Abstract
Background:
Crohn’s disease (CD) and ulcerative colitis (UC) arise from a dysregulation of the balance between commensal microbiota and mucosal-associated immune system, in patients with genetic and environmental predisposition. Different pathophysiological mechanisms have been reported to influence disease history, with impact on disease phenotype and risk of complications.
Objectives:
This review aims to summarize the definitions of early CD and UC, analyze the underlying immunological mechanisms, and evaluate the impact of recognizing and treating early inflammatory bowel disease (IBD) on patients’ prognosis (short- and long-term outcomes).
Design:
To address this issue, we have performed a scoping review.
Data sources and methods:
Three online databases (MEDLINE, Web of Science, and ScienceDirect) were searched and the results were independently screened by two reviewers.
Results:
From 683 records identified, 42 manuscripts evaluating early IBD in adult patients were included. The ‘early CD’ concept was first described in 2008. Four years later, an international consensus proposed the definition of diagnosis up to 18 months, in patients without previous or current need for disease-modifying therapies. Several other definitions have been proposed; the ‘2 years since diagnosis’ is the most used, regardless of disease characteristics or medication. The amount of evidence on early UC is lower and more recent. Regarding early disease pathogenesis, most theories emphasize the prominent role of innate immunity, followed by early-Th1 adaptive response.
Conclusion:
The treatment of early CD seems to be crucial for the management of CD patients, impacting short-, medium-, and long-term outcomes. On the other hand, the early treatment of UC appears to be less advantageous, yet evidence comes from only a few retrospective studies.
Keywords: Crohn’s disease, early disease, ulcerative colitis
Introduction
Inflammatory bowel diseases (IBDs), including Crohn’s disease (CD) and ulcerative colitis (UC), result from the dysregulated crosstalk between commensal microflora and the mucosal-associated immune system, in patients with a genetic predisposition and environmental exposure to risk factors. CD and UC pathogenesis are believed to have different yet interrelated immunological phases.1 From a pathophysiological point of view, several cytokines have been reported to be differentially expressed in distinct disease phases; particular attention shall be devoted to interleukin (IL)-12 and IL-17 during early and late stages, respectively.2 IBD-related inflammation may go unnoticed for prolonged periods delaying clinical presentation. Therefore, at the time of diagnosis, disease may be advanced, both immunologically and phenotypically.3 CD diagnosis is often delayed 5–9 months due to the variability of the initial manifestations, as opposed to UC, which is usually diagnosed earlier, due to the consistent and alarming initial symptoms and manifestations.4
Recognizing early disease as a clinically distinct entity may change the objectives of the treatment strategy and impact clinical outcomes, as it has been suggested for rheumatoid arthritis.3 The relevance of the transition from early to late disease in the management of IBD relies on the association of these states with disease phenotype, extension, severity, and prognosis.1 In fact, IBD progressively induced damage results from a continued phenomenon. Early CD phases have been reported to be more dependent on the breakdown of epithelial barrier function and on the impairment of innate immunity, triggering subacute inflammation. The subsequent compromise of bacterial clearance and the dysregulated adaptive immune responses perpetuate the inflammation state.1 The interest on UC early phases is more recent and, as in CD, innate immunity is also a key player. It has been described that early intervention with biologics may slow disease progression and improve long-term outcomes in IBD, reducing irreversible damage.2 This supports the existence of a ‘window opportunity’ for intervention, before severe inflammation and bowel damage become established.2,5
In this scoping review, we aimed to analyze the evidence regarding early CD and UC definition and the immunological mechanisms associated with early disease stages, as well as to summarize the available information on the impact of prompt treatment on prognosis, including its strengths and limitations.
Materials and methods
A scoping review was performed, following the PRISMA checklist for scoping reviews.6 Three databases [MEDLINE (through PubMed), ScienceDirect, and Web of Science] were searched, from inception to 12th August 2022, using the following terms: [Crohn Disease (MeSH terms) OR Crohn’s disease OR ulcerative colitis OR Inflammatory Bowel Diseases (MeSH terms) AND early disease].
The inclusion criteria were as follows: (i) including adults diagnosed with CD or UC using clinical, endoscopic, and/or pathological features and (ii) mentioning early disease and/or its immunopathogenesis and/or the impact of early treatment on clinical outcomes. Exclusion criteria were as follows: (i) including only patients without CD or UC and (ii) not referring to early disease.
The reference lists of the included studies and of important reviews on the topic were hand-searched to identify further relevant publications. The studies were independently screened by three reviewers (MM Estevinho, P Leão Moreira, and I Silva). Any study, whose title and abstract clearly indicated that it failed to meet the selection criteria, was excluded; for all others, the full text was carefully analyzed to decide for inclusion or exclusion. The following information was collected by three independent reviewers: authors’ name, publication year, study type, patients’ characteristics, intervention/exposure, comparator (posology and treatment duration), follow-up, assessed outcomes, and main results (regarding early disease).
Studies selection and data extraction
Overall, 683 results were identified (88 in PubMed, 502 in ScienceDirect, and 93 in Web of Science); from these, 39 studies were included in the scoping review: 28 regarding CD, 8 on UC, and one including both CD and UC. In addition, three reports on UC were identified through manual search, totalizing 42 included studies. Figure 1 depicts the studies’ selection process.
Figure 1.
Flow diagram of studies selection.
Definition of early disease
Crohn’s disease
The success of the early treatment of rheumatologic conditions, along with the diversification of CD treatment options, has brought the spotlight to the concept of ‘early disease’. Although the development of a clear definition has been challenging. The concept of ‘early CD’ emerged in 2008 and was first defined as a diagnosis within 4 years, in patients without exposure to corticosteroids, antimetabolites, or biological agents.7 The diagnosis-based time was selected to avoid recall bias associated with symptoms and to bypass the poor association between symptoms and inflammation. Since then, multiple definitions have been proposed, including not only time and medication, but also other characteristics like disease phenotype (Figure 2, Table 1). For example, in the SONIC trial, published in 2010, the 3 years cutoff was applied to stratify short/long disease duration.8 In the same year,4,9 the definition was upgraded to include parameters reflecting active disease (CD activity index >220, plus C-reactive protein >10 mg/L, ulceration of more than 10% in at least one bowel segment on endoscopy, bowel enhancement on computerized tomography or magnetic resonance imaging, or ‘positive’ fecal calprotectin), in patients in whom the diagnosis was performed less than 2 years before. In this definition,9 patients with early CD should not have fistula, abscess, stricture, prior CD-related surgery or endoscopic dilation, altered continence or need for enteral or parenteral supplementation or nutrition, exposure to immunomodulators, biologics, been steroid dependent or have ever received intravenous steroids. In 2012, the Paris definition was published by international opinion leaders. In this document, early CD was defined as a diagnosis up to 18 months,10 in patients without previous or current need for disease-modifying therapies (immunomodulators or biologics).1 Unlike the previous 2010 definition, corticosteroids were allowed, regardless of the posology. The Paris definition was independent of symptoms, presence of bowel damage (fistula, abscess, or stricture; prior CD-related surgery, perianal fistulas, or presence of anorectal strictures), and disease activity, yet such characteristics were recognized to have prognostic value. Due to the absence of objective analytical, endoscopic, and/or imagological markers, the application of the Paris definition was limited and the concept of ‘early disease’ remained mutable.11 The ‘2-years from diagnostic cutoff’ remained the most used definition throughout the years, due to its undeniable easiness of application.12–25 The fact that the same teams have used different definitions throughout time reinforces the need of adjusting translational research to the demanding circumstances of real-life clinical practice. A recent meta-analysis, including individual-patient data from 16 randomized controlled trials (RCT), used the cutoff of 18 months to define short-duration disease, regardless of other characteristics like phenotype or prior treatment.26 Besides that, some studies have focused on the concept of ‘very early’ CD, again with variable definitions (up to 627 or 1213,28,29 months since diagnosis). The current definitions of early CD have some main limitations: (i) they mostly rely on the time to a timely diagnosis, what is problematic considering the diagnostic delay that is frequently seen; (ii) inflammation may occur without symptoms; (iii) up to 20% of CD patients have stenosing and penetrating phenotypes without recalling prior symptomatology; and (iv) acute and chronic lesions may coexist, but as soon as irreversible bowel damage is detected, it appears legitimate to consider that the disease is no longer in an early stage. Indeed, patients with stenosing and penetrating phenotypes may present immunologically more advanced CD, and their labeling as ‘early’ may underestimate disease progression and postpone treatment introduction.
Figure 2.
