and infused in the morning over 2 h every third day. The treatment was administered after a washout period of 7–10 days. Both patients treated with IM neridronate in the double-blind phase of the study and patients treated with IV neridronate were followed up for 12 months.
Outcome measures
Outcome measures were assessed on the day of the first infusion of IV neridronate in previously IM placebo-treated patients and after 20 days, corresponding to 60 days from the start of IM neridronate, 180 days after the start of IM neridronate (140 days for IV neridronate), and 360 days after IM neridronate (320 days after starting IV neridronate).
The outcome measures employed were the same as those used in the double-blind phase of the study.6 The primary efficacy measure was the change in VAS pain score over the duration of the study. A decrease from the baseline value (before the IM or IV treatment) of at least 50% was considered clinically significant and qualified the patient as a responder.13 Clinical assessment included: allodynia tested as pain to light stroking with a small brush (the end of a Q-Tip) and hyperalgesia defined as a stimulus evoked by a pinprick being perceived as more painful or lasting longer than the duration of the stimulus in the affected limb compared with the contralateral limb, both rated as a dichotomous variable (present/absent).14 Local edema and pain at passive motion were also recorded: local edema scored as 0 = none, 1 = mild, 2 = moderate, 3 = severe was evaluated at the ankle and midfoot level for the foot involvement, wrist, centre of hand dorsum and finger for hand involvement; pain evoked by passive motion (ankle and finger joints for foot involvement and wrist and finger joints for hand involvement) was rated as 0 = none, 1 = mild, 2 = moderate, 3 = severe; both parameters were scored by the direct comparison with the contralateral unaffected limb. In each participating centre, the clinical evaluation was performed independently by two investigators; in case of discordance, the assessment was repeated by a third investigator and the score shared by at least two investigators was assigned. To assess QoL and functional status, Italian validated version of McGill Pain Questionnaire and 36-Item Short Form Health Survey (SF-36) questionnaire were evaluated.15,16 Both questionnaires were self-administered. All these outcome parameters were considered as secondary end points.
Safety
Physicians at the study sites reported adverse events (AEs) that were coded as preferred terms in the Medical Dictionary for Regulatory Activities system (MedDRA; version 22). According to investigators’ judgement, a drug-related AE was defined as definitely, probably, or possibly related to study treatment. Patients treated with IV neridronate were informed about a possible acute phase reaction (polyarthralgia and/or fever)17 occurring after parenteral aminobisphosphonate administration, and assumption of 500 mg acetaminophen tablets is recommended if these symptoms appeared.
Statistical analysis
Statistical analysis was carried out according to the intention-to-treat principle, including all patients who received at least one dose of the study medication. Missing data were replaced by LOCF (last observation carried forward). Data were evaluated for normal distribution using the Kolmogorov–Smirnov test. VAS scores were compared with the use of Student’s t test for unpaired data. VAS score changes were further evaluated using an analysis of covariance (ANCOVA) model for repeated measures, using the change from baseline as the dependent variable; treatment, visit, centre, and treatment by visit interaction as factors and baseline value as covariate. The proportion of responders (VAS reduction ⩾ 50%) as well as dichotomous variables (allodynia and hyperalgesia) were evaluated with the Fisher’s exact test for comparison between patients and with the McNemar test for comparisons within patient. The comparison of clinical parameters evaluated by means of rating scales (edema and pain at passive motion) was performed using the Wilcoxon rank sum test for comparisons between patients and the Wilcoxon signed-rank test for comparisons within patient. The results of the Mc Gill Pain questionnaire and SF-36 questionnaire were analysed by means of the t test for paired data or the Wilcoxon signed rank test accordingly with a previous analysis of normal distribution. The comparison between groups was performed by means of an ANCOVA model using the change from baseline as dependent variable, treatment and centre as factor of the model, and the baseline value as covariate. All statistical analyses were performed on changes in raw values, not transformed data (e.g. percentage change). Statistical analysis was performed using SAS Software (release 9.4) for Microsoft Windows. All tests were two-tailed, and a p value of < 0.05 was considered statistically significant.
