Abstract
Indonesian clove cigarettes—called “kretek” due to the crackling sound that can be heard when the product burns—are tobacco products containing clove and the "saus", a mixture of essential oils and plant extracts whose ingredients are mostly kept in secret. It is important to determine which ingredients those are to properly assess the effects that clove cigarettes can cause. An organoleptic, qualitative and quantitative analysis was made in 9 different brands of clove cigarettes obtained in Brazil. Nicotine, eugenol, menthol, and β-caryophyllene were quantified through gas chromatography coupled to mass spectrometry. The samples presented 20 different compounds, and all samples had a different combination of the compounds. Nicotine concentrations were generally higher than eugenol, and lower than nicotine concentration in a conventional cigarette. One sample had menthol even though the cigarette pack did not inform that it was a menthol product. There were traces of 2 unusual substances. Clindamycin is an antibiotic that can be used to treat bacterial infections in respiratory airways, and octodrine is an amphetaminic stimulant used in nutritional supplements, considered as a substance of doping by the World Anti-Doping Association. The presence of both substances was not tested using certified reference materials, but its possible presence raises concern about the compounds in kretek cigarettes. There should be more studies about the contents of clove cigarettes, to improve antitobacco legislations and regulations. This way it would be possible to properly inform the risks of smoking clove cigarettes and to diminish the number of tobacco users throughout the world.
Keywords: clove cigarettes, kretek, characterization, regulation, GC/MS
1. Introduction
Indonesian clove cigarettes are known as “kretek” due to the crackling sound that clove makes when it burns. The composition of kretek involves mostly tobacco and clove (Syzygium aromaticum [L.] Merrill & Perry). Indonesian popular culture says that a resident of the city of Kudus, Indonesia, suffering from asthma attacks, had the idea to add dried clove flower buds to the tobacco mix in his straw cigarette. The immediate relief that he felt led him to recommend the product to his friends and neighbors, and eventually to be commercialized by the pharmacies at the time.1
The sales of the product had taken great proportions, and an industry arised around 1906. From then on, different companies, brands, and forms of clove cigarettes were made: initially hand-rolled (“Sigaret Kretek Tangan”—or SKT in Bahasa Indonesia), the industry went through a boom during the end of the 1970s, with the objective to turn kretek into a national product, representing the Indonesian culture. From that came the machine-rolled cigarettes (“Sigaret Kretek Mesin”—or SKM in Bahasa Indonesia).2 Even so, the SKT industry is very much alive in the country: the taxation over the sales of SKM are much larger than the sales of SKT. Approximately one third of the Indonesian kretek are SKT, generating approximately 200 thousand jobs for the Indonesian people.3,4
However, it is not all roses—or in this case, cloves. The Indonesian people has the habit of adding seasoning to any species of food or substance that is ingested, and kretek did not pass through this tradition unscathed; essential oils, herbs, and flower extracts are added to the content of the clove cigarette. This mixture of components is called “saus”—which means “sauce” in Bahasa Indonesia.5 The concentration and the content of the saus, however, differs from each industry and brand, and is treated as Intellectual Propriety, usually know exclusively by the CEO and the chief-mixer, responsible for the insertion of ingredients in the formula.3,6 Despite containing substances that, separately, contain known pharmacological actions—for instance, eugenol, main component of the essential oil of clove, presents anesthetic, anti-inflammatory, and antiseptic activity—we do not know the drug interactions and mechanisms of action that can manifest when acting with other substances.7,8
The addition of ameliorating substances in the cigarette can bring greater consequences, especially for the young people. Tobacco products containing eugenol, menthol, and other substances that soften the severity of the smoke in general are better appreciated to new users, diminishing the perception of risk associated to smoking. Moreover, despite of consuming less frequently, users of flavored tobacco products tend to use a greater amount per interaction and require less contact to the surge of the first symptoms of dependence.9,10
To make matters worse, flavored tobacco control and legislation around the world tends to be vague and interpretative, opening precedents to loopholes which Transnational Tobacco Companies (TTCs) are ready to take advantage and push as much as possible without actually breaking the law.11,12 The exact knowledge and vigilance about the content in clove cigarettes can guarantee that the product, if not prohibited, at least have clear regulations about the ingredients and related side effects. With this objective, an organoleptic, qualitative and quantitative analysis was carried out in 9 different brands of clove cigarettes obtained in Brazil through gas chromatography coupled to mass spectrometry (GC/MS).
2. Materials and methods
2.1 Sample collection and storage
Nine clove cigarette samples were obtained in tobacco shops, newsstands, and supermarkets in Rio Grande do Sul state, Brazil. Each cigarette pack had 20 sticks and the word “kretek” or “clove,” informing the type of the cigarette. The exception was KTK_A1E, containing only 16 sticks and the phrase “Made in Indonesia” stamped in the pack, that was obtained in a tobacco shop specialized in imported products. The packs (n = 9) were labeled with an identification code, placed in zip-lock bags, and stored in a conventional freezer set at −4 °C, to avoid volatilization of the compounds of interest prior to the analysis. The samples were coded exclusively so that the brands are not identified through publication, to avoid any legal matters. Cigarette packs were stored still sealed, and before analysis, the packs were kept at room temperature for 1 h, and opened only in the moment of extraction, when 3 random sticks were chosen from the pack. The organoleptic properties of each pack were observed. Aroma, color of the pack, color of the stick, existence of health warnings, ingredients, presence of clove, presence of menthol, and product category were identified and registered. The tobacco contents were separated from the filter plug and the paper wrap, and the tobacco content was weighed.
2.2 Sample extraction
The tobacco samples (the equivalent to 1 cigarette) were weighed on an Atx 220 g × 0.0001 g Shimadzu Analytical Balance (São Paulo, Brazil). After weighing and measurement of the cigarette components (Table 1), the tobacco content was placed in an Erlenmeyer flask with 15 mL of methanol (analytical grade, Merck). The flask was placed in a magnetic stirrer for 30 min at a 4× speed, and after it the liquid content was filtered through a glass funnel with cotton. The filtered liquid was reserved in a glass beaker, the Erlenmeyer content was filled with 15 mL of methanol again and placed in the magnetic stirrer. These steps were repeated 3 times. After 3 filtrations, the tobacco content was discarded, and the liquid content was filtered through a glass funnel with a pleated filter paper. The filtered liquid was placed in a turned off fume hood for 24 h or until the content evaporated completely. The content in the glass beaker was then resuspended in 2 mL of methanol and collected with a 3-mL syringe. The content of the syringe was filtered through a 13-mm Nylon syringe filter with 0.45-μm pores (Ligand Scientific Co. Ltd, Muang, Nonthaburi, Thailand), to an Agilent 2 mL clear glass sample chromatography vial for GC/MS analysis. Tests were also made with hexane and ethyl acetate at the same volume of 15 mL taking the same steps as described before, to determine the best solvent.
Table 1.
Clove cigarettes and conventional cigarette design parameters measured in triplicate.
| Parameters/sample | Cigarette weight (mg) | Filler weight (mg) | Circumference (mm) | Cigarette length (mm) | Tobacco column length (mm) |
|---|---|---|---|---|---|
| KTK_A1 | 1,048 | 876 | 25.0 | 81.5 | 59.5 |
| KTK_A1E | 1,058 | 889 | 24.3 | 80.5 | 59.0 |
| KTK_B1 | 808 | 582 | 26.3 | 84.1 | 53.8 |
| KTK_B2 | 842 | 617 | 26.5 | 83.3 | 55.4 |
| KTK_B2M | 890 | 663 | 26.5 | 82.6 | 55.0 |
| KTK_C1 | 715 | 519 | 20.7 | 85.5 | 57.3 |
| KTK_D1 | 692 | 463 | 24.6 | 85.0 | 56.7 |
| KTK_D1M | 705 | 474 | 23.0 | 85.3 | 57.6 |
| KTK_D2M | 668 | 438 | 24.2 | 85.5 | 56.0 |
| CTRL | 818 | 551 | 26.3 | 83.4 | 52.3 |
2.3 Instrumental analysis
2.3.1 GC/MS analysis
Analytical measurements were performed using an Agilent 7980A gas chromatography system coupled to a 5975C mass-selective detector (Santa Clara, CA, United States). The GC oven was fitted with a DB-5 column (30 m × 0.25 mm × 0.5 μm). Aliquots of 1 μL of the samples were inserted with a CTC CombiPAL Autosampler (Leap Technologies, Carrboro, NC, United States). A blank sample of methanol was inserted before and after each sample to wash the column. Ultra-pure helium was used as a carrier gas in a flow rate of 1 mL/min. All injections were made in a split mode with a split ratio of 1:50. The methodology of analysis was adapted from Stanfill & Ashley.13 The GC oven had 3 different programming. Blank samples were held at 60 °C for 3 min, followed by an increase of 30 °C/min to 300 °C, with a total run time of 11 min. For cigarette extract samples, the initial oven temperature was 60 °C (held for 3 min), with an increase of 3 °C/min to 190 °C followed by an increase of 30 °C/min to 300 °C, with a total run time of 50 min. The initial oven temperature for the calibration curves samples was 60 °C (held for 3 min) followed by an increase of 3 °C/min to 135 °C for eugenol, 108 °C for menthol, 138 °C for β-caryophyllene, and 129 °C for nicotine, with a total run time of 28, 19, 29, and 26 min, respectively. The GC injector was set at 280 °C and the source heater was held at 280 °C. Peak areas were integrated using the ChemStation Integrator program in the HP Enhanced ChemStation Software (version B.04.01).
Identification of compounds was carried out by comparison of their relative retention times, calculated by linear interpolation relative to the retention time of a series of n-alkenes, and their mass spectra, with authentic samples or data taken from the literature,14 as well as by comparison with mass spectra recorded in the NIST 2006 (National Institute of Standards and Technology, Gaithersburg, MD, United States) mass spectral library.
2.3.2 Calibration curve
The analytical standards of nicotine, eugenol, menthol, and (−)-β-caryophyllene were purchased from Sigma-Aldrich (St. Louis, MO, United States). The standard solutions were used to construct calibration curves. Each standard solution was diluted at a concentration of 2% with methanol. The solutions were then diluted in enough analytical grade methanol to complete 3 mL of a mother solution. Dilutions of 1%, 0.5%, 0.25%, 0.125%, and 0.062% were used for the curves.
2.3.3 Data collection and normalization
Chromatogram peak areas were determined automatically, checked for proper integration, and reintegrated manually if needed. Areas were transferred to a spreadsheet and the concentration of eugenol, menthol, β-caryophyllene, and nicotine were determined. The quantification percentage was calculated using the concentration from the area under the curve and the dilution factor according to the equation:
 |
AC = area under the curve (mg/mL);
CW = cigarette weight (mg);
DF = dilution factor (mL).
From the percentage quantification, the concentration of the substances in milligrams per cigarette extraction (mg/cig) was obtained.
3. Results and discussion
3.1 Organoleptic properties
Samples were coded to avoid identification. Kretek cigarettes were given the code KTK, and the subsequent letter indicates the company (4 different companies from A to D). The number after the letters indicates the brand. Samples with the same number were from the same brand. In these cases, to avoid confusion, another letter was added (E for the Export sample, made in Indonesia, and M for menthol). A conventional cigarette was purchased to compare the results and label CTRL, as in “Control” (Fig. 1).
Fig. 1.

