Abstract
Legionella pneumophila does not induce apoptosis in the protozoan host, but induces pore formation-mediated cytolysis after termination of intracellular replication (L.-Y. Gao and Y. Abu Kwaik, Environ. Microbiol. 2:79–90, 2000). In contrast to this single mode of killing of protozoa, we have recently proposed a biphasic model by which L. pneumophila kills macrophages, in which the first phase is manifested through the induction of apoptosis during early stages of the infection, followed by an independent and temporal induction of necrosis during late stages of intracellular replication. Here we show that, similar to the protozoan host, the induction of necrosis and cytolysis of macrophages by L. pneumophila is mediated by the pore-forming toxin or activity. This activity is temporally and maximally expressed only upon termination of bacterial replication and correlates with cytolysis of macrophages and alveolar epithelial cells in vitro. We have identified five L. pneumophila mutants defective in the pore-forming activity. The phagosomes harboring the mutants do not colocalize with the late endosomal or lysosomal marker Lamp-1, and the mutants replicate intracellularly similar to the parental strain. Interestingly, despite their prolific intracellular replication, the mutants are defective in cytotoxicity and are “trapped” within and fail to lyse and egress from macrophages and alveolar epithelial cells upon termination of intracellular replication. However, the mutants are subsequently released from the host cell, most likely due to apoptotic death of the host cell. Data derived from cytotoxicity assays, confocal laser scanning microscopy, and electron microscopy confirm the defect in the mutants to induce necrosis of macrophages and the failure to egress from the host cell. Importantly, the mutants are completely defective in acute lethality (24 to 48 h) to intratracheally inoculated A/J mice. We conclude that the pore-forming activity of L. pneumophila is not required for phagosomal trafficking or for intracellular replication. This activity is expressed upon termination of bacterial replication and is essential to induce cytolysis of infected macrophages to allow egress of intracellular bacteria. In addition, this activity plays a major role in pulmonary immunopathology in vivo.
Recent studies have revealed new and exciting insights into the mechanisms of entry of intracellular pathogens into the host cell, their exploitation of the signal transduction and apoptotic pathways, and their modulation of the biogenesis of their vacuoles into idiosyncratic niches that evade fusion to the lysosomes, or their escape into the cytoplasm (22, 42). A fundamental step in the pathogenic cycle of intracellular pathogens is their ability to lyse and egress from the host cell after termination of intracellular replication, to infect other cells within the same host, or to be transmitted to a new susceptible host. The mechanisms by which vacuolar intracellular pathogens such as Mycobacterium, Chlamydia, Toxoplasma, Leishmania, Salmonella Coxiella, Brucella, Francisella, and Legionella induce cytolysis and egress from the host cell after its exploitation for intracellular proliferation are not known.
Legionella pneumophila is a gram-negative bacterium that is ubiquitous in the aquatic environment, where the bacterium invades and replicates within protozoa (8, 11, 31, 32, 54–56). Upon transmission to the human host, L. pneumophila invades alveolar macrophages and possibly epithelial cells (1, 7, 25). Within minutes of entry into the host cell, the bacteria modulate the biogenesis of their vacuole into a niche (16, 46) that evades maturation along the “default” endosomal-lysosomal degradation pathway (34) and is subsequently surrounded by the rough endoplasmic reticulum (3, 33, 53). Formation of this replicative niche is controlled by a type IV-like secretion machinery, designated Dot or Icm (48, 57), that functions in a cis-like manner to modulate the biogenesis of the phagosome, without affecting other endocytic fusion events within the infected cell (17). The bacteria replicate in this idiosyncratic niche, causing Legionnaires' disease, a potentially fatal pneumonia (1). Intracellular replication of L. pneumophila in the alveolar spaces is the hallmark of Legionnaires' disease (1).
Pulmonary histopathology of Legionnaires' disease patients and L. pneumophila-infected experimental animals is characterized by the extensive cytolysis of inflammatory cells in the alveolar spaces and necrosis of the alveolar epithelium (13, 36), which has been proposed to be mediated by a cytotoxin (58). In addition, bacterial growth-independent acute death (i.e., within 48 h) of mice is manifested following intratracheal inoculation with a high dose of the bacteria, and this acute lethality is thought to be mediated by a cytotoxin (14). A pore-forming toxin or activity has been described recently for L. pneumophila (35, 39). Upon contact with cellular membranes, this activity is manifested in insertion of pores <3 nm in diameter that allow the passage of molecules <1,500 Da (35, 39). However, the role of this activity in the intracellular infection and its potential role in the pulmonary immunopathology of Legionnaires' disease are not known (59).
Along with other vacuolar intracellular pathogens, the prolific intracellular replication of L. pneumophila culminates in filling the host cell with bacteria and subsequent killing of the host cell to allow egress of intracellular bacteria (1). The mechanisms of killing of the host cell and release of intracellular bacteria after termination of intracellular replication are not known for L. pneumophila or any other vacuolar intracellular pathogen. It has been presumed that the physical and metabolic burden on the host cell by a large number of intracellular bacteria is sufficient to kill the host cell by nonspecific means. We have recently shown that L. pneumophila does not induce apoptosis in the protozoan host, but does induce pore formation-mediated cytolysis after termination of intracellular replication, and mutants defective in pore formation fail to egress from the protozoan host (21, 31). In contrast to this single mode of killing of protozoa, we have recently proposed a model of biphasic death of mammalian cells by L. pneumophila (19, 20). The first phase is mediated by a growth-phase-independent induction of caspase-3-mediated apoptosis during early stages of the infection (19, 20, 43), while the second phase is mediated by necrotic damage upon growth transition of the bacteria into the postexponential phase (15). We have speculated (20) that this growth-phase-dependent necrotic damage is mediated by the pore-forming toxin or activity (35, 39).
In this report, we provide evidence that the pore-forming activity of L. pneumophila is temporally and maximally expressed upon termination of bacterial replication in vitro and intracellularly and is essential for cytolysis of macrophages and for the release of intracellular bacteria. Importantly, the pore-forming toxin plays a major role in the pathogenesis of Legionnaires' disease in experimental animals. This fascinating L. pneumophila-regulated cytolysis of macrophages may illuminate a similar mechanism utilized by other vacuolar intracellular pathogens to spare killing of the host cell during intracellular proliferation and to kill and egress from the spent host cell upon termination of intracellular replication.
MATERIALS AND METHODS
Bacterial strains and host cells.
The virulent parental strain AA100 of L. pneumophila and construction of the mini-Tn10::kan bank of mutants have been described previously (24). Bacteria were grown on artificial media as previously described (24, 51). The parental strain AA100 was grown on buffered charcoal-yeast extract (BCYE) agar plates or in buffered yeast extract (BYE) broth, and for the mutants, the media were supplemented with 50 μg of kanamycin per ml (24).
The human macrophage-like cell line U937 was maintained and differentiated into macrophage-like cells as described previously (24). Human type I alveolar epithelial cells (American Type Culture Collection; WI-26 VA4) were maintained as described previously (25).
Cytopathogenicity to and intracellular growth of L. pneumophila within U937 macrophages and WI-26 alveolar epithelial cells.
