Abstract
Background:
Rare protein-altering variants in SCN5A, KCNQ1, and KCNH2 are major causes of Brugada Syndrome (BrS) and the congenital Long QT Syndrome (LQTS). While splice-altering variants lying outside 2-bp canonical splice sites can cause these diseases, their role remains poorly described. We implemented two functional assays to assess 12 recently reported putative splice-altering variants of uncertain significance (VUS) and 1 likely pathogenic (LP) variant without functional data observed in BrS and LQTS probands.
Methods:
We deployed minigene assays to assess the splicing consequences of 10 variants. Three variants incompatible with the minigene approach were introduced into control induced pluripotent stem cells (iPSCs) by CRISPR genome editing. We differentiated cells into iPSC-derived cardiomyocytes (iPSC-CMs) and studied splicing outcomes by reverse transcription-polymerase chain reaction (RT-PCR). We used the American College of Medical Genetics and Genomics functional assay criteria (PS3/BS3) to reclassify variants.
Results:
We identified aberrant splicing, with presumed disruption of protein sequence, in 8/10 variants studied using the minigene assay and 1/3 studied in iPSC-CMs. We reclassified 8 VUS to LP, 1 VUS to Likely Benign, and 1 LP variant to pathogenic.
Conclusions:
Functional assays reclassified splice-altering variants outside canonical splice sites in BrS- and LQTS-associated genes.
Keywords: Brugada syndrome, long QT Syndrome, splicing, minigene, CRISPR-Cas9, iPSC-cardiomyocyte, ACMG, functional genomics
Introduction
The arrhythmia syndromes Brugada Syndrome (BrS) and congenital Long QT Syndrome (LQTS) are rare autosomal dominant Mendelian diseases, mainly involving variants in cardiac ion channels. BrS is associated with loss-of-function (LoF) variants in the SCN5A sodium channel gene in 20% of patients, while LoF variants in the potassium channel genes KCNQ1 and KCNH2 and gain-of-function (GoF) variants in SCN5A are found in 80% of patients with congenital LQTS 1,2. These diseases contribute to the >250,000 cases of sudden cardiac death (SCD) each year in the US through fatal ventricular arrhythmias. When affected heterozygotes are recognized early, BrS and LQTS can be clinically managed through medications and/or interventional therapies. However, when not recognized through medical or genetic screening, SCD may present as the sentinel disease manifestation3. Genetic sequencing therefore offers the opportunity to uncover risk in 1) clinically unrecognized heterozygotes identified in unascertained population sequencing studies, or 2) family members of a recognized proband. Multiple approaches are in development to support genome-first approaches and cascade screening, but these efforts are still hampered by issues of clinical interpretability4,5. The American College of Medical Genetics and Genomics (ACMG) has provided a framework for variant interpretation, spanning benign (B) to pathogenic (P) based on criteria including variant functional evidence, population minor allele frequencies, segregation within families, and computational predictions, among others6. While panel sequencing and whole exome/genome sequencing are improving our broader understanding of the genetic basis of disease, a large proportion of detected variants are classified as Variants of Uncertain Significance (VUS) and are therefore not clinically actionable7.
Protein-altering variants (including nonsense, frameshift, and missense variants) are a major focus of clinical attention. Variants affecting RNA splicing occupy a comparatively less explored fraction of the genome, despite estimates that they contribute to up to 10% of pathogenic variants in Mendelian diseases8. Aberrant splicing from rare variants that alter the 2-bp canonical splice sites flanking each exon is typically characterized as meeting the PVS1 criterion, and therefore often these variants are often classified as P/LP. However, rare splice-altering variants falling outside of these 2-bp sites are more difficult to interpret. Aberrant splicing may arise from more distant intronic or exonic variation by introducing or ablating splice acceptors or donor sequences or by affecting regulatory splicing enhancers or silencers (Figures 1A and 1B; see Supplemental Figure I for position nomenclature)9. In silico predictors of splicing consequences have historically had only modest predictive ability.10,11 However, recent tools leveraging advances in machine learning and large RNA-seq datasets raise the possibility that in silico splicing predictors may be increasingly used to facilitate interpretation of suspected splice-altering variation8.
Figure 1. Aberrant splicing from cis-genetic variation and previous variant prioritization.
A) Variants in an exon near the 2-bp splice acceptor (AG) or donor (GT) can disrupt the recognition of the canonical site and lead to exon skipping. Alternatively, variants within the exon may introduce a new splice acceptor or donor site and lead to truncation of the exon, which may introduce frameshifts or damage protein function. This schematic features canonical AG-GT splice sites; rare alternate splice sites are possible. B) Intronic variants adjacent to the 2-bp splice acceptor or donor may disrupt spliceosome recognition of the canonical splice site and lead to exon skipping. Deeper intronic variants may introduce cryptic splice acceptor or donor sites that may lead to transcription of intronic regions (pseudo-exons) that may disrupt the reading frame or compromise protein function. This schematic features canonical AG-GT splice sites; rare alternate splice sites are possible. C) A recent variant interpretation effort curated LQTS and BrS heterozygotes and reported variants. 17 putative splice-altering variants were reported, of which 15 were Single Nucleotide Variants. Of these, 13 were previously not functionally characterized. We analyzed 10 of 13 using a standard minigene approach and 3 using RT-PCR assays from iPSC-CMs.
In this study, we used minigene assays in Human Embryonic Kidney 293 (HEK293) cells to study variant consequences on splicing for 10 putative splice-altering variants in arrhythmia genes identified in a recent cohort of BrS and LQTS patients (Figure 1C). For three variants incompatible with minigene assays, we examined the impact on splicing in induced pluripotent stem cell-derived cardiomyocytes (iPSC-CMs) that were edited with CRISPR-Cas912. We applied these functional splicing assays to reclassify a total of 10 putative splice-altering variants within the ACMG framework, including 8 VUS to Likely Pathogenic.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. All data drawn from previously published studies used in this analysis received IRB/REC approval. Stem cell work was completed under IRB #9047. Full methods are available online as Supplemental Data.