Clove cigarette sticks samples and a conventional cigarette as control. From left to right: KTK_A1, KTK_A1E, KTK_B1, KTK_B2, KTK_B2M, KTK_C1, KTK_D1, KTK_D1M, KTK_D2M, and CTRL.
The first sample, KTK_A1, had a strong scent of clove, with another sweet smell, like incense (Table 2). The pack had a metallic color pallet in red and gold, very shiny, and exotic, with an “expensive” look. There were 2 health warnings, 1 in each side of the pack, but even though the pack was purchased in a tobacco shop in Brazil, the warnings were written in Spanish and Guarani. There was no indication that the product was a clove cigarette, but it is a very common brand in Brazil, probably the most traditional kretek brand in the country. There were also no indications of the ingredients, and only a small indication that the product was a SKM. The cigarette stick was mostly white, with exception of the filter, which had the same color pallet from the pack. The famous clove oil stains were visible in the paper wrap, with a caramel color (Fig. 1). Allegedly, the more stains the cigarette have, the better its quality.1
Table 2.
Organoleptic properties of clove cigarettes according to the perception of authors.
| No. | Scent | Company A | Company B | Company C | Company D | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KTK_A1 | KTK_A1E | KTK_B1 | KTK_B2 | KTK_B2M | KTK_C1 | KTK_D1 | KTK_D1M | KTK_D2M | CTRL | ||
| 1 | Clove / spices | ++++ | ++ | + | ++ | + | ++ | ++ | + | + | − |
| 2 | Menthol | − | − | − | − | + | ++++ | − | ++ | ++ | − |
| 3 | Incense | ++ | + | − | − | − | + | − | − | − | − |
| 4 | Caramelic | + | − | + | − | − | + | − | − | − | + |
Sample KTK_A1E was from the same company and brand of the first sample. The color pallet of the pack was identical, and the health warning was resumed to a single phrase in Bahasa Indonesia, almost imperceptible. The pack had the indication of “Kretek cigarettes” in the front, and it was thinner and elongated, with a stamp, apparently from Indonesia, since the price printed was in Rupiah, Indonesia’s currency (Rp 1,050, which is equivalent to ~US$ 0.074, in the exchange rate of 1st September 2021—oddly enough, the price of a cigarette pack from the same brand in Brazil is ~R$ 35, which is equivalent to US$ 6.74 in the same exchange rate). The scent of KTK_A1E was essentially the same of the first sample, but noticeably weaker (Table 2). There were only 16 sticks on the pack, with the same colors and clove oil stains, but the pack was not as shiny as the first sample. There was no indication as to the category of the cigarette, although it looks like a SKM.
KTK_B1 had a strong smell of nicotine, and the scent of clove or any flavoring was very weak, almost unnoticeable (Table 2). The pack was black and orange, with patterns that in Brazil are called “tribal,” which resembles the body paintings of the Austronesian Peoples. The health warnings occupied the entire back of the pack and a strip on the front. There was the word “Kretek” written in the front, and the ingredients were listed in the bottom (although there was only the indication of “flavorings,” without indicate which flavorings those are). The sticks were almost identical to a conventional cigarette stick, all white, including the filter, with the brand and the word “kretek” printed in the filter. There were no clove oil stains whatsoever (Fig. 1).
KTK_B2 had a pack very similar to a deck of cards, white, with a big red label in the center, and the indication of “20 kretek” and “100% Indonesian clove.” The health warnings were placed in the same distribution of KTK_B1. There were no indications as to the category of kretek, and the ingredients were listed identically to KTK_B1. The cigarette stick was all white, with a gold striped band at the end of the filter, with the brand label in red, and a few clove oil stains, almost imperceptible. The cigarette had a weak smell of clove, and the smell of nicotine was a bit stronger (Table 2).
KTK_B2M pack was identical to KTK_B2 with 2 differences: the label was green instead of red, and although there were the indications of “20 kretek” and “100% Indonesian clove,” there was also the indication of “menthol” above the label. There was a green striped band at the end of the filter of each stick, with almost imperceptible clove oil stains (Fig. 1). There was the smell of something refreshing, supposedly menthol, although not very strong, and an even weaker scent of clove (Table 2).
The pack of KTK_C1 was the most different from the others. The pack was shorter, all black with a fluorescent print, and the edges were rounded. The health warnings were equal to the others, and the ingredients were the same, with a single difference: this was the only pack that listed clove nominally as an ingredient. There was an indication that this product was a SKM—for export only—, and the indication that it was an “Indonesian kretek". Although this was not a menthol cigarette according to the pack, there was a really strong smell of mint, almost numbing, that masked other scents—there was a trace of a sweetish smell, almost like candy, but very faint (Table 2). The stick was considerably thinner than the others, all white with a single red strip at the end of the filter.
KTK_D1 had a black, thin, and long pack, almost square-shaped, with a red triangle in the center. Health warnings and ingredients were identical to the others, and there was an indication of “KRETEK” and “Clove cigarettes” at the front of the pack, and the indication of “SKM” near the barcodes. There was a sweet smell, like incense, a bit “spicy” (Table 2). The stick was thin, although not as thin as KTK_C1, and it was all black with a single silver band at the end of the filter below the name of the brand. KTK_D1M was identical to KTK_D1, but the triangle was green instead of red, and there was an indication of “KRETEK MENTHOL". There was a strong scent of mint, also a bit “spicy” like KTK_D1, and the cigarette stick was also all black and thin with a single silver band.
The last kretek cigarette, KTK_D2M, had a white pack with green and silver details. The dimensions were the same as KTK_D1 and KTK_D1M, and although it was not the same brand, it was from the same company. There was the indication of a SKM cigarette, “KRETEK” and “Clove Cigarettes.” The name of the brand indicated that there was menthol in the cigarette, agreeing with the smell, although not very strong, and not any other smells (Table 2). The cigarette stick was all white with a single thin silver stripe near the end of the filter, and the name of the brand. It was as thin as KTK_D1 and KTK_D1M, with slightly perceptible clove oil stains.
As to the conventional cigarette, CTRL, there was a strong smell of the tobacco plant, not just nicotine, with a “sugary” touch (Table 2). The stick was almost identical to KTK_D2M, a bit thicker. There was no visible correlation between the 2 companies. The ingredients were the same as the other, with the difference that there were no flavorings or ameliorates.
3.2 Developing the method
To define the best method to be used, some tests were made using a sample from the same brand of KTK_A1. The extractions were made with methanol, hexane, and ethyl acetate, and a fractionated extraction with these 3 solvents, from the lowest to the highest polarity. However, no matter the solvent used, it was not possible to observe the presence of nicotine, something much unexpected since sample KTK_A1 was supposed to be a tobacco product. Figure 2 shows the chromatogram of a methanol extract with an increase of 30 °C/min in the oven temperature, a total of 11-min run time. These conditions were used initially to all tests.
Fig. 2.

