Abstract
Premature ejaculation (PE) is reported to be the most common sexual dysfunction in men and is defined as the inability to control or delay ejaculation. Steady Freddy is a lidocaine-based ejaculation-delaying spray. This article examines the effects of Steady Freddy on the sexual experience of men that have self-reported to suffer from PE. Under the conditions of a randomized single-blind placebo-controlled clinical trial, 150 participants received either placebo or treatment for the duration of 12 weeks and completed an internet-based questionnaire for the quality of their sexual experience. Prior to product usage, participant average latency time was <1 min, 70% claimed to be very sexually dissatisfied, and 67% claimed to be very dissatisfied with ejaculation control. Upon product usage, sexual experience was significantly improved (p<.01). Participant average latency time increased to >2 min, 80% claimed to be sexually satisfied, and 70% claimed to be satisfied with ejaculation control. These effects were not present in the placebo group throughout the trial. These findings provide evidence for the effectiveness of Steady Freddy in significantly improving the quality of sexual experience and suggest that Steady Freddy can assist with PE.
Keywords: premature ejaculation (PE), lidocaine, men sexual health
Introduction
Premature ejaculation (PE) is reported to be the most common sexual dysfunction in men, affecting 30% to 50% of the male population (Mohamed et al., 2021). In accordance with the International Society for Sexual Medicine, PE is defined as a male sexual dysfunction in which ejaculation always or almost always occurs prior to or within about 1 min of sexual penetration, the inability to delay ejaculation during sexual activity, and negative personal consequences, such as distress, frustration, and/or the avoidance of sexual intimacy (Serefoglu et al., 2014). PE has been classified by Waldinger and colleagues into four subtypes being distinguished by the duration of the intravaginal ejaculatory latency time (IELT), frequency of complaints, and course in life (Waldinger & Schweitzer, 2006). These subtypes were classified as lifelong, acquired, variable, and subjective PE. Lifelong and acquired PE present as a persistent ejaculatory problem with an IELT of <1 min for lifelong and <3 min for acquired. conversely, both variable and subjective PE are inconsistent and present with short, normal or prolonged IELT (Serefoglu et al., 2014). The assessment of PE mainly relies on the usage of questionnaires with five validations that have been developed and published (El-Hamd et al., 2019). These questionnaires cover 4 main areas of PE including index of prematurity (IPE), PE diagnostic tool (PEDT), PE profile (PEP), and IELT.
Treatment options for PE include a variety of options across psychological, behavioral, surgical, and pharmaceutical therapies (Pu et al., 2013). Among these, topical aesthetic agents pose the least risks for patients and are characterized by minimal systemic side effects and the ability to be used on demand. Lidocaine is an amide class local anesthetic that acts via the blockade of voltage-gated sodium channels leading to a reversible blockage of action potential propagation (Garmon & Huecker, 2022). Lidocaine is commonly used as a local and topical anesthetic and was previously used for the treatment of PE in various concentrations (El-Hamd, 2021; Mark & Kerner, 2016).
Steady Freddy is an over-the-counter lidocaine-based topical spray that is applied to the penile skin prior to sexual interaction for the purpose of delaying ejaculation and enhancing the quality of sexual experience (QSE). To date, no studies have evaluated the effectiveness of Steady Freddy, nor any clinical trials investigated the effectiveness of 9.6% lidocaine topical spray formulation with 45% ethanol on PE or QSE.
Aim of the Study
Utilizing electronic reporting this study sought to examine the impact of the Steady Freddy lidocaine-based pump spray on the sexual experience of men that have self-reported to suffer from PE or who ejaculate sooner than they desire. We specifically aimed to study the extent to which Steady Freddy affected participants’ sexual experience. To do so, we explored the impact on the experience of IELT, ejaculatory control and overall QSE and hypothesize that by using Steady Freddy, those scores will increase.