Results
Study population
A total of 73 patients (40 in the IM neridronate group and 33 previously treated with IM placebo and then treated with IV neridronate) started the follow-up phase of the study which was completed by 60 patients (82.2%), 35 in the IM group (87.5%), and 25 in the IV group (75.8%). Three patients in the previous IM placebo group who did not attend the last visit of the double-blind phase started the open phase and were treated with IV neridronate. Seven patients (5 in the IV group and 2 in the IM group) discontinued the study due to consent withdrawal, and 6 patients (3 in both groups) were lost to follow-up. The flow chart illustrating the patients’ disposition is presented in Figure 1. The mean pain VAS score before the first IV neridronate administration (57.8 ± 20.0) was very similar to the mean VAS score found in patients starting the open-extension phase of a previous study in which patients were treated with IV neridronate after IV placebo5 (55.4 ± 24.2; p = 0.66).
Figure 1.
Flow chart illustrating the disposition of patients.
Pain VAS
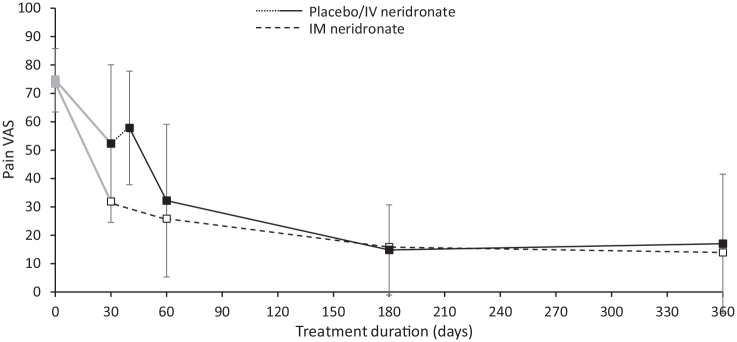
A non-significant increase in mean VAS score (from 52.3 ± 27.8 to 57.8 ± 20; p = 0.36) was observed between the end of IM placebo treatment in the double-blind phase and the start of the IV neridronate course. After treatment, mean VAS pain score decreased progressively up to day 360 with an overlapping trend in both IM and IV patients (Figure 2). Of note, at day 60 after starting IM neridronate (corresponding to 20 days after initiating IV neridronate), VAS was not significantly different between groups both in terms of absolute values (25.8 ± 28.3 versus 32.2 ± 26.9; p = 0.34) or in terms of adjusted mean changes from measures assessed at the day of the first drug administration (−47.2 ± 25.5 versus −43.2 ± 29.6; p = 0.54). Consistent with these results, at day 60 the percentage of patients achieving a ⩾50% reduction in VAS pain, such as patients referred to as ‘responders’, was not significantly different between the two treatment arms (IM neridronate 79.5% versus IV neridronate 64.5%; p = 0.18). Similar values were observed at days 180 and 360 with no statistical difference between IM and IV treatment. In IM neridronate group, the rate of patients achieving a ⩾50% reduction in pain VAS was observed in 27 of 41 (65.9%) patients at the end of the double-blind phase. This percentage progressively increased up to day 360 to 32 of 35 (91.4%) patients.
Figure 2.
Change in VAS pain score in CRPS-1 patients treated with intramuscular (IM) or intravenous (IV) neridronate from baseline to day 360.
Patients treated with IM placebo in the double-blind phase of the study (in grey) were treated with IV neridronate after a washout period of 7–10 days.
It is worth highlighting that among the 27 patients treated with IM neridronate showing a VAS decrease ⩾50% at the end of the double-blind phase, 26 reported the same result at day 360, with only one patient complaining of pain worsening to be no longer recognized as responder. An akin result was observed at day 360 in IV neridronate patients with a responder rate of 22 of 25 (88%; p = 0.66 between groups). These results did not change after adjusting for mean changes by the ANCOVA model.