Summary of early CD definitions (parameters included and studies using such definition).
CD, Crohn’s disease.
Table 1.
Evidence regarding therapeutic intervention on early CD.
| Authors | Study type | Patients’ characteristics | Follow-up | Early disease/therapy definition | Intervention/exposure | Outcomes | Main results |
|---|---|---|---|---|---|---|---|
| D’Haens et al.7 | Open-label randomized trial | Newly diagnosed and treatment-naïve CD patients | 24 months | ⩽4 years since diagnosis | Infliximab (episodic treatment with 5 mg/kg) + azathioprine (2–2.5 mg/kg) (n = 67); azathioprine (n = 66) | CFR (CDAI < 150 + absence of CCT + no intestinal resection) | Absolute difference of 19.4% (95% CI: 2.4–36.43) in CFR between early combined immunosuppression versus azathioprine. Longer time to relapse and lower CCT exposure in patients assigned to early combined immunosuppression |
| Baert et al.30 | Extension study (follow-up of an RCT) | Newly diagnosed and treatment-naïve CD patients | 24 months | ⩽4 years since diagnosis | Infliximab 5 mg/kg (weeks 0, 2, and 6) in combination with azathioprine 2–2.5 mg/kg (n = 26) versus CCT (n = 23) | Stable remission (CDAI < 150 or HBI < 3) and no use of CCT; complete mucosal healing (SES-CD = 0) | In patients with early-stage CD, CFR was more frequent in those with complete mucosal healing [70.8% versus 27.3% (patients with endoscopic activity)]; this supports that a top-down approach in early CD may alter disease course |
| Sandborn et al.12 | Open-label induction; RCT maintenance | Moderate to severe CD (CDAI 220–450), with secondary failure to infliximab | 26 weeks | ⩽2 years since diagnosis | Induction – certolizumab pegol 400 mg weeks 0, 2, and 4; maintenance – if clinical response at week 6, certolizumab 400 mg every 2 (n = 161) or 4 weeks (n = 168) | Clinical response (⩾100-point reduction in CDAI) and remission (CDAI score ⩽150 points) | Clinical response after induction was not superior in patients with early disease (60.0% versus 64.1%), nor for patients receiving maintenance certolizumab, every 2 weeks. Maintenance certolizumab every 4 weeks was associated with higher clinical response in those with longer disease (42.2% for > 5 years, 31.6 for 2–5 years from diagnosis) |
| Schreiber et al.13 | RCT – posthoc analysis | Moderate-to-severe CD (CDAI 220–450) + clinical response (⩾100-point reduction in CDAI) after certolizumab induction | 26 weeks | Very early disease defined as <12 months after diagnosis; early as 12–24 months after diagnosis | Certolizumab pegol 400 mg every 4 weeks (n = 151) versus placebo (n = 109) | Clinical response (⩾100-point reduction in CDAI) and remission (CDAI score ⩽150 points) | Response during maintenance inversely related to disease duration [89.5% (up to 12 months of disease duration), 75.0% (between 12 and 24 months), 62.2% (24–60 months)] |
| Ramadas et al.28 | Observational | CD incident cases | Median follow-up 92 months (range 41–261) | ⩽1 year since diagnosis | Azathioprine (~2 mg/kg; n = 117) or 6-mercaptopurine (~1 mg/kg; n = 12) | Need for intestinal surgery (resection of bowel, stricturoplasty, stoma formation) | Early thiopurines start was independently associated with a significant reduction in the cumulative probability of intestinal surgery (HR: 0.47, 95% CI 0.27–0.79) |
| Kato et al.31 | Observational | CD patients admitted for induction therapy with infliximab | 14 weeks | ⩽19 months since diagnosis | Infliximab 5 mg/kg (weeks 0, 2, and 6) (n = 22) | Effect of infliximab on patients’ immune profiles (Treg, serum cytokines and chemokines) | Modulation of the peripheral immune system of CD patient in very early stage induced a differentiation toward Th17, alleviating the inflammatory drive |
| Rubin et al.32 | Observational | CD patients | 24 months | Early-TNF treatment (initiated anti-TNF within 30 days of the first prescription for CD) | ‘Step-up’ (5-ASA, CCT and/or IS prior to anti-TNF; n = 1396); ‘IS-to TNF’ (IS prior to anti-TNF; n = 1031); ‘Early-TNF’ (n = 1321) | Concomitant CCT use, CD surgery, anti-TNF dose escalation, and anti-TNF discontinuation/switch | Earlier use of anti-TNF is associated with a lower risk of concomitant CCT use, anti-TNF dose escalation, discontinuation/switch of anti-TNF therapy, and CD-related surgery compared to conventional models of step-up or IS to anti-TNF strategies |
| Lakatos et al.33 | Observational | CD incident cases | At least 52 weeks | Very early disease defined as < 18 months after diagnosis; early as < 36 months + at least 6 months before the first surgery | Azathioprine (⩾1.5 mg/kg) (n = 224) | Need for surgery | Early azathioprine use was significantly associated with a reduction in the CD-related surgical rates (HR: 0.43, 95% CI: 0.28–0.65) |
| Schreiber et al.14 | RCT and open-label extension | Moderate-to-severe CD (CDAI 220–450 points) + clinical response (⩾100-point reduction in CDAI) after adalimumab induction | 56 weeks | ⩽2 years since diagnosis | Adalimumab 40 mg (eow or weekly) (n = 517); placebo (n = 260) | Clinical response (⩾100-point reduction in CDAI) and remission (CDAI score ⩽150 points), hospitalizations | Shorter disease duration was identified as a significant predictor for higher remission rate, lower hospitalization, and less adalimumab-related serious adverse events |
| Feagan et al.34 | RCT | CD patients who had initiated prednisone induction therapy (15–40 mg/day) within the preceding 6 weeks | 50 weeks | ⩽2 years since diagnosis | Infliximab (5 mg/kg at weeks 1, 3, 7, 14, 22, 30, 38, and 46) + methotrexate (gradual increase up to 25 mg/week) (n = 63) versus infliximab + placebo (n = 63) | Time to treatment failure [lack of CFR (CDAI < 150) at week 14 or failure to maintain remission through week 50] | Although patients who had been diagnosed within 2 years had a lower rate of treatment failure in both treatment groups, the between-group differences were not significant |
| Peters et al.35 | Observational | CD patients | Median follow-up of 2.0 years (IQR: 1.1–2.7) | No definition | Adalimumab [160 mg (week 0) and 80 mg (week 2) for induction, maintenance with 40 mg eow] (n = 438) | Clinical response (ongoing adalimumab or intended discontinuation), CFR, clinical remission (no diarrhea, abdominal pain, or draining fistulae) | Longer duration of disease before adalimumab start increased the risk to failure to induction therapy (OR: 1.05; 95% CI: 1.02–1.09, p = 0.01) |
| Colombel et al.15 | RCT | Moderate-to-severe CD (CDAI of 220–450), with secondary failure to infliximab | 52 weeks | ⩽2 years since diagnosis | Adalimumab 40 mg (eow) (n = 64); adalimumab induction + placebo maintenance (n = 65) | Deep remission: CDAI < 150 + absence of mucosal ulceration) | Attainment of deep remission was superior in patients with disease diagnosed up to 2 years prior starting (33.0%) than in those with longer disease duration (20.0% if 2–5 years, 16.0% if >5 years) |
| Juillerat et al.36 | Observational | CD patients exposed at least once to infliximab | Median follow-up 13 months (range 2–43) | No definition | Infliximab exposure (n = 1014) | Predictive clinical characteristics for the successful ‘long-term use’ (5 years) of infliximab | Initiation of infliximab earlier in the disease course influenced short-term but not long-term response |
| Nuij et al.37 | Observational | 413 IBD patients were included, (201 CD, 188 UC, and 24 IBDU) | Median follow-up 39 months (range: 0.2–47.9) | Early treatment defined as starting anti-TNF at most 16 months diagnosis; very early (12 months) | Anti-TNF therapy (n = 66; infliximab n = 51, adalimumab n = 10, certolizumab n = 5) | Mucosal healing, complications, surgery | Starting anti-TNF early was not associated with higher achievement of mucosal healing, lower occurrence of disease complications or surgery |