Results
Minigene Assay Reveals Aberrant Splicing.
We used a minigene construct previously deployed by our group13 and others14,15 to study the effects of SCN5A potential splice-altering variants that have been observed in one or more patients with BrS (Figure 2A). This assay allows the direct comparison of WT versus variant splicing outcomes by studying a specific exon and flanking intronic regions of interest (primers in Supplemental Table I). We first studied the known likely pathogenic (LP) exon-truncating SCN5A synonymous variant c.4719C>T in our system. This variant was previously shown to disrupt splicing in both a minigene assay and RNA isolated from patient peripheral blood lymphocytes16. Compared to the respective WT minigene construct, the size of the RT-PCR product from the c.4719C>T minigene was consistent with cDNA truncation (Figure 2B). This was confirmed by Sanger sequencing (Figure 2C). All major bands observed by gel electrophoresis were subjected to characterization by Sanger sequencing. For the bands that could be unambiguously identified, we inferred the splice product. For each major band, we inferred the splicing consequence based on reconstruction of the minigene vector and native genomic sequence (Supplemental Table II). We next studied the SCN5A VUS c.1890G>A located at the terminal coding nucleotide position of exon 12 and its WT counterpart. In the minigene assay, c.1890G>A resulted in retention of intron 10 by a splice donor loss as evidenced by RT-PCR product gel band size and confirmatory Sanger sequencing (Figure 2D and 2E). Quantification of the gel band intensity showed significant changes in the WT exon cassette percent spliced in (PSI) in both cases (Figure 2F; all minigene PSI in Supplemental Table III). The effects on transcript composition of these two variants are shown schematically in Figure 2G. This approach was attempted for two additional SCN5A VUS, c.4299G>C and c.4299+6T>C, but the results of the minigene assay were inconclusive due to poor splicing in of the WT exon (Supplemental Figure II)
Figure 2. Minigene assay and studies on SCN5A.
A) Schematic of minigene assay. The PCR product of an exon and segments of flanking introns of interest is cloned into a multiple cloning site (MCS) between two known exons. Mutagenesis inserts the variant. After transfection of the WT and variant plasmids into HEK cells, RNA is isolated, and RT-PCR provides cDNA for analysis of transcript composition. B) Gel of PCR products for the known exon truncating variant c.4719C>T and WT plasmid. The WT band is consistent with higher molecular weight compared to the truncated form induced by the aberrant splice donor gain. All minigene assays are presented in triplicate. C) Sanger sequencing of the exon-exon junctions of WT and truncated RT-PCR products. D) Gel of the WT exon 12 and c.1890G>A RT-PCR products implicating a pseudo-exon gain induced by the variant. E) Confirmatory Sanger sequencing of RT-PCR products for the WT and c.1890G>A VUS. F) Quantification of WT and variant Percent Spliced In (PSI) for 3 independent replicates, respectively. * indicates p < 0.05, independent 2-group Mann-Whitney U Test. G) Schematic of assay results. SCN5A c.4719C>T truncates exon in our assay, whereas the SCN5A variant c.1890G>A induces intron retention.
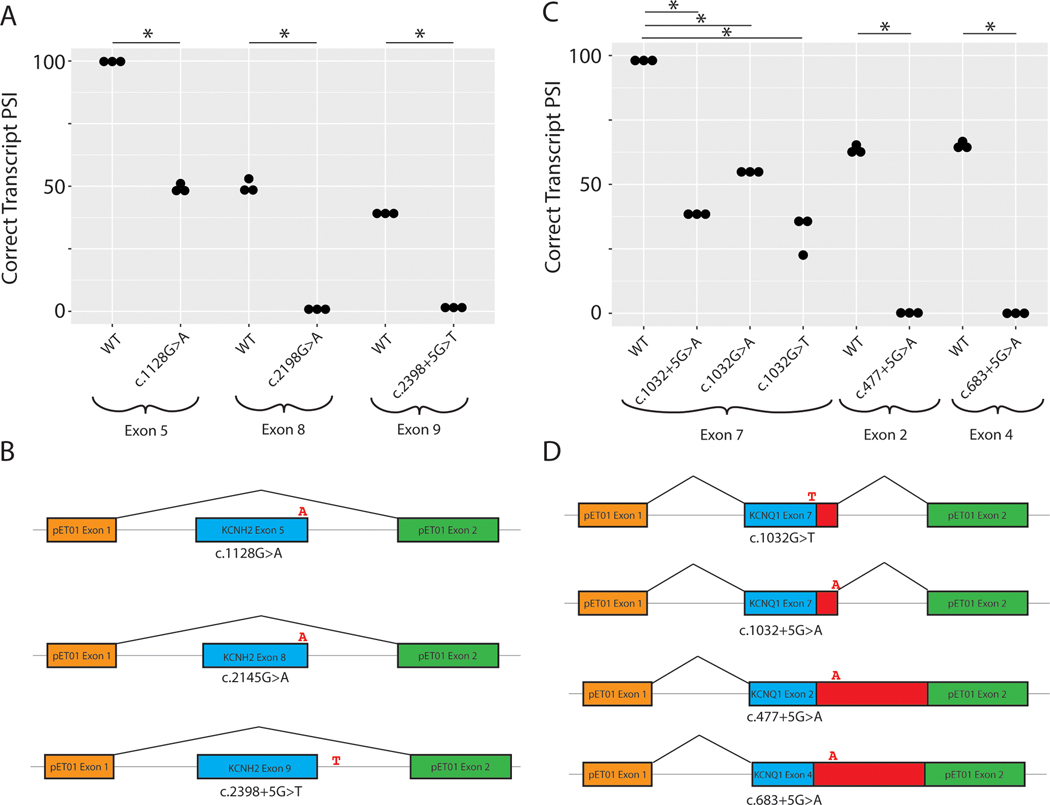
We studied three KCNH2 VUS c.1128G>A, c.2145G>A, and c.2398+5G>T discovered in the LQTS cohort17. The variant c.1128G>A induced exon skipping, which is predicted to disrupt the downstream reading frame based on exon size (Supplemental Figure III). The variant c.2145G>A led to an exon skipping event at exon 8 (Supplemental Figure III), similarly resulting in a predicted frameshift. Both nucleotides are located at the last nucleotide position of the coding exon. The intronic variant c.2398+5G>T behaved similarly, leading to exon skipping (Supplemental Figure III). This exon 9 skipping event is also predicted to lead to a frameshift. Quantification of the respective WT exon cassette PSI showed significant changes in all cases (p < 0.05, Mann-Whitney U Test) (Figure 3A). Schematic depictions of the major splicing outcomes on the transcript are shown in Figure 3B. Raw gels and Sanger traces for all major splicing outcomes are shown in Supplemental Figure III.