Clinical signs and symptoms
During the follow-up phase of the study, both swelling and pain at passive motion continued to progressively improve from baseline up to day 360, with a similar trend in IM and IV neridronate treated patients. On day 360 in IM neridronate patients swelling was absent in 27 (77.2%), mild in 6 (17.1%), moderate in 2 (5.7%), and severe in no patients. An almost identical result was seen in IV neridronate patients, without any statistically significant difference in mean edema score (p = 0.88) (Figure 3(a)). Likewise, pain in passive motion showed a marked improvement over the duration of the study. On day 360 in IM neridronate group pain at passive motion was absent in 30 (85.7%), mild in 3 (8.6%), moderate in 2 (5.7%), and severe in none of the patients. In IV neridronate group similar results were seen with no statistically significant difference between groups (p = 0.33; Figure 3(b)).
Figure 3.
Change in clinical signs and symptoms. (a) Edema, (b) Passive motion, (c) Hyperalgesia, (d) Allodinia in CRPS-1 patients treated with intramuscular or intravenous neridronate at 360 days.
Data are presented as mean score for clinical signs evaluated by means of rating scales (edema and pain at passive motion), or the proportion of patients presenting with symptoms assessed as a dichotomous variable (present/absent) (allodynia and hyperalgesia). Values of p denote the level of statistical significance between groups.
During the study, the number of patients with hyperalgesia continued to progressively decrease up to day 360 in both IM neridronate and IV neridronate patients. On day 360, hyperalgesia was absent in 32 patients (91.4%) in the IM neridronate group and in 22 patients (95.7%) in the IV neridronate group with no significant difference observed between groups (p = 1.0; Figure 3(c)).
Similar to hyperalgesia, the number of patients with allodynia continued to progressively decrease. On day 360, allodynia was absent in 33 patients (94.3%) in the IM neridronate group and in 22 (95.7%) IV treated patients (p = 1.0; Figure 3(c)). A similar result was seen on day 180 (p = 1.0). Conversely, on day 60, corresponding to 20 days after starting IV neridronate treatment, a significant difference between the groups was found with a higher rate of patients complaining of allodynia observed in IV treated patients (p = 0.002).
Short-form 36
In this open phase of the study, the mean scores of SF-36 domains progressively improved up to day 360 in both IM and IV neridronate groups. In the IM Neridronate group, the mean score of SF-36 Physical Component Summary score continued to increase from 35.4 ± 5.6 at baseline to 48.4 ± 7.3 at the end of the study (p < 0.0001). In addition, in the IV neridronate group a significant increase was observed, with a mean score of 37.0 ± 7.2 on the day of the first neridronate infusion to 49.3 ± 8.5 on day 360 (p < 0.0001). The extent of the mean increase from baseline during the follow-up phase was similar in the two groups.
Similarly, the mean score for SF-36 Mental Component Summary progressively increased from basal values to day 360 in both groups. In the IM neridronate group, the mean value increased from 38.5 ± 9.9 to 47.7 ± 10.0 (p < 0.0001), and in the IV neridronate group from 37.4 ± 11.6 to 51.8 ± 7.3 (p < 0.0001). The extent of the mean increase was similar in the two groups.
Every domain showed overlapping results with a statistically significant increase (data not shown). Only the General Health domain just failed to reach statistical significance in the IM neridronate group (p = 0.06).
McGill Pain Questionnaire Short-Form
The mean score of SF-MPQ Sensory, Affective, Present Pain Intensity, and VAS Pain Intensity continued to progressively decrease up to day 360 in both IM and IV groups, with a statistically significant difference between the pre-treatment scores and values assessed at the end of the study (Figure 4). The extent of the mean decrease during the study was similar in the two groups. These results did not change after adjusting for mean changes by the ANCOVA model.
Figure 4.
Change in McGill pain questionnaire items in CRPS-1 treated with intramuscular or intravenous neridronate from baseline to 360 days.
Data are presented as mean ± SD for the following items: sensory, affective, PPI, and VAS. Values of p denote the level of statistical significance between groups.
PPI, present pain intensity; SF-MPQ, Short-Form McGill Pain Questionnaire; VAS, visual analogue scale.
