Abstract
Background:
Oestrogen receptor positive, human epidermal growth factor receptor-2 (HER2) negative breast cancer (BC) is the most frequently diagnosed BC subtype. Combinations of cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) with anti-oestrogen therapy have led to improved survival compared with anti-oestrogen therapy alone for advanced/metastatic BC. The evaluation of CDK4/6i in the real-world facilitates treatment planning, insights into the incidence of drug toxicities, dose modifications including dose delays (DDs) and dose reductions (DRs) and improves prognostic accuracy in subgroups, for example geriatric patients, who are under-represented in clinical trials.
Methods:
This multi-centre study analysed retrospective and prospective data from 456 patients treated with CDK4/6i between January 2015 and December 2020. We examined patient characteristics, variation in prescribing practices, efficacy and toxicity outcomes.
Results:
In all, 456 patients were included in this study. The median age was 59 (range: 24–92). In total, 85 (19%) were ⩾70 years old. In all, 122 (27%) and 119 (26%) of patients were treated in the first-line and second-line settings, respectively. In total, 25 (5%), 31 (7%) and 145 (32%) of patients had brain, peritoneum and liver metastasis, respectively, at the time of CDK4/6i initiation. On univariate analysis, heavily pre-treated patients and those with distant metastases, involving the liver, brain or peritoneum, had significantly shorter progression-free survival (PFS) and 24-month overall survival (OS). Elderly patients (⩾70) had a shorter PFS; OS results were not mature. Majority of patients (n = 362, 80%) initiated treatment with the United States FDA-approved starting dose of CDK4/6i. In all, 330 (72%) had at least one DD and 217 (48%) patients required at least one DR, but these dose modifications were not associated with poorer survival outcomes. Patients age ⩾70 were more likely to require dose modifications leading to a lower treatment dose. The most common reason for DD/DR was neutropenia (60%) and the incidence of febrile neutropenia was only 2%.
Conclusions:
Our study indicates CDK4/6i is effective and safe. Age ⩾ 70, distant metastases to liver, peritoneal or brain were negative prognostic factors. Age ⩾ 70 was associated with significantly increased requirement for dose modification; however, this did not impact survival outcomes. These findings provide reassurance that survival outcomes are not adversely affected in elderly patients when DD/DR is indicated.
Keywords: advanced/metastatic breast cancer, Asia, CDK4/6 inhibitors, real world
Introduction
Breast cancer (BC) is the most frequently diagnosed cancer and the leading cause of cancer deaths in women.1 Over two-thirds of advanced BC patients have hormone receptor positive (HR+), human epidermal growth factor receptor 2 negative (HER2−) disease.2,3 Cyclin-dependent kinase 4/6 inhibitors (CDK4/6i) in combination with anti-oestrogen therapy is a cornerstone of therapy in the treatment of advanced/metastatic HR+, HER2− BC.4 The CDK4/6-Retinoblastoma pathway is a key regulator in the progression from G1 to S phase in a cell cycle. CDK4/6i target the CDK4/6 enzyme complex and disrupt cell cycle progression, preventing uncontrolled cellular proliferation.5,6
CDK4/6i currently approved in the treatment of advanced/metastatic HR+ HER2− BC include palbociclib, ribociclib and abemaciclib, which have labelled indications in combination with aromatase inhibitors,7–11 fulvestrant10,12–16 or as monotherapy (abemaciclib)17 based on landmark randomised controlled trials (RCT) demonstrating progression-free survival (PFS)7–9,11,13,16 and overall survival (OS)10,12,14,15 benefit compared to anti-oestrogen therapy plus placebo, in both pre- and post-menopausal patient populations.10,12,14,15
Real-world evidence offers important insights into efficacy endpoints such as PFS, OS and patient toxicities in the real-world setting, and importantly allow for analysis of patient subgroups, including elderly patients, those with poor performance status or the presence of brain metastases, that are often not adequately captured by RCTs. The objective of our study was to conduct a real-world analysis on the use of CDK4/6i in Asian advanced HR+/HER2− BC patients.
Methods
Patients and treatment
A multi-centre cohort study was carried out for all patients receiving palliative intent CDK4/6i in any line of palliative systemic therapy for advanced/metastatic BC between 2015 and 2020 in Singapore. Data were retrospectively collected from 1 January 2015 to 31 July 2020 and prospectively collected from 1 August 2020 to 31 December 2020.
The study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB) (Reference number: 2018/01081) and Parkway Independent Ethics Committee (PIEC/2019/040) and was conducted in accordance with the Declaration of Helsinki provision. Informed consent was waived for patients recruited retrospectively and written informed consent was obtained for patients recruited prospectively.
We included patients from three major academic health institutions in Singapore – National University Cancer Institute, Singapore, National Cancer Centre, Singapore and Tan Tock Seng Hospital, that together see the majority of cancer patients in the public sector in Singapore, as well as two private oncology groups – Icon Cancer Centre and OncoCare Cancer Centre. Clinical patient characteristics, oncologic and treatment history including CDK4/6i indication, duration and reasons for CDK4/6i discontinuation were extracted from electronic medical records. Endocrine-resistant BC was defined as recurrence while on or ⩽12 months from end of adjuvant endocrine therapy. Endocrine-sensitive BC was defined as recurrence >12 months from end of adjuvant endocrine therapy. Treatment was stopped in the following situations: disease progression, unacceptable toxicities, death or patient’s decision to stop treatment.