Figure 3. Minigene analysis of KCNH2 and KCNQ1 splice variants.
A) Quantification of KCNH2 band intensity corresponding to the PSI of the WT and variant bands. 3 independent replicates per group (* indicates p < 0.05, independent 2-group Mann-Whitney U Test). B) Schematic depiction of the splicing outcome associated with the highest intensity band for each KCNH2 variant. C) Quantification of normal exon PSI across all samples. 3 independent replicates per group (* indicates p < 0.05, independent 2-group Mann-Whitney U Test). D) Schematic depictions of the major splicing outcome for each variant.
We studied 5 candidate LQTS splicing variants in KCNQ1. 3 KCNQ1 VUS (c.683+5G>A, c.1032+5G>A, c.1032G>T) and 2 KCNQ1 LP variants (c.477+5G>A and c.1032G>A) were studied. The c.1032 position is the terminal coding position of this exon and is a hotspot for variation. As a control, we first studied c.1032G>A, one of the most common causes of LQTS among Japanese probands (29 cases, 0 gnomAD controls).18 This variant has been previously shown to affect splicing in vitro and did so in our assay as well, leading to 2 alternative pseudoexon inclusions (Supplemental Figure IV). The adjacent intronic VUS, c.1032+5G>A, had a similar gel electrophoresis profile and led to multiple pseudoexon inclusions with variable respective cryptic donor sites. A second VUS near this splice junction, c.1032G>T, was also functionally assayed. In this case, 3 pseudoexons and an exon skipping event were observed, with a small amount of WT transcript overlapping with a pseudoexon (Supplemental Figure IV). These consequences are predicted to have similarly deleterious effects on the as those previously studied for c.1032G>A18. These results show that the KCNQ1 exon 9 splice donor site is highly intolerant to variation. The previously unstudied LP variant, c.477+5G>A, was assayed and showed nearly complete loss of WT splicing, with inclusion of 2 unambiguously defined pseudoexons (Supplemental Figure IV). The retained intron sequence contains a stop codon that would disrupt protein function. In our functional studies, introduction of the VUS c.683+5G>A variant completely abrogated WT splicing, while also introducing a pseudoexon and retaining an exon skipping event moderately observed in the WT (Supplemental Figure IV). The retained intronic sequence would also lead to a stop codon inclusion, which is predicted to disrupt protein function. Quantifications of splicing outcomes for the WT PSI are shown in Figure 3C (p < 0.05, Mann-Whitney U Test). Major splicing outcomes for each variant are depicted schematically in Figure 3D. Raw gels and Sanger traces for all major splicing outcomes are shown in Supplemental Figure IV.
CRISPR/Cas9 and iPSC-CMs Reveal Aberrant Splicing.
Many non-standard splice sites exist at a small percentage of splice sites throughout the human genome19. Although the minigene assay can reliably assess many variant effects on splicing, it is an in vitro assay that best performs with splicing to an exon on both sides with the canonical AG-GT splice sites. Therefore, outcomes may be confounded when assaying the first or last exon of a gene or assaying rare splice sites that do not use the standard AG-GT sites. Three candidate splice-altering VUSs were incompatible with minigene assays: KCNQ1 c.386+6T>G (first exon), SCN5A c.393–5C>T (AC-GT splice site), and SCN5A c.4437+5G>A (AG-AT splice site). These three variants were therefore studied by introducing each variant with CRISPR-Cas9 into healthy control iPSCs (PAM site variant SpliceAI scores in Supplemental Table IV), differentiating into iPSC-CMs, and then assessing splicing consequences by RT-PCR of isolated RNA20.
The RT-PCR products of the SCN5A VUS c.4437+5G>A and WT RNA were approximately the same size by gel electrophoresis (Figure 4A); however, Sanger sequencing of relevant exon junctions showed aberrant splicing of the edited allele (Figure 4B). We used negative and positive controls in all RT-PCR experiments corresponding to no PCR input and a WT SCN5A or KCNQ1 cDNA construct. TA cloning and sequencing revealed 2 major transcripts corresponding to the WT and predominant edited allele product, inducing a frameshifting variant (Figure 4B). Splicing predictions suggested a cryptic donor site that would lead to a frameshift of the transcript, matching the experimentally observed transcript (Figure 4C). The variant SCN5A c.393–5C>T was previously studied by minigene assays and shown to induce exon skipping21; however, due to the non-standard splice site usage, this specific implementation of the assay may lead to inappropriate conclusions. In the CRISPR-Cas9 iPSC-CM model, we observed no change in splicing by RT-PCR (Figures 4D and 4E). RT-PCR of the KCNQ1 VUS c.386+6T>G also showed no disruption of splicing by gel electrophoresis or by Sanger sequencing (Figures 4F and 4G). Further investigations into this variant using primers to detect large pseudoexons and NMD inhibition by cycloheximide failed to show aberrant splicing (Supplemental Figure V). Altogether, these studies implicated SCN5A c.4437+5G>A as splice-altering, and SCN5A c.393–5C>T and KCNQ1 C.386+6T>G as not splice-altering.