Methanol extraction of clove cigarette with total run time of 11 min. Figure shows the peak of eugenol (10), β-caryophyllene (13), α-humulene (14), acetophenone (19), and isophytol (20).
The method run time was altered to verify if the peak of other substance could be overlapping the nicotine peak. The increase of oven temperature was raised to 3 °C/min, with a total run time of 83 min. Using the same methanol extract, it became clear that the nicotine peak was overlapped by the eugenol peak, due to the proximity of their retention time (Fig. 3). The tests with a fractionated extraction were performed again, using hexane, ethyl acetate, and methanol, with relative polarity of 0.009, 0.228, and 0.762, respectively.15 Still, the nicotine peak did not appear at hexane and ethyl acetate extractions, although traces of α-pinene and β-pinene were observed in the ethyl acetate extract (Fig. 4). To confirm these results, an extraction with each solvent separately was performed. The hexane extract chromatogram had too many impurities, and the ethyl acetate extract had only eugenol and β-caryophyllene peaks, with a small peak of caryophyllene oxide. Thus, the methanol extraction with an increase of oven temperature in 3 °C/min was defined as the ideal, since there was the separation of eugenol and nicotine peaks, and all compounds observed in the hexane and ethyl acetate extracts were also observed in the methanol extract. The methanol extract run with 83 min had no quantifiable peaks beyond 45 min, so the increase of the oven temperature was sped up to 30 °C/min after approximately 46 min of run time—equivalent to 190 °C—until the oven temperature reached 300 °C and total run time reached 50 min (Fig. 3).
Fig. 3.
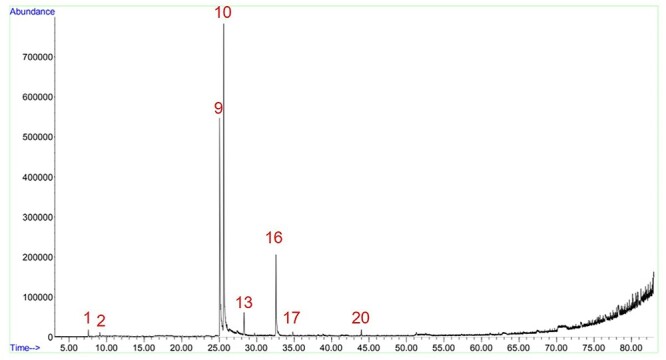
Methanol extraction of clove cigarette with total run time of 83 min. Figure shows the peaks of α-pinene (1), β-pinene (2), nicotine (9), eugenol (10), β-caryophyllene (13), acetyleugenol (16), caryophyllene oxide (17), and isophytol (20).
Fig. 4.

Clove cigarette fractionated extraction with A) methanol, B) hexane, and C) ethyl acetate with total run time of 83 min. Extraction with methanol shows nicotine (9), acetyleugenol (16), acetophenone (19), and isophytol (20), not observed in other extracts.
3.3 Qualitative analysis
With the methodology steps established, the samples were extracted, and the results were evaluated. Each sample was extracted in triplicate, and all the compounds found are listed in Table 3.
Table 3.
List of name, molecular weight, chemical formula, and chemical structure of 20 compounds found in 9 different brands of clove cigarettes by GC/MS.
| No. | Compound | RT a | Molecular weight | Chemical formula | Chemical structure |
|---|---|---|---|---|---|
| 1 | α-pinene | 7.539 | 136.23 | C10H16 |
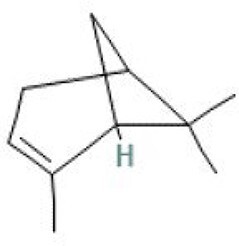
|
| 2 | β-pinene | 9.078 | 136.23 | C10H16 |

|
| 3 | Clindamycin | 13.315 | 424.98 | C18H33ClN2O5S |

|
| 4 | DDMP | 16.176 | 144.12 | C6H8O4 |

|
| 5 | Menthol | 17.454 | 156.26 | C10H20O |

|
| 6 | HMFCA | 19.971 | 142.11 | C6H6O4 |

|
| 7 | Chavicol | 21.202 | 134.17 | C9H10O |

|
| 8 | Octodrine | 22.515 | 129.24 | C8H19N |

|
| 9 | Nicotine | 25.107 | 162.23 | C10H14N2 |
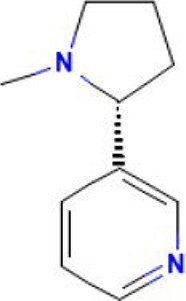
|
| 10 | Eugenol | 25.740 | 164.20 | C10H12O2 |

|
| 11 | δ-Cadinene | 26.494 | 204.35 | C15H24 |

|
| 12 | Aromadendrene | 27.792 | 204.35 | C15H24 |
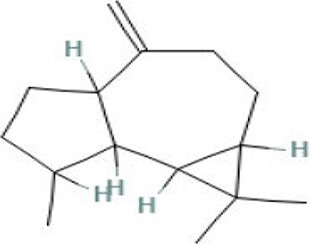
|
| 13 | β-caryophyllene | 28.335 | 204.35 | C15H24 |