Efficacy outcomes
Chest and/or abdominal computed tomography scans and/or bone scans were performed by clinicians every 8–12 weeks as part of routine clinical care, to evaluate patient’s response and assess for disease progression. PFS was measured from time of initiation of drug to disease progression or death due to any cause. OS was measured from time of initiation of drug to death due to any cause. Safety analysis examined the incidence of adverse events (AEs) as recorded by clinicians. A dose delay (DD) was defined as the discrete number of times the CDK4/6i was not started at the planned date of a 28-day cycle (±2 days). A dose reduction (DR) was defined as a decrease in the dose of drug prescribed. All patients who have received ⩾1 cycle of CDK4/6i were included in the survival analysis.
Statistical analysis
Continuous and categorical variables were summarised as median (interquartile range) and frequency (percentage), respectively. Survival analysis was performed using Kaplan–Meier survival curves for PFS and OS. Univariate and multivariable Cox-proportional hazard regression models were applied to survival outcomes. Quantitative association from Cox regression was expressed as hazard ratio (HR) with its corresponding 95% confidence interval (CI). All statistical tests utilised were two-sided and a p value < 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS statistics version 22.
Results
Demographics, tumour and treatment characteristics
In all, 456 patients received CDK4/6i for advanced/metastatic BC from January 2015 to December 2020. Patient characteristics at the time of CDK4/6i initiation are shown in Table 1. The median age of diagnosis was 59 (range: 24–92). In all, 85 (19%) patients were ⩾70 years old. Majority of the patients were ethnically Chinese (n = 326, 72%), female (n = 454, 99%), had an Eastern Cooperative Oncology Group (ECOG) status of 0/1 (n = 356, 78%) and were post-menopausal (n = 344, 75%). Of 107 (24%) pre-menopausal patients, 85 (79%) were receiving ovarian function suppression with surgery or gonadotrophin-releasing hormone analogues concurrent to endocrine therapy.
Table 1.
Patient demographics.
| Total number of patients | 456 | |
|---|---|---|
| Median age of diagnosis of metastatic BC | 59 (range: 24–92) | |
| Age | <70 years old | 371 (81%) |
| ≥70 years old | 85 (19%) | |
| Ethnicity | Chinese | 326 (72%) |
| Malay | 49 (11%) | |
| Indian | 29 (6%) | |
| Others ➢ Asiansa ➢ Non-Asians |
52 (11%) ➢ N = 24 (5%) ➢ N = 28 (6%) |
|
| Gender | Female | 454 (99%) |
| Male | 2 (1%) | |
| ECOG | 0–1 | 356 (78%) |
| 2 | 27 (6%) | |
| 3–4 | 16 (4%) | |
| Unknown | 57 (12%) | |
| Menopausal status | Post-menopausal | 344 (75%) |
| Pre-menopausal ➢ On ovarian suppression |
107 (24%) ➢ N = 85 (79%) |
|
| Unknown | 5 (1%) | |
Middle east (n = 8), Filipino (n = 4) and Indonesian (n = 12).
BC, breast cancer; ECOG, Eastern Cooperative Oncology Group.
Patient tumour and treatment characteristics are summarised in Table 2. Most of the BCs were invasive ductal carcinomas (327, 72%) and oestrogen receptor positive (448, 98%). In all, 17 (4%) patients had HER2+ BC. Half the patients had de novo metastatic disease (226, 50%). Out of the 228 (50%) patients with disease relapse following initial early-stage cancer diagnosis, 85 (37%), 86 (38%) and 27 (12%) patients had endocrine-resistant, endocrine-sensitive disease and no endocrine therapy in the adjuvant setting, respectively. In total, 17 (4%) patients had HER2+ BC; 25 (5%), 31 (7%), 145 (32%), 244 (54%) and 342 (75%) patients had brain, peritoneum, liver, lung and bone metastasis at the time of CDK4/6i initiation, respectively.
Table 2.
Tumour and treatment characteristics.
| Treatment setting | De novo metastatic | 226 (50%) |
| Locoregional recurrence | 37 (8%) | |
| Distant recurrence | 191 (42%) | |
| Others | 2 (1%) ➢ neoadjuvant (1) ➢ PD on neoadjuvant chemo (1) |
|
| Hormone sensitivity of patients with recurrent BC | Hormone resistant ➢ Recurred while on adjuvant hormone therapy ➢ Recurred ⩽ 12 months from end of adjuvant hormone therapy |
85 (37%) ➢ 67 (79%) ➢ 18 (21%) |
| Hormone sensitive ➢ Recurred 13–24 months from end of adjuvant endocrine therapy ➢ Recurred > 24 months from end of adjuvant endocrine therapy |
86 (38%) ➢ 8 (9%) ➢ 78 (91%) |
|
| Did not take endocrine therapy in adjuvant setting | 27 (12%) | |
| Unknown | 30 (13%) | |
| Histology | Invasive ductal carcinoma | 327 (72%) |
| Invasive lobular carcinoma | 34 (7%) | |
| Others ➢ Colloid ➢ Medullary ➢ Papillary ➢ Mucinous ➢ No special type |
46 (10%) ➢ 1 (2%) ➢ 1 (2%) ➢ 4 (9%) ➢ 12 (26%) ➢ 25 (54%) |
|
| Unknown | 49 (11%) | |
| Biomarker status | Oestrogen receptor ➢ Positive ➢ Negative ➢ Unknown |
448 (98%) 3 (1%) 5 (1%) |
| Progesterone receptor ➢ Positive ➢ Negative ➢ Unknown |
369 (81%) 72 (16%) 15 (3%) |
|
| Her2Neu ➢ Positive ➢ Negative |
17 (4%) 439 (96%) |
|
| Site of metastasis | Brain metastasis | 25 (5%) |
| Peritoneum | 31 (7%) | |
| Liver | 145 (32%) | |
| Lung | 244 (54%) | |
| Bone | 342 (75%) | |
| Type of CDK4/6 inhibitor | Palbociclib | 435 (95%) |
| Ribociclib | 21 (5%) | |
| Partner anti-oestrogen therapy with CDK4/6 inhibitor | Fulvestrant | 128 (28%) |
| Aromatase inhibitor ➢ Letrozole ➢ Anastrozole ➢ Exemestane |
290 (64%) ➢ 236 (81%) ➢ 16 (6%) ➢ 38 (13%) |
|
| Tamoxifen | 38 (8%) | |
| Line of treatment | First line | 122 (27%) |
| Second line | 119 (26%) | |
| Third line | 70 (15%) | |
| Fourth line and beyond | 145 (32%) |
BC, breast cancer; CDK4, CDK4/6i, cyclin-dependent kinase 4.