Figure 4. Variant functional analysis through CRISPR and iPSC-CMs.
A) RT-PCR analysis of population control iPSC-CM (designated C2) vs SCN5A c.4437+5G>A iPSC-CM. Negative control indicates no PCR input, and positive control indicates SCN5A cDNA construct. B) Sanger trace of exon 25 junction shows normal splicing of WT allele and intron retention in the CRISPR-edited allele. * indicates variant DNA base from CRISPR edit. C) Schematic depiction of predicted splicing outcome of c.4437+5G>A, consistent with experimental findings. D) RT-PCR analysis of population control iPSC-CM (designated C2) vs SCN5A c.393–5C>T iPSC-CM.E) Sanger trace of exon 3/exon 4 junction shows WT splicing of both SCN5A alleles c.393–5C and c.393–5T. F) RT-PCR analysis of population control iPSC-CM (designated C2) vs KCNQ1 c.386+6T>G iPSC-CM. G) Sanger trace of exon 1/exon 2 junction shows canonical splicing of both KCNQ1 alleles c.386+6T>G.
SpliceAI Scores of Affected Variants.
Aggregate SpliceAI scores are shown in Table 1 (individual scores are provided in Supplemental Table V). SpliceAI provides probabilities of losing or gaining a splice acceptor or donor site due to cis-genetic variation. Most variants were predicted to introduce and/or ablate a donor site as their major consequence, consistent with their position at the 3’ end of each exon (12/13). SpliceAI results were generally concordant with our experimental findings. For example, the SCN5A c.393–5C>T VUS was not predicted to alter splicing, matching the experimental findings (Figure 4C). 7/11 variants that disrupted splicing in vitro had SpliceAI scores >0.5. One exception is KCNH2 c.2145G>A, which was not highly predicted to disrupt a splice site by SpliceAI (0.25 aggregate score), but was observed in 2 LQTS patients17 and caused exon skipping in vitro. SpliceAI also successfully identified the exonic cryptic donor splice site introduced by the LP variant SCN5A c.4719C>T (0.96 aggregate score). Plotting the difference between WT and variant PSI for each minigene construct pair versus SpliceAI score revealed a modest but non-significant correlation (Supplemental Figure VI).
Table 1.
Summary of variants with aggregate SpliceAI scores, assay results, case and control frequencies, and ACMG reclassifications.
| Gene | Variant | Aggregate SpliceAI | Assay Type | Assay Result | Case / gnomAD | ACMG Post-Assay | Reclassification |
|---|---|---|---|---|---|---|---|
| SCN5A | c.393–5C>T | 0.21 | iPSC-CM | Normal | 1 / 0 | PM2, BS3, BP4 | VUS → LB |
| SCN5A | c.1890G>A | 0.72 | Minigene | Aberrant | 1 / 0 | PM2, PS3, | VUS → LP |
| SCN5A | c.4299+6T>C | 0.75 | Minigene | N/A | 1 / 0 | PM2 | VUS → VUS |
| SCN5A | c.4299G>C | 0.80 | Minigene | N/A | 1 / 0 | PM2 | VUS → VUS |
| SCN5A | c.4437+5G>A | 0.69 | iPSC-CM | Aberrant | 2 / 0 | PM2, PS3 | VUS → LP |
| SCN5A | c.4719C>T | 0.96 | Minigene | Aberrant | 2 / 2 | PM2, PS3, PS4_strong, PP3 | LP → P |
| KCNH2 | c.1128G>A | 0.34 | Minigene | Aberrant | 2 / 0 | PM2, PS3 | VUS → LP |
| KCNH2 | c.2145G>A | 0.25 | Minigene | Aberrant | 2 / 0 | PM2, PS3, BP4 | VUS → LP |
| KCNH2 | c.2398+5G>T | 0.45 | Minigene | Aberrant | 2 / 0 | PM2, PS3 | VUS → LP |
| KCNQ1 | c.386+6T>G | 0.96 | iPSC-CM | Benign | 1 / 0 | PM2, BS3, PP3 | VUS → VUS |
| KCNQ1 | c.477+5G>A | 0.75 | Minigene | Aberrant | 7 / 3 | PM2, PS3, PS4_strong | LP → P |
| KCNQ1 | c.683+5G>A | 0.29 | Minigene | Aberrant | 3 / 4 | PM2, PS3, PS4_moderate | VUS → LP |
| KCNQ1 | c.1032+5G>A | 0.95 | Minigene | Aberrant | 2 / 0 | PM2, PS3, PP3 | VUS → LP |
| KCNQ1 | c.1032G>T | 0.74 | Minigene | Aberrant | 1 / 0 | PM2, PS3 | VUS → LP |
| KCNQ1 | c.1032G>A | 0.68 | Minigene | Aberrant | 9 / 0 | PM2, PS3, PS4_strong | LP → P |
ACMG Reclassification of Putative Splice-altering Variants.
Following functional assays, we applied the PS3 criteria to 8 variants and the BS3 to 2 variants. Computational predictions from SpliceAI were also included: 2 variants meeting BP4 and 3 meeting PP3 criteria. After integrating these functional and computational findings with the previous ACMG criteria calculated by Walsh et al.17 (Supplemental Table VI), we reclassified 8/12 VUS to LP, 1 VUS to LB, and 1 LP variant to P (Table 1).
Discussion
Splicing as Disease Mechanism.