L. pneumophila strains were grown on BCYE plates for 3 days prior to infection. Monolayers in 96-well plates containing 105 cells/well were infected, in triplicates, for 1 h followed by washing and killing of extracellular bacteria with 50 μg of gentamicin per ml. Cytopathogenicity was determined by using the vital dye Alamar blue (Alamar Bioscience, Inc., Sacramento, Calif.), as previously described (6). The number of bacteria in the monolayers at several time intervals after the 1-h infection period was determined, as previously described (23).
Analysis of contact-dependent pore formation by L. pneumophila.
Monolayers were infected by strain AA100 or the rib mutants at a multiplicity of infection (MOI) of 500, as previously described (39). Permeability to propidium iodide (PI) (0.5 μg/ml) was determined by epifluorescence microscopy. At least 100 cells/sample were counted for multiple independent samples. To examine pore formation in membranes of infected cells by intracellular bacteria, the monolayers were infected by strain AA100 or the rib mutants at an MOI of 5, followed by washing and killing of extracellular bacteria, exactly as described above. At the indicated time points postinfection, the cells were stained with PI and examined as described above.
Contact-dependent pore formation in plasma membrane was also determined by examining hemolysis of sheep erythrocytes (sRBCs) by L. pneumophila at an MOI of 25 following 20 to 60 min of bacterial-sRBC contact, as previously described (39). To examine pore formation by intracellular bacteria, U937 macrophages were infected by strain AA100 at an MOI of 5 for 1 h, followed by killing of extracellular bacteria with gentamicin as described above. At several time intervals postinfection, intracellular bacteria were isolated by Percoll density gradient, as we previously described (6), and hemolytic activity was examined at an MOI of 25, as described above.
Analysis of apoptosis in L. pneumophila-infected U937 macrophages and WI-26 alveolar epithelial cells.
Monolayers of U937 macrophages and WI-26 alveolar epithelial cells were infected at an MOI of 10, exactly as described above. Terminal deoxytransferase-mediated dUTP nick-end labeling (TUNEL) assays were performed with the cell death detection kit (Boehringer Mannheim Corporation, Indianapolis, Ind.) and examined on a Leica TCS NT confocal laser microscope, exactly as we described previously (19, 20, 26). Apoptosis in U937 macrophages was also examined by detection of the surface exposure of phosphatidylserine (PS) by using the Annexin-V-FLUOS staining kit (Boehringer Mannheim GmbH, Mannheim, Germany), and examined by confocal laser microscopy, exactly as we described previously (20). A minimum of 100 cells per sample were counted by epifluorescence microscopy, and multiple independent samples were examined.
Confocal laser scanning and transmission electron microscopy.
Colocalization of L. pneumophila with Lamp-1 in infected U937 macrophages was performed and samples were examined by confocal laser scanning microscopy, exactly as described previously (28) (at least 100 infected cells were examined). Samples were analyzed with a Leica TCS SP laser scanning confocal microscope (Leica Microscopy and Scientific Instruments Group, Heerburg, Switzerland), equipped with three lasers: an argon laser (488-nm excitation line), a krypton laser (568-nm excitation line), and a helium neon laser (633-nm excitation line).
For transmission electron microscopy, U937 macrophages were infected with L. pneumophila at an MOI of 1 for 1 h, followed by extensive washing of extracellular bacteria and further incubation for several time intervals. Preparation of ultrathin sections was performed, and the sections were examined with a Hitachi H-7000/STEM electron microscope (Hitachi, Inc., Japan) at 75 KV, as described previously (23).
Inoculation of A/J mice.
Female pathogen-free A/J mice 8 to 9 weeks of age were inoculated intratracheally, as described previously (25), with the exception that a suspension of approximately 109 CFU of bacteria in 50 μl of water was injected directly into the trachea. The mice were observed twice daily for lethality.
DNA manipulations.
Transfections, restriction enzyme digestions, and DNA ligations were performed as described elsewhere, unless specified otherwise (10). Restriction enzymes and T4 DNA ligase were purchased from Promega (Madison, Wis.). Plasmid and cosmid DNA preparations were performed with the Bio-Rad Quantum miniprep kit (Bio-Rad Laboratories, Hercules, Calif.) and the polyethylene glycol DNA extraction protocol, as described elsewhere (47). Electroporations were performed with a Bio-Rad Gene Pulser, as recommended by the manufacturer. DNA probes for Southern hybridization were generated by PCR amplification with a Perkin-Elmer Gene Amp PCR system 2400 (Perkin-Elmer, Norwalk, Conn.). Purification of DNA fragments from agarose gels for subcloning or labeling was carried out with a QIAquick gel purification kit (Qiagen Inc., Chatsworth, Calif.). Fluorescein labeling of DNA probes for Southern hybridization was performed with the ECL (enhanced chemiluminescence) Random Prime Labeling System, version II (Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). Transfer of DNA from agarose gels onto membranes, fluorescein labeling of DNA probes, hybridizations, and detection were performed as previously described (10). Oligonucleotide synthesis for PCR and sequencing was performed by Integrated DNA Technologies, Inc. (Coralville, Calif.). Sequencing was carried out by the University of Kentucky Macromolecular Structure Analysis Facility (Lexington, Ky.). Sequence analysis and comparisons were performed with MacVector (Oxford Molecular Group, Inc., Campbell, Calif.), AssemblyLign, BlastX, GCG SeqWeb, and ProfileScan.
Genetic characterization of the rib mutants.
The L. pneumophila chromosomal cosmid library has been described previously (49). The plasmid pBC-SK+ was used to subclone L. pneumophila DNA (Stratagene, La Jolla, Calif.), and Escherichia coli strain DH5α (BRL, Gaithersburg, Md.) was used for the majority of cloning experiments. The plasmid pUC-4K was purchased from Pharmacia (Piscataway, N.J.), and was the source of the kanamycin resistance gene used as a probe for Southern hybridization. L. pneumophila chromosomal DNA was prepared by using the Puregene DNA isolation kit (Gentra Systems, Minneapolis, Minn.).
Clones harboring all of the dot and icm genes were obtained from H. Shuman and R. Isberg and were used as probes. The kan insertion and flanking genomic DNA from the mutants was cloned into pBC and used to probe the cosmid library, as we described previously (49). These constructs were also introduced into the parental strain AA100 of L. pneumophila by the natural transformation procedure to reconstruct the insertion, exactly as we described previously (50). The reconstruction of the insertion was confirmed by Southern hybridizations with the flanking L. pneumophila DNA and the kan cassette as probes.
RESULTS
Identification of L. pneumophila mutants competent for intracellular replication, but defective in cytotoxicity and cytolysis of macrophages and alveolar epithelial cells.