Safety
The safety results observed after IV neridronate administration were consistent with the known safety profile of this drug. Adverse events judged as treatment-related were observed in 12 patients (36.4%) and in 9 cases expressing the so-called ‘acute phase reaction’17 (polyarthralgia). This AE was graded moderate in 4, mild in 3, and severe in 2 patients, Fever (never exceeding 38°C) was reported in 4 patients. These events were treated with acetaminophen (less than 2 g/day) and in all cases disappeared within 3 days. These AEs were generally well tolerated, and no patient discontinued the study due to these AEs.
There were no clinically significant abnormal changes in laboratory parameters (haematology and blood chemistry) performed in all patients at each clinical evaluation.
No AEs considered to be related to treatment were observed during the entire follow-up period. In both treatment groups, there were no clinically important changes at any post-baseline time-point in safety laboratory parameters and vital signs. No patients complained of osteonecrosis of the jaw or other serious dental problems. During the study, serious AEs were reported in 3 patients, all originally randomized to IM Neridronate. One patient reported a distal radius fracture after a fall, and one patient reported a skull fracture with a subarachnoid haemorrhage. A third patient was hospitalized for acute hypertension. All these AEs were not considered related to neridronate administration.
Discussion
The results of this open-label extension study provide evidence that patients with CRPS-1 treated with neridronate administered either IM or IV experienced a permanent remission of the disease. The benefit in pain, clinical, and functional measures observed a few weeks after treatment were maintained and further improved over 12 months of follow-up, regardless of the administration route.
Due to the short follow-up duration of the studies published so far, a legitimate concern could have been whether neridronate would confer only a temporary benefit. This possibility is also justified by the pharmacokinetic characteristics of all bisphosphonates.18 After administration, serum levels of these drugs rapidly decline and disappeared in a few hours for bone-adsorbed fraction and renal clearance. Despite this, a long-lasting pharmacological effect of bisphosphonates on bone turnover exerted by osteoclast inhibition is explained by the amount of bone-bound drug.18
The mechanisms of action of bisphosphonates in the treatment of CRPS remain conjectural.19,20 The greater efficacy observed in the early stages of the disease is consistent with the hypothesis that these drugs act by interfering with the early inflammatory phase of the disease, when they reduce local cytokine increase.21–23 This effect is likely achieved through local macrophage cell inhibition, which represents a specific target of bisphosphonates.19,24 The permanent and curative result we observed could be due to an acute and robust effect on macrophages, interrupting the cascade of events leading to later stages/chronicity of the disease, from neurogenic inflammation to central sensitization and cortical reorganization.25 Alternately, this prolonged effect may be due to the subsequent slow release of a relevant bone-bound drug fraction that can only be achieved in the early stage of the disease as inferred by scintigraphic studies using a bisphosphonate as the carrier of the radiotracer and showing a locally increased uptake early after the disease onset. This hypothesis is also consistent with the demonstration that bone-bound bisphosphonates can act on adjacent non-bone cells.26 Accordingly, bisphosphonates can be effective only in the early phase of the disease, during a time-limited pro-inflammatory macrophage enhanced activity, similar to that observed in the first stage of the bone repair process after a fracture.27,28
In a CRPS animal model, bisphosphonates showed a significant effect in decreasing tumor necrosis factor alpha, interleukin 1, interleukin 6, and nerve growth factor from skin specimens.29 Keratinocytes are recognized as a cellular source involved in pro-inflammatory cytokine production, leading to pain and cutaneous clinical features of CRPS30 and a frequent event inducing CRPS, such as a fracture, induces keratinocyte expression of pro-nociceptive and inflammatory mediators.31 Bisphosphonates reach the maximal concentration on the bone surface, but this structure cannot be close to skin tissue; so, a direct effect on keratinocytes may be possible as suggested by the results of studies on the pathophysiology of the oesophageal side effects of these drugs when orally administered.32
When specifically evaluating the high rate of patients reaching clinical remission after 12 months (about 90%), this result should be seen together with those who achieve spontaneous CRPS remission or an improvement of some symptoms and signs even without treatment, as can be sometimes observed in the first months after the onset of the disease.33 Even if the more recent longitudinal studies showed a significant number of patients experiencing persistent pain and dysfunction at 12 months from disease onset,34,35 not even the more optimistic reports36,37 showed rates of clinical remission as high as rates that we observed 1 year after neridronate treatment.