|
| 14 | α-humulene | 29.711 | 204.35 | C15H24 |
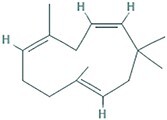
|
| 15 | Oxalic acid b | 30.817 | 284.39 | C16H28O4 |
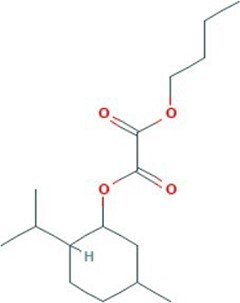
|
| 16 | Acetyleugenol | 32.560 | 206.24 | C12H14O3 |

|
| 17 | Caryophyllene oxide | 34.819 | 220.35 | C15H24O |
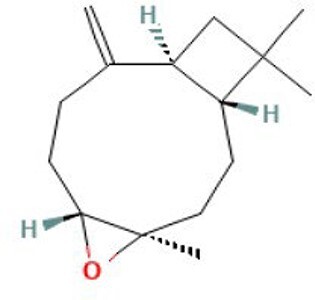
|
| 18 | Pentanoic acid c | 35.335 | 286.41 | C16H30O4 |

|
| 19 | Acetophenone d | 38.712 | 210.23 | C11H14O4 |

|
| 20 | Isophytol e | 43.933 | 296.50 | C20H40O |

|
aRetention time (in minutes) based on a DB-5 column.
bButyl 1-menthyl ester oxalic acid.
c2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester pentanoic acid.
d2′,3′,4′-Trimethoxyacetophenone.
e3,7,11,15-Tetramethylhexadec-2-en-1-ol.
3.3.1 Eugenol
Eugenol is a very well-known compound, the main substance of the essential oil extracted from the leaves, flower buds, and stems of clove (S. aromaticum [L.] Merrill & Perry). The composition of clove oil depends on the geographic location and the part of the plant used, but eugenol presents itself as the main compound in all cases.16 The compound has analgesic, anti-inflammatory, antioxidant, and cardiovascular characteristics and is generally rapidly absorbed by the gastrointestinal tract. Blood concentration of eugenol is higher according to the form of ingestion: oral ingestion causes a higher concentration in the body than cutaneous or inhaled absorption.17
The eugenol molecule presents similar structure to the monoamine neurotransmitters (dopamine, norepinephrine, epinephrine, and serotonin; Table 3). Although there are no studies demonstrating hallucinogenic effects due to eugenol, it was indicated that the substance may inhibit monoamine oxidase A (MAOA), suggesting an antidepressant effect, analogous to the action of monoamines.18
As expected, all samples presented eugenol (Table 4). Sample KTK_A1 was the only sample that had more eugenol than nicotine, which was noticeable even prior to the quantification, due to the size of the peak. Although sample KTK_A1E was from the same company and brand, the eugenol content was very different, and considerably lower than nicotine (Figs. 5 and 6).
Table 4.
Presence and concentration of eugenol in each of the 9 brands of clove cigarettes analyzed (mg/cig).
| No. | Compound name | Company A | Company B | Company C | Company D | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KTK_A1 | KTK_A1E | KTK_B1 | KTK_B2 | KTK_B2M | KTK_C1 | KTK_D1 | KTK_D1M | KTK_D2M | CTRL | ||
| 10 | Eugenol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Concentration (mg/cig) | 27.218 | 3.416 | 3.788 | 5.957 | 10.011 | 9.699 | 3.266 | 3.223 | 2.733 | – | |
Fig. 5.
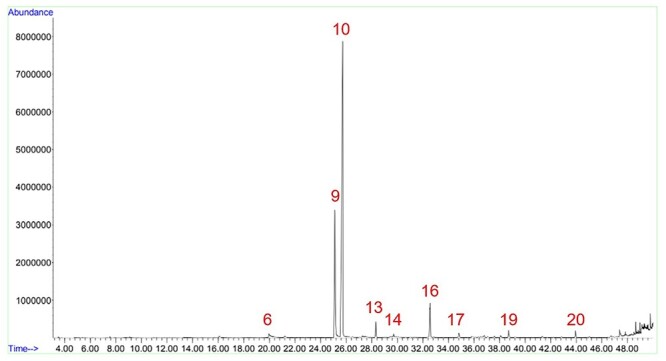
Sample KTK_A1 chromatogram. Figure shows the peaks of HMFCA (6), nicotine (9), eugenol (10), β-caryophyllene (13), α-humulene (14), acetyleugenol (16), caryophyllene oxide (17), acetophenone (19), and isophytol (20).
Fig. 6.

Sample KTK_A1E chromatogram. Figure shows the peaks of nicotine (9), eugenol (10), and acetophenone (19).
3.3.2 Nicotine
Nicotine is a psychoactive, yellow-colored liquid alkaloid that constitutes the active ingredient of Nicotiana tabacum L. (Solanaceae), a plant whose leaves produce most of the tobacco sold worldwide. It was isolated from the plant for the first time in 1828 by Posselt and Reimann, being considered a poison at the time.19 When tobacco smoke reaches the airways and alveoli in the lungs, nicotine is rapidly absorbed due to the extension of the alveolar surface. Inhaling tobacco smoke causes rapid distribution of nicotine to the nervous system, activating the dopamine-releasing nicotinic acetylcholine receptors; the increased release of this neurotransmitter acts directly on the reward system of the mesolimbic pathway.20,21
According to Brazilian law, the sale of smoked tobacco products with a nicotine content equal to or greater than 1 mg/cigarette in the mainstream smoke should be prohibited; however, due to a legal loophole, the resolution was suspended for 5 years, being re-established at the end of 2018. This judicial maneuver opened precedent to the allowance of sales.22 Through content analysis of clove cigarettes, all products presented nicotine and, apart from KTK_A1, the nicotine content was higher than eugenol (Table 5). Sample KTK_B1 had the greatest difference of peak size between nicotine and eugenol (Fig. 7).
Table 5.
Presence and concentration of nicotine in each brand of clove cigarettes analyzed and in the conventional cigarettes, used as control (CTRL) (mg/cig).
| No. | Compound name | Company A | Company B | Company C | Company D | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KTK_A1 | KTK_A1E | KTK_B1 | KTK_B2 | KTK_B2M | KTK_C1 | KTK_D1 | KTK_D1M | KTK_D2M | CTRL | ||
| 9 | Nicotine | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Concentration (mg/cig) | 12.563 | 5.438 | 14.070 | 9.226 | 12.487 | 13.704 | 10.345 | 6.235 | 4.629 | 19.169 | |
Fig. 7.

