Abstract
TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) is a ubiquitous environmental toxicant and a notable teratogenic agent for cleft palate (CP), a common congenital structural malformation that can result from abnormalities during palatal shelf connection and/or fusion. The development of the palate requires precise coordination between mesenchymal and epithelial cells. Exosomes are vesicles secreted by cells and participate in organ development by transferring various bioactive molecules between cells and regulating cell proliferation, migration, apoptosis, and epithelial–mesenchymal transition (EMT); these vesicles represent a new method of intercellular communication. To explore how TCDD could influence palatal cell behaviors and communication, we treated mesenchymal cells with TCDD, collected the exosomes secreted by the cells, assessed the 2 types of palatal cells, and then observed the effects of TCDD-induced exosomes. We found that the effects of TCDD-induced exosomes were equal to those of TCDD. Thus, TCDD might change the genetic materials of palatal cells and exosomes to cause dysregulated gene expression from parental cells, affect cellular information communicators, and induce abnormal cellular behaviors that could lead to CP.
Keywords: 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin, exosomes, proliferation, migration, apoptosis, epithelial–mesenchymal transition
Introduction
TCDD is a highly toxic chemical and a dangerous environmental pollutant that can cause anatomical abnormalities and developmental defects.1 TCDD is widely accepted as a teratogenic effector of cleft palate (CP) and has been revealed to cause high rates of CP.2 CP is a common congenital anomaly that occurs due to variations in genes associated with craniofacial development and may occur as part of a syndrome or more commonly in isolated forms.3 Both genetic variation and environmental factors play important roles in nonsyndromic cases.4 Because it consists of both epithelium and mesenchyme, the formation of the palate requires these two types. Palatal development requires delicate coordination during programs of palatal cell migration, growth, differentiation, and apoptosis, which are precisely regulated by genes. MicroRNAs (miRNAs) are a family of small noncoding RNAs (ncRNAs) that inhibit the expression of target messenger RNAs (mRNAs) by binding to complementary sequences in these molecules. MiRNAs are also key regulators in embryological development and play pivotal roles in palatogenesis.5,6 Abnormal changes in any signal molecules in time, space, and quantity during the morphogenesis, growth, and fusion of the palate can lead to cleft lip and palate.7 However, how TCDD, as an environmental toxicant, induces CP remains unclear. Previous studies focused on only one type of palatal cell and never combined both epithelial cells and mesenchymal cells to clarify the process of palatal formation. Understanding the interaction between palatal cells will facilitate the development of targeted drugs and clinical treatment for CP in the future.
Exosomes are vesicles that can be secreted by almost all mammalian cells and are widely distributed in many body fluids.8 These vesicles represent a new mode of intercellular communication and play an important role in the human transcriptome, which can be selectively packed, secreted, and transferred to exosomes to regulate cell proliferation, migration, apoptosis, and epithelial–mesenchymal transition (EMT) and thus plays a key role in a variety of pathophysiological processes.9 Exosomes contain bioactive molecules derived from parental cells, including nucleoid acids, lipids, and proteins. Extensive studies have revealed that miRNAs are selectively sorted into exosomes that participate in cellular communication.10
We hypothesized that TCDD might exert detrimental side effects on cellular processes via exosomes. To date, the role of exosomes during palate formation is unclear. The purpose of this experiment was to elucidate the potential mechanism of TCDD-mediated CP and the role of TCDD-induced exosomes in this condition.
Materials and methods
Animals and cells
After receiving approval from Children’s Hospital of Chongqing Medical University, 30 pregnant C57BL/6 female mice were purchased from Chongqing Medical University Animal Center and propagated. The mice were fed under conditions consisting of a sustainable temperature (22 2°C), 50
2°C), 50 relative humidity and a 12:12-h alternating light–dark cycle. All experiments were supported by the Experimental Animal Center Guide for the Care and Use of Laboratory Animals.
relative humidity and a 12:12-h alternating light–dark cycle. All experiments were supported by the Experimental Animal Center Guide for the Care and Use of Laboratory Animals.