A possible change of pain measure associated with placebo effect is another issue that deserves to be considered. As observed in studies on many diseases in which pain measure had been assessed by VAS, significant decreases after parenteral placebo administration were reported. Supporting this, we observed a reduction in VAS pain score after IM placebo administration in the double-blind arm of the study,6 namely before IV neridronate administration reported in the present study. However, this decrease did not reach the value of 30 mm considered the minimum level reflecting a clinically relevant difference.38 Conversely, the decreases in VAS score observed along this open-label extension arm largely overcame this cutoff, and this finding should be viewed together with the results of a meta-analysis showing a lack of placebo analgesia response in long-standing CRPS trials.39
A translational study using a rat fracture model treated with alendronate or zoledronate did not observe a persistent beneficial effect after stopping orally administered zoledronate.29 Unfortunately, this study did not assess the parenteral alendronate effect over time. Beyond the route of zoledronate administration approved only for IV infusion, we elsewhere speculated on the unsuitability of zoledronate in the treatment of CRPS6 due to its high hydroxyapatite affinity and then unable to reach an adequate local concentration instead of being captured by the whole skeleton.
Looking at the results longitudinally achieved throughout the study, no difference was found between IM and IV administration at 6 and 12 months. In a shorter time, namely 60 days after starting IM administration, corresponding to 20 days from the start of IV administration, we found a very similar therapeutic effect although allodynia showed a significantly higher prevalence in IV-treated patients. This result could be associated to a faster therapeutic effect by IV administration.
A defining feature of CRPS is the extensive variability of symptoms and signs severity that often fluctuates over the course of the disease. Besides patients developing a disabling chronic pain syndrome, pain tends to improve spontaneously over time.40 Often, an impaired functional outcome with weakness, stiffness, and a restricted range of motion represents the long-term complaints that limit the functional status and activity of daily living in CRPS patients.41 The long-term improvement of instruments exploring the functional status and the quality of daily living as the SF-36 and the McGill Pain Questionnaire strengthens the result that neridronate administered in the early stage of the disease allows preventing the long-term disability.
As a limitation, this study was not designed to assess the long-term safety profile of the drug. However, no drug-related AEs and no change in laboratory parameters at each clinical evaluation were observed up to 12 months. Moreover, no major side effects more frequently observed in long-term bisphosphonate users (e.g. osteonecrosis of the jaw and atypical fractures) were reported. Periodic safety post-marketing reports issued from the first marketing authorization of neridronate in Italy (April 2002) do not refer to date reports of these AEs despite thousands of patients treated for osteogenesis imperfecta, Paget’s disease of bone, and CRPS. This favourable long-term safety profile is most likely due to the short schedule treatment length for all diseases for which neridronate is licenced.
Conclusion
In conclusion, this open-label extension study that follows a randomized, placebo-controlled trial has shown significant, clinically relevant, and persistent benefit in patients with acute CRPS-1 following both an IM and IV neridronate treatment regimen. The short-term efficacy and safety has proven to be sustained and enhanced after 1 year. These results provide evidence that the use of parenteral neridronate induces a permanent disease remission preventing chronic pain and motor dysfunction.
Acknowledgments
None.
Footnotes
ORCID iDs: Massimo Varenna  https://orcid.org/0000-0002-8644-0909
https://orcid.org/0000-0002-8644-0909
Chiara Crotti  https://orcid.org/0000-0001-7015-269X
https://orcid.org/0000-0001-7015-269X
Giovanni Iolascon  https://orcid.org/0000-0002-0976-925X
https://orcid.org/0000-0002-0976-925X
Maurizio Rossini  https://orcid.org/0000-0001-9692-2293
https://orcid.org/0000-0001-9692-2293
Contributor Information
Massimo Varenna, Bone Diseases Unit, Department of Rheumatology, Gaetano Pini Institute, Via Pini, 9, 20122 Milan, Italy.