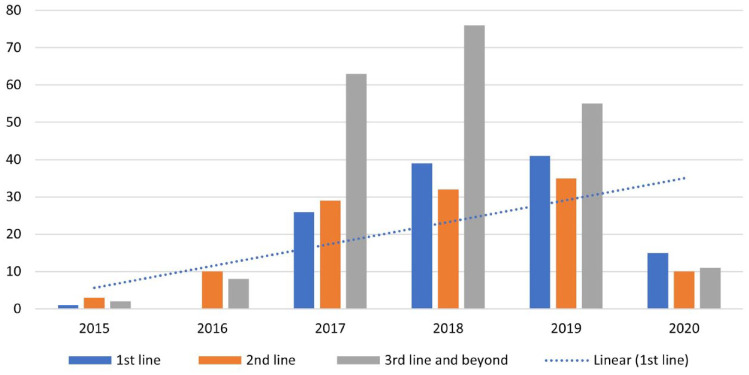
In our cohort, 435 (95%) of patients received the CDK4/6i palbociclib. The most common partner anti-oestrogen drug used was aromatase inhibitors (n = 290, 64%), followed by fulvestrant (n = 128, 28%). Letrozole was the most common aromatase inhibitor used (n = 236, 81%). Patients received CDK4/6i most commonly in the first-line (n = 122, 27%) and second-line setting (n = 119, 26%). Figure 1 demonstrates the increasing trend of CDK4/6i usage as first-line treatment with time from 2015 to 2020.
Figure 1.
Time trend of the line of use of CDK4/6i from 2015 to 2020.
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor.
Survival analysis
All patients were included in the survival analysis. Median follow-up duration was 30.1 months.
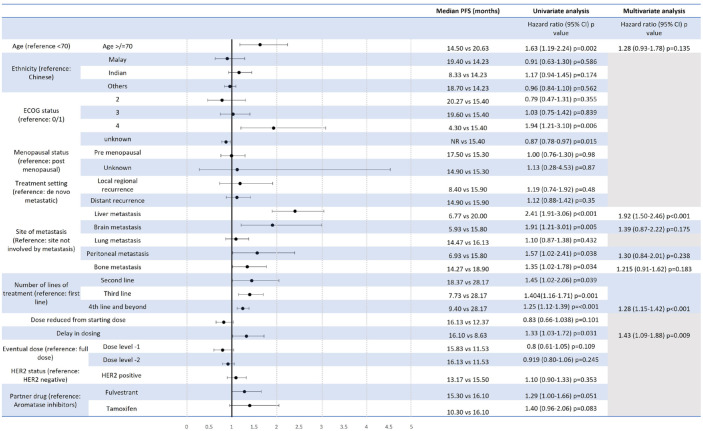
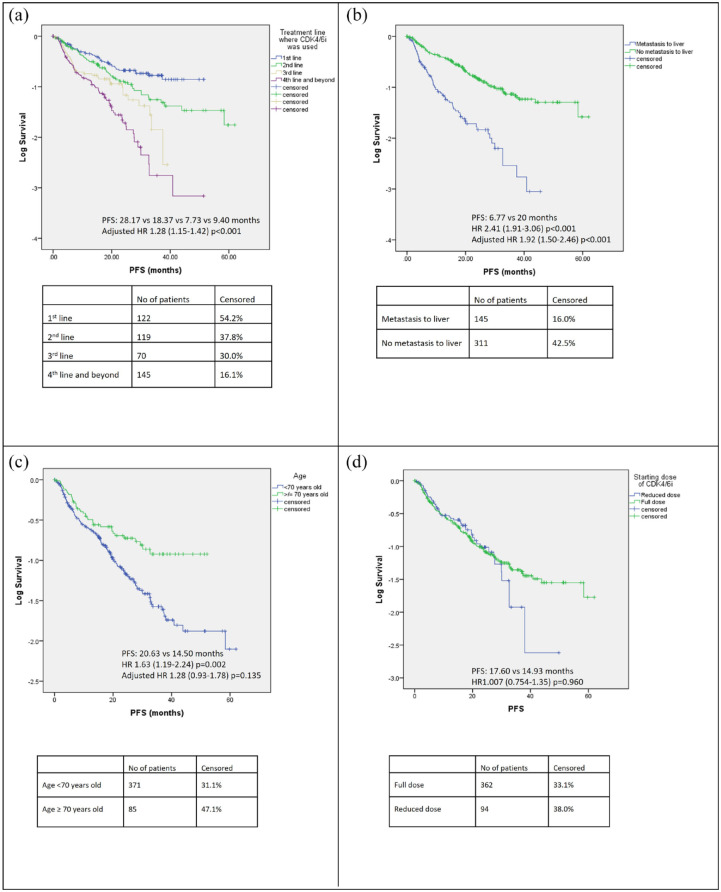
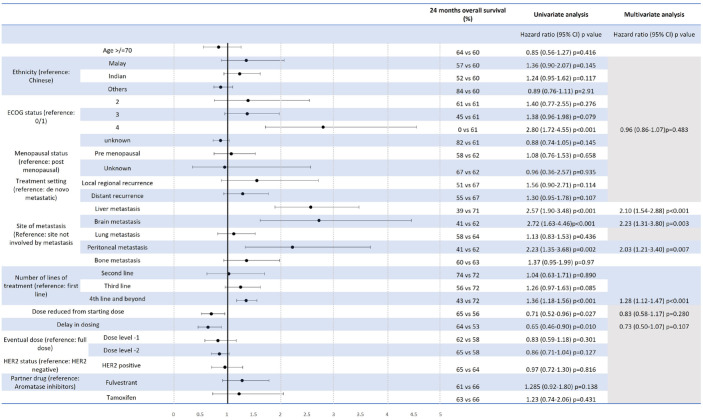
Median PFS was 28.17, 18.37, 7.73 and 9.40 months in the first line, second line, third line and fourth line of treatment and beyond, respectively (Figures 2 and 4(a)), while the corresponding proportions of patients alive at 24 months were 72%, 74%, 56%, 43%, respectively (Figure 3).
Figure 2.
Univariate and multivariate Cox-proportional hazards model for PFS of patients receiving CDK4/6i.
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; PFS, progression-free survival.
Figure 4.
PFS of patients stratified by (a) line of treatment where CDK4/6i was used, (b) presence of liver metastasis, (c) age and (d) starting dose of CDK4/6i.
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; PFS, progression-free survival.
Figure 3.
Univariate and multivariate Cox-proportional hazards model for OS of patients receiving CDK4/6i.
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; OS, overall survival.