It is estimated that up to 10% of pathogenic variants may arise from aberrant splicing8. In addition to the widespread annotation of variants disrupting the canonical 2-bp splice sites as meeting the PVS1 criterion, the importance of splice-altering variants outside the canonical 2-bp splice sites has also been shown for Mendelian diseases22,23. While not all variation close to the exon/intron junction should be assumed to affect splicing, functional assays provide expedient evidence for prospective variant reclassification when observed. Although we did not use SpliceAI to help decide which variants to investigate, SpliceAI scores correlated well with functional assay results (Supplemental Figure VI). This recently developed convolutional deep neural network appears to predict aberrant splicing with much higher accuracy than previous computational predictors8. Future in vitro splicing studies might therefore prioritize variants that are predicted to be splice-altering by this algorithm. Notably, the LQTS/BrS consortium dataset did not include any examples of deep intronic variation, commonly defined as >100 bp away from the exon/intron junction24.
Functional Assays Reveal Aberrant Splicing.
We applied functional studies to reclassify a set of VUS that have been observed in clinical cohorts of BrS and LQTS patients. The minigene remains a standard assay to address the fidelity of splicing and has been deployed in many applications25,26. Although a few reports of high-throughput adaptations of the minigene system have been published, these often remain restricted to a small genomic area27 or are limited by in-frame exon triplet cassettes28. A complementary tool is RT-PCR of primary patient tissue or a secondary tissue developed from patient-derived iPSCs when primary tissue is unavailable. Studying candidate arrhythmia variants in a cardiomyocyte context naturally provides more information than the in vitro minigene assays alone. For example, investigators found many more splicing aberrations in an iPSC-CM model than in minigene assays alone for the LQTS-associated splice variant c.1032G>A18. We observed aberrant splicing in 1/3 of our CRISPR edited iPSC-CM assays. We did not detect aberrant splicing for the SCN5A c.393–5C>T variant in iPSC-CMs, which was discordant with a previous study that showed exon skipping in a minigene assay21. As mentioned above, however, the use of non-canonical splice sites in minigene assays may have caused the previously observed exon skipping events. Further supporting our results is the high frequency of the related c.393–5C>A variant: an allele count of 45 in gnomAD, ~8x higher than the 2.5e-5 cutoff that we and others have used29.
Aberrant Splicing-associated Cardiac Morbidity.
Aberrant splicing is increasingly recognized as a driver of cardiovascular disease. Most of such variation has been implicated in cardiomyopathy genes22,23,30–32. A recent functional genomics variant reclassification effort examined aberrant splicing from a set of 56 hypertrophic cardiomyopathy (HCM) probands with MYBPC3 variants. The investigators performed RNA analysis of 9 such variants outside the canonical splice sites from venous blood or myocardial tissue, and were able to reclassify 6 variants (4 VUS -> LP, and 2 LP -> P)32. In a follow up study, the investigators used patient-derived iPSC-CMs followed by RNA-seq to identify 2 known and 1 novel deep intronic splice variants in HCM probands30. The investigators also found that rationally designed antisense oligonucleotides (ASOs) could correct the aberrant splicing of one of the three variants. In a similar study, investigators combined SpliceAI with cohort WGS to identify known splice-altering variants and a novel splice-altering variant in MYBPC3 from studies of peripheral blood and RT-PCR31. A complementary study jointly investigated splice-altering variants in LMNA, a dilated cardiomyopathy (DCM) associated gene, and MYBPC3 using computational tools to prioritize variants, and a high-throughput sequencing-based minigene platform23. The authors also extended this system to study non-canonical splice-altering variants in TTN22. Aberrant splicing in the cardiac arrhythmias is comparatively less studied. A seminal study identified a branch point-altering variant in KCNH2 that elicited the LQTS phenotype in a multigenerational family using patient tissue and minigene assays33. More recently, a deep intronic splice-altering variant in KCNH2 was identified in a genotype-negative LQTS family and functionally characterized using a similar CRISPR iPSC-CM framework as used in this study34.
In silico Prediction Concordance.
We found that SpliceAI agreed with our functional assays in most cases (Table 1). For example, our RT-PCR studies of the SCN5A c.393–5C>T variant agreed with the benign predictions of SpliceAI to have little effect. The SCN5A c.4719C>T variant was also strongly predicted by SpliceAI to introduce a new cryptic exonic donor site, consistent with the truncation event observed in our study. In contrast, we observed divergent outcomes with the KCNQ1 c.386+6T>G variant, where we observed no splicing abnormality despite high predictions of disruption (0.96 aggregate score). Further studies excluding large pseudoexon retention and NMD inhibition experiments did not yield evidence of aberrant splicing. SpliceAI also was highly predictive of disrupted splicing at the +5 position (4/5 variant studied had >0.5 aggregate SpliceAI score). The +5 position has frequently been invoked in disease and is particularly intolerant to variation in evolutionary studies35. Experimentally, a recent high-throughput study of non-canonical splice-altering TTN variants also found a large enrichment of splice-altering variants at this position22.
Therapeutic Opportunities.
Splice-altering variants comprise an eminently targetable class of genetic variation, with therapeutic modalities spanning ASOs, traditional small molecules, and emerging gene editing platforms. ASO technologies that leverage coding variation benefit by retaining pharmacokinetic and pharmacodynamic properties over a broad range of possible sequence targets36. Indeed, ‘N of 1’ drug discovery has already been implemented clinically37, and has even precipitated new regulatory processes in anticipation of increased translational opportunities38. Complementary mechanism-agnostic gene therapy approaches such as the ‘suppression-replacement’ strategy may be capable of treating a variety of variant classes in a specified gene, including those that alter splicing39. Altogether, the characterization of splice-altering variants may prioritize therapeutic opportunities in the genetic arrhythmias.
Future Directions.
Future studies can apply advances multiplexed assays to enable rapid high-throughput prospective evaluation of splice-perturbing variants. Further characterizing the fraction of splice-altering variants responsible for the Mendelian channelopathies may inspire future investigations in this area.
Limitations.