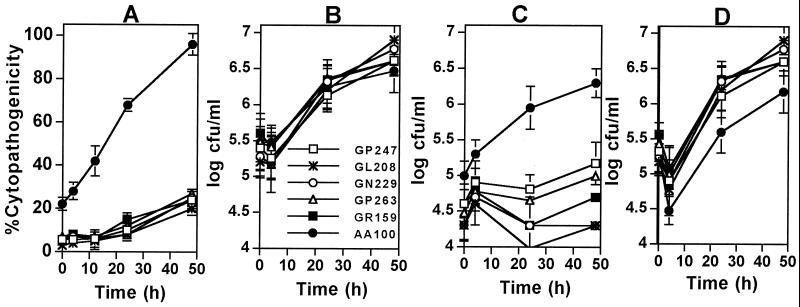
We have previously identified the pmi and mil mutants of L. pneumophila that are defective in both cytotoxicity and intracellular replication, and the degree of both defects are correlated (23, 24). During our screening of the miniTn10::kan mutant library (∼5,000 clones) of L. pneumophila (23, 24) for the pmi and mil mutants, we discovered five mutants (GP247, GL208, GN229, GP263, and GR159) that were severely defective in their cytotoxicity, but replicated similar to the parental strain AA100 within U937 macrophages and type I alveolar epithelial cells (MOI of 5) (Fig. 1A and B) (data not shown). All of the intracellular bacteria belonging to the parental strain were released into the tissue culture medium within 24 to 48 h postinfection (Fig. 1C and D) (data not shown). In contrast, despite the prolific intracellular replication of the five mutants, they were “trapped” within and failed to egress from macrophages and epithelial cells during the 48-h infection, and the majority of the infected cells remained viable and intact (Fig. 1C and D) (data not shown). Phase-contrast images of the infection showed that the AA100-infected cells underwent complete cytolysis within 24 to 48 h postinfection, concomitant with termination of intracellular replication, while cells infected by the mutants were intact during this period (data not shown). However, after 72 h postinfection, the viability of the cells infected by the mutants declined gradually, and the bacteria were subsequently released (see below).
FIG. 1.
The L. pneumophila mutants are defective in killing and exiting U937 macrophages, but not in intracellular replication. (A) Cytopathogenicity to infected cells (MOI of 5) was determined by Alamar blue assays and compared to that of the noninfected cells. (B) Growth kinetics within U937 macrophages. The indicated numbers of bacteria represent the combined numbers of intracellular bacteria and bacteria released into the supernatant. (C) Bacteria released into the tissue culture medium. (D) Intracellular bacteria. Values are the means of triplicate samples, and error bars represent standard deviations.
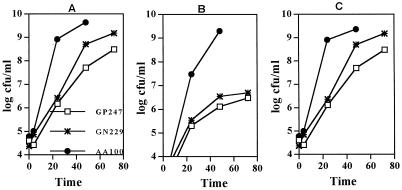
The data predicted that if an infection by the mutants is carried out at a low MOI, the mutants would be successful in intracellular replication during the primary infection, but would fail to initiate a secondary infection due to the defect in cytolysis of the primary host cell. Interestingly, when U937 macrophages were infected by two of the mutants (GP247 and GN229) at an MOI of 0.5, approximately 600-fold less mutant bacteria were recovered by 24 and 48 h postinfection compared to the parental strain (Fig. 2). Importantly, 100- and 1,000-fold more wild-type bacteria were recovered from the supernatant at 24 and 48 h postinfection, respectively, compared to the mutants (Fig. 2). Compared to the remarkable destruction and cytolysis of macrophages and epithelial cells infected by the wild-type strain by 48 h postinfection, the cells infected by the mutants remained viable and intact for at least 48 h, despite the prolific intracellular bacterial replication (Fig. 3). However, after 72 h postinfection, the viability of the cells infected by mutants started to decline.
FIG. 2.
Intracellular growth kinetics and egress of U937 macrophages infected at an MOI of 0.5. (A) Growth kinetics within U937 macrophages. The indicated numbers of bacteria represent the combined numbers of intracellular bacteria and bacteria released into the supernatant. (B) Bacteria released into the tissue culture medium. (C) Intracellular bacteria. Values are the means of triplicate samples, and standard deviations are not shown due to their small values.
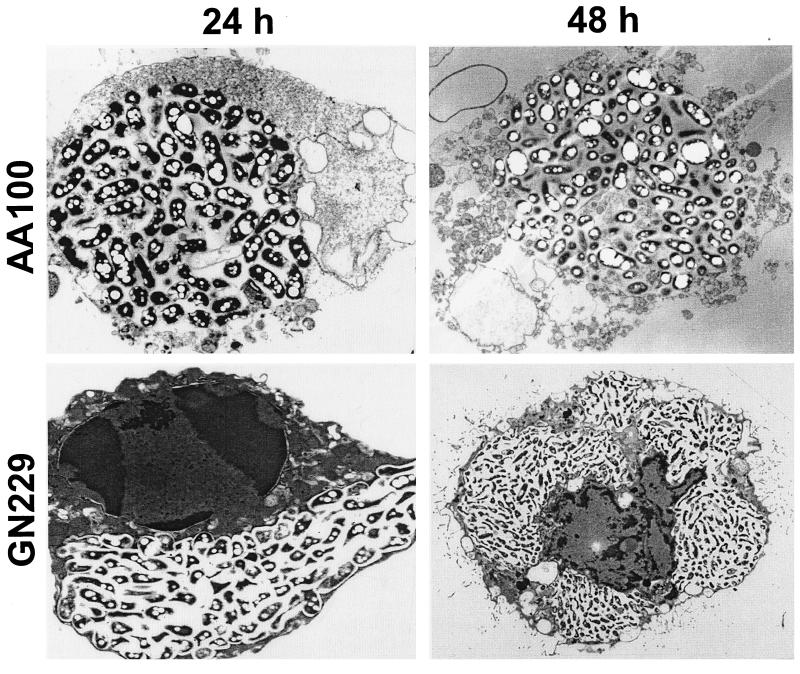
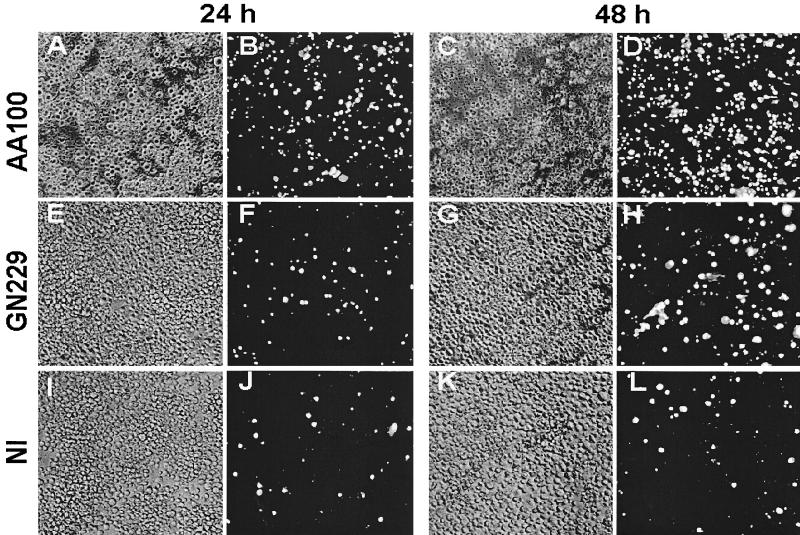
FIG. 3.
The rib mutants' defect in cytolysis of the host cell is due to a defect in necrosis-mediated killing. Representative transmission electron micrographs of infected U937 macrophages at 24 h and 48 h postinfection by the wild-type strain AA100 and the GN229 mutant. The original magnifications were ×7,000 and ×5,000 for the 24- and 48-h infections, respectively.
Confocal laser scanning microscopy showed that, similar to strain AA100, the mutants did not colocalize with the late endosomal or lysosomal marker Lamp-1 at 2 to 4 h postinfection (data not shown). In contrast, heat-killed cells of L. pneumophila, or a dotA mutant (46), used as controls, exhibited predominant colocalization with Lamp-1 (more than 80%) (data not shown). Taken together, these data showed that the mutants were defective in killing and exiting the host cell, despite their prolific intracellular replication.