Davide Gatti, Rheumatology Unit, Department of Medicine, University of Verona, Verona, Italy.
Francesca Zucchi, Bone Diseases Unit, Department of Rheumatology, Gaetano Pini Institute, Milan, Italy.
Chiara Crotti, Bone Diseases Unit, Department of Rheumatology, Gaetano Pini Institute, Milan, Italy.
Vania Braga, ULSS 9, Verona, Italy.
Giovanni Iolascon, Department of Medical and Surgical Specialties, University of Campania ‘Luigi Vanvitelli’, Naples, Italy.
Bruno Frediani, Unit of Rheumatology, University of Siena, Siena, Italy.
Fabrizio Nannipieri, Clinical Research, Abiogen Pharma, Pisa, Italy.
Maurizio Rossini, Rheumatology Unit, Department of Medicine, University of Verona, Verona, Italy.
Declarations
Ethics approval and consent to participate: This study was conducted under the provisions of the Declaration of Helsinki and in accordance with the International Conference on Harmonization Consolidated Guideline on Good Clinical Practice. The study was approved by the Milano B Ethics committee (28 October 2014). All patients provided written informed consent to participate in the study.
Consent for publication: Not applicable.
Author contributions: Massimo Varenna: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Visualization; Writing – original draft; Writing – review & editing.
Davide Gatti: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Francesca Zucchi: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Chiara Crotti: Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Vania Braga: Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Giovanni Iolascon: Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Bruno Frediani: Data curation; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Fabrizio Nannipieri: Formal analysis; Project administration; Writing – original draft; Writing – review & editing.
Maurizio Rossini: Investigation; Methodology; Writing – original draft; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Abiogen Pharma S.p.A, Pisa, Italy.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Massimo Varenna has received advisory board honoraria from UCB and Kyowa Kirin, consultancy fees from Abiogen and Amgen, and speaker fees from Sandoz. Davide Gatti has received advisory board honoraria from Pfizer, consultancy fees from Abiogen and UCB, and speaker fees from Celgene, Eli Lilly, and Neopharmed Gentili. Francesca Zucchi has received speaker fees from Sandoz. Chiara Crotti has received speaker fees from Kyowa Kirin and Sandoz. Giovanni Iolascon has received speaker fees from Eli Lilly and UCB. Maurizio Rossini has received advisory board honoraria from Abbvie, Sandoz, UCB, and Menarini and speaker fees from AbbVie, Amgen, BMS, Galapagos, Eli Lilly, Novartis, Pfizer, Theramex, and UCB. Fabrizio Nannipieri is an employee of Abiogen Pharma S.p.A. All other authors have no conflict of interest to declare.
Availability of data and materials: Data are available from the authors upon reasonable request and with permission of Abiogen S.p.A.