Sample KTK_B1 chromatogram. Figure shows the peaks of α-pinene (1), β-pinene (2), DDMP (4), nicotine (9), eugenol (10), β-caryophyllene (13), acetyleugenol (16), acetophenone (19), and isophytol (20).
3.3.3 Menthol
Menthol is a naturally occurring substance in several plants of the genus Mentha L., such as mint. It is primarily responsible for the plant’s characteristic minty flavor and aroma; for this reason, it is widely used by the food and hygiene industry.23 Applications of the substance in the nasal route activate the transient receptor potential cation channel melastatin-8 (TrpM8), causing a sensation of cooling in the inhaled air, which is therefore associated with an increase in the opening of airways.24,25
Due to this refreshing property, menthol is another substance widely used as an ameliorant in the manufacture of cigarettes. It is estimated that about 30% of the tobacco smoking population in the Americas and Europe consume menthol cigarettes; due to its milder effect, this type of cigarette may be responsible for the increase on the number of smokers, working as some kind of precursor to nicotine addiction.10,26
To this analysis, 3 menthol cigarette packs were purchased, all of them containing cloves also, according to the information printed. However, 4 cigarette packs had menthol (Table 6). Sample KTK_C1 did not inform the presence of menthol on the pack in any way, but through our analysis we have identified the substance in all triplicates (Fig. 8). As reported in the organoleptic properties of the sample, there was an almost numbing mint scent when opening the box. Chromatographic analysis confirmed that the compound is, in fact, menthol.
Table 6.
Presence and concentration of menthol in each of the 9 brands of clove cigarettes analyzed (mg/cig).
| No. | Compound name | Company A | Company B | Company C | Company D | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KTK_A1 | KTK_A1E | KTK_B1 | KTK_B2 | KTK_B2M | KTK_C1 | KTK_D1 | KTK_D1M | KTK_D2M | CTRL | ||
| 5 | Menthol | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ |
| Concentration (mg/cig) | – | – | – | – | 0.166 | 0.720 | – | 0.427 | 0.190 | – | |
Fig. 8.
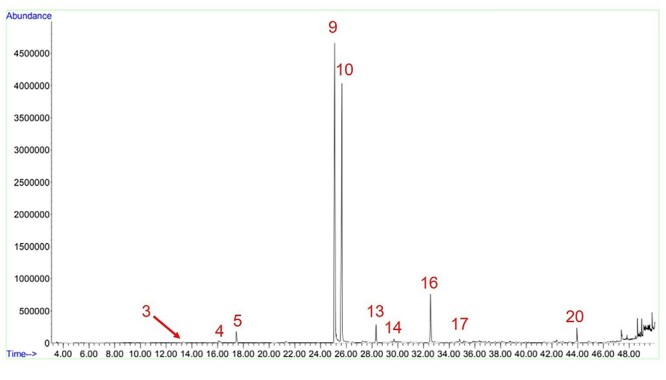
Sample KTK_C1 chromatogram. Figure shows the peaks of , DDMP (4), menthol (5), nicotine (9), eugenol (10), β-caryophyllene (13), α-humulene (14), acetyleugenol (16), caryophyllene oxide (17), and isophytol (20).26 Peak 3 shows trace of a different substance, possibly clindamycin.
3.3.4 Caryophyllane sesquiterpenes
Terpenes are compounds that occur in living organisms, mostly plants, essential for their metabolism, growth, and development. The most prevalent terpenic compounds in essential oils are monoterpenes and sesquiterpenes, which can be acyclic, monocyclic, or bicyclic depending on their structure.27
Caryophyllane sesquiterpenes are bicyclic sesquiterpenes widely used in cosmetics and in the food industries, as a scent and flavoring, respectively. Clove oil is one of the main sources of caryophillane sesquiterpenes in nature (clove may also be known as Eugenia caryophyllus [Spreng.] Bullock & S.G. Harrison), containing compounds such as β-caryophyllene, α-humulene, and caryophyllene oxide.8 It is believed that β-caryophyllene gives the “spicy” aroma in essential oils.28 The pharmacological activity of these compounds is well-documented. β-caryophyllene has an analgesic, anti-inflammatory, and antidepressant activity, whereas α-humulene presents anti-inflammatory activity, and caryophyllene oxide presents an analgesic, and anti-inflammatory activity.8
During analysis, all cigarettes presented caryophyllene oxide in a quantifiable amount, and at least traces of β-caryophyllene, but not all samples presented α-humulene (Table 7). Sample KTK_B2 (Fig. 9) was one of the few cigarettes that had visible peaks of the 3 compounds.
Table 7.
Presence of caryophyllane sesquiterpenes and concentration of β-caryophyllene in each of the 9 brands of clove cigarettes analyzed (mg/cig).
| No. | Compound name | Company A | Company B | Company C | Company D | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KTK_A1 | KTK_A1E | KTK_B1 | KTK_B2 | KTK_B2M | KTK_C1 | KTK_D1 | KTK_D1M | KTK_D2M | CTRL | ||
| 13 | β-caryophyllene | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Concentration (mg/cig) | 1.678 | 0.641 | 0.965 | 1.600 | 1.795 | 1.359 | 0.719 | 0.848 | 0.826 | – | |
| 14 | α-humulene | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ |
| 17 | Caryophyllene oxide | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
Fig. 9.

Sample KTK_B2 chromatogram. Figure shows the peaks of DDMP (4), nicotine (9), eugenol (10), aromadendrene (12), β-caryophyllene (13), α-humulene (14), acetyleugenol (16), caryophyllene oxide (17), acetophenone (19), and isophytol (20).
3.3.5 Other flavoring compounds
Clove oil can present several compounds, in different concentrations depending on the place of harvest, the part of the plant used or even the method of extraction.29 Some of these compounds could be seen through this analysis. Acetyleugenol, the second most present compound in clove oil, was present in all samples, as expected.30 Cadinene—another bicyclic sesquiterpene—was present in only 2 samples from the same brand, KTK_B2 (Fig. 9) and KTK_B2M (Fig. 10), but only in traces. Aromadendrene was absent from all samples but KTK_B2M. With antibacterial activity,29 2′,3′,4′-Trimethoxyacetophenone was absent from sample KTK_C1 (Fig. 8), but all the other samples had at least traces (Table 8). It is unknown if these compounds are added by design or only as a coincidence due to its presence in clove oil.
Fig. 10.