The infection of U937 macrophages by one of the mutants was examined at the ultrastructural level. At 24 h postinfection, at least 50% of the cells in the AA100-infected monolayers were lysed, and the remaining cells exhibited necrotic morphology (Fig. 3) (data not shown). At 48 h postinfection, >95% of the AA100-infected cells were lysed, and the remaining cells exhibited severe signs of necrosis (an example is shown in Fig. 3). In contrast, the mutant-infected cells were still intact at 48 h postinfection and were not necrotic, but apoptotic nuclei with condensed chromatin were readily detectable (Fig. 3). These data were consistent with the substantial intracellular replication by the mutants and the defect in cytolysis of macrophages. The data suggested that the mutants are not defective in induction of apoptosis, but are defective in induction of necrosis.
The mutants are defective in pore-forming toxin or activity.
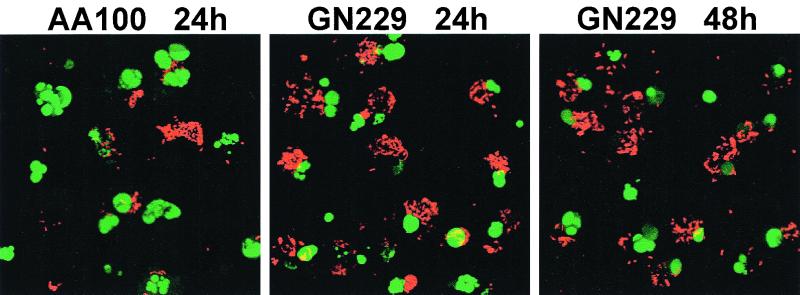
We have recently proposed a model of biphasic death of the host cell by L. pneumophila (20) initiated by caspase-3-dependent apoptosis followed by necrosis (19), which is most probably mediated by the pore-forming toxin (39). We confirmed the ability of the mutants to induce apoptosis, similar to the parental strain, by using agarose gel electrophoresis of the host cell DNA, TUNEL assays, and surface exposure of PS (Fig. 4) (data not shown). Consistent with the above data, TUNEL assays showed that the AA100-infected cells completely underwent cytolysis by 48 h postinfection and were left with bare apoptotic nuclei. In contrast, most cells infected by the mutants were intact, but apoptotic at 24 and 48 h postinfection, despite the presence of large numbers of intracellular bacteria (Fig. 4). These data showed that the mutants induced apoptosis and confirmed their defect in the ability to lyse and egress from the host cell during the 48-h infection period, despite their intracellular replication. Thus, the defective loci were designated rib (release of intracellular bacteria).
FIG. 4.
The defect in the rib mutants to induce cytolysis of macrophages is not due to a defect in induction of apoptosis. Representative TUNEL assays were performed for infections by strain AA100 or the GN229 mutant at 24 and 48 h postinfection. The apoptotic nuclei are shown in green, while the bacteria were detected with an antibody and are shown in red. The 48-h time point is not shown for AA100, due to complete lysis and loss of the monolayers. Stacked images of multiple 0.5-μm confocal z-sections are shown.
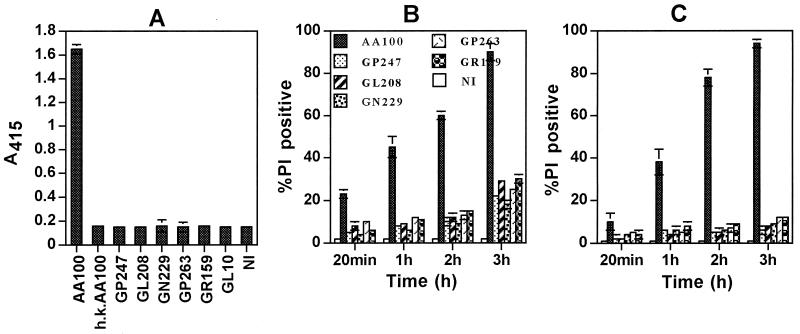
We next examined the rib mutants for pore-forming activity by using two different strategies (39). In contrast to the wild-type strain, all of the five rib mutants were completely defective in contact-dependent lysis of sRBCs, at a bacterium/RBC ratio of 25:1 (Fig. 5A). Hemolysis was not detected by the mutants, even when the bacterium/RBC ratio was increased by 200-fold, to 5,000:1 (data not shown). Heat-killed bacteria, a dotA mutant (39) (Fig. 5A), or bacterial culture supernatants did not cause hemolysis of sRBCs (data not shown). In the second strategy, we examined permeability of the plasma membrane of macrophages and epithelial cells to PI (molecular mass of 668 Da) upon infection with an MOI of 500 (39). In contrast to the parental strain, all of the five rib mutants were severely defective in pore formation in macrophages and epithelial cells (Fig. 5B and C and Fig. 6). No alteration in permeability to PI was detected when the cells were incubated with heat-killed bacteria, bacterial culture supernatants, or supernatants of AA100-infected cells obtained 3 h postinfection (data not shown). Taken together, the data indicated that the five rib mutants were defective in expression of the pore-forming toxin or activity (39).
FIG. 5.
The rib mutants of L. pneumophila are defective in expression of the pore-forming toxin or activity. (A) Contact-dependent hemolysis of sRBCs. GL10 is a dotA icmWXYZ mutant derivative of AA100 (24). h.k., heat-killed AA100; NI, noninfected; A415, measurement of optical density of the released hemoglobin at a wavelength of 415 nm. (B and C) Rapid contact-dependent pore formation in U937 macrophages and WI-26 alveolar epithelial cells (C) at an MOI of 500, measured by permeability to PI and expressed as percent PI positive. At least 100 cells were examined for each of the multiple samples. Values are the means of triplicate samples, and error bars represent standard deviations.
FIG. 6.
The mutants are defective in cytotoxicity. Phase-contrast images and PI staining of U937 macrophages examined at 24 h (left two columns) and 48 h (right two columns) postinfection are shown. Phase-contrast images are shown in panels A, C, E, G, I, and K, and the corresponding PI staining is shown in panels B, D, F, H, J, and L, respectively. NI, noninfected.
Temporal expression of the pore-forming activity.
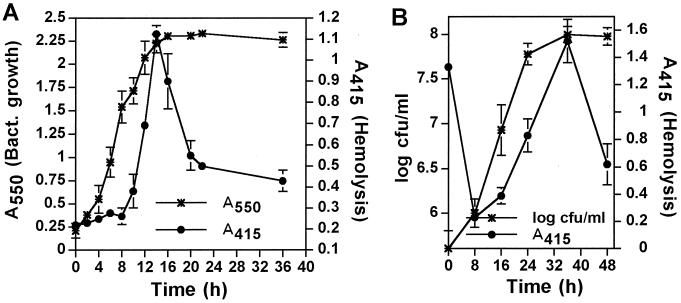
We hypothesized that due to the potency of the pore-forming toxin or activity to disrupt biological membranes and to cause necrosis and cytolysis of the host cell, its expression by intracellular bacteria is incompatible with viability of the host cell, which is essential for intracellular bacterial proliferation. Therefore, we examined the kinetics of expression of the pore-forming activity by in vitro-grown and intracellular bacteria at several stages of growth, by using contact-dependent hemolysis of sRBCs. Our data showed that expression of the pore-forming toxin or activity by L. pneumophila grown in vitro and within macrophages was completely repressed during exponential growth, but was temporally activated to a maximal level upon entry into the postexponential phase and declined rapidly afterwards (Fig. 7). Although the infecting bacteria were competent for pore formation (Fig. 7B, time zero), this capacity was abolished within 4 to 8 h of invasion (early exponential phase) (Fig. 7B). Our data showed that the phenotypic transition of L. pneumophila into the cytotoxic phenotype upon entering the postexponential phase, both in vitro (15) and intracellularly, was associated with expression of the pore-forming toxin or activity.