| Colombel et al.38 | RCT and open-label extension | Moderate-to-severe CD (CDAI of 150–450) + ulcerations on endoscopy + no previous biologics or immunomodulators | 26 weeks | ⩽18 months after diagnosis, no previous use of IS or biologics, and no fistulas | Infliximab (5 mg/kg, n = 62), azathioprine (2.5 mg/kg, n = 51), or combination (infliximab + azathioprine, n = 72) | Clinical remission (CDAI < 150), mucosal healing (no ulcerations), composite endpoints (clinical remission ± mucosal healing ± normal CRP) | Early disease was associated with higher rates of clinical remission (81.0% versus 80.0%, p = 0.036) and clinical remission plus mucosal healing (63.0% versus 53.3%, p = 0.004), when compared with non-early disease |
| Safroneeva et al.17 | Observational | CD patients | Up to 96 months | ⩽2 years since diagnosis | Immunomodulators (n = 188), TNF antagonist (n = 55), or combination of both (n = 49) | Occurrence of bowel strictures, perianal fistulas, internal fistulas, intestinal surgery, perianal surgery | Early use of immunomodulators or anti-TNF was associated with a reduced risk of bowel strictures (HR: 0.496, p = 0.004), reduced risk of intestinal surgery (HR: 0.322, p = 0.005), perianal surgery (HR: 0.361, p = 0.042), and of developing any complication (HR 0.567, p = 0.006) |
| Khanna et al.39
(REACT trial) |
Open-label cluster RCT | CD patients | Up to 24 months | No definition | Combined immunosuppression (adalimumab + antimetabolite; n = 1084) or conventional therapy (n = 898) | Proportion of patients in CFR (HBI ⩽ 4) | Patients treated with early combination therapy had a significant reduction in serious adverse events such as surgery, hospital admission, or serious disease-related complications |
| Kotze et al.16 | Observational | Anti-TNF naïve patients | 17.3 (±12.4) months | ⩽2 years since diagnosis | Infliximab (5 mg/kg, n = 175) or adalimumab (160 mg, 80 mg and then 40 mg eow, n = 58) | Loss of efficacy (need for CCT, surgery, dose increase, interval shortening or switching to other anti- TNF) | The rates of loss of efficacy were similar among patients with early and non-early disease |
| Ogata et al.19 | Observational | Moderate-to-severe active CD | 24 weeks | ⩽2 years since diagnosis | Adalimumab (160 mg at week 0, 80 mg at week 2, and 40 mg eow thereafter, n = 1693) | Clinical remission (CDAI < 150), adverse events | Clinical remission rates were significantly higher in patients with shorter disease duration compared with those with longer duration, at weeks 4 and 24 |
| Ma et al.18 | Observational | CD patients with primary response to anti-TNF therapy | Median follow-up 154 weeks (IQR: 106.4–227.8) | ⩽2 years since diagnosis | Infliximab (5 mg/kg, n = 100) or adalimumab (160 mg, 80 mg and then 40 mg, n = 90) | Occurrence of surgical resection or clinical secondary loss of response | Less patients in the early initiation group required surgery (5.7% versus 30.7%, p = 0.001) or experienced secondary loss of response (45.3% versus 67.2%, p = 0.006) |
| Oh et al.21 | Observational | CD patients naïve to both intestinal surgery and intestinal complications | Mean follow-up 103 months (±53.92) | ⩽2 years since diagnosis | Early anti-TNF group (n = 79); early IS group (n = 286); late therapy group (n = 305) | Intestinal surgery, stricturing complications, penetrating complications, colorectal cancer, and mortality | Late anti-TNF/IS treatment was independently associated with higher risks of intestinal surgery (HR: 2.32, p < 0.001), behavioral progression (HR: 2.00, p < 0.001), strictures (HR: 1.73, p = 0.003), and penetrating complications (HR: 3.31, p < 0.001) than early treatment |
| Colombel et al.20
(CALM trial) |
RCT | Moderate-to-severe CD (CDAI of 150–450) + active endoscopic disease (CDEIS > 6) + CRP 5 mg/L or more, FC of 250 μg/g or more, or both | 48 weeks | ⩽2 years since diagnosis | Tight control (n = 122) versus conventional management (n = 122) (adalimumab 160 mg at week 0, 80 mg at week 2 and 40 mg eow thereafter) | Mucosal healing (CDEIS < 4 and no deep ulcers); deep remission (CDAI < 150, CDEIS < 4 and no deep ulcers, no draining fistula, no CCT for ⩾ 8 weeks); biological remission (FC < 250 μg/g, CRP < 5 mg/L, CDEIS < 4) | Patients with recent disease onset benefit from early biological treatment (higher mucosal healing and CFR) |
| Faleck et al.22 | Observational | CD diagnosis + active symptoms attributed to CD | 6 months | ⩽2 years since diagnosis | Vedolizumab (n = 650) | Clinical remission, CFR, or endoscopic remission | Early treatment is associated with higher rates of clinical, CFR and endoscopic remission than later treatment |
| Frei et al.23 | Observational | CD patients | 10 years | ⩽2 years since diagnosis | Early anti-TNF group (infliximab, adalimumab, or certolizumab pegol; n = 246); late anti-TNF group (n = 696) | Stenosis, perianal fistula, any fistula, perianal surgery, intestinal surgery, extraintestinal manifestations | Early anti-TNF treatment was associated with reduced risks of bowel stenosis, osteoporosis, and anemia |
| Panaccione et al.29 | RCT – post-hoc analysis | Moderate-to-severe CD (CDAI 220–450 or HBI ⩾ 7 | 52/56 weeks | Four different subgroups of disease duration: <1 year; ⩾1–<2 years; ⩾2–⩽5 years; and > 5 years | Placebo (n = 263), or adalimumab (160 mg, followed by 80 mg and then 40 mg eow, n = 377), followed by maintenance therapy with adalimumab | Clinical remission defined as CDAI < 150; clinical response defined: CR-70 and CR-100 | Induction – highest remission rates in patients with shortest disease duration (45.8% if disease duration < 12 months, 31.0% for 12–24 months, 23.1% for 24–60 months); similarly, response rates were highest in the < 1 year subgroup. Maintenance – patients with < 1 year disease duration had the highest remission rates (p = 0.002) |
| Loftus et al.24 | Observational | Moderately to severely active CD with or without prior adalimumab experience | 6 years | ⩽2 years since diagnosis | Adalimumab (160 mg, 80 mg and then 40 mg eow) (n = 2057) | Physician’s Global Assessment, clinical remission (HBI < 5), SIBDQ | Patients with early disease achieved numerically higher HBI remission rates than patients with longer disease duration (84% versus 68%) |
| Zhu et al.40 | Observational | CD patients | Median follow-up 17 months | ⩽18 months since diagnosis, no previous treatment with disease-modifying agents | Infliximab (5 mg/kg) + azathioprine (1.5 or 2.0 mg/kg/d) (n = 154) | Lémann index, based on MRE; predictors of short-term bowel resection | ‘Early therapy’ group had higher decrease in the Lémann Index (61.4% versus 42.9%) as well as lower increase (20% versus 35.7%); drug therapy reversed bowel damage to a greater extent in early CD patients |
| Jung et al.41 | Observational | CD patients | 5–7 years | ⩽1 year since diagnosis | Infliximab (n = 410) or adalimumab (n = 199) for at least 6 months | Abdominal surgery, CD-related ER visit or hospitalization, new CCT use | Late anti-TNF initiation associated with an increased risk of surgery (HR: 1.64; 95% CI: 1.05–2.55) and was associated with increased risk of ER visit (HR: 1.38, 95% CI: 0.99–1.94) |
| Ungaro et al.25 | Observational | Moderate to severe CD (CDAI of 150–450) | 3 years | ⩽2 years since diagnosis | Tight control (n = 61) versus conventional management (n = 61) (adalimumab 160 mg at week 0, 80 mg at week 2 and then 40 mg eow) | Deep remission (CD endoscopic index of severity scores below 4, with no deep ulcerations or CCT treatment, for 8 or more weeks) | Induction of deep remission in early CD with decreased risk of disease progression over a median time of 3 years, regardless of tight control or conventional management strategy |
5-ASA, 5-aminosalicylic acid; 95% CI, 95% confidence interval; CCT, corticosteroid; CD, Crohn’s disease; CDAI, Crohn’s Disease Activity Index; CDEIS, Crohn’s Disease Endoscopic Index of Severity; CFR, corticosteroid-free remission; CRP, C-reactive protein; eow, every other week; ER, emergency room; FC, fecal calprotectin; HBI, Harvey Bradshaw Index; HR, hazard ratio; IQR, interquartile range; IS, immunosuppressants; MRE, magnetic resonance enterography; N, number; RCT, randomized controlled trials; SES-CD, Simple Endoscopic Score for CD; SIBDQ, Short Inflammatory Bowel Disease Questionnaire; TNF, tumor necrosis factor; Treg, regulatory T-cells.