Sample KTK_B2M chromatogram. Figure shows the peaks of DDMP (4), nicotine (9), eugenol (10), β-caryophyllene (13), α-humulene (14), acetyleugenol (16), caryophyllene oxide (17), acetophenone (19), and isophytol (20). There are also traces of menthol (5), and chavicol (7).
Table 8.
Presence of other flavoring compounds in each of the 9 brands of clove cigarettes analyzed (mg/cig).
| No. | Compound name | Company A | Company B | Company C | Company D | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KTK_A1 | KTK_A1E | KTK_B1 | KTK_B2 | KTK_B2M | KTK_C1 | KTK_D1 | KTK_D1M | KTK_D2M | CTRL | ||
| 1 | α-pinene | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ |
| 2 | β-pinene | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ |
| 3 | Clindamycin | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ |
| 4 | DDMP | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ |
| 5 | Menthol | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ |
| 6 | HMFCA | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 7 | Chavicol | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ |
| 8 | Octodrine | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ |
| 9 | Nicotine | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| 10 | Eugenol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| 11 | Cadinene | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 12 | Aromadendrene | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ | ✗ |
| 13 | β-caryophyllene | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| 14 | α-humulene | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ |
| 15 | Oxalic acid | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ |
| 16 | Acetyleugenol | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| 17 | Caryophyllene oxide | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| 18 | Pentanoic acid | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ |
| 19 | Acetophenone | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| 20 | Isophytol | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
There are other compounds used as flavoring for the industry. The kretek industry emerged using herbs, spices, and plant extracts as saus, but the continuing growth of the industry made companies specialized in food flavoring to establish branch offices in Indonesia.1 Chavicol was present in 3 samples, KTK_A1, KTK_C1, and KTK_B2M, but only in traces. Two bicyclic monoterpenes, α-pinene and β-pinene, are found in the essential oils of many species of coniferous trees in the genus Pinus, notably pine. Both can be used as precursor substances to other aroma compounds, in addition to having their own “woody” scent.31 Both appeared together in almost all samples, except in the menthol cigarettes (KTK_B2M, KTK_D1M, and KTK_D2M). KTK_C1 have menthol but did show both pinene compounds (Table 8). In addition, KTK_A1E and KTK_D1 did not show any of both, although they did not have menthol (Fig. 11). Both menthol brands of company D (KTK_D1M and KTK_D2M) were the only samples to present butyl 1-menthyl ester oxalic acid. According to its chemical structure, this compound can be a precursor to menthol, by hydrolyzation of the oxalic acid.
Fig. 11.
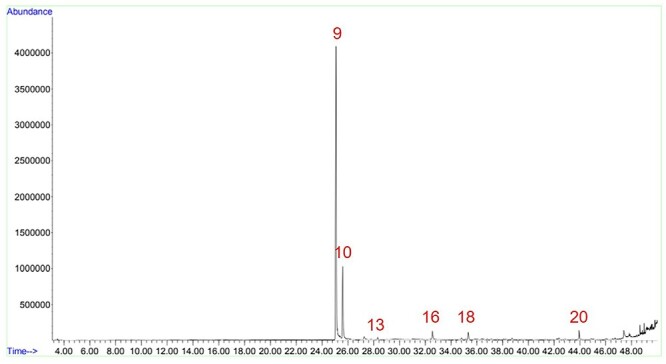
Sample KTK_D1 chromatogram. Figure shows the peaks of nicotine (9), eugenol (10), β-caryophyllene (13), acetyleugenol (16), pentanoic acid (18), and isophytol (20).
One of the most common compounds in the analysis was 3,5-dihydroxy-6-methyl-2,3-dihydro-4H-pyran-4-one (DDMP). During tobacco curing process, the reduction of sugar can form flavoring precursors, such as DDMP, presenting a sugary scent, like caramel.32 Since it is characteristic to tobacco, it was present in almost all samples, including the control sample, CTRL (Fig. 12)—it could also be the reason behind the sugary scent of CTRL observed in the organoleptic properties. However, it was not present in the KTK_A1E sample and all the samples from company D (KTK_D1, KTK_D1M, and KTK_D2M), which could indicate that this company uses a different tobacco curing process (Table 8). The absence in KTK_A1E might be understandable due to the complexity of Indonesian tobacco.1 Company D had another peculiarity, since it was the only one to present pentanoic acid, 2,2,4-trimethyl-3-carboxyisopropyl, isobutyl ester, in all samples. This ester might be involved with the tobacco curing process also, since its presence is reported in studies about the flavorings of tobacco in conventional cigarettes.33
Fig. 12.
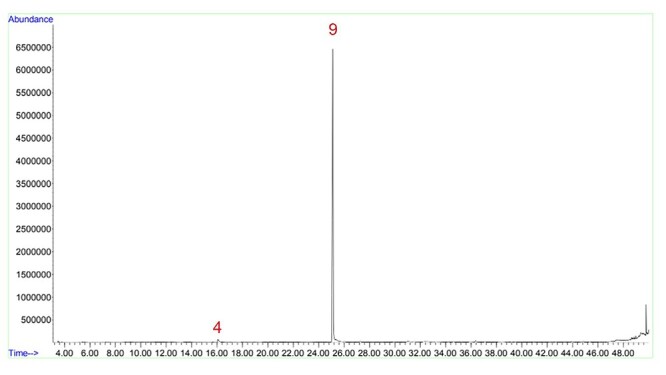
Chromatogram of the control sample, CTRL. Figure shows peaks of DDMP (4) and nicotine (9).
The 5-hydroxymethyl-2-furancarboxylic acid (HMFCA) is formed by the incomplete oxidation of 5-hydroxymethylfurfural (HMF), main product of fructose pyrolysis. HMF has a sweet and floral scent, but the role of HMFCA in flavoring is not reported, since the substance itself is not very well-known.34 The only substance that had HMFCA was KTK_A1. On the other hand, all samples had 3,7,11,15-Tetramethylhexadec-1-en-3-ol, also known as isophytol. The isophytol is a product of degradation of the chlorophyll phytyl side chain, reported in the essential oil of terrestrial plants like feather moss, Ginkgo biloba L. and sweet potato leaves.35
3.3.6 Substances of forensic interest
Clindamycin is an antibiotic used as a treatment for a series of bacterial infections, including streptococcal pharyngitis and pneumonitis. Case reports of clindamycin administration suggests that, when administered with neuromuscular blocking agents, clindamycin can cause impaired muscle function, resulting in excessive relaxation. The authors suggested that the similarity between the molecular structures of nicotine and clindamycin could explain this action.36 Traces of the substance were identified in all triplicates of sample KTK_C1 (Fig. 8; Table 9) using the NIST 2006 database. Although the substance was not compared with a certified reference material, its alleged presence raises concern due to the unknown drug interactions that can be developed without the knowledge of the user. More studies with different methods of analysis and using certified standards should be developed to ensure that this is not a simple artifact.
Table 9.
Presence of clindamycin and octodrine in traces in each of the 9 brands of clove cigarettes analyzed (mg/cig) (data not verified through a certifiable method).
| No. | Compound name | Company A | Company B | Company C | Company D | Control | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KTK_A1 | KTK_A1E | KTK_B1 | KTK_B2 | KTK_B2M | KTK_C1 | KTK_D1 | KTK_D1M | KTK_D2M | CTRL | ||
| 3 | Clindamycin | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✗ | ✗ | ✗ |
| 8 | Octodrine | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ |
Octodrine, also known as 1,5-dimetylhexamine, 2-amino-6-methylheptane or DMHA, is an aliphatic amine that acts as a vasoconstrictor. It was a candidate to substitute amphetamines in the aerosols for treatment of bronchitis and laryngitis in the 1940s. Studies found that octodrine can increase blood pressure and cardiac output in large animals, and there are suggestions of central nervous system action in rodents, increasing the uptake of certain neurotransmitters.37
Recently, octodrine appeared in nutritional supplements as a fat-burning pre-workout, treated as a natural occurring substance. There are indications of possible sources, such as jimson weed (Datura stramonium L.) and sausage tree (Kigelia africana [Lam.] Benth), although those are not confirmed.38 A very recent study reported that octodrine can be formed through the process of pyrolysis of rice husks.39 The substance was listed as a specified stimulant in the World Anti-Doping Association (WADA) list of prohibited substances for the first time in 202040.