Univariate analysis of PFS (Figure 2) and 24-month OS (Figure 3) for the use of CDK4/6i showed that age ⩾70 years old, presence of liver, brain or peritoneal metastasis, and use of CDK4/6i in a later line of treatment were associated with a significantly shorter PFS. PFS was 6.77 versus 20.00 months (HR: 2.41, CI: 1.91–3.06, p < 0.001) and 14.50 versus 20.63 months (HR: 1.63, CI: 1.19–2.24, p = 0.002) for presence versus absence of liver metastasis and age ⩾70 versus <70, respectively (Figure 4(b) and (c)). A starting dose of CDK4/6i at a reduced dose was not significantly associated with a reduced PFS. PFS for starting dose of CDK4/6i at full dose and reduced dosed was 17.60 and 14.93 months, respectively (HR: 1.01, CI: 0.75–1.35, p = 0.960) (Figure 4(d)). The presence of liver, brain, peritoneal metastasis and use of CDK4/6i in a later line of treatment were also associated with a significantly shorter 24-month OS (Figure 3).
On multivariate analysis for PFS and OS, both liver metastasis and use of CDK4/6i in a later line of treatment continued to be associated with a significantly shorter PFS, while the presence of liver, brain, peritoneal and use of CDK4/6i in a later line of treatment remained significantly associated with a shorter OS (Figures 2 and 3).
Dose modifications
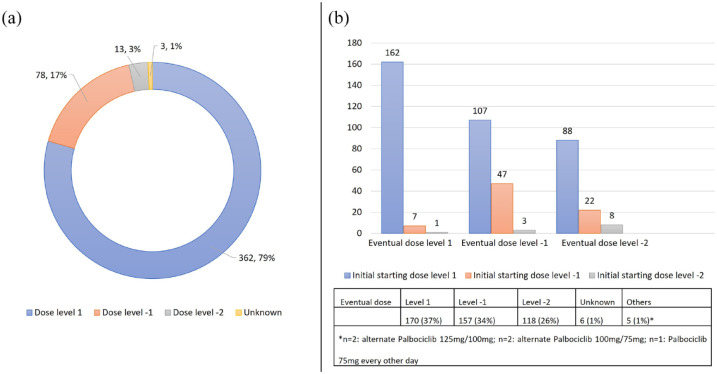
In all, 362 (80%) of patients received the US FDA recommended starting dose of CDK4/6i (dose level 1). In total, 78 (17%) received a lower starting dose of palbociclib 100 mg or ribociclib 400 mg (dose level 1), and 13 (3%) receiving a starting dose of palbociclib 75 mg or ribociclib 200 mg (dose level 2) (Figure 5(a)). Age ⩾ 70 and ECOG ⩾ 2 were significantly associated with a lower starting dose of CDK4/6i (Table 3).
Figure 5.
(a) Starting dose of CDK4/6i and (b) eventual dose of CDK4/6i and their starting dose.
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor.
Table 3.