Method
Study Design and Data
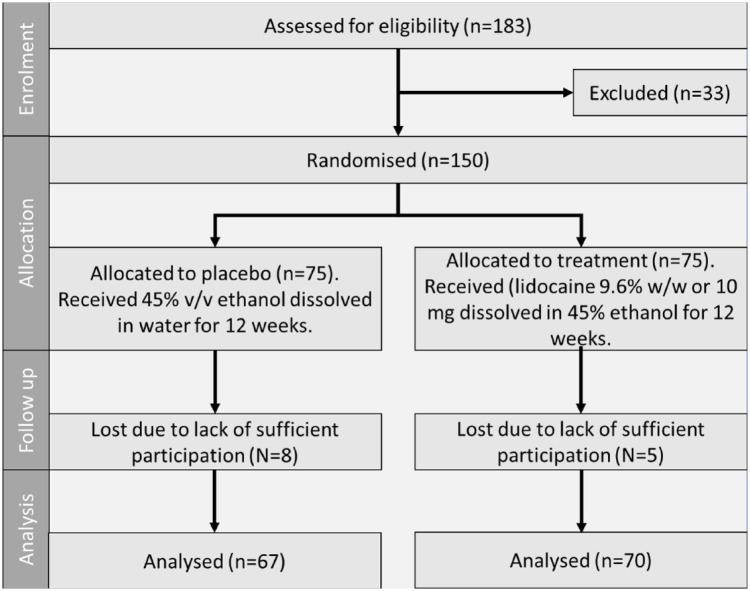
Data were collected as part of an online study of men who self-reported that they suffer from PE or who ejaculate sooner than they desire. The participation of human subjects for this study was approved by the ethics committee, ethics number 2021.ETH.00056. Eligibility was assessed via a brief survey. A total of 175 participants were recruited online via the company’s website and social media websites (Facebook and Twitter). Of those participants, 150 men met the eligibility criteria and agreed to participate, and 137 completed the trial. Eligibility criteria included being in the age group of 18 to 45 years old, currently in a sexual relationship, and with the following medical history: no allergy to lidocaine or topical anesthetics, no history of liver disease, no history of heart rhythm disease, no lesions on their own or their partner’s genitals and oral tissue and having a partner who is not currently pregnant. Once eligible, participants were assigned a number and requested to provide their mailing address for the product sample to be sent. This study was designed as a randomized single-blind placebo-controlled clinical trial. As such, participant numbers were randomized and allocated to either a placebo (45% v/v ethanol dissolved in water) or treatment (lidocaine 9.6% w/w or 10 mg dissolved in 45% ethanol) group. The product sample was blinded, and participants were not aware of what group they were assigned to. Product was sent with clear instructions for usage and was requested to be used every time the participants engaged in sex (defined as oral, anal or vaginal). They were also asked to report on their sexual behavior and relationship context every 4 weeks through an electronic survey that was provided via a unique link sent to them via email. An electronic-based data collection method was used due to the opportunity to create a more favorable environment to collect sensitive data such as sexual behavior, encouraging more accurate reporting and continuous participation.
Main Outcome Measures
In the beginning of the study, participants completed the PEDT assessment, which has been demonstrated to have strong validity and reliability in assessing PE (Symonds et al., 2007). The PEDT asked participants to answer five questions on a 1 to 5-point scale about difficulty delaying ejaculation (response options: “very easy,” ‘somewhat easy “neither easy nor difficult,” “somewhat difficult” and “very difficult”), ejaculating before desired, ejaculation with very little stimulation (response options: “almost never or never,” “less than half the time,” “about half the time,” “more than half the time” and “almost always or always”), frustration with ejaculating before desired, and perception of whether time to ejaculation affected sexual fulfillment of their partner (response options: “not at all,” “slightly,” “moderately,” “very” and “extremely”). In accordance with the validation of the PEDT assessment, a score of 11 or more was associated with the diagnosis of PE, a score of 9 and 10 was considered as probable PE, and a score of 8 or less indicated no PE (Symonds et al., 2007). In addition, to the PEDT, participants provided data once every 4 weeks on the product impact on their sexual experience. This included IELT that was measured by the question “Approximately how much time (in min) passed between the start of penetration with your partner and ejaculation?.” It also included data collected about the importance of ejaculatory control, extent of impact on satisfaction, and confidence.