Ulcerative colitis
The main differences between UC and CD are related to bowel involvement, disease progression, and response to therapeutic intervention.42 However, as CD, UC is also a progressive disease, and its standard therapeutic approach has been questioned. It has been hypothesized that controlling the disease in earlier stages would be the most adequate approach to manage its progression. Identifying the disease in early stages allows taking advantage of the pathophysiological ‘window of opportunity’, in which distinct treatment interventions may potentially modify the natural history of the disease and reach better outcomes. Yet, unlike CD, the concept of ‘early UC’ is still to be clarified. According to the available literature, summarized in Table 2, the early disease definition for UC is variable. In four published studies22,43–45 and one ongoing trial,46 the cutoff to define early disease was 2 years or less since diagnosis. However, other studies have stretched the time range to 318,47 or even 548,49 years. A Canadian study45 evaluating early anti-tumor necrosis factor (TNF) treatment verified that the impact of the timing of anti-TNF therapy, on long-term outcomes of UC patients, was not significant. This trend was also confirmed in a Korean study.44 Information on the immune profile, including cytokine patterns, could provide a more solid basis for a clearer definition of ‘early disease’ in UC.
Table 2.
Evidence regarding therapeutic intervention on early UC.
| Authors | Study type | Patients’ characteristics | Follow-up | Early disease/therapy definition | Intervention/exposure | Outcomes | Main results |
|---|---|---|---|---|---|---|---|
| Oussalah et al.50 | Observational | UC patients without prior anti-TNF exposure | 18 months | ⩽50 months since diagnosis | Infliximab (n = 191) | Predictors of short- and long-term outcomes of infliximab | Short duration at infliximab initiation predicts first hospitalization (HR = 2.14, 95% CI = 1.25–3.66, p = 0.006) |
| Mandel et al.47 | Observational | Clinical response to anti-TNF induction (>70 point decrease in CDAI, or > 3 point decrease in Mayo) | 8.5 years | ⩽3 years since diagnosis | Infliximab or adalimumab (n = 42) | Hospitalization rates and predictors | Hospitalization rate was not associated with time to therapy |
| Murthy et al.51 | Observational | Patients with active CCT-refractory or CCT-dependent UC | 5 years | No definition | Infliximab (n = 213) | Annual CFR (pMayo score of 0 without systemic CCT); infliximab failure; colectomy | Longer disease was associated with higher odds of 1-year SFR; lesser risks of infliximab failure and colectomy (OR = 2.1, p = 0.061; HR = 0.59, p = 0.0198, HR = 0.49, p = 0.048, respectively) |
| Ma et al.18 | Observational | UC patients with primary response to induction therapy (decrease in partial Mayo score > 2 points) | 16 weeks | ⩽3 years since diagnosis | Infliximab (n = 78) or adalimumab (n = 37) | Colectomy; UC-related hospitalization; secondary loss of response | Earlier treatment does not prevent hospitalization, colectomy, or secondary loss of response. Early initiators versus late initiators: 6:100 versus 2.7:100 people/year, p = 0.13; 43.9% versus 27.6%, p = 0.7; 49.1% versus 58.6%, p = 0.31 |
| Faleck et al.22 | Observational | Active clinical symptoms attributed to UC before starting vedolizumab | 6 months | ⩽2 years since diagnosis | Vedolizumab (n = 437) | Clinical remission rates; CFR; endoscopic remission | Disease duration does not associate with response to vedolizumab in patients with UC. Early-stage versus late-stage CD: HR = 1.59, p < 0.20; HR = 3.39, p < 0.20; HR = 1.90, p < 0.20 |
| Nguyen et al.43 | Single-center retrospective cohort study | Biologic treated patients with UC | 24 months | ⩽2 years since diagnosis | New exposure to TNFα antagonists (infliximab, adalimumab or golimumab) or vedolizumab n = 160 | Time to treatment failure | Each 1-year increase in disease duration was associated with a 5% lower risk of treatment failure (HR = 0.95; 95% CI, 0.91–0.99) |
| Han et al.44 | Observational | UC subjects treated with anti-TNF for more than 6 months | Not available | ⩽2 years | Anti-TNF therapy [infliximab (n = 420), adalimumab (n = 242), golimumab (n = 36)] | Colectomy, ER visit, hospitalization, new steroid use | No significant differences in the risk of colectomy (HR = 0.41; 95% CI, 0.04–3.90), ER visits (HR = 0.98; 95% CI, 0.50–1.92), hospitalization (HR = 0.76; 95% CI, 0.57–1.01), and corticosteroid use (HR = 1.04; 95% CI, 0.71–1.50) between early and late initiators of anti-TNF therapy |
| Kariyawasam et al.48 | Cohort study | UC subjects treated with thiopurines | Not available | ⩽5 years since diagnosis | Early thiopurine maintenance (azathioprine or mercaptopurine for at least 6 months) n = 982 | Colectomy rate and endoscopic proximal disease extension | Decreased colectomy (HR = 0.10, p = 0.002) and proximal progression of disease extent (HR = 0.26; 95% CI, 0.10–0.78; p = 0.015) |
| Targownik et al.45 | Observational | New diagnosis of IBD | 5 years | ⩽2 years | Anti-TNF therapy (n = 318) | Hospitalization and surgery | There was no impact of the timing of anti-TNF therapy on the rates of hospitalization and surgery |
| D’Amico et al.49 | Retrospective cohort study | Diagnosis of UC for at least 6 months | 24 months (median) | 5 years | Impact of disease clearance on long-term negative outcomes | Disease clearance: simultaneous clinical (pMayo score ⩽2), endoscopic (pMayo score = 0), and histological (Nancy = 0) remission | Early disease clearance had significant lower risk for hospitalization and surgery. Patients who achieved disease clearance had a shorter duration of disease than those without (5 versus. 9 years p < 0.001). |
| SPRINT study group | Phase IV RCT, EudraCT number: 2020-003420-16 | Patients with moderate to severe UC | 52 weeks | ⩽2 years since diagnosis | Top-down approach (starting treatment with adalimumab) versus standard step-up approach | Histological improvement, no colectomy and, no UC-related hospitalization; histological remission and histological healing in the short term (week 16) | Ongoing |
95% CI, 95% confidence interval; CCT, corticosteroid; CFR, corticosteroid-free remission; ER, emergency room; HR, hazard ratio; N, number; RCT, randomized controlled trials; TNF, tumor necrosis factor; UC, ulcerative colitis.