Factors affecting the starting and eventual dose of CDK4/6i.
| Starting dose | p Value | Eventual dose | p Value | |||
|---|---|---|---|---|---|---|
| Dose level 1 | Reduced dose | Dose level 1 | Reduced dose | |||
| Age | ||||||
| ⩾70 | 56 (66%) | 29 (34%) | p = 0.003 | 18 (21%) | 67 (79%) | p = 0.001 |
| <70 | 306 (82%) | 65 (18%) | 152 (41%) | 219 (59%) | ||
| ECOG | ||||||
| 0/1 | 293 (82%) | 63 (18%) | p = 0.004 | 134 (38%) | 222 (62%) | p = 0.816 |
| ⩾2 | 69 (69%) | 31 (31%) | 36 (36%) | 64 (64%) | ||
| Liver metastasis | ||||||
| Present | 117 (81%) | 28 (19%) | p = 0.659 | 58 (40%) | 87 (60%) | p = 0.430 |
| Absent | 245 (79%) | 66 (21%) | 112 (36%) | 199 (64%) | ||
| Brain metastasis | ||||||
| Present | 19 (76%) | 6 (24%) | p = 0.659 | 12 (48%) | 13 (52%) | p = 0.259 |
| Absent | 343 (80%) | 88 (20%) | 158 (40%) | 273 (60%) | ||
| Lung metastasis | ||||||
| Present | 195 (80%) | 49 (20%) | p = 0.779 | 83 (34%) | 161 (66%) | p = 0.100 |
| Absent | 166 (78%) | 46 (22%) | 87 (41%) | 125 (59%) | ||
| Peritoneal metastasis | ||||||
| Present | 23 (74%) | 8 (26%) | p = 0.455 | 14 (45%) | 17 (55%) | p = 0.345 |
| Absent | 338 (80%) | 87 (20%) | 156 (37%) | 269 (63%) | ||
| Bone metastasis | ||||||
| Present | 269 (79%) | 73 (21%) | p = 0.451 | 125 (37%) | 217 (63%) | p = 0.451 |
| Absent | 93 (82%) | 21 (18%) | 45 (39%) | 69 (61%) | ||
| Line of treatment | ||||||
| First line | 104 (85%) | 18 (15%) | p = 0.258 | 51 (42%) | 70 (58%) | p = 0.424 |
| Second line | 96 (81%) | 23 (19%) | 43 (36%) | 76 (64%) | ||
| Third line | 51 (73%) | 19 (27%) | 20 (29%) | 50 (71%) | ||
| Fourth line and beyond | 111 (77%) | 34 (23%) | 56 (39%) | 89 (61%) | ||
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; ECOG, Eastern Cooperative Oncology Group.
Overall, 217 (48%) of patients required at least one DR and 88 (19%) had two DRs. Of the patients who were prescribed full dose (dose level 1) at the start, 195 (54%) required at least one DR. Of the 101 (20%) of patients who were started at a reduced dose of CDK4/6i, 22 (22%) of patients required a further DR, while 11 (11%) patients had their dose increased eventually. Approximately one-third of patients each had an eventual dose of dose level 1 (37%) and dose level 1 (34%). Five (1%) of our patients had an eventual dose of alternating palbociclib 125 mg/100 mg (n = 2), alternating palbociclib 100 mg/75 mg (n = 1) and palbociclib 75 mg every other day (Figure 5(b)). Only age ⩾ 70 was significantly associated with a lower eventual dose of CDK4/6i (Table 3).
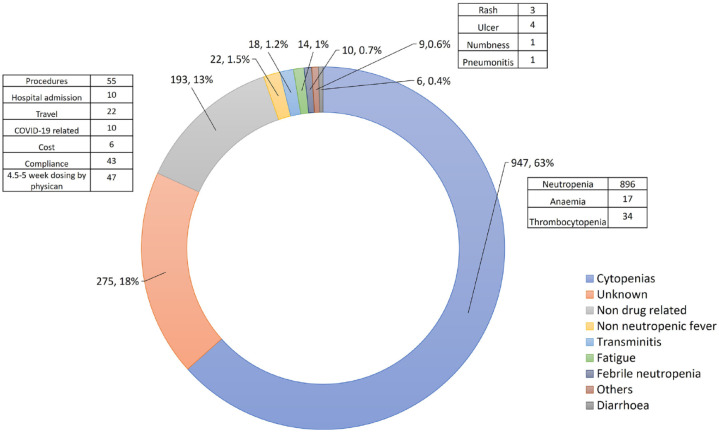
In total, 330 (72%) of all patients and 63 (74%) of patients ⩾70 years old had at least one DD. The total number of DD episodes experienced by patients in our study were 1466 (median number of DDs per patient 2, range: 0–37). Some patients had more than one reason for a DD. The most common reason for DDs was neutropenia (60%). There were 10 (0.7%) febrile neutropenia episodes out of the total number of DDs, and this occurred in nine patients or 2% of the entire population (Figure 6).18
Figure 6.
Reasons for CDK4/6i DDs.
CDK4/6i, cyclin-dependent kinase 4/6 inhibitor; DDs, dose delays.
Discussion
Previous studies have published real-world use of CDK4/6i19–23 with the largest being the retrospective observational analysis from the Flatiron Health Analytic Database comprising 1430 patients of which 1.6% were Asians.20 To our knowledge, our study is the largest real-world study in Asia evaluating the use of CDK4/6i in the treatment of advanced/metastatic BC. Our study indicates that CDK4/6i is an effective and safe treatment, with the increasing trend of CDK4/6i usage as first-line treatment with time from 2015 to 2020, and efficacy results consistent with those seen in clinical trials.