Statistical Analyses
Participants who only used the product and did not fill in the survey were removed from the analytic data set as no estimate of product effect could be obtained from these individuals. All statistical analyses were performed using the GraphPad Prism software. After the participant exclusions, the analytic sample consisted of 150 subjects. To analyze the difference between before and after product usage, we conducted a series of random effect mixed models. Since data were collected from the same men over the course of 12 weeks, data points were not independent of one another in these models. In addition, we conducted a comparison of the placebo group against the treatment group across all time points using two-way analysis of variance (ANOVA) for the determination of statistical significance.
Results
Baseline Results
Written and verbal consent was received from 150 male participants that were eligible in accordance with the specified criteria, with 75 being allocated to each group. The PEDT score at baseline was 15 ± 0.5 for the placebo and 14.9 ± 0.5 for the treatment group, indicating a clinical diagnosis of PE in accordance with the PEDT scoring scale (Symonds et al., 2007). Cronbach’s alpha in the current sample was 0.89, indicating high consistency. Prior to product usage, IELT was <1 min for 90 (60%) participants. Satisfaction of ejaculatory control was very low as indicated by 100 (67.3%) of the participants, which also expressed being almost always or always distressed or concerned about ejaculating sooner. These results can be seen in Table 1.
Table 1.
Baseline Values of Participants in Both the Placebo and Treatment Group. Values Were Self-Reported and Recorded Via Online Participant Questionnaire Before the Usage of the Allocated Treatments. Error Is Represented as Standard Error of the Mean (SEM).
| Item | Placebo | Treatment |
|---|---|---|
| Sample size | 75 | 75 |
| PEDT | 15.0 ± 0.5 | 14.9 ± 0.5 |
| IELT (<1 min) | 59% | 61% |
| Satisfied from sexual life | 2% | 2% |
| Frustrated from intercourse duration | 83% | 80% |
Note. PEDT = premature ejaculation diagnostic tool; IELT = intravaginal ejaculatory latency time.
Electronic Report Results
Once treatment has commenced, IELT significantly increased (p>.001) in the treatment group resulting in 43 (61%) of participants lasting more than 2 min and 20 (28%) lasting between 1.5 and 2 min. Compared with the baseline results, product usage has resulted in an increase of 59% in participants lasting >2 min (Figure 1). Similarly, sexual satisfaction was also significantly increased (p>.001) upon product usage, as 37 (53%) of participants reported to be very satisfied with their sexual life and sexual relationship with their partner (Figure 2). No changes were observed in the placebo group. These results were consistent throughout the whole trial period.
Figure 1.
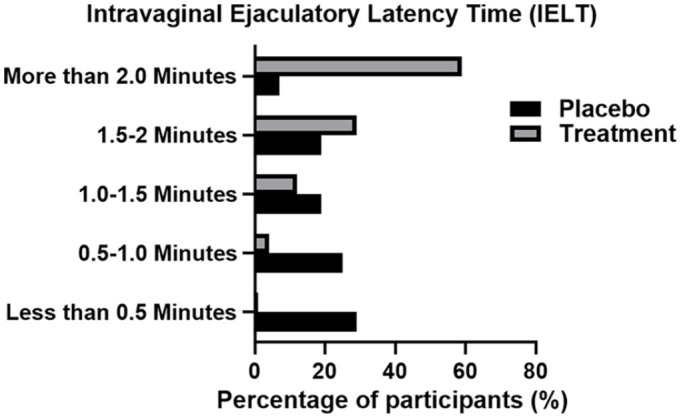
Intravaginal Ejaculatory Latency Time (IELT) in Both Placeo and Treatment Groups Post 4 Weeks of Treatment. IELT Was Self-Reported and Recorded Via Online Participant Questionnaire
Figure 2.
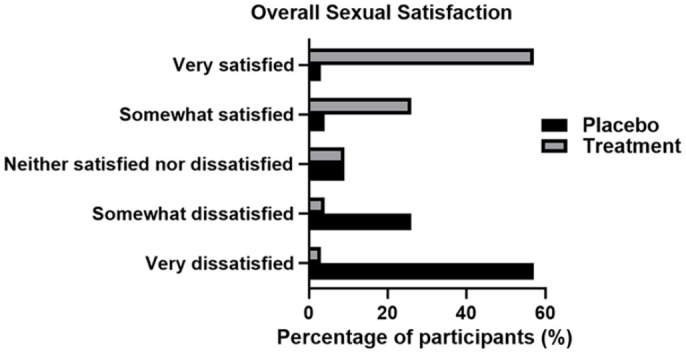
Overall Sexual Satisfaction in Both Placebo and Treatment Groups Post 4 Weeks of Treatment. IELT Was Self-Reported and Recorded Via Online Participant Questionnaire
Note. IELT = intravaginal ejaculatory latency time.