References
- 1. Adami S, Fossaluzza V, Gatti D, et al. Bisphosphonate therapy of reflex sympathetic dystrophy syndrome. Ann Rheum Dis 1997; 56: 201–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Varenna M, Zucchi F, Ghiringhelli D, et al. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol 2000; 27: 1477–1483. [PubMed] [Google Scholar]
- 3. Robinson JN, Sandom J, Chapman PT. Efficacy of pamidronate in complex regional pain syndrome type I. Pain Med 2004; 5: 276–280. [DOI] [PubMed] [Google Scholar]
- 4. Manicourt DH, Brasseur JP, Boutsen Y, et al. Role of alendronate in therapy for posttraumatic complex regional pain syndrome type I of the lower extremity. Arthritis Rheum 2004; 50: 3690–3697. [DOI] [PubMed] [Google Scholar]
- 5. Varenna M, Adami S, Rossini M, et al. Treatment of complex regional pain syndrome type I with neridronate: a randomized, double-blind, placebo-controlled study. Rheumatology 2013; 52: 534–542. [DOI] [PubMed] [Google Scholar]
- 6. Varenna M, Braga V, Gatti D, et al. Intramuscular neridronate for the treatment of complex regional pain syndrome type 1: a randomized, double-blind, placebo-controlled study. Ther Adv Musculoskelet Dis 2021; 13: 1759720X211014020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wertli MM, Kessels AG, Perez RS, et al. Rational pain management in complex regional pain syndrome 1 (CRPS 1) – a network meta-analysis. Pain Med 2014; 15: 1575–1589. [DOI] [PubMed] [Google Scholar]
- 8. Chevreau M, Romand X, Gaudin P, et al. Bisphosphonates for treatment of complex regional pain syndrome type 1: a systematic literature review and meta-analysis of randomized controlled trials versus placebo. Joint Bone Spine 2017; 84: 393–399. [DOI] [PubMed] [Google Scholar]
- 9. Fassio A, Mantovani A, Gatti D, et al. Pharmacological treatment in adult patients with CRPS-I: a systematic review and meta-analysis of randomised controlled trials. Rheumatology 2022; 61: 3534–3546. [DOI] [PubMed] [Google Scholar]
- 10. Harden NR, Bruehl S, Perez RSGM, et al. Validation of proposed diagnostic criteria (the ‘Budapest criteria’) for complex regional pain syndrome. Pain 2010; 150: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harden RN, Bruehl S, Stanton-Hicks M, et al. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med 2007; 8: 326–331. [DOI] [PubMed] [Google Scholar]
- 12. Cappello ZJ, Kasdan ML, Louis DS. Meta-analysis of imaging techniques for the diagnosis of complex regional pain syndrome type I. J Hand Surg Am 2012; 37: 288–296. [DOI] [PubMed] [Google Scholar]
- 13. Forouzanfar T, Weber WE, Kemler M, et al. What is a meaningful pain reduction in patients with complex regional pain syndrome type 1. Clin J Pain 2003; 19: 281–285. [DOI] [PubMed] [Google Scholar]
- 14. Harden RN, Oaklander AL, Burton AW, et al. Complex regional pain syndrome: practical diagnostic and treatment guidelines, 4th edition. Pain Med 2013; 14: 180–229. [DOI] [PubMed] [Google Scholar]
- 15. Maiani G, Sanavio E. Semantics of pain in Italy: the Italian version of the McGill pain questionnaire. Pain 1985; 22: 399–405. [DOI] [PubMed] [Google Scholar]
- 16. Apolone G, Mosconi P. The Italian SF-36 health survey: translation, validation and norming. J Clin Epidemiol 1998; 51: 1025–1036. [DOI] [PubMed] [Google Scholar]
- 17. Adami S, Bhalla AK, Dorizzi R, et al. The acute-phase response after bisphosphonate administration. Calcif Tissue Int 1987; 41: 326–331. [DOI] [PubMed] [Google Scholar]
- 18. Sinigaglia L, Varenna M, Casari S. Pharmacokinetic profile of bisphosphonates in the treatment of metabolic bone disorders. Clin Cases Miner Bone Metab 2007; 4: 30–36. [PMC free article] [PubMed] [Google Scholar]
- 19. Tzschentke TM. Pharmacology of bisphosphonates in pain. Br J Pharmacol 2021; 178: 1973–1994. [DOI] [PubMed] [Google Scholar]
- 20. Varenna M. Bisphosphonates beyond their anti-osteoclastic properties. Rheumatology 2014; 53: 965–967. [DOI] [PubMed] [Google Scholar]