Palbociclib was the most frequently prescribed CDK4/6i in this cohort. This is similar to other CDK4/6i real-world studies,20,24,25 and is likely due to palbociclib being the first CDK4/6i to be approved for use in HR+/HER2− advanced/metastatic BC. The median PFS for first-line CDK4/6i in our study is 28.17 months, and this is similar to the PALOMA-2 and MONALEESA-2 study for first-line advanced/metastatic BC 7–9 which reported a PFS of 24.80 and 25.30 months, respectively. The PFS reported in our study is numerically longer than the 20.00 and 21.50 months in the Flatiron study20 and the PALOMA-4 study of palbociclib and letrozole in Asian postmenopausal woman, respectively.26 Unlike the PALOMA, MONALESSA and Flatiron studies, most of the patients in our study are Asians (n = 440, 97%) with predominantly Chinese (n = 326, 72%), providing important real-world data validating the efficacy of CDK4/6i in Asian, particularly Chinese patients.
While CDK4/6i has been approved by the US FDA in metastatic HR+ HER2− advanced/metastatic BC, several important data gaps exist for patient groups which are not well represented in RCTs such as elderly patients or patients with brain metastasis.27 The MONALEESA-228 and PALOMA-27 included 295 (44%) and 262 (39%) of patients ⩾65 years old. While the benefit from addition of ribociclib to letrozole was reported in patients ⩾65 years old,28 the proportion of patients ⩾70 years old and benefit derived from addition of CDK4/6i to aromatase inhibitor in this elderly population was not reported in the randomised trials. Almost 20% patients in our cohort are ⩾70, and PFS was significantly shorter for this elderly population on univariate analysis in our study. Both PFS and 24-month OS were also significantly shorter for patients with brain, liver or peritoneal metastasis. This offers important insights into treatment planning and prognostication in special subgroups of patients in the clinic.
The toxicities of CDK4/6i reported in this real-world study are similar to those reported in prospective clinical trials. The most common AE is neutropenia with only 2% of the cohort experiencing febrile neutropenia. In all, 217 (48%) of our patients required at least one DR which is higher than the 35.5–36.9% reported in phase II/III trials evaluating the use of CDK4/6i.29,30 This could be explained by our more heterogeneous group of patients including elderly and patients with visceral metastasis. Furthermore, most patients in the initial RCTs leading to the approval of CDK4/6i were Whites while our study has a predominantly Asian population. It is known that toxicity varies in different ethnic groups.31–34 Pharmacoethnicity may account for inter-racial variation in anticancer drug toxicity due to allelic variants of genes encoding drug-metabolising enzymes.35 Reassuringly, a reduced starting dose, DRs, DDs and the eventual dose of CDK4/6i were all not associated with an inferior PFS or 24-month OS.
Our study has its limitations. Certain treatment combinations or indications in our study were not aligned with the approved US FDA indication. For instance, 17 (4%) of patients who had HER2+ BC was prescribed CDK4/6i. While there was no difference in PFS and 24-month OS for HER2+ versus HER2− patients on univariate analysis, the number of patients was small, and no definitive conclusions can be made. Only 80% patients were started on CDK4/6i at the approved dose level in our real-world study. Moreover, the retrospective nature of the study, differing baseline characteristics, and imbalances in number of patients receiving the various CDK4/6i and limited sample size do not allow for valid efficacy comparison among different CDK4/6i and in subpopulations.
Conclusion
Our study shows that CDK4/6i is an effective and safe treatment in the real world, consistent with results seen in clinical trials. Age ⩾70 and presentation with liver, peritoneal or brain metastasis are negative prognostic factors. Significant dose modifications were observed but this did not impact on survival. This will help assure clinicians and patients that their outcomes will not be adversely affected when dose modifications are clinically required.
Acknowledgments
None.
Contributor Information
Jia Li Low, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Elaine Lim, Division of Medical Oncology, National Cancer Center Singapore, Singapore.
Lavina Bharwani, Department of Medical Oncology, Tan Tock Seng Hospital, Singapore.
Andrea Wong, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Karmen Wong, Icon Cancer Centre, Singapore.
Samuel Ow, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Siew Eng Lim, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Matilda Lee, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Joan Choo, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Joline Lim, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Gloria Chan, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Robert John Walsh, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Vaishnavi Muthu, Department of Medical Oncology, Tan Tock Seng Hospital, Singapore.
Natalie Ngoi, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Wanqin Chong, Department of Hematology-Oncology, National University Cancer Institute (NCIS), Singapore.
Sing Huang Tan, OncoCare Cancer Centre, Singapore.
Soo Chin Lee, Department of Hematology-Oncology, National University Cancer Institute (NCIS), 1E Lower Kent Ridge Road, Singapore 119228; Cancer Science Institute, Singapore; Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore.
Declarations
Ethics approval and consent to participate: The study was approved by the National Healthcare Group Domain Specific Review Board (NHG DSRB) (Reference number: 2018/01081) and Parkway Independent Ethics Committee (PIEC/2019/040) and was conducted in accordance with the Declaration of Helsinki provision. Informed consent was waived for patients recruited retrospectively and written informed consent was obtained for patients recruited prospectively.
Consent for publication: Not applicable.