FIG. 7.
Growth-phase-dependent expression of the pore-forming activity by L. pneumophila in vitro and intracellularly. (A and B) Contact-dependent hemolysis of sRBCs by in vitro-grown L. pneumophila (A) or intracellular bacteria isolated from U937 macrophages (B). Infection of the cells in panel B was performed with in vitro-grown bacteria that had reached their maximal growth and hemolysis (14 h in panel A). At the indicated time points, the bacterial growth was determined by the A550 in panel A or by the CFU in panel B (left y axis), and hemolytic activity was determined (right y axis) with an equivalent number of bacteria at all time points. Values are the means of triplicate samples, and error bars represent standard deviations.
rib mutants are defective in acute cytotoxic lethality to mice.
Pulmonary histopathology of Legionnaires' disease patients and L. pneumophila-infected experimental animals is characterized by extensive lysis of inflammatory cells and necrosis of the alveolar epithelium (13, 36), which has been proposed to be mediated by a cytotoxin (58). In addition, intratracheal inoculation of A/J mice with >108 CFU of L. pneumophila results in bacterial growth-independent acute death (i.e., within 48 h) that is thought to be mediated by a cytotoxin (14). Intratracheal inoculation of A/J mice (52) with 2.7 × 109 or 2.7 × 108 CFU (five mice each) of the parental strain resulted in 100 and 60% death, respectively, within 24 to 48 h. In contrast, all five GN229-infected animals survived a similar dose, similar to animals infected by heat-killed AA100. Moreover, when 10 mice were infected by 4 × 109 CFU of the parental strain or the GN229 mutant, all 10 of the AA100-infected animals died within 24 to 48 h, but all 10 of the GN229-infected animals survived for the 7-day observation period. These data showed that the pore-forming activity played a major role in acute lethality and in the pathophysiology of Legionnaires' disease.
Are the kan insertions in the rib mutants responsible for the rib defect?
Southern hybridization analyses showed that the insertions in the rib mutants were located in different chromosomal regions distinct from the two regions containing the 23 dot and icm genes (data not shown) (48, 57). Sequence analysis of the flanking regions of the kan insertion in the mutants showed that all five of the mutants had insertions within open reading frame (ORF) with no similarity to other genes in genetic database, with the exception of the GN229 mutant, in which the insertion was within an ORF with a three-dimensional motif with similarity to that coding for the pore-forming toxin RtxA. The flanking DNA sequence of the kan insertion in each of the mutants was used to probe the cosmid library of L. pneumophila. Several cosmid clones that hybridized to the probe were isolated for each of the mutants. Southern hybridizations showed that the cosmids corresponding to each of the mutants were overlapping, but distinct from cosmids corresponding to the other mutants. By electroporation, we introduced two overlapping cosmids corresponding to each of the mutants into the respective mutant. Functional complementation of the defective Rib phenotype by the cosmids was performed by using the cytotoxicity assays of infected U937 macrophages and by contact-dependent hemolysis of sRBCs. The data clearly indicated that none of the cosmids complemented the phenotype of the mutants (data not shown). Therefore, it was essential to reconstruct the mutations in the parental strain background. The cloned kan insert and the flanking L. pneumophila DNA from each of the five rib mutants cloned into pBC were used to introduce the kan insertion into the parental strain AA100 by natural transformation (50). This procedure allows for 100% fidelity in the allelic exchange with the chromosomal locus of the colonies that arise on kan-supplemented agar plates (50). We were able to reconstruct all five of the kan insertions, individually, in the parental strain AA100, which was confirmed by Southern hybridizations probed with the kan cassette alone and with the DNA flanking regions in each of the mutants. The reconstructed mutants were examined for the defect in cytotoxicity to U937 macrophages by using cytotoxicity assays and for contact-dependent hemolysis of sRBCs. All of the reconstructed mutants exhibited a wild-type phenotype in both assays. Taken together, these data clearly showed that the kan insertions were not responsible for the defective phenotype in the pore-forming activity in the five mutants and that the mutations were spontaneous. These findings were surprising, but not unusual, since many L. pneumophila insertion mutations have been found to be spontaneous, where the insertion is not responsible for the defective phenotype, including the dotA mutant (12).
DISCUSSION
The mechanisms by which L. pneumophila or other vacuolar intracellular pathogens lyse and egress the host cell after its exploitation for intracellular proliferation are not known. In contrast to all previously isolated mutants of L. pneumophila (23, 24, 28, 29, 48, 51, 57; O. S. Harb and Y. Abu Kwaik, submitted for publication), the rib mutants are defective in killing and exiting the host cell upon termination of intracellular replication, but are not defective in modulating the biogenesis of their vacuole, nor in intracellular replication. The rib mutants are defective in expression of the pore-forming toxin or activity, which is only expressed in the parental strain upon termination of replication. Our studies provide the first example of a fascinating strategy by which a vacuolar intracellular pathogen regulates cytolysis of the host cell to ensure maximal exploitation for intracellular proliferation. During intracellular replication, L. pneumophila undergoes a dramatic phenotypic modulation (2, 4–6, 9, 10, 30), but the signals that trigger this modulation are not known. The signal that triggers expression of the pore-forming toxin by intracellular bacteria is also not known, but starvation (15, 27), quorum sensing, or deterioration of cellular processes may contribute. It is intriguing that other vacuolar intracellular pathogens, such as Mycobacterium tuberculosis, Mycobacterium haemophilum, Salmonella, Leishmania, and Chlamydia, also exhibit contact-mediated hemolysis or cytotoxicity, but its role in pathogenesis is not known (18, 37, 40, 41, 44, 45). Interestingly, the pore-forming activity of Leishmania is triggered to a maximal level upon entry into the stationary phase of growth (45) and has been proposed to play a role in egress of the parasites from the host cell (44). It is therefore possible that temporal pore formation-mediated cytolysis of the host cell is a strategy utilized by other vacuolar intracellular pathogens to kill and egress from the spent host cell after exploitation for intracellular proliferation.