Samples KTK_D1M and KTK_D2M, both from the same company and sold as “Kretek Menthol Cigarettes” (Fig. 13; Table 9), had a substance that was identified as octodrine by the NIST 2006 library, but its presence was not tested with a certified reference material to confirm the identity of the substance. However, the possible presence of octodrine is noteworthy, especially due to its alleged effect on the uptake of neurotransmitters, which could aggravate the lightheadedness effects of clove cigarettes. Interestingly enough, both substances—clindamycin and octodrine—have their origins as treatment for respiratory airways diseases, reminiscing to the first use of kretek as a relief from chest pains.1 This alone should be sufficient reason to encourage further studies to confirm or discard the presence of these substances.
Fig. 13.
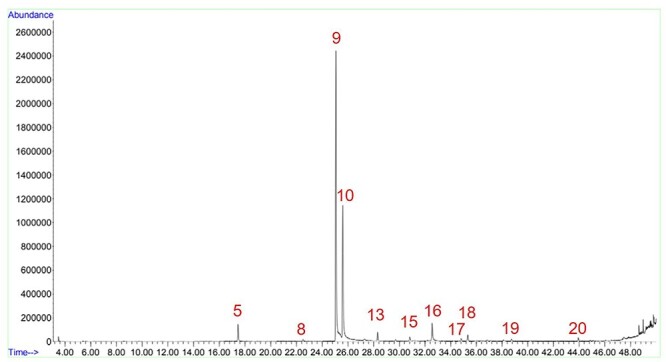
Sample KTK_D1M chromatogram. Figure shows the peaks of menthol (5), nicotine (9), eugenol (10), β-caryophyllene (13), oxalic acid (15), acetyleugenol (16), pentanoic acid (18), acetophenone (19), and isophytol (20). There are also traces of caryophyllene oxide (17) and possibly octodrine (8).
3.4 Quantitative analysis
In possession of the qualitative results, calibration curves were designed to quantify some of the main substances. Eugenol, menthol, β-caryophyllene, and nicotine were chosen, to assess if the levels of 3 major flavoring compounds were high, and to verify if the nicotine content met the criteria specified in the law. At least 4 points of each substance were chosen to construct the curve. All analytes exhibited linearity (R2) greater than 0.997. Eugenol equation was y = 4.820.580,575x − 4.082.817,013 (R2 = 0.999971), menthol equation was y = 1.793.728,817x − 345.720,458 (R2 = 0.995765), β-caryophyllene equation was y = 2.958.234,227x − 906.579,880 (R2 = 0.999891) and nicotine equation was y = 3.118.714,667x − 676.581,982 (R2 = 0.997). The quantity in mg per cigarette extraction (mg/cig) is listed in Table 10.
Table 10.
Concentration of eugenol, nicotine, menthol, and β-caryophyllene in milligrams per cigarette extraction (mg/cig).
| Sample/compound | Eugenol | Nicotine | Menthol | β-caryophyllene |
|---|---|---|---|---|
| Concentration (mg/cig) | ||||
| KTK_A1 | 27.218 | 12.563 | – | 1.678 |
| KTK_A1E | 3.416 | 5.438 | – | 0.641 |
| KTK_B1 | 3.788 | 14.070 | – | 0.965 |
| KTK_B2 | 5.957 | 9.226 | – | 1.600 |
| KTK_B2M | 10.011 | 12.487 | 0.166 | 1.795 |
| KTK_C1 | 9.699 | 13.704 | 0.720 | 1.359 |
| KTK_D1 | 3.266 | 10.345 | – | 0.719 |
| KTK_D1M | 3.223 | 6.235 | 0.427 | 0.848 |
| KTK_D2M | 2.733 | 4.629 | 0.190 | 0.826 |
| CTRL | – | 19.169 | – | – |
As observed in the qualitative analysis, all samples had lower levels of eugenol compared with the levels of nicotine, except for sample KTK_A1, with levels of eugenol 2 times higher than levels of nicotine. Apart from the CTRL, the sample with more nicotine concentration was indeed KTK_B1, as theorized in the analysis of the organoleptic properties, almost 4 times higher than the levels of eugenol—the smell of nicotine overcomes any other scent. This was also the cheapest product, equivalent to approximately US$ 1.25, using the exchange rate of 1st September 2021 (R$ 6.50). Brazillian law prohibit the sale of product with a nicotine content equal to or greater than 1 mg/cigarette in the mainstream smoke. This methodology does not evaluate the quantity in smoke, but all samples, including CTRL, had levels much greater than 1 mg/cigarette using a solvent extract. That raises the question of how much nicotine concentration in the cigarette content is equivalent to 1 mg/cigarette in the mainstream smoke.
The qualitative analysis of the chromatogram peaks indicated that KTK_C1 had menthol even though it was not displayed on the cigarette pack. Quantitative analysis not only confirmed this information, but also evaluated that the menthol concentration was higher than the content of menthol cigarettes. All the other samples indicating menthol content had at least a low concentration of the compound. β-caryophyllene concentration seems to be high in samples with high eugenol concentration, and low in samples with low eugenol concentration; however, the relation did not seem to be linear. Sample KTK_B2M had the highest content of β-caryophyllene, but its eugenol content was the second highest, and the second highest level of β-caryophyllene was present in KTK_A1, which has the highest content of eugenol.
4. Conclusions
The organoleptic properties were important to indicate the probable contents present in each sample. Kretek cigarette packs have a beautiful and exotic design, a lot more attractive than conventional cigarette packs. This could be a strategy to draw the attention of teenagers and young adults, through visual appeal and the implementation of new trends. All of the packs were either pitch-black, shiny, elegant, mysterious, or a combination of these traits.
One of the packs did not have the indication of a menthol product, but the scent of mint coming from the pack, confirmed by qualitative and quantitative analysis proved that the compound was there, and with a higher concentration than the other menthol cigarettes. All menthol cigarettes had low concentrations of the compound, but the minty scent was very perceptible, showing that even a small concentration can have an important effect on flavoring. The cheapest product (KTK_B1) had the highest amount of nicotine comparing with eugenol, and the most expensive (KTK_A1) had the highest concentration of eugenol. KTK_A1 was also the only brand that had more eugenol than nicotine; all the other brands had a higher concentration of nicotine than eugenol, but none of them had a higher concentration than the conventional cigarette, CTRL. The concentration of β-caryophyllene had some correlation with the concentration of eugenol, although not linear.
Through the methodology of methanol extraction, several compounds were identified; although a methodology using headspace extraction could identify more volatile compounds, the solvent extraction was efficient especially with substances derived from essential oils. Some of these were expected to be found in the samples, such as eugenol, nicotine, menthol, and β-caryophyllene. A few others could be predicted, like other terpenes (cadinene, aromadendrene, acetyleugenol, α-pinene, and β-pinene) or other flavoring substances (acetophenone, pentanoic acid, and isophytol). However, 2 compounds were unexpected: clindamycin and octodrine. These 2 substances were not confirmed by a certified method, but the mechanisms of action that these substances have on nicotinic receptor could lead to important development on the user’s well-being, due to drug interaction and unknown effects or intolerance. It is crucial to realize more studies that can confirm or rule out the presence of clindamycin and octodrine, along with other substances that could be used as ameliorants or stimulants in cigarettes. The determination of the content in kretek cigarettes should be more studied to adjust the courses of action regarding the treatment of users and tobacco control and regulation around the world.
Acknowledgments
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support and the Universidade Federal do Rio Grande do Sul (UFRGS) for the opportunity to use its facilities to perform this analysis.
Contributor Information
João Marcelo Astolfi Picanço, Faculty of Pharmacy, Universidade Federal do Rio Grande do Sul (UFRGS), BrazilAv. Ipiranga 2752, Porto Alegre, 90160-093, Brazil.
Renata Pereira Limberger, Faculty of Pharmacy, Universidade Federal do Rio Grande do Sul (UFRGS), BrazilAv. Ipiranga 2752, Porto Alegre, 90160-093, Brazil.
Miriam Anders Apel, Faculty of Pharmacy, Universidade Federal do Rio Grande do Sul (UFRGS), BrazilAv. Ipiranga 2752, Porto Alegre, 90160-093, Brazil.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 88887.356837/2019-00.
Conflict of interests
The authors declare none.
Authors’ contributions
João Marcelo Astolfi Picanço conceived the study, conducted the investigation, the formal analysis, the data curation, and the writing of the original draft. Miriam Anders Apel supervised the study, administered the project, developed the methodology, provided the resources, and conducted the formal analysis, the review and editing of the writing. Renata Pereira Limberger also supervised the study, provided the resources, acquired the funding, and conducted the validation, the review and the editing of the writing.
References