HEK293 cells do not capture the full environment of a native cardiomyocyte. Trans-acting proteins may also affect splicing outcomes in native cardiomyocytes. Although minigene assays offer clear functional readouts, the exact consequences of the reading frame in a human gene may remain cryptic due to the limited intronic window of the assay. Predicted termination codons beyond the 100 bp of cloned intronic DNA are therefore necessarily inferred from the observed reading frames. The variants in this study represent only a small fraction of the total quantity of possible splice site-adjacent variants in these three arrythmia-associated genes. Splicing is a complex and stochastic process with a continuum of effect. The current ACMG guidelines do not capture the nuance of this behavior, and only provide a dichotomous (yes/no) framework for variant effect interpretation in this context. An additional challenge is the use of in silico predictors for variants adjacent to exons that use non-canonical splice sites, given the relative paucity of training data. A limitation of the CRISPR iPSC-CM approach is the necessity of an additional PAM site-disrupting variant to prevent Cas9 re-cutting. Although the SpliceAI predictions were low for each variant, we cannot exclude the possibility that a PAM variant could influence the splicing outcome (Supplemental Table IV). In the future, technologies such as base editors or prime editors that do not require a PAM-disrupting variant may be preferred40.
Conclusions
We deployed functional assays to reclassify 10 variants near canonical splice sites observed in BrS and LQTS probands. This work suggests that splice-altering variation may play a prominent role in the genetic etiology of Mendelian arrhythmias, consistent with recent work defining splice-altering substrates in the cardiomyopathies22,23,30–32. Methods to rapidly assess suspected splice-altering variation will play an important role in the continued realization of personalized medicine for diagnostic, and potentially therapeutic purposes.”
Supplementary Material
Sources of Funding:
Flow Cytometry experiments were performed in the VMC Flow Cytometry Shared Resource. The VMC Flow Cytometry Shared Resource is supported by the Vanderbilt Ingram Cancer Center (P30 CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404). This research was funded by NIH grants T32GM007347 and American Heart Association 907581 (MJO), the Heart Rhythm Society Clinical Research Award in Honor of Mark Josephson and Hein Wellens (Y.W.), American Heart Association 830951 (Y.W.), R00 HG010904 (AMG), and R01 HL149826 (DMR).
Nonstandard Abbreviations and Acronyms
- ACMG
American College of Medical Genetics and Genomics
- ASO
Antisense Oligonucleotide
- BrS
Brugada Syndrome
- DCM
Dilated Cardiomyopathy
- GoF
Gain-of-Function
- HCM
Hypertrophic Cardiomyopathy
- iPSC-CM
induced Pluripotent Stem Cell-Cardiomyocyte
- LoF
Loss-of-Function
- LQTS
Long QT Syndrome
- NMD
Nonsense Mediated Decay
- PAM
Protospacer Adjacent Motif
- PSI
Percent Spliced In
- SCD
Sudden Cardiac Death
- VUS
Variant of Uncertain Significance
Footnotes
Disclosures: None
References:
- 1.Adler A, Novelli V, Amin AS, Abiusi E, Care M, Nannenberg EA, Feilotter H, Amenta S, Mazza D, Bikker H, et al. An International, Multicentered, Evidence-Based Reappraisal of Genes Reported to Cause Congenital Long QT Syndrome. Circulation. 2020;141:418–428. doi: 10.1161/circulationaha.119.043132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hosseini SM, Kim R, Udupa S, Costain G, Jobling R, Liston E, Jamal SM, Szybowska M, Morel CF, Bowdin S, et al. Reappraisal of Reported Genes for Sudden Arrhythmic Death: Evidence-Based Evaluation of Gene Validity for Brugada Syndrome. Circulation. 2018;138:1195–1205. doi: 10.1161/circulationaha.118.035070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwartz PJ, Ackerman MJ, Antzelevitch C, Bezzina CR, Borggrefe M, Cuneo BF, Wilde AAM. Inherited cardiac arrhythmias. Nat Rev Dis Primers. 2020;6:58. doi: 10.1038/s41572-020-0188-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glazer AM, Davogustto G, Shaffer CM, Vanoye CG, Desai RR, Farber-Eger EH, Dikilitas O, Shang N, Pacheco JA, Yang T, et al. Arrhythmia variant associations and reclassifications in the eMERGE-III sequencing study. medRxiv. 2021:2021.2003.2030.21254549. doi: 10.1101/2021.03.30.21254549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parikh VN. Promise and Peril of Population Genomics for the Development of Genome-First Approaches in Mendelian Cardiovascular Disease. Circ Genom Precis Med. 2021;14:e002964. doi: 10.1161/circgen.120.002964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581:434–443. doi: 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, Kosmicki JA, Arbelaez J, Cui W, Schwartz GB, et al. Predicting Splicing from Primary Sequence with Deep Learning. Cell. 2019;176:535–548.e524. doi: 10.1016/j.cell.2018.12.015 [DOI] [PubMed] [Google Scholar]
- 9.Sibley CR, Blazquez L, Ule J. Lessons from non-canonical splicing. Nat Rev Genet. 2016;17:407–421. doi: 10.1038/nrg.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pertea M, Lin X, Salzberg SL. GeneSplicer: a new computational method for splice site prediction. Nucleic Acids Res. 2001;29:1185–1190. doi: 10.1093/nar/29.5.1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yeo G, Burge CB. Maximum entropy modeling of short sequence motifs with applications to RNA splicing signals. J Comput Biol. 2004;11:377–394. doi: 10.1089/1066527041410418 [DOI] [PubMed] [Google Scholar]