Since several dot or icm mutants that are defective in trafficking and intracellular replication are also defective in pore-forming activity (39), Kirby et al. proposed that the pore-forming activity is required for export of effector molecules that are required for phagosomal trafficking to evade maturation along the “default” endosomal-lysosomal pathway (38, 39). It is important to note that these dot or icm mutants are defective in components of the Dot or Icm secretion apparatus, and thus, their defect in the pore-forming activity may be due to a defect in a Dot- or Icm-mediated export of the molecules responsible for this pore-forming activity as well as molecules responsible for trafficking. Another class of dot or icm mutants that are defective in trafficking and intracellular replication, but retain the pore-forming activity, have been also isolated (59). Based on the phenotypes of this new class of mutants, Zuckman et al. (59) concluded that the pore-forming activity is not sufficient for phagosomal trafficking, but proposed that the pore may be a vehicle to deliver effector molecules into the host cell cytoplasm. However, these observations do not exclude a role for the pore-forming activity in phagosomal trafficking. In this study, we have identified a novel class of mutants that do not colocalize with Lamp-1, similar to the parental strain, and are capable of intracellular replication, but are defective in the pore-forming activity. Our data show that if the pore-forming activity is required to export bacterially derived effector molecules into the host cell cytoplasm (59), the exported molecules play no detectable role in the intracellular replication. Importantly, our data clearly show that the pore-forming activity is not required for evasion of acquisition of Lamp-1 or intracellular replication. However, our data do not exclude the presence of another “less cytotoxic” pore utilized by L. pneumophila to export effector molecules required for phagosomal trafficking and intracellular replication.
Our data are consistent with the recent model that we have proposed by which L. pneumophila kills the host cell through two independent mechanisms manifested in two phases (Fig. 8) (19, 20). During early stages of the infection, L. pneumophila induces apoptosis (20, 43) in the host cell in a dose-dependent, but growth-phase-independent fashion (19, 20). In contrast, expression of the pore-forming toxin or activity is completely repressed during exponential replication, but is temporally expressed upon entry into the postexponential phase. We speculate that upon termination of intracellular replication, the bacteria exhibit the contact-dependent pore formation in the phagosomal membrane that results in its disruption, followed by access of the bacteria to the cytoplasm and subsequent contact-dependent pore formation in organelles and in the plasma membrane, followed by osmotic cytolysis of the host cell (see the model in Fig. 8).
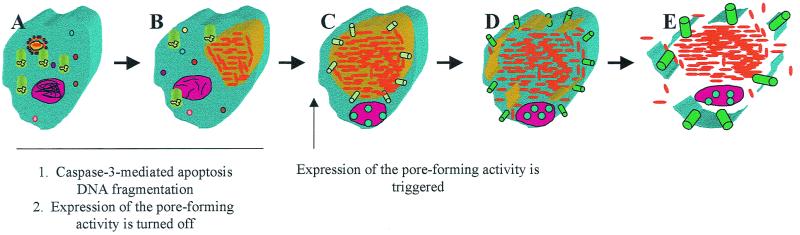
FIG. 8.
A model of growth-phase-dependent cytolysis of mammalian cells by L. pneumophila upon termination of intracellular bacterial replication to egress from the spent host cell. During early stages of formation of the mitochondria and rough endoplasmic reticulum-surrounded phagosome (A) and during exponential intracellular replication (B), expression of the pore-forming activity is turned off, but caspase-3-mediated apoptosis is triggered. Upon transition to the postexponential phase of growth, expression of the pore-forming activity is triggered, which results in insertions of pores in the phagosomal membrane first (C), leading to its disruption (D). This is followed by insertions of the pores in the plasma membrane (E), leading to osmotic lysis of the cell and release of the intracellular bacteria.
It is interesting that apoptosis by itself is not sufficient to release the rib mutants from the host cell within 48 h, which may suggest that L. pneumophila may interfere with late stages of apoptosis that include changes in permeability of the plasma membrane. It is important to note that release of the rib mutants from the host cells is initiated approximately 72 h postinfection, which is probably due to late stages of apoptosis and changes in the permeability of the plasma membrane. Interestingly, we have recently shown that although the protozoan host Acanthamoeba polyphaga is capable of undergoing apoptosis upon proper induction, L. pneumophila does not induce apoptosis in this host, but preferentially kills this amoeba by pore formation-mediated cytolysis (21, 31). Remarkably, the rib mutants are completely defective in cytolysis of the protozoan host, despite their prolific intracellular replication (21, 31). Thus, Legionella utilizes the pore-forming activity to induce cytolysis of two evolutionarily distant phagocytic cells (21, 31). Interestingly, the intracellular rib mutants gradually lose their viability within amoebae after termination of intracellular proliferation. Therefore, since the rib mutants subsequently egress from mammalian cells after 72 h, presumably as a result of apoptosis, utilization of two independent mechanisms by L. pneumophila to kill mammalian cells may be an efficient bacterial strategy to ensure egress from the spent host cell.
Pulmonary histopathology of Legionnaires' disease patients and L. pneumophila-infected experimental animals is characterized by extensive cytolysis of inflammatory cells in the alveolar spaces and necrosis of the alveolar epithelium (13, 36), which has been proposed to be mediated by a cytotoxin (58). In addition, bacterial growth-independent acute death (i.e., within 48 h) of mice is manifested following intratracheal inoculation with a high dose of the bacteria, and this acute lethality is thought to be mediated by a cytotoxin (14). Our data show that this heat-sensitive pore-forming toxin plays a major role in acute death after pulmonary infection of A/J mice. We predict that this toxin or activity is the major factor involved in rapid lysis of the phagosomal membrane, followed by that of the plasma membrane of the host cell and subsequent release of intracellular bacteria (see the model in Fig. 8). The release of intracellular bacteria, which express high levels of the pore-forming activity, is expected to induce cytolysis of neighboring cells in the alveolar spaces. Therefore, this toxin or activity is likely to be a major factor contributing to the extensive necrosis and cytolysis of alveolar epithelial cells and inflammatory cells in the alveolar space during the pulmonary infection of humans and experimental animals. Further characterization of the Rib proteins and the mechanisms of their action in disrupting the integrity of biological membranes will contribute to our understanding of an intriguing aspect of the host-parasite interaction of L. pneumophila and the associated inflammatory response in the alveolar spaces.
In summary, we have identified mutants of L. pneumophila with a novel phenotype that has never been described before for any vacuolar intracellular pathogen. The mutants are defective or delayed in egress from mammalian cells due to a defect in the pore-forming activity, which is triggered only upon termination of bacterial replication. Our data clearly show that the pore-forming activity is not required for phagosomal trafficking or for intracellular replication, but is essential for egress from the host cell upon termination of intracellular replication and is a major factor in acute death in the animal model. Future characterization of the rib loci and their growth phase regulation will yield important and interesting knowledge about the fine-tuning of this fascinating host-parasite interaction.
ACKNOWLEDGMENTS
We thank A. Kaplan, C. Snow, Anthony Sinai, and Subbarao Bondada for comments on the manuscript. We thank H. Shuman and R. Isberg for their kind gifts of the dot or icm clones. We also thank Omar Harb and Barbara Stone for technical assistance.
Y.A.K. is supported by Public Health Service Awards R29AI38410 and R01AI43965.
O.A.T.A. and L.-Y.G. contributed equally to this work.