Immunological mechanisms underlying early IBD pathogenesis
Crohn’s disease
Accumulating evidence supports that early CD pathogenesis relies on a ‘permeability-to-inflammation’ pathway, with a ‘defective-to-overactive’ imbalanced immune response to stimuli. In early disease phases, following the activation of innate immunity, mucosal T cells mount a Th1 response, with higher production of IL-12,52 interferon-gamma (IFN γ), TNF-α,1,53 as well as higher expression of IL-12 receptor b2 (IL-12Rb2) chain, resembling an acute infectious process. The reverse seems to occur in late CD, in which IL-12Rb2 chain expression and IL-12-induced IFN-γ production are decreased. Indeed, later in the disease course, the immunological background changes, with a shift toward a strong Th2 response1 and higher expression of IL-23, IL-33, IL-13, IL-5, and IL-17 (whose production is mediated by IL-23).53 Besides this ‘T-cell signature’, the dysregulation of microRNAs has also been reported to play a role in early disease phases.54 A study on gene and microRNA expression profiles in ileal mucosal biopsies from CD patients showed that microRNA dysregulation was more relevant in post-operative recurrent CD and newly diagnosed patients, suggesting an important role in the early stage of CD. Folate Hydrolase 1 emerged as the most dysregulated gene either in newly diagnosed CD or postoperative recurrent CD.54
Ulcerative colitis
It has been proposed that the immunopathogenesis of UC changes during the disease course, with a transition from a Th1- in early UC into a Th2-driven disease later in disease course.55,56,57. Also, higher mucosal mRNA expression of IL-23 has been detected in newly diagnosed patients, in comparison with those with longstanding disease.55 As the disease evolves, other cells such as Th-17, Th-9 (possibly due to the polarization of naïve T cells in the presence of IL-4 and transforming growth factor beta), and other cytokines like IFN γ, IL-17A, and IL-955,58 may increase. The expression of genes associated with T cell differentiation has also been postulated to vary; TNF-α and suppressor of cytokine signaling 1 expression are increased during early disease, while IL-4 receptor, growth factor independence 1, IL-1 receptor like 1, peroxisome proliferator-activated receptor gamma, and IL-5 expression are elevated later during UC course.55,57
Early intervention and impact on prognosis
Crohn’s disease
The acknowledgment of ‘early disease’ is the key to a prompt therapeutic action. The rationale for treating early disease, using a treat-to-target approach, is based on the evolving immunological milieu, and on the accumulating damage and disability associated with later CD stages. Throughout the years, several authors have evaluated the impact of targeting early CD on disease outcomes (Table 1). Most of the available evidence comes from the use of anti-TNF drugs, either alone (2212–21,23–25,29,31,32,34,35,36,37,38,41 out of 29 studies) or in combination with immunosuppressants (77,17,30,34,38,39,40); the single use of immunosuppressants was evaluated in five studies,17,21,28,33,38 while vedolizumab was studied once.22 Although ‘early disease’ definition and the outcomes sought varied widely, data from observational studies and from post-hoc analysis of RCTs have shown that patients who received therapy in the early stage of disease have better outcomes than those who received treatment later. Short-term and medium-term outcomes were evaluated in nearly all studies regarding early CD management. In general, individual studies suggested that prompt medical intervention may improve the possibility of reaching clinical response and/or remission,7,13,14,19,20,22,24,29,30,32,35,36,38 reduce inflammatory biomarkers (C-reactive protein and fecal calprotectin),20 and increase endoscopic response and/or remission.15,20,22,25 Also, in a recent meta-analysis,26 which pooled individual patient data from 16 RCT evaluating biologic-treated CD patients, disease duration impacted the rate of clinical remission (odds ratio = 0.75; 95% confidence interval = 0.61–0.92) in patients with a diagnosis more than 18 months prior treatment start; yet, the placebo effect was also higher in that subset of patients. On the other hand, conflicting results were reported twice; Sandborn et al.12 did not find acceptable clinical outcomes in patients who started anti-TNF in the first 2 years since diagnosis, while Nuij et al.49 described that early anti-TNF treatment was not associated with higher mucosal healing. These discrepancies may be due to the low number of patients in the ‘early’ groups, as well as to inappropriate selection of patients for therapy, which may have led to suboptimal treatment and outcomes. The meta-analysis published by Ben-Horin et al.26 did not find an association between early therapeutics with biologics and higher reduction in C-reactive therapy. In addition, these authors were not able to define a specific time point on CD history that could clearly impact the proportional biologic/placebo risk for remission (defined as the treatment effect), suggesting that disease progression may be influenced more strongly by other variables besides disease duration.
Concerning long-term outcomes, early introduction of immunosuppressants17,21,28,33,39 or anti-TNF drugs17,18,21,39,44 was associated with a significant reduction in the probability of requiring CD-related surgeries. Also, patients receiving treatment early in disease course had fewer disease-related complications17,21,39 and need for hospitalization.14,21,41 Besides that, some authors have stated that intervention within 18 months after CD diagnosis may prevent and even reverse bowel damage.40 On the other hand, the impact of early treatment on patients’ quality of life, work productivity, and disability remains largely unexplored.
Postoperative recurrence is a setting that mimics early CD. According to the literature, up to 90% of the patients experience recurrence within 1 year of surgical removal of all macroscopically identifiable disease.59 Although no accurate biomarkers of recurrence exist, some studies have described the association with higher preoperative C-reactive protein levels and neutrophil-to-lymphocyte60 ratio, while others highlight the role of postoperative fecal calprotectin.61 Despite this, the relevance of postoperative prophylactic therapy in high-risk patients (e.g. several CD-related surgeries, smoking habits, penetrating disease) is well stated, as is the need for early screening colonoscopy after 1 year, in which evidence of macroscopic disease (Rutgeerts score of i2 or more) usually prompts therapeutic institution or escalation.62 The drugs usually recommended as prophylactic therapy are thiopurines and/or anti-TNF. Although the body of evidence is relatively small, the prophylactic use of anti-TNF has been reported to prevent clinical and endoscopic recurrence in the first 2 years, yet the effect on long-term outcomes remains elusive.63 The immunological mechanisms underlying recurrence after macroscopic resetting are still being investigated but may involve upregulation of several genes (TNF-α, IFNγ, IL23A, and IL17A) and pathways (mitochondrial dysfunction and JAK-STAT).64
Ulcerative colitis
Little evidence is available regarding early intervention in UC. The existing data (Table 2) derive from retrospective observational studies, which evaluated different endpoints (rate of colectomy, secondary loss response, UC-related hospitalization, among others), at diverse time points. Overall, these studies18,22,44,45,47,50,51 failed to identify the differences in treatment response and prognosis, in patients receiving early therapeutic interventions, mostly biologics. The effect of aminosalicylates in early versus non-early disease has not been explored. Furthermore, a retrospective cohort study43 showed that shorter disease duration is independently associated with increased risk of treatment failure in biologic-treated UC patients. On the other hand, biologic therapy early in the course of the disease was a negative prognostic marker. Likewise, another retrospective study observed that disease duration was not associated with response to vedolizumab in patients with UC.22 In line with this, a recent meta-analysis,26 which explored early biologic treatment (⩽18 months), concluded that early treatment of UC patients was not associated with higher rates of clinical remission after induction therapy. Some individual studies have analyzed long-term outcomes,2,18,47,48,50 such as hospitalization and surgery. However, once again, earlier treatment did not have a positive impact. Although most of these assumptions are not encouraging regarding the benefit of early interventions in UC, considering the retrospective nature of the studies, the conclusions to be drawn are limited. In this context, prospective studies comparing outcomes from patients treated at different time points, after UC diagnosis (early versus late disease) would provide relevant information for more generalized conclusions.
Tools to estimate disease progression
Crohn’s disease
Considering that preventing structural changes is the cornerstone of CD therapeutics, the introduction of objective tools to quantify that damage is of utmost importance. The Lémann index (Crohn’s Disease Digestive Damage Score), which incorporates clinical, endoscopic, imaging, and surgical features from all segments of the digestive tract, was the first and unique instrument to do so.65 This index, whose score ranges from 0 to 140, considers damage location (upper tract, small bowel, colon/rectum, and anus), extent (each organ is divided into several segments, totalizing 30), severity (grade 0–3) and reversibility (concerning stricturing, penetrating and perianal lesions), and was recently validated.66 This index is usually calculated using data from magnetic resonance imaging and colonoscopy, yet recent preliminary studies have shown that bowel ultrasound may be a reliable alternative for transmural evaluation.67 Since its development, several reports have explored its ability to predict long-term disability. Gradual elevations in the Lémann index have been reported to occur in at least 60% of the patients in the first decade after diagnosis,68 being associated with a significant increase in need for steroids and healthcare utilization, including surgeries and hospitalization.69 Likewise, Lémann index at diagnosis has been reported to be a reliable predictor of the risk of abdominal surgery in the first years after diagnosis.70 On the other hand, Lémann index’s responsiveness to therapeutic interventions was evaluated in less and smaller studies,71,72 requiring further confirmation. In a study of 30 patients treated with anti-TNF, a Lémann index score of 4.8 was the best cutoff to define the presence of bowel damage, while receiving this therapeutic agent was effective in stopping the progression of bowel damage in 83% of the subjects.71 In another study enrolling 35 patients under anti-TNF, the early introduction of this biologic was associated with lower bowel damage scores.73 Although this score has some limitations, particularly its complexity and need for trained gastroenterologists and radiologists, it has good predictive performance and should be incorporated in all disease-modification CD trials as a secondary endpoint, as pointed out by the SPIRIT initiative.66 Conversely, its application into routine clinical practice will rely on further simplification or widespread training initiatives.