- 12.Hanses U, Kleinsorge M, Roos L, Yigit G, Li Y, Barbarics B, El-Battrawy I, Lan H, Tiburcy M, Hindmarsh R, et al. Intronic CRISPR Repair in a Preclinical Model of Noonan Syndrome-Associated Cardiomyopathy. Circulation. 2020;142:1059–1076. doi: 10.1161/circulationaha.119.044794 [DOI] [PubMed] [Google Scholar]
- 13.Bastarache L, Hughey JJ, Hebbring S, Marlo J, Zhao W, Ho WT, Van Driest SL, McGregor TL, Mosley JD, Wells QS, et al. Phenotype risk scores identify patients with unrecognized Mendelian disease patterns. Science. 2018;359:1233–1239. doi: 10.1126/science.aal4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laššuthová P, Gregor M, Sarnová L, Machalová E, Sedláček R, Seeman P. Clinical, in silico, and experimental evidence for pathogenicity of two novel splice site mutations in the SH3TC2 gene. J Neurogenet. 2012;26:413–420. doi: 10.3109/01677063.2012.711398 [DOI] [PubMed] [Google Scholar]
- 15.Cho SY, Lau EY, Luk DC, Law CY, Lai CK, Lam CW. Novel PPOX exonic mutation inducing aberrant splicing in a patient with homozygous variegate porphyria. Clin Chim Acta. 2021;512:117–120. doi: 10.1016/j.cca.2020.10.033 [DOI] [PubMed] [Google Scholar]
- 16.Bardai A, Amin AS, Blom MT, Bezzina CR, Berdowski J, Langendijk PN, Beekman L, Klemens CA, Souverein PC, Koster RW, et al. Sudden cardiac arrest associated with use of a non-cardiac drug that reduces cardiac excitability: evidence from bench, bedside, and community. Eur Heart J. 2013;34:1506–1516. doi: 10.1093/eurheartj/eht054 [DOI] [PubMed] [Google Scholar]
- 17.Walsh R, Lahrouchi N, Tadros R, Kyndt F, Glinge C, Postema PG, Amin AS, Nannenberg EA, Ware JS, Whiffin N, et al. Enhancing rare variant interpretation in inherited arrhythmias through quantitative analysis of consortium disease cohorts and population controls. Genet Med. 2021;23:47–58. doi: 10.1038/s41436-020-00946-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuriyanghai Y, Makiyama T, Sasaki K, Kamakura T, Yamamoto Y, Hayano M, Harita T, Nishiuchi S, Chen J, Kohjitani H, et al. Complex aberrant splicing in the induced pluripotent stem cell-derived cardiomyocytes from a patient with long QT syndrome carrying KCNQ1-A344Aspl mutation. Heart Rhythm. 2018;15:1566–1574. doi: 10.1016/j.hrthm.2018.05.028 [DOI] [PubMed] [Google Scholar]
- 19.Burset M, Seledtsov IA, Solovyev VV. Analysis of canonical and non-canonical splice sites in mammalian genomes. Nucleic Acids Res. 2000;28:4364–4375. doi: 10.1093/nar/28.21.4364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, Zhang F. Genome engineering using the CRISPR-Cas9 system. Nat Protoc. 2013;8:2281–2308. doi: 10.1038/nprot.2013.143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frisso G, Detta N, Coppola P, Mazzaccara C, Pricolo MR, D’Onofrio A, Limongelli G, Calabrò R, Salvatore F. Functional Studies and In Silico Analyses to Evaluate Non-Coding Variants in Inherited Cardiomyopathies. Int J Mol Sci. 2016;17. doi: 10.3390/ijms17111883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel PN, Ito K, Willcox JAL, Haghighi A, Jang MY, Gorham JM, DePalma SR, Lam L, McDonough B, Johnson R, et al. Contribution of Noncanonical Splice Variants to TTN Truncating Variant Cardiomyopathy. Circ Genom Precis Med. 2021;14:e003389. doi: 10.1161/circgen.121.003389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ito K, Patel PN, Gorham JM, McDonough B, DePalma SR, Adler EE, Lam L, MacRae CA, Mohiuddin SM, Fatkin D, et al. Identification of pathogenic gene mutations in LMNA and MYBPC3 that alter RNA splicing. Proc Natl Acad Sci U S A. 2017;114:7689–7694. doi: 10.1073/pnas.1707741114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaz-Drago R, Custódio N, Carmo-Fonseca M. Deep intronic mutations and human disease. Hum Genet. 2017;136:1093–1111. doi: 10.1007/s00439-017-1809-4 [DOI] [PubMed] [Google Scholar]
- 25.Gaildrat P, Killian A, Martins A, Tournier I, Frébourg T, Tosi M. Use of splicing reporter minigene assay to evaluate the effect on splicing of unclassified genetic variants. Methods Mol Biol. 2010;653:249–257. doi: 10.1007/978-1-60761-759-4_15 [DOI] [PubMed] [Google Scholar]
- 26.Gao D, Morini E, Salani M, Krauson AJ, Chekuri A, Sharma N, Ragavendran A, Erdin S, Logan EM, Li W, et al. A deep learning approach to identify gene targets of a therapeutic for human splicing disorders. Nat Commun. 2021;12:3332. doi: 10.1038/s41467-021-23663-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gergics P, Smith C, Bando H, Jorge AAL, Rockstroh-Lippold D, Vishnopolska SA, Castinetti F, Maksutova M, Carvalho LRS, Hoppmann J, et al. High-throughput splicing assays identify missense and silent splice-disruptive POU1F1 variants underlying pituitary hormone deficiency. Am J Hum Genet. 2021. doi: 10.1016/j.ajhg.2021.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheung R, Insigne KD, Yao D, Burghard CP, Wang J, Hsiao YE, Jones EM, Goodman DB, Xiao X, Kosuri S. A Multiplexed Assay for Exon Recognition Reveals that an Unappreciated Fraction of Rare Genetic Variants Cause Large-Effect Splicing Disruptions. Mol Cell. 2019;73:183–194.e188. doi: 10.1016/j.molcel.2018.