REFERENCES
- 1.Abu Kwaik Y. Fatal attraction of mammalian cells to Legionella pneumophila. Mol Microbiol. 1998;30:689–696. doi: 10.1046/j.1365-2958.1998.01092.x. [DOI] [PubMed] [Google Scholar]
- 2.Abu Kwaik Y. Induced expression of the Legionella pneumophila gene encoding a 20-kilodalton protein during intracellular infection. Infect Immun. 1998;66:203–212. doi: 10.1128/iai.66.1.203-212.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abu Kwaik Y. The phagosome containing Legionella pneumophila within the protozoan Hartmanella vermiformis is surrounded by the rough endoplasmic reticulum. Appl Environ Microbiol. 1996;62:2022–2028. doi: 10.1128/aem.62.6.2022-2028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abu Kwaik Y, Eisenstein B I, Engleberg N C. Phenotypic modulation by Legionella pneumophila upon infection of macrophages. Infect Immun. 1993;61:1320–1329. doi: 10.1128/iai.61.4.1320-1329.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abu Kwaik Y, Engleberg N C. Cloning and molecular characterization of a Legionella pneumophila gene induced by intracellular infection and by various in vitro stress stimuli. Mol Microbiol. 1994;13:243–251. doi: 10.1111/j.1365-2958.1994.tb00419.x. [DOI] [PubMed] [Google Scholar]
- 6.Abu Kwaik Y, Gao L-Y, Harb O S, Stone B J. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol Microbiol. 1997;24:629–642. doi: 10.1046/j.1365-2958.1997.3661739.x. [DOI] [PubMed] [Google Scholar]
- 7.Abu Kwaik Y, Gao L-Y, Stone B J, Harb O S. Invasion of mammalian and protozoan cells by Legionella pneumophila. Bull Inst Pasteur. 1998;96:237–247. [Google Scholar]
- 8.Abu Kwaik Y, Gao L-Y, Stone B J, Venkataraman C, Harb O S. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl Environ Microbiol. 1998;64:3127–3133. doi: 10.1128/aem.64.9.3127-3133.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abu Kwaik Y, Harb O S. Phenotypic modulation by intracellular bacterial pathogens. Electrophoresis. 1999;20:2248–2258. doi: 10.1002/(SICI)1522-2683(19990801)20:11<2248::AID-ELPS2248>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 10.Abu Kwaik Y, Pederson L L. The use of differential display-PCR to isolate and characterize a Legionella pneumophila locus induced during the intracellular infection of macrophages. Mol Microbiol. 1996;21:543–556. doi: 10.1111/j.1365-2958.1996.tb02563.x. [DOI] [PubMed] [Google Scholar]
- 11.Abu Kwaik Y, Venkataraman C, Harb O S, Gao L-Y. Signal transduction in the protozoan host Hartmannella vermiformis upon attachment and invasion by Legionella micdadei. Appl Environ Microbiol. 1998;64:3134–3139. doi: 10.1128/aem.64.9.3134-3139.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berger K H, Isberg R R. Two distinct defects in intracellular growth complemented by a single genetic locus in Legionella pneumophila. Mol Microbiol. 1993;7:7–19. doi: 10.1111/j.1365-2958.1993.tb01092.x. [DOI] [PubMed] [Google Scholar]
- 13.Blackmon J A, Hicklin M D, Chandler F W. Legionnaires' disease: pathological and historical aspects of a “new” disease. Arch Pathol Lab Med. 1978;102:337–343. [PubMed] [Google Scholar]
- 14.Brieland J, Freeman P, Kunkel R, Chrisp C, Hurley M, Fantone J, Engleberg N C. Replicative Legionella pneumophila lung infection in intratracheally inoculated A/J mice: a murine model of human Legionnaires' disease. Am J Pathol. 1994;145:1537–1546. [PMC free article] [PubMed] [Google Scholar]
- 15.Byrne B, Swanson M S. Expression of Legionella pneumophila virulence traits in response to growth conditions. Infect Immun. 1998;66:3029–3034. doi: 10.1128/iai.66.7.3029-3034.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemens D L, Horwitz M A. Characterization of the Mycobacterium tuberculosis phagosome and evidence that phagolysosomal maturation is inhibited. J Exp Med. 1995;181:257–270. doi: 10.1084/jem.181.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coers J, Monahan C, Roy C R. Modulation of phagosome biogenesis by Legionella pneumophila creates an organelle permissive for intracellular growth. Nat Cell Biol. 1999;1:451–453. doi: 10.1038/15687. [DOI] [PubMed] [Google Scholar]
- 18.Fischer L J, Quinn F D, White E H, King C H. Intracellular growth and cytotoxicity of Mycobacterium haemophilum in a human epithelial cell line (Hec-1-B) Infect Immun. 1996;64:269–276. doi: 10.1128/iai.64.1.269-276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao L-Y, Abu Kwaik Y. Activation of caspase 3 in Legionella pneumophila-induced apoptosis in macrophages. Infect Immun. 1999;67:4886–4894. doi: 10.1128/iai.67.9.4886-4894.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao L-Y, Abu Kwaik Y. Apoptosis in macrophages and alveolar epithelial cells during early stages of infection by Legionella pneumophila and its role in cytopathogenicity. Infect Immun. 1999;67:862–870. doi: 10.1128/iai.67.2.862-870.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gao L-Y, Abu Kwaik Y. The mechanism of killing and exiting the protozoan host Acanthamoeba polyphaga by Legionella pneumophila. Environ Microbiol. 2000;2:79–90. doi: 10.1046/j.1462-2920.2000.00076.x. [DOI] [PubMed] [Google Scholar]
- 22.Gao L-Y, Abu Kwaik Y. The modulation of host cell apoptosis by intracellular bacterial pathogens. Trends Microbiol. 2000;8:306–313. doi: 10.1016/s0966-842x(00)01784-4. [DOI] [PubMed] [Google Scholar]
- 23.Gao L-Y, Harb O S, Abu Kwaik Y. Identification of macrophage-specific infectivity loci (mil) of Legionella pneumophila that are not required for infectivity of protozoa. Infect Immun. 1998;66:883–892. doi: 10.1128/iai.66.3.883-892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao L-Y, Harb O S, Abu Kwaik Y. Utilization of similar mechanisms by Legionella pneumophila to parasitize two evolutionarily distant hosts, mammalian macrophages and protozoa. Infect Immun. 1997;65:4738–4746. doi: 10.1128/iai.65.11.4738-4746.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao L-Y, Stone B J, Brieland J K, Abu Kwaik Y. Different fates of Legionella pneumophila pmi and mil mutants within human-derived macrophages and alveolar epithelial cells. Microb Pathog. 1998;25:291–306. doi: 10.1006/mpat.1998.0237. [DOI] [PubMed] [Google Scholar]
- 26.Gao L-Y, Susa M, Ticac B, Abu Kwaik Y. Heterogeneity in intracellular replication and cytopathogenicity of Legionella pneumophila and Legionella micdadei in mammalian and protozoan cells. Microb Pathog. 1999;27:273–287. doi: 10.1006/mpat.1999.0308. [DOI] [PubMed] [Google Scholar]
- 27.Hammer B K, Swanson M S. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol Microbiol. 1999;33:721–731. doi: 10.1046/j.1365-2958.1999.01519.x. [DOI] [PubMed] [Google Scholar]
- 28.Harb O S, Abu Kwaik Y. Characterization of a macrophage-specific infectivity locus (milA) of Legionella pneumophila. Infect Immun. 2000;68:368–376. doi: 10.1128/iai.68.1.368-376.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harb O S, Abu Kwaik Y. Identification of the aspartate-β-semialdehyde dehydrogenase gene of Legionella pneumophila and characterization of a null mutant. Infect Immun. 1998;66:1898–1903. doi: 10.1128/iai.66.5.1898-1903.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harb O S, Abu Kwaik Y. Probing the microenvironment of intracellular bacterial pathogens. Microb Infect. 1999;1:445–453. doi: 10.1016/s1286-4579(99)80048-3. [DOI] [PubMed] [Google Scholar]