Author contribution(s): Jia Li Low: Conceptualisation; Data curation; Formal analysis; Investigation; Methodology; Project administration; Writing – original draft; Writing – review & editing.
Elaine Lim: Data curation; Project administration; Writing – review & editing.
Lavina Bharwani: Data curation; Project administration; Writing – review & editing.
Andrea Wong: Data curation; Writing – review & editing.
Karmen Wong: Data curation; Writing – review & editing.
Samuel Ow: Data curation; Writing – review & editing.
Siew Eng Lim: Data curation; Writing – review & editing.
Matilda Lee: Data curation; Writing – review & editing.
Joan Choo: Data curation; Writing – review & editing.
Joline Lim: Data curation; Writing – review & editing.
Gloria Chan: Data curation; Writing – review & editing.
Robert John Walsh: Data curation; Writing – review & editing.
Vaishnavi Muthu: Data curation; Writing – review & editing.
Natalie Ngoi: Data curation; Writing – review & editing.
Wanqin Chong: Data curation; Writing – review & editing.
Sing Huang Tan: Data curation; Writing – review & editing.
Soo Chin Lee: Conceptualisation; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study is supported by a research grant from Pfizer (ID#56272553) and by the Singapore National Medical Research Council (NMRC/CSA-SI/0004/2015).
Andrea Wong:
Advisory board: Novartis, Pfizer, Eisai, Ostuka
Research collaborations: Otsuka pharmaceuticals
Karmen Wong:
Advisory board: Pfizer, Novartis
Samuel Ow:
Honararia/Consulting: Pfizer, Astra Zeneca, Roche, Novartis, Eli Lily
Joline Lim:
Advisory/Consulting: Pfizer, Novartis, MSD, Everest Medicine
Research funding: Synthon
Travel/accommodation/expenses: Novartis, Astra Zeneca, MSD
Robert John Walsh:
Honorarium: Pfizer
Advisory board: Pfizer
Natalie Ngoi:
Honorarium: Astra Zeneca, Pfizer, Merck
Travel: Astra Zeneca
Wan Qin Chong:
Conference support: Ipsen Pharma
Soo Chin Lee:
Grant support/Research collaborations: Pfizer, Eisai, Taiho, ACT Genomics, Bayer, MSD, Karyopharm, Adagene
Advisory board/Speaker Invitation: Pfizer, Novartis, Astra Zeneca, ACT Genomics, Eli Lilly, MSD, Roche
Conference support: Amgen, Pfizer, Roche
Availability of data and materials: Not applicable.
References
- 1. Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer 2019; 144: 1941–1953. [DOI] [PubMed] [Google Scholar]
- 2. Reinert T, Barrios CH. Overall survival and progression-free survival with endocrine therapy for hormone receptor-positive, HER2-negative advanced breast cancer: review. Ther Adv Med Oncol 2017; 9: 693–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lim E, Metzger-Filho O, Winer EP. The natural history of hormone receptor-positive breast cancer. Oncology (Williston Park, N.Y.) 2012; 26: 688–694. [PubMed] [Google Scholar]
- 4. Cardoso F, Paluch-Shimon S, Senkus E, et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann Oncol 2020; 31: 1623–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scott SC, Lee SS, Abraham J. Mechanisms of therapeutic CDK4/6 inhibition in breast cancer. Semin Oncol 2017; 44: 385–394. [DOI] [PubMed] [Google Scholar]
- 6. Finn RS, Aleshin A, Slamon DJ. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res 2016; 18: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Finn RS, Martin M, Rugo HS, et al. Palbociclib and letrozole in advanced breast cancer. N Engl J Med 2016; 375: 1925–1936. [DOI] [PubMed] [Google Scholar]
- 8. O’Shaughnessy J, Petrakova K, Sonke GS, et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2− advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat 2018; 168: 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hortobagyi GN, Stemmer SM, Burris HA, et al. Ribociclib as first-line therapy for HR-positive, advanced breast cancer. N Engl J Med 2016; 375: 1738–1748. [DOI] [PubMed] [Google Scholar]
- 10. Im SA, Lu YS, Bardia A, et al. Overall survival with ribociclib plus endocrine therapy in breast cancer. N Engl J Med 2019; 381: 307–316. [DOI] [PubMed] [Google Scholar]
- 11. Johnston S, Martin M, di Leo A, et al. MONARCH 3 final PFS: a randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019; 5: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Turner NC, Slamon DJ, Ro J, et al. Overall survival with palbociclib and fulvestrant in advanced breast cancer. N Engl J Med 2018; 379: 1926–1936. [DOI] [PubMed] [Google Scholar]
- 13. Slamon DJ, Neven P, Chia S, et al. Phase III randomized study of ribociclib and fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer: MONALEESA-3. J Clin Oncol 2018; 36: 2465–2472. [DOI] [PubMed] [Google Scholar]
- 14. Slamon DJ, Neven P, Chia S, et al. Overall survival with ribociclib plus fulvestrant in advanced breast cancer. N Engl J Med 2020; 382: 514–524. [DOI] [PubMed] [Google Scholar]
- 15. Slamon DJ, Neven P, Chia S, et al. Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: updated overall survival. Ann Oncol 2021; 32: 1015–1024. [DOI] [PubMed] [Google Scholar]
- 16. Sledge GW, Toi M, Neven P, et al. MONARCH 2: abemaciclib in combination with fulvestrant in women with HR+/HER2-advanced breast cancer who had progressed while receiving endocrine therapy. J Clin Oncol 2017; 35: 2875–2884. [DOI] [PubMed] [Google Scholar]