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whiffin N, Minikel E, Walsh R, O’Donnell-Luria AH, Karczewski K, Ing AY, Barton PJR, Funke B, Cook SA, MacArthur D, et al. Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med. 2017;19:1151–1158. doi: 10.1038/gim.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Holliday M, Singer ES, Ross SB, Lim S, Lal S, Ingles J, Semsarian C, Bagnall RD. Transcriptome Sequencing of Patients With Hypertrophic Cardiomyopathy Reveals Novel Splice-Altering Variants in MYBPC3. Circ Genom Precis Med. 2021;14:e003202. doi: 10.1161/circgen.120.003202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lopes LR, Barbosa P, Torrado M, Quinn E, Merino A, Ochoa JP, Jager J, Futema M, Carmo-Fonseca M, Monserrat L, et al. Cryptic Splice-Altering Variants in MYBPC3 Are a Prevalent Cause of Hypertrophic Cardiomyopathy. Circ Genom Precis Med. 2020;13:e002905. doi: 10.1161/circgen.120.002905 [DOI] [PubMed] [Google Scholar]
- 32.Singer ES, Ingles J, Semsarian C, Bagnall RD. Key Value of RNA Analysis of MYBPC3 Splice-Site Variants in Hypertrophic Cardiomyopathy. Circ Genom Precis Med. 2019;12:e002368. doi: 10.1161/circgen.118.002368 [DOI] [PubMed] [Google Scholar]
- 33.Crotti L, Lewandowska MA, Schwartz PJ, Insolia R, Pedrazzini M, Bussani E, Dagradi F, George AL Jr., Pagani F. A KCNH2 branch point mutation causing aberrant splicing contributes to an explanation of genotype-negative long QT syndrome. Heart Rhythm. 2009;6:212–218. doi: 10.1016/j.hrthm.2008.10.044 [DOI] [PubMed] [Google Scholar]
- 34.Tobert KE, Tester DJ, Zhou W, Haglund-Turnquist CM, Giudicessi JR, Ackerman MJ. Genome Sequencing in a Genetically Elusive Multi-Generational Long QT Syndrome Pedigree Identifies a Novel LQT2-Causative Deeply Intronic KCNH2 Variant. Heart Rhythm. 2022. doi: 10.1016/j.hrthm.2022.02.004 [DOI] [PubMed] [Google Scholar]
- 35.Lord J, Gallone G, Short PJ, McRae JF, Ironfield H, Wynn EH, Gerety SS, He L, Kerr B, Johnson DS, et al. Pathogenicity and selective constraint on variation near splice sites. Genome Res. 2019;29:159–170. doi: 10.1101/gr.238444.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crooke ST, Baker BF, Crooke RM, Liang XH. Antisense technology: an overview and prospectus. Nat Rev Drug Discov. 2021;20:427–453. doi: 10.1038/s41573-021-00162-z [DOI] [PubMed] [Google Scholar]
- 37.Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, Pendergast MK, Goldkind SF, Lee EA, Kuniholm A, et al. Patient-Customized Oligonucleotide Therapy for a Rare Genetic Disease. N Engl J Med. 2019;381:1644–1652. doi: 10.1056/NEJMoa1813279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brierley J, Aylett S, Archard D. Framework for “N-of-1” Experimental Therapies. N Engl J Med. 2020;382:e7. doi: 10.1056/NEJMc1915778 [DOI] [PubMed] [Google Scholar]
- 39.Dotzler SM, Kim CSJ, Gendron WAC, Zhou W, Ye D, Bos JM, Tester DJ, Barry MA, Ackerman MJ. Suppression-Replacement KCNQ1 Gene Therapy for Type 1 Long QT Syndrome. Circulation. 2021. doi: 10.1161/circulationaha.120.051836 [DOI] [PubMed] [Google Scholar]
- 40.Anzalone AV, Koblan LW, Liu DR. Genome editing with CRISPR-Cas nucleases, base editors, transposases and prime editors. Nat Biotechnol. 2020;38:824–844. doi: 10.1038/s41587-020-0561-9 [DOI] [PubMed] [Google Scholar]
- 41.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Concordet JP, Haeussler M. CRISPOR: intuitive guide selection for CRISPR/Cas9 genome editing experiments and screens. Nucleic Acids Res. 2018;46:W242–w245. doi: 10.1093/nar/gky354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wada Y, Yang T, Shaffer CM, Daniel LL, Glazer AM, Davogustto GE, Lowery BD, Farber-Eger E, Wells QS, Roden DM. Common Ancestry-specific Ion Channel Variants Predispose to Drug-induced Arrhythmias. Circulation. 2022. doi: 10.1161/circulationaha.121.054883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burridge PW, Matsa E, Shukla P, Lin ZC, Churko JM, Ebert AD, Lan F, Diecke S, Huber B, Mordwinkin NM, et al. Chemically defined generation of human cardiomyocytes. Nat Methods. 2014;11:855–860. doi: 10.1038/nmeth.2999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blakes AJM, Wai H, Davies I, Moledian HE, Ruiz A, Thomas T, Bunyan D, Thomas NS, Burren CP, Greenhalgh L, et al. A systematic analysis of splicing variants identifies new diagnoses in the 100,000 Genomes Project. medRxiv. 2022:2022.2001.2028.22270002. doi: 10.1101/2022.01.28.22270002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Valenzuela-Palomo A, Bueno-Martínez E, Sanoguera-Miralles L, Lorca V, Fraile-Bethencourt E, Esteban-Sánchez A, Gómez-Barrero S, Carvalho S, Allen J, García-Álvarez A, et al. Splicing predictions, minigene analyses, and ACMG-AMP clinical classification of 42 germline PALB2 splice-site variants. J Pathol. 2021. doi: 10.1002/path.5839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fraile-Bethencourt E, Díez-Gómez B, Velásquez-Zapata V, Acedo A, Sanz DJ, Velasco EA. Functional classification of DNA variants by hybrid minigenes: Identification of 30 spliceogenic variants of BRCA2 exons 17 and 18. PLoS Genet. 2017;13:e1006691. doi: 10.1371/journal.pgen.1006691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kleinberger J, Maloney KA, Pollin TI, Jeng LJ. An openly available online tool for implementing the ACMG/AMP standards and guidelines for the interpretation of sequence variants. In: Genet Med. 2016:1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.