- 1. Hanusz M. Kretek: the culture and heritage of Indonesia’s clove cigarettes. Jakarta, Indonesia: Equinox Publishing; 2000. p. 203 [Google Scholar]
- 2. Djajadi D. Tobacco diversity in Indonesia. J Biol Res. 2015:20(2):27–32. Available from. http://berkalahayati.org/index.php/jurnal/article/view/106. [Google Scholar]
- 3. Arnez M. Tobacco and Kretek: Indonesian drugs in historical change. ASEAS - Austrian J South-East Asian Stud. 2009:2(1):49–69. [Google Scholar]
- 4. Roemer E, Dempsey R, Schorp MK. Toxicological assessment of kretek cigarettes: part 1: background, assessment approach, and summary of findings. Regul Toxicol Pharmacol. 2014:70(S1):S2–S14. [DOI] [PubMed] [Google Scholar]
- 5. Cloves and Kretek . Bull Indones Econ Stud. 1965; 1(2):49–59. 10.1080/00074916512331339849 [DOI] [Google Scholar]
- 6. Achadi A, Soerojo W, Barber S. The relevance and prospects of advancing tobacco control in Indonesia. Health Policy (New York). 2005:72(3):333–349. [DOI] [PubMed] [Google Scholar]
- 7. Polzin GM, Stanfill SB, Brown CR, Ashley DL, Watson CH. Determination of eugenol, anethole, and coumarin in the mainstream cigarette smoke of Indonesian clove cigarettes. Food Chem Toxicol. 2007:45(10):1948–1953. [DOI] [PubMed] [Google Scholar]
- 8. Sotto A, Mancinelli R, Gullì M, Eufemi M, Mammola CL, Mazzanti G, di Giacomo S. Chemopreventive potential of caryophyllane sesquiterpenes: an overview of preliminary evidence. Cancers (Basel). 2020:12(10):3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Huh J, Timberlake DS. Do smokers of specialty and conventional cigarettes differ in their dependence on nicotine? Addict Behav. 2009:34(2):204–211. [DOI] [PubMed] [Google Scholar]
- 10. Eijk Y, Lee JK, Ling PM. How menthol is key to the tobacco industry’s strategy of recruiting and retaining young smokers in Singapore. J Adoles Health. 2019:64(3):347–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amul GGH, Tan GPP, Eijk Y. A systematic review of tobacco industry tactics in southeast asia: lessons for other low-and middle-income regions. Int J Health Policy Manag. 2021:10(6):324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Erinoso O, Clegg Smith K, Iacobelli M, Saraf S, Welding K, Cohen JE. Global review of tobacco product flavour policies. Tob Control. 2021:30(4):373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stanfill SB, Ashley DL. Solid phase microextraction of alkenylbenzenes and other flavor-related compounds from tobacco for analysis by selected ion monitoring gas chromatography–mass spectrometry. J Chromatogr A. 1999:858(1):79–89. [DOI] [PubMed] [Google Scholar]
- 14. Adams RP. Identification of essential oil components by gas chromatography/mass spectroscopy. 4th ed. Carol Strem, IL, USA: Allured; 2017. p. 804 [Google Scholar]
- 15. Reichardt C, Welton T. Solvents and solvent extracts in organic chemistry. 4th ed. Weinheim, Germany: Wiley-VCH Verlag & Co; 2011. p. 677 [Google Scholar]
- 16. Bainard LD, Isman MB, Upadhyaya MK. Phytotoxicity of clove oil and its primary constituent eugenol and the role of leaf epicuticular wax in the susceptibility to these essential oils. Weed Sci. 2006:54(05):833–837. [Google Scholar]
- 17. Sellamuthu R. Eugenol. In: Encyclopedia of toxicology. Third ed. Cambridge, Massachusetts: Academic Press; 2014. pp. 539–541
- 18. Tao G, Irie Y, Li DJ, Wing MK. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorg Med Chem. 2005:13(15):4777–4788. [DOI] [PubMed] [Google Scholar]
- 19. Henningfield JE, Zeller M. Nicotine psychopharmacology research contributions to United States and global tobacco regulation: a look back and a look forward. Psychopharmacology. 2006:184(3–4):286–291. [DOI] [PubMed] [Google Scholar]
- 20. Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. Berlin, Heidelberg: Springer; 2010:35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. D’Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract. 2011:6(1):4–16. [PMC free article] [PubMed] [Google Scholar]
- 22. Picanço JMA, Limberger RP, Apel MA. The risk associated to the lack of information about clove cigarettes. Biomed J Sci Tech Res. 2019:18(5):13863–13865. [Google Scholar]
- 23. Galeotti N, Di Cesare ML, Mazzanti G, Bartolini A, Ghelardini C. Menthol: a natural analgesic compound. Neurosci Lett. 2002:322(3):145–148. [DOI] [PubMed] [Google Scholar]
- 24. Eccles R. Menthol and related cooling compounds. J Pharm Pharmacol. 1994:46:618–630. [DOI] [PubMed] [Google Scholar]
- 25. Henderson BJ. Linking nicotine, menthol, and brain changes. In: Neuroscience of nicotine. 2019. pp. 87–95 [Google Scholar]
- 26. Henningfield JE, London ED, Pogun S. Nicotine psychopharmacology. Cambridge, Massachusetts: Academic Press; Vol. 192; 2009. p. 544 [Google Scholar]
- 27. Dewick PM. Medicinal natural products: a biosynthetic approach. In: Medicinal natural products: a biosynthetic approach: third edition. Vol. 5. third ed. Hoboken, USA: John Wiley & Sons; 2009. p. 539 [Google Scholar]
- 28. Amaral MSS, Marriott PJ. The blossoming of technology for the analysis of complex aroma bouquets—a review on flavour and odorant multidimensional and comprehensive gas chromatography applications. Molecules. 2019:24(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma YN, Xu FR, Chen CJ, Li QQ, Wang MZ, Cheng YX, Dong X. The beneficial use of essential oils from buds and fruit of Syzygium aromaticum to combat pathogenic fungi of Panax notoginseng. Ind Crop Prod. 2019:133(February):185–192. [Google Scholar]
- 30. Chaieb K, Hajlaoui H, Zmantar T, Kahla-Nakbi AB, Rouabhia M, Mahdouani K, Bakhrouf A. The chemical composition and biological activity of clove essential oil, Eugenia caryophyllata (Syzigium aromaticum L. Myrtaceae): a short review. Phytother Res. 2007:21(6):501–506. [DOI] [PubMed] [Google Scholar]
- 31. Vespermann KAC, Paulino BN, Barcelos MCS, Pessôa MG, Pastore GM, Molina G. Biotransformation of α- and β-pinene into flavor compounds. Appl Microbiol Biotechnol. 2017:101(5):1805–1817. [DOI] [PubMed] [Google Scholar]
- 32. Yin F, Karangwa E, Song S, Duhoranimana E, Lin S, Cui H, Zhang X. Contribution of tobacco composition compounds to characteristic aroma of Chinese faint-scent cigarettes through chromatography analysis and partial least squares regression. J Chromatogr B. 2019:1105(November 2018):217–227. [DOI] [PubMed] [Google Scholar]
- 33. Hou Y, Yang L, Wang B, Xu J, Yang Y, Yang Y, Cao Q, Xie X. Analysis of chemical components in tobacco flavors using stir bar sorptive extraction and thermal desorption coupled with gas chromatography-mass spectrometry. Chin J Chromatogr. 2006:24(6):601–605. [DOI] [PubMed] [Google Scholar]
- 34. Sayed M, Pyo SH, Rehnberg N, Hatti-Kaul R. Selective oxidation of 5-hydroxymethylfurfural to 5-hydroxymethyl-2-furancarboxylic acid using gluconobacter oxydans. ACS Sustain Chem Eng. 2019:7(4):4406–4413. [Google Scholar]
- 35. Rontani JF, Galeron MA. Autoxidation of chlorophyll phytyl side chain in senescent phototrophic organisms: a potential source of isophytol in the environment. Org Geochem. 2016:97:35–40. [Google Scholar]
- 36. Schulze J, Toepfer M, Schroff KC, Aschhoff S, Remien J, Müller-Felber W, Endres S. Clindamycin and nicotinic neuromuscular transmission. Lancet. 1999:354(9192):1792–1793. [DOI] [PubMed] [Google Scholar]
- 37. Dib J, Bosse C, Tsivou M, Glatt AM, Geisendorfer T, Geyer H, Gmeiner G, Sigmund G, Thevis M. Is heptaminol a (major) metabolite of octodrine? Drug Test Anal. 2019:11(11–12):1761–1763. [DOI] [PubMed] [Google Scholar]
- 38. Cohen PA, Travis JC, Keizers PHJ, Deuster P, Venhuis BJ. Four experimental stimulants found in sports and weight loss supplements: 2-amino-6-methylheptane (octodrine), 1,4-dimethylamylamine (1,4-DMAA), 1,3-dimethylamylamine (1,3-DMAA) and 1,3-dimethylbutylamine (1,3-DMBA). Clin Toxicol. 2018:56(6):421–426. [DOI] [PubMed] [Google Scholar]
- 39. Tian B, Xu L, Jing M, Liu N, Tian Y. A comprehensive evaluation on pyrolysis behavior, kinetics, and primary volatile formation pathways of rice husk for application to catalytic valorization. Fuel Process Technol. 2021:214(January):106715. [Google Scholar]
- 40. World Anti-Doping Agency (WADA) . Standard prohibited list. The World Anti-Doping Code; 2020 [Google Scholar]