- 31.Harb O S, Gao L-Y, Abu Kwaik Y. From protozoa to mammalian cells: a new paradigm in the life cycle of intracellular bacterial pathogens. Environ Microbiol. 2000;2:251–265. doi: 10.1046/j.1462-2920.2000.00112.x. [DOI] [PubMed] [Google Scholar]
- 32.Harb O S, Venkataraman C, Haack B J, Gao L-Y, Abu Kwaik Y. Heterogeneity in the attachment and uptake mechanisms of the Legionnaires' disease bacterium, Legionella pneumophila, by protozoan hosts. Appl Environ Microbiol. 1998;64:126–132. doi: 10.1128/aem.64.1.126-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horwitz M A. Formation of a novel phagosome by the Legionnaires' disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horwitz M A. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J Exp Med. 1983;158:2108–2126. doi: 10.1084/jem.158.6.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Husmann L K, Johnson W. Cytotoxicity of extracellular Legionella pneumophila. Infect Immun. 1994;62:2111–2114. doi: 10.1128/iai.62.5.2111-2114.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Katz S M, Hashemi S. Electron microscopic examination of the inflammatory response to Legionella pneumophila in guinea pigs. Lab Investig. 1982;46:24–32. [PubMed] [Google Scholar]
- 37.King C H, Mundayoor S, Crawford J T, Shinnick T M. Expression of contact-dependent cytolytic activity by Mycobacterium tuberculosis and isolation of the genomic locus that encodes the activity. Infect Immun. 1993;61:2708–2712. doi: 10.1128/iai.61.6.2708-2712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kirby J E, Isberg R R. Legionnaires' disease: the pore macrophage and the legion of terror within. Trends Microbiol. 1998;6:256–258. doi: 10.1016/s0966-842x(98)01310-9. [DOI] [PubMed] [Google Scholar]
- 39.Kirby J E, Vogel J P, Andrews H L, Isberg R R. Evidence for pore-forming ability by Legionella pneumophila. Mol Microbiol. 1998;27:323–336. doi: 10.1046/j.1365-2958.1998.00680.x. [DOI] [PubMed] [Google Scholar]
- 40.Kuo C C. Immediate cytotoxicity of Chlamydia trachomatis for mouse peritoneal macrophages. Infect Immun. 1978;20:613–618. doi: 10.1128/iai.20.3.613-618.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Libby S J, Goebel W, Ludwig A, Buchmeier N, Bowe F, Fang F C, Guiney D G, Songer J G, Heffron F. A cytolysin encoded by Salmonella is required for survival within macrophages. Proc Natl Acad Sci USA. 1994;91:489–493. doi: 10.1073/pnas.91.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meresse S, Steele-Mortimer O, Moreno E, Desjardins M, Finlay B, Gorvel J P. Controlling the maturation of pathogen-containing vacuoles: a matter of life and death. Nat Cell Biol. 1999;1:E183–E188. doi: 10.1038/15620. [DOI] [PubMed] [Google Scholar]
- 43.Müller A, Hacker J, Brand B C. Evidence for apoptosis of human macrophage-like HL-60 cells by Legionella pneumophila infection. Infect Immun. 1996;64:4900–4906. doi: 10.1128/iai.64.12.4900-4906.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noronha F S M, Cruz J S, Beirão P S L, Horta M F. Macrophage damage by Leishmania amazonensis cytolysin: evidence of pore formation on cell membrane. Infect Immun. 2000;68:4578–4584. doi: 10.1128/iai.68.8.4578-4584.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Noronha F S M, Ramalho-Pinto F J, Horta M F. Cytolytic activity in the genus Leishmania: involvement of a putative pore-forming protein. Infect Immun. 1996;64:3975–3982. doi: 10.1128/iai.64.10.3975-3982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roy C R, Berger K H, Isberg R R. Legionella pneumophila DotA protein is required for early phagosome trafficking decisions that occur within minutes of bacterial uptake. Mol Microbiol. 1998;28:663–674. doi: 10.1046/j.1365-2958.1998.00841.x. [DOI] [PubMed] [Google Scholar]
- 47.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual, 2 ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 48.Segal G, Purcell M, Shuman H A. Host cell killing and bacterial conjugation require overlapping sets of genes within a 22-kb region of the Legionella pneumophila chromosome. Proc Natl Acad Sci USA. 1998;95:1669–1674. doi: 10.1073/pnas.95.4.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stone B J, Abu Kwaik Y. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect Immun. 1998;66:1768–1775. doi: 10.1128/iai.66.4.1768-1775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stone B J, Abu Kwaik Y. Natural competency for DNA transformation by Legionella pneumophila and its association with expression of type IV pili. J Bacteriol. 1999;181:1395–1402. doi: 10.1128/jb.181.5.1395-1402.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stone B J, Brier A, Kwaik Y A. The Legionella pneumophila prp locus: required during infection of macrophages and amoebae. Microb Pathog. 1999;27:369–376. doi: 10.1006/mpat.1999.0311. [DOI] [PubMed] [Google Scholar]
- 52.Susa M, Ticac T, Rukavina T, Doric M, Marre R. Legionella pneumophila infection in intratracheally inoculated T cell depleted or non-depleted A/J mice. J Immunol. 1998;160:316–321. [PubMed] [Google Scholar]
- 53.Swanson M S, Isberg R R. Association of Legionella pneumophila with the macrophage endoplasmic reticulum. Infect Immun. 1995;63:3609–3620. doi: 10.1128/iai.63.9.3609-3620.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Venkataraman, C., and Y. Abu Kwaik. Signal transduction in the protozoan host Hartmannella vermiformis upon attachment to Legionella pneumophila. Microb. Infect., in press. [DOI] [PubMed]
- 55.Venkataraman C, Gao L-Y, Bondada S, Abu Kwaik Y. Identification of putative cytoskeletal protein homologues in the protozoan Hartmannella vermiformis as substrates for induced tyrosine phosphatase activity upon attachment to the Legionnaires' disease bacterium, Legionella pneumophila. J Exp Med. 1998;188:505–514. doi: 10.1084/jem.188.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Venkataraman C, Haack B J, Bondada S, Abu Kwaik Y. Identification of a Gal/GalNAc lectin in the protozoan Hartmannella vermiformis as a potential receptor for attachment and invasion by the Legionnaires' disease bacterium, Legionella pneumophila. J Exp Med. 1997;186:537–547. doi: 10.1084/jem.186.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vogel J P, Andrews H L, Wong S K, Isberg R R. Conjugative transfer by the virulence system of Legionella pneumophila. Science. 1998;279:873–876. doi: 10.1126/science.279.5352.873. [DOI] [PubMed] [Google Scholar]
- 58.Winn W C, Glavin F L, Perl D P, Keller J L, Andres T L, Brown T M, Coffin C M, Sensecqua J E, Roman L N, Craighead J E. The pathology of Legionnaires' disease: fourteen fatal cases from the 1977 outbreak in Vermont. Arch Pathol Lab Med. 1978;102:344–350. [PubMed] [Google Scholar]
- 59.Zuckman D M, Hung J B, Roy C R. Pore-forming activity is not sufficient for Legionella pneumophila phagosome trafficking and intracellular replication. Mol Microbiol. 1999;32:990–1001. doi: 10.1046/j.1365-2958.1999.01410.x. [DOI] [PubMed] [Google Scholar]