- 21. Huygen FJ, De Bruijn AG, De Bruin MT, et al. Evidence for local inflammation in complex regional pain syndrome type 1. Mediators Inflamm 2002; 11: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Uçeyler N, Eberle T, Rolke R, et al. Differential expression patterns of cytokines in complex regional pain syndrome. Pain 2007; 132: 195–205. [DOI] [PubMed] [Google Scholar]
- 23. Roelofs AJ, Thompson K, Ebetino FH, et al. Bisphosphonates: molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr Pharm Des 2010; 16: 2950–2960. [DOI] [PubMed] [Google Scholar]
- 24. Moreau MF, Guillet C, Massin P, et al. Comparative effects of five bisphosphonates on apoptosis of macrophage cells in vitro. Biochem Pharmacol 2007; 73: 718–723. [DOI] [PubMed] [Google Scholar]
- 25. Littlejohn G. Bisphosphonates for early complex regional pain syndrome. Nat Rev Rheumatol 2013; 9: 199–200. [DOI] [PubMed] [Google Scholar]
- 26. Cornish J, Bava U, Callon KE, et al. Bone-bound bisphosphonate inhibits growth of adjacent non-bone cells. Bone 2011; 49: 710–716. [DOI] [PubMed] [Google Scholar]
- 27. Loi F, Córdova LA, Pajarinen J, et al. Inflammation, fracture and bone repair. Bone 2016; 86: 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu AC, Raggatt LJ, Alexander KA, et al. Unraveling macrophage contributions to bone repair. Bonekey Rep 2013; 2: 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang L, Guo T-Z, Wei T, et al. Bisphosphonates inhibit pain, bone loss, and inflammation in a rat tibia fracture model of complex regional pain syndrome. Anesth Analg 2016; 123: 1033–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Birklein F, Drummond PD, Li W, et al. Activation of cutaneous immune responses in complex regional pain syndrome. J Pain 2014; 15: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li WW, Guo TZ, Li XQ, et al. Fracture induces keratinocyte activation, proliferation, and expression of pro-nociceptive inflammatory mediators. Pain 2010; 151: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Reszka AA, Halasy-Nagy J, Rodan GA. Nitrogen-bisphosphonates block retinoblastoma phosphorylation and cell growth by inhibiting the cholesterol biosynthetic pathway in a keratinocyte model for esophageal irritation. Mol Pharmacol 2001; 59: 193–202. [DOI] [PubMed] [Google Scholar]
- 33. Goebel A, Birklein F, Brunner F, et al. The Valencia consensus-based adaptation of the IASP complex regional pain syndrome diagnostic criteria. Pain 2021; 162: 2346–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bean DJ, Johnson MH, Heiss-Dunlop W, et al. Extent of recovery in the first 12 months of complex regional pain syndrome type-1: a prospective study. Eur J Pain 2016; 20: 884–894. [DOI] [PubMed] [Google Scholar]
- 35. Beerthuizen A, Stronks DL, Van’t Spijker A, et al. Demographic and medical parameters in the development of complex regional pain syndrome type 1 (CRPS1): prospective study on 596 patients with a fracture. Pain 2012; 153: 1187–1192. [DOI] [PubMed] [Google Scholar]
- 36. Bickerstaff DR, Kanis JA. Algodystrophy: an under-recognized complication of minor trauma. Br J Rheumatol 1994; 33: 240–248. [DOI] [PubMed] [Google Scholar]
- 37. Zyluk A. The natural history of post-traumatic reflex sympathetic dystrophy. J Hand Surg Br 1998; 23: 20–23. [DOI] [PubMed] [Google Scholar]
- 38. Lee JS, Hobden E, Stiell IG, et al. Clinically important change in the visual analog scale after adequate pain control. Acad Emerg Med 2003; 10: 1128–1130. [DOI] [PubMed] [Google Scholar]
- 39. Mbizvo GK, Nolan SJ, Nurmikko TJ, et al. Placebo responses in long-standing complex regional pain syndrome: a systematic review and meta-analysis. J Pain 2015; 16: 99–115. [DOI] [PubMed] [Google Scholar]
- 40. Bean DJ, Johnson MH, Kydd RR. The outcome of complex regional pain syndrome type 1: a systematic review. J Pain 2014; 15: 677–690. [DOI] [PubMed] [Google Scholar]
- 41. Johnson S, Cowell F, Gillespie S, et al. Complex regional pain syndrome what is the outcome? – a systematic review of the course and impact of CRPS at 12 months from symptom onset and beyond. Eur J Pain 2022; 26: 1203–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]