In addition, 39 (56%) participants who used the product have indicated that they were more satisfied with their overall sexual intercourse and additional 23 (33%) have indicated that they were very satisfied. Participants largely felt the use of the product positively affected their sexual experience, with 42 (61%) indicating that sexual intercourse was more than half the time or always satisfactory and they were more satisfied with the length (49, 71%). Conversely, placebo participants indicated that they were very dissatisfied (42, 63%), indicated that their sexual intercourse was not satisfactory (53, 78%), and nor was the length of the intercourse (55, 81%). These results can be seen in Figures 3 and 4.
Figure 3.
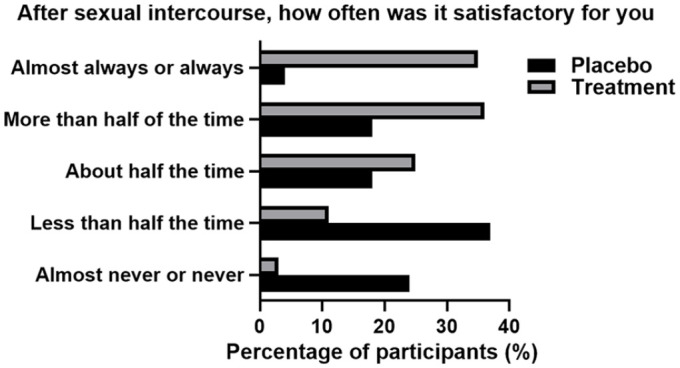
Satisfaction in Sexual Intercourse in Both Placebo and Treatment Groups Post 4 Weeks of Treatment. Results Were Self-Reported and Recorded Via Participant Patient Questionnaire
Figure 4.
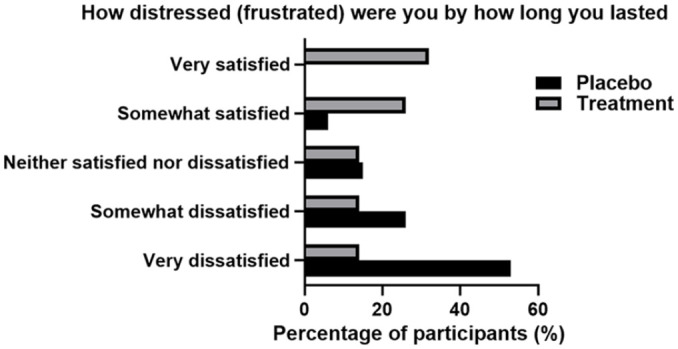
Frustration in Duration of Sexual Intercourse in Both Placebo and Treatment Groups Post 4 Weeks of Treatment. Results Were Self-Reported and Recorded Via Participant Patient Questionnaire
Moreover, the usage of the product resulted with 48 (68%) of the participants claiming to be satisfied with their control over ejaculation. While the placebo group has indicated that 53 (79%) of participants were dissatisfied or very dissatisfied (Figure 5).
Figure 5.