- 17. Dickler MN, Tolaney SM, Rugo HS, et al. MONARCH 1, a phase II study of abemaciclib, a CDK4 and CDK6 inhibitor, as a single agent, in patients with refractory HR+/HER2- metastatic breast cancer. Clin Cancer Res 2017; 23: 5218–5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Turner NC, Ro J, André F, et al. Palbociclib in hormone-receptor–positive advanced breast cancer. N Engl J Med 2015; 373: 209–219. [DOI] [PubMed] [Google Scholar]
- 19. García-Trevijano Cabetas M, Lucena Martínez P, Jiménez Nácher I, et al. Real-world experience of palbociclib and ribociclib: novel oral therapy in metastatic breast cancer. Int J Clin Pharm 2021; 43: 893–899. [DOI] [PubMed] [Google Scholar]
- 20. DeMichele A, Cristofanilli M, Brufsky A, et al. Comparative effectiveness of first-line palbociclib plus letrozole versus letrozole alone for HR+/HER2− metastatic breast cancer in US real-world clinical practice. Breast Cancer Res 2021; 23: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gogia A, Arora S, Choudhary P, et al. Real-world Indian scenario of CDK4/6 inhibitors (palbociclib and ribociclib) with endocrine therapy in upfront hormone positive metastatic breast cancer. J Clin Oncol 2021; 39 (Suppl. 15): e13028. [Google Scholar]
- 22. Fountzilas E, Koliou GA, Vozikis A, et al. Real-world clinical outcome and toxicity data and economic aspects in patients with advanced breast cancer treated with cyclin-dependent kinase 4/6 (CDK4/6) inhibitors combined with endocrine therapy: the experience of the Hellenic Cooperative Oncology Group. ESMO Open 2020; 5: e000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Palumbo R, Torrisi R, Sottotetti F, et al. Patterns of treatment and outcome of palbociclib plus endocrine therapy in hormone receptor-positive/HER2 receptor-negative metastatic breast cancer: a real-world multicentre Italian study. Ther Adv Med Oncol 2021; 13: 1758835920987651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Harbeck N, Bartlett M, Spurden D, et al. CDK4/6 inhibitors in HR+/HER2- advanced/metastatic breast cancer: a systematic literature review of real-world evidence studies. Future Oncol 2021; 17: 2107–2122. [DOI] [PubMed] [Google Scholar]
- 25. Harbeck N, Bartlett M, Spurden D, et al. PCN28 cyclin-dependent kinase 4/6 inhibitors (CDK4/6I) in hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) advanced/metastatic breast cancer (A/MBC): a systematic literature review of real-world evidence (RWE) studies. Value Health 2020; 23: S27. [Google Scholar]
- 26. Xu B, Hu X, Li W, et al. 228MO PALOMA-4: primary results from a phase III trial of palbociclib (PAL) + letrozole (LET) vs placebo (PBO) + LET in Asian postmenopausal women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative (ER+/HER2–) advanced breast cancer (ABC). Ann Oncol 2021; 32: S457. [Google Scholar]
- 27. Waller J, Mitra D, Taylor-Stokes G, et al. Abstract P6-18-21: real world treatment patterns and outcomes of patients receiving palbociclib plus fulvestrant in the United States: sub-groups analysis based on age, performance status and sites of metastases. Cancer Res 2019; 79: P6-18-21. [Google Scholar]
- 28. Sonke GS, Hart LL, Campone M, et al. Ribociclib with letrozole vs letrozole alone in elderly patients with hormone receptor-positive, HER2-negative breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res Treat 2018; 167: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verma S, Im SA, Ro J, et al. Hematologic adverse events following palbociclib (PAL) dose reduction in patients (pts) with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2-) advanced breast cancer (ABC): pooled analysis from randomized phase 2 and 3 studies. J Clin Oncol 2018; 36: 1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ettl J, Im SA, Ro J, et al. Hematologic adverse events following palbociclib dose reduction in patients with hormone receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer: pooled analysis from randomized phase 2 and 3 studies. Breast Cancer Res 2020; 22: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu J. JCSE01.03 any difference on efficacy and toxicity between east and west? J Thorac Oncol 2019; 14: S125. [Google Scholar]
- 32. Phan VH, Moore MM, McLachlan AJ, et al. Ethnic differences in drug metabolism and toxicity from chemotherapy. Expert Opin Drug Metab Toxicol 2009; 5: 243–257. [DOI] [PubMed] [Google Scholar]
- 33. Li LJ, Chong Q, Wang L, et al. Different treatment efficacies and side effects of cytotoxic chemotherapy. J Thorac Dis 2020; 12: 3785–3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hasegawa Y, Kawaguchi T, Kubo A, et al. Ethnic difference in hematological toxicity in patients with non-small cell lung cancer treated with chemotherapy: a pooled analysis on Asian versus non-Asian in phase II and III clinical trials. J Thorac Oncol 2011; 6: 1881–1888. [DOI] [PubMed] [Google Scholar]
- 35. O’Donnell PH, Dolan ME. Cancer pharmacoethnicity: ethnic differences in susceptibility to the effects of chemotherapy. Clin Cancer Res 2009; 15: 4806–4814. [DOI] [PMC free article] [PubMed] [Google Scholar]