Ulcerative colitis
To date, as far as the authors know, there are no indexes to quantify colonic damage associated with UC. Some tools have been developed for cross-sectional imaging in UC (e.g. Tsuga’s colorectal ultrasound criteria, and a simplified magnetic resonance colonography index), but these tools lack validated cutoff values for extent of disease severity, being their use very restricted and not validated.74
Future perspectives
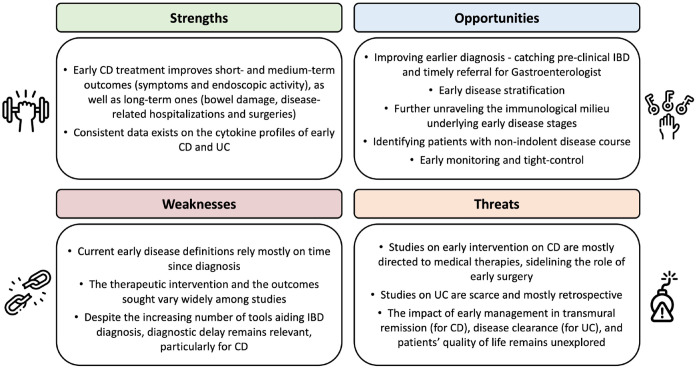
The strengths and weaknesses of the current evidence on early IBD, the anticipated treats and opportunities are summarized in Figure 3. The definition of early disease based not only on time, but also on immunological characteristics is critical to guide therapeutic decisions, particularly in CD, in which the impact of prompt therapy on prognosis has been described. However, to avoid overtreatment, it is also essential to identify the patients with early disease that will present an indolent course. Indeed, considering that up to 20% of the CD patients will present a ‘benign’ and non-progressive disease course, it is important to select biomarkers to recognize the patients that would benefit from close monitoring and, if needed, from an accelerated ‘step-up’ approach, rather than early therapeutic ‘top-down’ intervention. In a recent study, Yanai et al.75 demonstrated that routine measurements, such as body mass index, vitamin B12, white blood cells count, and alanine aminotransferase, at the time of diagnosis may predict the likelihood of an indolent course with a 90% accuracy, on the following 12 months. The validation of this model in longer follow-ups is pending. Also, it remains unknown whether drug de-escalation shall be considered in CD patients that started treatment in early disease stages; the results of ongoing trials, like the CURE trial (NCT03306446) may shed some light on this topic.
Figure 3.
Strengths and weaknesses of current evidence on early management of IBDs, anticipated opportunities, and threats (SWOT analysis).
IBDs, inflammatory bowel diseases.
Concerning UC, the concept of early disease is less defined and the evidence of the benefits of early therapeutic interventions is very scarce and pertains, in general, to retrospective studies. The available evidence shows no relevant benefit of early treatment in UC patients. It is expected that upcoming studies, such as the SPRINT (ongoing randomized multicenter study, EudraCT number: 2020-003420-16), which will compare the efficacy of top-down and step-up approaches in UC patients diagnosed up to 2 years and followed-up for 3 years, will bring relevant data on this regard. Also, it remains to be clarified whether early treatment impacts more ambitious UC targets, such as ‘disease clearance’, which combines clinical, endoscopic, and histological remission.2,76
Conclusions
This scoping review summarizes the definition, pathophysiology, and data on early therapeutic intervention in patients with CD and UC, discussing its strengths and limitations. Since 2008, the interest in early CD increased, and continuous efforts to uniformize its definition resulted in the publication, in 2012, of the Paris consensus, which incorporates the concept of ‘time since diagnosis’ as well as medication data. However, the adherence to this definition has been modest. In fact, more than half of the studies published thereafter still define early CD studies only in a time-based fashion (disease diagnosed up to 2 years). Regarding UC, the definition of early disease is still not consensual. Although definitions are strictly related to time since diagnosis, it must be stressed that ‘true’ early disease comes from an early diagnosis free from diagnostic delay.
From a pathophysiological point of view, the expression profile of several cytokines is different in early phases of disease. Particular attention shall be devoted to IL-12, IFN γ, TNF-α (in the case of CD), and IL-23 (for UC). Therefore, further studies on cytokines profile may help to clarify definitions from an immunological perspective.
Overall, the available evidence supports that recognizing earlier stages of CD and adopting an early therapeutic intervention improves symptoms’ control, endoscopic activity (short- and medium-term outcomes), and reduces bowel disability, disease-related hospitalizations, and surgeries (long-term outcomes). However, these trends cannot be extended to UC, as, to date, no comparative studies have shown that prompt treatment impacts patients’ prognosis. Long-term high-quality RCTs are needed to further clarify these aspects. Also, moving the focus away from medical therapy, it is important to acknowledge that, for certain cases, early surgery may be the first therapeutic option, offering the best outcomes for a particular patient, either in short term or in long term.
This scoping review has some limitations; first, different ‘early disease’ definitions have been applied by the included studies; second, the therapeutic interventions and the outcomes sought varied widely; third, no formal assessments of literature quality were performed. Further studies are needed to explore the immunological signature of early disease, which will guide the identification of biomarkers to predict non-indolent progressive disease that may benefit from a ‘watch-and-wait’ strategy (particularly in CD). Immunological data may also be crossed with other patient-related characteristics with influence on disease progression, guiding decision-making and, gradually, stepping into tailored treatment schemes for each patient’s profile.
Acknowledgments
The authors would like to thank Paula Pinto, PharmD, PhD (PMA – Pharmaceutical Medicine Academy) for providing medical writing and editorial assistance.
Footnotes
ORCID iD: Fernando Magro  https://orcid.org/0000-0003-2634-9668
https://orcid.org/0000-0003-2634-9668
Contributor Information
Maria Manuela Estevinho, Department of Gastroenterology, Vila Nova de Gaia Espinho Hospital Center, Vila Nova de Gaia, Portugal; Department of Biomedicine, Unit of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto, Porto, Portugal.
Paula Leão Moreira, Unidade de Farmacologia Clínica, São João Hospital University Centre, Porto, Portugal.
Isabel Silva, Unidade de Farmacologia Clínica, São João Hospital University Centre, Porto, Portugal.
João Laranjeira Correia, Department of Gastroenterology, Vila Nova de Gaia Espinho Hospital Center, Vila Nova de Gaia, Portugal.
Mafalda Santiago, Portuguese Group of Studies in Inflammatory Bowel Disease (Grupo de Estudos da Doença Inflamatória Intestinal - GEDII), Porto, Portugal; Center for Health Technology and Services Research (CINTESIS), Porto, Portugal.
Fernando Magro, Department of Biomedicine, Unit of Pharmacology and Therapeutics, Faculty of Medicine, University of Porto, Rua Dr. Plácido Costa, 3, Porto 4200-450, Portugal; Unidade de Farmacologia Clínica, São João Hospital University Centre, Porto, Portugal; Center for Health Technology and Services Research (CINTESIS), Porto, Portugal; Department of Gastroenterology, São João Hospital University Centre, Porto, Portugal.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contribution(s): Maria Manuela Estevinho: Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Paula Leão Moreira: Formal analysis; Methodology; Writing – original draft.
Isabel Silva: Formal analysis; Investigation; Writing – original draft.
João Laranjeira Correia: Investigation; Writing – original draft.
Mafalda Santiago: Methodology; Visualization; Writing – review & editing.
Fernando Magro: Conceptualization; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
FM served as a speaker and received honoraria from Abbvie, Biogen, Falk, Ferring, Hospira, Janssen, Laboratórios Vitoria, Lilly, Pfizer, Merck Sharp & Dohme, Sandoz, Takeda, UCB, and Vifor. HTS received fees for serving as a speaker for Takeda, AbbVie, Janssen, Pfizer, Ferring, and Biogen. The other authors have no conflicts of interest to disclose.
Availability of data and materials: Available at reasonable request to the corresponding author.