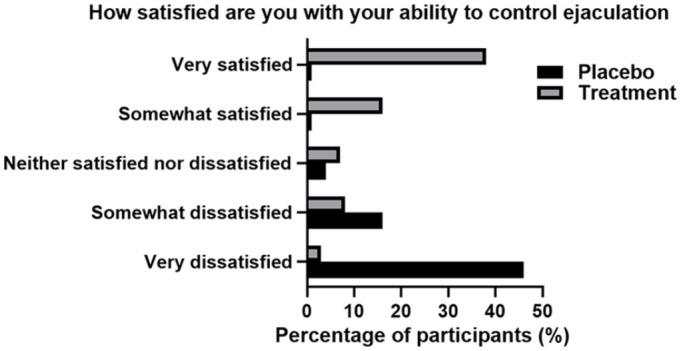
Satisfaction of Ejaculation Control in Both Placebo and Treatment Groups Post 4 Weeks of Treatment. Results Were Self-Reported and Recorded Via Participant Patient Questionnaire
Analysis comparing the placebo and product usages indicated a significant impact of product usage on QSE (p > .001) with PEDT scores reducing from 15.3 ± 4.3 to 14.2 ± 4.5. In addition to enhancing QSE, we observed that frustration and anxiety was decreased with the use of the product, as 41 (58%) of participants claimed they were content, compared with placebo where 53 (79%) of participants claimed to be very frustrated and anxious. Ejaculatory control and anxiety related to ejaculation has also improved upon product usage, as 46 (65%) of participants claimed to be never distressed versus 46 (68%) of placebo participants that claimed to be more distressed.
Discussion
To our knowledge, this is the first study to be done as a randomized single-blind placebo-controlled clinical trial setting testing sexual satisfaction and experience using Steady Freddy a lidocaine-based pump spray for the treatment of PE. From the obtained results, we found that the usage of Steady Freddy significantly increased IELT, sexual satisfaction, ejaculation control, and QSE. In our sample, the average IELT prior to product usage was 1 min or less. Although we did not rely on stopwatch-based IELT because this was an internet-based rather than clinic-based study, in accordance with the SSMI, IELT time of 1 min or less is an indication of lifelong PE (Serefoglu et al., 2014). As such, this in addition to the PEDT scoring (15.3) confirmed that the men used in this study have met the criteria of PE. Our results demonstrated observed improvements in QSE with the usage of Steady Freddy. We observed increases in sexual satisfaction, partner satisfaction, and sexual relationship with partners. Together with this, we saw increases in IELT and a decrease in PEDT scoring. These suggested that Steady Freddy is effective in PE and can be used as a topical treatment. In addition to enhancing QSE, we observed that frustration and anxiety were decreased with the use of the product. In addition, no adverse events or product dissatisfaction was reported by the user. Previous studies have demonstrated that men with PE often suffer from significant psychological distress including anxiety, depression, lack of sexual confidence, poor self-esteem, impaired quality of life, sexual dissatisfaction, and interpersonal difficulties (Fiala et al., 2021; Rowland, 2011). With the use of Steady Freddy, participants claimed they were content, compared with the initial claim of being very frustrated and anxious prior to product use. Anxiety related to ejaculation has also improved upon product usage as participants claimed to be distressed almost never or less than half of the time. Those that used the placebo did not present with this improvement and claimed to be anxious and distressed more than half of the time or almost always. Studies examining emerging therapies for PE have recently demonstrated a fold change in IELT ranging between 1.1 and 4.4 when compared with placebo (Gul et al., 2022). The usage of Steady Freddy resulted in a similar effect with an increase of 3 to 3.5-fold in IELT. As such, we suggest that Steady Freddy can assist men suffering from PE with psychological distress, although we recognize that further studies will be required to study the exact effect of Steady Freddy on mental health and PE.
Study Limitations
This study’s limitations include the inclusion of medical history for the participants. While our screening process and eligibility criteria have ensured no interaction with the product or effect on PE, all medical history was entirely self-reported. Future studies should take this factor into account. In addition, the recording of any present medical conditions or medications taken that could affect sexual response time beside PE, should also be recorded and taken into consideration. While this study included a modest sample size and a long-time course, in a single-blind placebo-controlled environment, we were limited with the study conditions. As such, a future study with a randomized double-blind, placebo-controlled, cross-over trial will further prove the effects that have been demonstrated in this study.
Conclusion
Steady Freddy is a lidocaine-based pump spray that acts as an ejaculation-delaying topical agent. This study has demonstrated for the first time that Steady Freddy can improve ejaculation control, QSE, and sexual satisfaction in men with PE. Furthermore, by doing so Steady Freddy may reduce psychological distress associated with PE.
Acknowledgments
The authors thank Medical Symbiosis for the funding to conduct this research.
Appendix
Figure A1.