References
- 1. Bamias G, Cominelli F. Exploring the early phase of Crohn’s disease. Clin Gastroenterol Hepatol 2021; 19: 2469–2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Solitano V, D’Amico F, Zacharopoulou E, et al. Early intervention in ulcerative colitis: ready for prime time? J Clin Med 2020; 9: 2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Louis E. Epidemiology of the transition from early to late Crohn’s disease. Dig Dis 2012; 30: 376–379. [DOI] [PubMed] [Google Scholar]
- 4. Fiorino G, Danese S. Diagnostic delay in Crohn’s disease: time for red flags. Dig Dis Sci 2016; 61: 3097–3098. [DOI] [PubMed] [Google Scholar]
- 5. Chateau T, Peyrin-Biroulet L. Evolving therapeutic goals in Crohn’s disease management. United European Gastroenterol J 2020; 8: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. [DOI] [PubMed] [Google Scholar]
- 7. D’Haens G, Baert F, van Assche G, et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: an open randomised trial. Lancet 2008; 371: 660–667. [DOI] [PubMed] [Google Scholar]
- 8. Colombel JF, Sandborn WJ, Reinisch W, et al. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med 2010; 362: 1383–1395. [DOI] [PubMed] [Google Scholar]
- 9. Peyrin-Biroulet L, Loftus EV, Colombel JF, et al. Early Crohn disease: a proposed definition for use in disease-modification trials. Gut 2010; 59: 141–147. [DOI] [PubMed] [Google Scholar]
- 10. Peyrin-Biroulet L, Billioud V, D’Haens G, et al. Development of the Paris definition of early Crohn’s disease for disease-modification trials: results of an international expert opinion process. Am J Gastroenterol 2012; 107: 1770–1776. [DOI] [PubMed] [Google Scholar]
- 11. Ben-Horin S, Zhao Y, Guo J, et al. Efficacy of biological drugs in short-duration versus long-duration inflammatory bowel disease: a protocol for a systematic review and an individual-patient level meta-analysis of randomised controlled trials. BMJ Open 2019; 9: e024222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sandborn WJ, Abreu MT, D’Haens G, et al. Certolizumab pegol in patients with moderate to severe Crohn’s disease and secondary failure to infliximab. Clin Gastroenterol Hepatol 2010; 8: 688–695.e2. [DOI] [PubMed] [Google Scholar]
- 13. Schreiber S, Colombel JF, Bloomfield R, et al. Increased response and remission rates in short-duration Crohn’s disease with subcutaneous certolizumab pegol: an analysis of PRECiSE 2 randomized maintenance trial data. Am J Gastroenterol 2010; 105: 1574–1582. [DOI] [PubMed] [Google Scholar]
- 14. Schreiber S, Reinisch W, Colombel JF, et al. Subgroup analysis of the placebo-controlled CHARM trial: increased remission rates through 3 years for adalimumab-treated patients with early Crohn’s disease. J Crohns Colitis 2013; 7: 213–221. [DOI] [PubMed] [Google Scholar]
- 15. Colombel JF, Rutgeerts PJ, Sandborn WJ, et al. Adalimumab induces deep remission in patients with Crohn’s disease. Clin Gastroenterol Hepatol 2014; 12: 414–422.e5. [DOI] [PubMed] [Google Scholar]
- 16. Kotze PG, Ludvig JC, Teixeira FV, et al. Disease duration did not influence the rates of loss of efficacy of the anti-TNF therapy in Latin American Crohn’s disease patients. Digestion 2015; 91: 158–163. [DOI] [PubMed] [Google Scholar]
- 17. Safroneeva E, Vavricka SR, Fournier N, et al. Impact of the early use of immunomodulators or TNF antagonists on bowel damage and surgery in Crohn’s disease. Aliment Pharmacol Ther 2015; 42: 977–989. [DOI] [PubMed] [Google Scholar]
- 18. Ma C, Beilman CL, Huang VW, et al. Anti-TNF therapy within 2 years of Crohn’s disease diagnosis improves patient outcomes: a retrospective cohort study. Inflamm Bowel Dis 2016; 22: 870–879. [DOI] [PubMed] [Google Scholar]
- 19. Ogata H, Watanabe M, Matsui T, et al. Safety of adalimumab and predictors of adverse events in 1693 Japanese patients with Crohn’s disease. J Crohns Colitis 2016; 10: 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Colombel JF, Panaccione R, Bossuyt P, et al. Effect of tight control management on Crohn’s disease (CALM): a multicentre, randomised, controlled phase 3 trial. Lancet 2017; 390: 2779–2789. [DOI] [PubMed] [Google Scholar]
- 21. Oh EH, Oh K, Han M, et al. Early anti-TNF/immunomodulator therapy is associated with better long-term clinical outcomes in Asian patients with Crohn’s disease with poor prognostic factors. PLoS One 2017; 12: e0177479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Faleck DM, Winters A, Chablaney S, et al. Shorter disease duration is associated with higher rates of response to vedolizumab in patients with Crohn’s disease but not ulcerative colitis. Clin Gastroenterol Hepatol 2019; 17: 2497–2505.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frei R, Fournier N, Zeitz J, et al. Early initiation of anti-TNF is associated with favourable long-term outcome in Crohn’s disease: 10-year-follow-up data from the swiss IBD cohort study. J Crohns Colitis 2019; 13: 1292–1301. [DOI] [PubMed] [Google Scholar]
- 24. Loftus EV, Reinisch W, Panaccione R, et al. Adalimumab effectiveness up to six years in adalimumab-naïve patients with Crohn’s disease: results of the PYRAMID registry. Inflamm Bowel Dis 2019; 25: 1522–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ungaro RC, Yzet C, Bossuyt P, et al. Deep remission at 1 year prevents progression of early Crohn’s disease. Gastroenterology 2020; 159: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ben-Horin S, Novack L, Mao R, et al. Efficacy of biologic drugs in short-duration versus long-duration inflammatory bowel disease: a systematic review and an individual-patient data meta-analysis of randomized controlled trials. Gastroenterology 2022; 162: 482–494. [DOI] [PubMed] [Google Scholar]
- 27. Dias CC, Rodrigues PP, Coelho R, et al. Development and validation of risk matrices for Crohn’s disease outcomes in patients who underwent early therapeutic interventions. J Crohn’s Colitis 2016; 11: 445–453. [DOI] [PubMed] [Google Scholar]
- 28. Ramadas AV, Gunesh S, Thomas GA, et al. Natural history of Crohn’s disease in a population-based cohort from Cardiff (1986–2003): a study of changes in medical treatment and surgical resection rates. Gut 2010; 59: 1200–1206. [DOI] [PubMed] [Google Scholar]
- 29. Panaccione R, Löfberg R, Rutgeerts P, et al. Efficacy and safety of adalimumab by disease duration: analysis of pooled data from Crohn’s disease studies. J Crohns Colitis 2019; 13: 725–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baert F, Moortgat L, Van Assche G, et al. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn’s disease. Gastroenterology 2010; 138: 463–468. [DOI] [PubMed] [Google Scholar]
- 31. Kato K, Fukunaga K, Kamikozuru K, et al. Infliximab therapy impacts the peripheral immune system of immunomodulator and corticosteroid naïve patients with Crohn’s disease. Gut Liver 2011; 5: 37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rubin DT, Uluscu O, Sederman R. Response to biologic therapy in Crohn’s disease is improved with early treatment: an analysis of health claims data. Inflamm Bowel Dis 2012; 18: 2225–2231. [DOI] [PubMed] [Google Scholar]
- 33. Lakatos PL, Golovics PA, David G, et al. Has there been a change in the natural history of Crohn’s disease? Surgical rates and medical management in a population-based inception cohort from Western Hungary between 1977–2009. Am J Gastroenterol 2012; 107: 579–588. [DOI] [PubMed] [Google Scholar]
- 34. Feagan BG, McDonald JW, Panaccione R, et al. Methotrexate in combination with infliximab is no more effective than infliximab alone in patients with Crohn’s disease. Gastroenterology 2014; 146: 681–688.e1. [DOI] [PubMed] [Google Scholar]
- 35. Peters CP, Eshuis EJ, Toxopeüs FM, et al. Adalimumab for Crohn’s disease: long-term sustained benefit in a population-based cohort of 438 patients. J Crohns Colitis 2014; 8: 866–875. [DOI] [PubMed] [Google Scholar]
- 36. Juillerat P, Sokol H, Froehlich F, et al. Factors associated with durable response to infliximab in Crohn’s disease 5 years and beyond: a multicenter international cohort. Inflamm Bowel Dis 2015; 21: 60–70. [DOI] [PubMed] [Google Scholar]
- 37. Nuij V, Fuhler GM, Edel AJ, et al. Benefit of earlier anti-TNF treatment on IBD disease complications? J Crohns Colitis 2015; 9: 997–1003. [DOI] [PubMed] [Google Scholar]
- 38. Colombel JF, Reinisch W, Mantzaris GJ, et al. Randomised clinical trial: deep remission in biologic and immunomodulator naïve patients with Crohn’s disease - a SONIC post hoc analysis. Aliment Pharmacol Ther 2015; 41: 734–746. [DOI] [PubMed] [Google Scholar]
- 39. Khanna R, Bressler B, Levesque BG, et al. Early combined immunosuppression for the management of Crohn’s disease (REACT): a cluster randomised controlled trial. Lancet 2015; 386: 1825–1834. [DOI] [PubMed] [Google Scholar]
- 40. Zhu M, Feng Q, Xu X, et al. Efficacy of early intervention on the bowel damage and intestinal surgery of Crohn’s disease, based on the Lémann index. BMC Gastroenterol 2020; 20: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jung YS, Han M, Park S, et al. Impact of early anti-TNF use on clinical outcomes in Crohn’s disease: a nationwide population-based study. Korean J Intern Med 2020; 35: 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Finkelstein SD, Sasatomi E, Regueiro M. Pathologic features of early inflammatory bowel disease. Gastroenterol Clin North Am 2002; 31: 133–145. [DOI] [PubMed] [Google Scholar]
- 43. Nguyen NH, Kurnool S, Dulai PS, et al. Short disease duration is associated with increased risk of treatment failure in biologic-treated patients with ulcerative colitis. Inflamm Bowel Dis 2020; 26: 1429–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han M, Jung YS, Cheon JH, et al. Similar clinical outcomes of early and late anti-TNF initiation for ulcerative colitis: a nationwide population-based study. Yonsei Med J 2020; 61: 382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Targownik LE, Bernstein CN, Benchimol EI, et al. Earlier anti-TNF initiation leads to long-term lower health care utilization in Crohn’s disease but not in ulcerative colitis. Clin Gastroenterol Hepatol 2022; 20: 2607–2618.e14. [DOI] [PubMed] [Google Scholar]
- 46. Register ECT. SPRINT, https://www.clinicaltrialsregister.eu/ctr-search/trial/2020-003420-16/ES2022 (2022, accessed 20 July 2022).
- 47. Mandel MD, Balint A, Golovics PA, et al. Decreasing trends in hospitalizations during anti-TNF therapy are associated with time to anti-TNF therapy: results from two referral centres. Dig Liver Dis 2014; 46: 985–990. [DOI] [PubMed] [Google Scholar]
- 48. Kariyawasam VC, Mourad FH, Mitrev N, et al. Early thiopurine maintenance is associated with reduced proximal disease progression and colectomy rate in ulcerative colitis. Eur J Gastroenterol Hepatol 2021; 33: 1524–1532. [DOI] [PubMed] [Google Scholar]
- 49. D’Amico F, Fiorino G, Solitano V, et al. Ulcerative colitis: impact of early disease clearance on long-term outcomes - a multicenter cohort study. United European Gastroenterol J 2022; 10: 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Oussalah A, Evesque L, Laharie D, et al. A multicenter experience with infliximab for ulcerative colitis: outcomes and predictors of response, optimization, colectomy, and hospitalization. Am J Gastroenterol 2010; 105: 2617–2625. [DOI] [PubMed] [Google Scholar]
- 51. Murthy SK, Greenberg GR, Croitoru K, et al. Extent of early clinical response to infliximab predicts long-term treatment success in active ulcerative colitis. Inflamm Bowel Dis 2015; 21: 2090–2096. [DOI] [PubMed] [Google Scholar]
- 52. Zorzi F, Monteleone I, Sarra M, et al. Distinct profiles of effector cytokines mark the different phases of Crohn’s disease. PLoS One 2013; 8: e54562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Danese S, Fiorino G, Peyrin-Biroulet L. Early intervention in Crohn’s disease: towards disease modification trials. Gut 2017; 66: 2179–2187. [DOI] [PubMed] [Google Scholar]
- 54. Verstockt S, De Hertogh G, Van der Goten J, et al. Gene and mirna regulatory networks during different stages of Crohn’s disease. J Crohns Colitis 2019; 13: 916–930. [DOI] [PubMed] [Google Scholar]
- 55. Mavroudis G, Magnusson MK, Isaksson S, et al. Mucosal and systemic immune profiles differ during early and late phases of the disease in patients with active ulcerative colitis. J Crohn’s Colitis 2019; 13: 1450–1458. [DOI] [PubMed] [Google Scholar]
- 56. Kałużna A, Olczyk P, Komosińska-Vassev K. The role of innate and adaptive immune cells in the pathogenesis and development of the inflammatory response in ulcerative colitis. J Clin Med 2022; 11: 400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nakase H, Sato N, Mizuno N, et al. The influence of cytokines on the complex pathology of ulcerative colitis. Autoimmunity Reviews 2022; 21(3): 103017. [DOI] [PubMed] [Google Scholar]
- 58. Shohan M, Sabzevary-Ghahfarokhi M, Bagheri N, et al. Intensified Th9 response is associated with the immunopathogenesis of active ulcerative colitis. Immunol Invest 2018; 47: 700–711. [DOI] [PubMed] [Google Scholar]
- 59. Hamilton AL, De Cruz P, Wright EK, et al. Non-invasive serological monitoring for Crohn’s disease post-operative recurrence. J Crohn’s Colitis. Epub ahead of print June 2022. DOI: 10.1093/ecco-jcc/jjac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mullin G, Zager Y, Anteby R, et al. Inflammatory markers may predict post-operative complications and recurrence in Crohn’s disease patients undergoing gastrointestinal surgery. ANZ J Surg 2022; 92: 2538–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wright EK, Kamm MA, De Cruz P, et al. Measurement of fecal calprotectin improves monitoring and detection of recurrence of Crohn’s disease after surgery. Gastroenterology 2015; 148: 938–947.e1. [DOI] [PubMed] [Google Scholar]
- 62. van Lent AU, D’Haens GR. Management of postoperative recurrence of Crohn’s disease. Digestive Diseases 2013; 31: 222–228. [DOI] [PubMed] [Google Scholar]
- 63. Uchino M, Ikeuchi H, Hata K, et al. Does anti-tumor necrosis factor alpha prevent the recurrence of Crohn’s disease? Systematic review and meta-analysis. J Gastroenterol Hepatol 2021; 36: 864–872. [DOI] [PubMed] [Google Scholar]
- 64. Ngollo M, Perez K, Hammoudi N, et al. Identification of gene expression profiles associated with an increased risk of post-operative recurrence in Crohn’s disease. J Crohns Colitis 2022; 16: 1269–1280. [DOI] [PubMed] [Google Scholar]
- 65. Kopylov U, Eliakim R. The lemann index—a glance through the window of opportunity? J Crohn’s Colitis 2016; 11: 261–262. [DOI] [PubMed] [Google Scholar]
- 66. Pariente B, Torres J, Burisch J, et al. Validation and update of the Lémann index to measure cumulative structural bowel damage in Crohn’s disease. Gastroenterology 2021; 161: 853–864.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Allocca M, Dell’Avalle C, Radice S, et al. P230 ultrasonography-based and magnetic resonance-based Lémann index: two sides of the same coin. J Crohn’s Colitis 2022; 16: i278–i279. [Google Scholar]
- 68. Gilletta C, Lewin M, Bourrier A, et al. Changes in the Lémann index values during the first years of Crohn’s disease. Clin Gastroenterol Hepatol 2015; 13: 1633–1640.e3. [DOI] [PubMed] [Google Scholar]
- 69. Rao BB, Koutroubakis IE, Ramos Rivers C, et al. Delineation of Crohn’s disease trajectories using change in Lémann index: a natural history study. J Clin Gastroenterol 2016; 50: 476–482. [DOI] [PubMed] [Google Scholar]
- 70. Liu W, Zhou W, Xiang J, et al. Lémann index at diagnosis predicts the risk of early surgery in Crohn’s disease. Dis Colon Rectum 2018; 61: 207–213. [DOI] [PubMed] [Google Scholar]
- 71. Fiorino G, Bonifacio C, Allocca M, et al. Bowel damage as assessed by the Lémann index is reversible on anti-TNF therapy for Crohn’s disease. J Crohns Colitis 2015; 9: 633–639. [DOI] [PubMed] [Google Scholar]
- 72. Panchal H, Wagner M, Chatterji M, et al. Earlier anti-tumor necrosis factor therapy of Crohn’s disease correlates with slower progression of bowel damage. Dig Dis Sci 2019; 64: 3274–3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lauriot Dit Prevost C, Azahaf M, Nachury M, et al. Bowel damage and disability in Crohn’s disease: a prospective study in a tertiary referral centre of the Lémann index and inflammatory bowel disease disability index. Aliment Pharmacol Ther 2020; 51: 889–898. [DOI] [PubMed] [Google Scholar]
- 74. Peyrin-Biroulet L, Panés J, Sandborn WJ, et al. Defining disease severity in inflammatory bowel diseases: current and future directions. Clin Gastroenterol Hepatol 2016; 14: 348–354.e17. [DOI] [PubMed] [Google Scholar]
- 75. Yanai H, Goren I, Godny L, et al. Early indolent course of Crohn’s disease in newly diagnosed patients is not rare and possibly predictable. Clin Gastroenterol Hepatol 2021; 19: 1564–1572.e5. [DOI] [PubMed] [Google Scholar]
- 76. Magro F, Alves C, Lopes J, et al. Histologic features of colon biopsies (geboes score) associated with progression of ulcerative colitis for the first 36 months after biopsy. Clin Gastroenterol Hepatol 2021; 19: 2567–2576.e9. [DOI] [PubMed] [Google Scholar]