Flow Diagram of the Progress Through the Phases of a Placebo Controlled Parallel Randomized Trial of Two Treatment Groups (i.e., Enrolment, Intervention Allocation, Follow-Up, and Data Analysis)
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Funding for this study was solemnly provided by Medical Symbiosis who are the Australian sponsor of Steady Freddy, the product presented in this paper. Yet, Medical Symbiosis did not have any involvement in the study design, outcomes, or this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Artur Shariev  https://orcid.org/0000-0002-7991-6323
https://orcid.org/0000-0002-7991-6323
References
- El-Hamd M. A. (2021). Effectiveness and tolerability of lidocaine 5% spray in the treatment of lifelong premature ejaculation patients: A randomized single-blind placebo-controlled clinical trial. International Journal of Impotence Research, 33(1), 96–101. 10.1038/s41443-019-0225-9 [DOI] [PubMed] [Google Scholar]
- El-Hamd M. A., Saleh R., Majzoub A. (2019). Premature ejaculation: An update on definition and pathophysiology. Asian Journal of Andrology, 21(5), 425–432. 10.4103/aja.aja_122_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiala L., Lenz J., Konecna P., Zajicova M., Cerna J., Sajdlova R. (2021). Premature ejaculation and stress. Andrologia, 53(7), Article e14093. 10.1111/and.14093 [DOI] [PubMed] [Google Scholar]
- Garmon E. H., Huecker M. R. (2022). Topical, local, and regional anesthesia and anesthetics. StatPearls. https://www.ncbi.nlm.nih.gov/pubmed/28613644 [PubMed] [Google Scholar]
- Gul M., Bocu K., Serefoglu E. C. (2022). Current and emerging treatment options for premature ejaculation. Nature Reviews Urology, 19(11), 659–680. 10.1038/s41585-022-00639-5 [DOI] [PubMed] [Google Scholar]
- Mark K. P., Kerner I. (2016). Event-level impact of promescent on quality of sexual experience in men with subjective premature ejaculation. International Journal of Impotence Research, 28(6), 216–220. 10.1038/ijir.2016.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed A. H., Mohamud H. A., Yasar A. (2021). The prevalence of premature ejaculation and its relationship with polygamous men: A cross-sectional observational study at a tertiary hospital in Somalia. BioMed Central Urology, 21(1), Article 175. 10.1186/s12894-021-00942-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu C., Yang L., Liu L., Yuan H., Wei Q., Han P. (2013). Topical anesthetic agents for premature ejaculation: A systematic review and meta-analysis. Urology, 81(4), 799–804. 10.1016/j.urology.2012.12.028 [DOI] [PubMed] [Google Scholar]
- Rowland D. L. (2011). Psychological impact of premature ejaculation and barriers to its recognition and treatment. Current Medical Research and Opinion, 27(8), 1509–1518. 10.1185/03007995.2011.590968 [DOI] [PubMed] [Google Scholar]
- Serefoglu E. C., McMahon C. G., Waldinger M. D., Althof S. E., Shindel A., Adaikan G., Becher E. F., Dean J., Giuliano F., Hellstrom W. J., Giraldi A., Glina S., Incrocci L., Jannini E., McCabe M., Parish S., Rowland D., Segraves R. T., Sharlip I., Torres L. O. (2014). An evidence-based unified definition of lifelong and acquired premature ejaculation: Report of the second international society for sexual medicine ad hoc committee for the definition of premature ejaculation. Sexual Medicine, 2(2), 41–59. 10.1002/sm2.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds T., Perelman M. A., Althof S., Giuliano F., Martin M., May K., Abraham L., Crossland A., Morris M. (2007). Development and validation of a premature ejaculation diagnostic tool. European Urology, 52(2), 565–573. 10.1016/j.eururo.2007.01.028 [DOI] [PubMed] [Google Scholar]
- Waldinger M. D., Schweitzer D. H. (2006). Changing paradigms from a historical DSM-III and DSM-IV view toward an evidence-based definition of premature ejaculation. Part II—Proposals for DSM-V and ICD-11. Journal of Sexual Medicine, 3(4), 693–705. 10.1111/j.1743-6109.2006.00276.x [DOI] [PubMed] [Google Scholar]