Primary mesenchymal cells originated from the palatal tissue of gestational day (GD) 14 embryos from C57BL/6 mice. Mesenchymal cells were isolated and cultured according to a previous study.11,12 The culture medium was basic (1X) DMEM/F12 (1:1) (Gibco, USA) with 10% fetal calf bovine serum (FBS, Sijiqing, China). Primary epithelial cells were also derived from the palatal tissue of GD14 embryos from C57BL/6 mice. Both epithelial cells and medium were acquired from Shanghai Haling Biotechnology Company, Limited. A specific medium was used to cultivate the epithelial cells. Their morphologies were confirmed by microscopic analysis. The palatal cell species were identified as described previously.11
Exosome isolation and identification
Exosome isolation
Third- to seventh-generation mouse embryonic palatal mesenchymal (MEPM) cells were cultured in Gibco Dulbecco’s modified Eagle’s medium:F-12 (1:1) supplemented with 10% exosome-free serum (FBS, Sijiqing, Hangzhou, China) by ultracentrifugation. The mesenchymal cells were placed in a humidified incubator at 37°C with a 5% CO2 atmosphere in medium. The cells of the TCDD group (T group) were treated with 10 nmol/L TCDD (Sigma–Aldrich, MO, USA) diluted in DMSO, whereas the cells of the control group (C group) were treated with DMSO (≤0.05%). The dose of TCDD used was determined based on a previous study that identified the proper TCDD dose.13
Supernatants were collected after 48 h. After removal of nonadherent cells and debris, extracellular vesicles (EVs) were further purified using a total exosome isolation kit (Qiagen, USA) according to the manufacturer’s instructions. Briefly, the supernatants were filtered to exclude particles >0.8 μm using syringe filters (EMD Millipore, Burlington, MA, USA). Prefiltered samples were then mixed with Buffer XBP (EMD) and bound to an exoEasy membrane affinity spin column. The bound EVs were washed with Buffer XWP (EMD), eluted with 1,000 μL of Buffer XE (an aqueous buffer containing primarily inorganic salts, EMD), and identified with a nanoparticle tracking system, Western blots, and electron microscopy.
Transmission electron microscopy
The isolated multivehicular bodies from MEPM cells were identified via electron microscopy to verify the size and characteristic cup-shaped morphology. In short, purified exosomes were resuspended in 2% paraformaldehyde, loaded onto formvar carbon-coated grids and then dried for 20 min at 40°C. The grids were washed in PBS, fixed in 1% glutaraldehyde, and then rinsed several times in distilled water. The grids were transferred to 4% uranyl oxalate solution for 5 min and then embedded in a mixture of uranyl acetate (4%) and methyl cellulose (2%) on ice. The mounted exosomal samples were examined with an electron microscope.
Western blot analysis for exosome identification
The protein contents of MEPM cell-derived exosomes were determined using a Bicinchoninic Acid Assay analysis kit (Solarbio, China), and Western blot was then performed using 10 μg of protein that was separated on 10% polyacrylamide gels. The following exosome-specific antibodies were used: CD63 (Wanlei Bio, 1:1,000), Alix (Wanlei Bio, 1:1,000), and TSG101 (Wanlei Bio, 1:1,000).
Nanoparticle tracking analysis
A NanoSight model LE14 (NanoSight) equipped with a blue (404 nm, 70 mW) laser and SCMOS camera was used to analyze the purified EV samples. The samples were diluted 10 times with PBS, and three 90-s videos were recorded using a camera level 13–14. Nanoparticle tracking analysis (NTA) software 2.3 with the detection threshold optimized for each sample was used to analyze the data, and a screen gain of 10 was used to track as many particles as possible with minimal background.
Exosomal miRNA sequencing
miRNA sequencing of MEPM cell-derived exosomes was performed. Total miRNA was purified using the miRNeasy Serum/Plasma Kit (50) (Qiagen #217184). The quality of the isolated miRNA was tested using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA, USA), and the miRNAs were quantified using a Qubit®3.0 Fluorometer and NanoDrop One spectrophotometer. The purified miRNAs were converted into first-strand complementary DNA (cDNA) using reverse transcriptase and random primers. These DNAs were purified and then enriched by PCR to create a final cDNA library. The purified library was quantified using a Qubit™ 3.0 Fluorometer (Thermo Fisher, USA) and validated with an Agilent Technologies 2100 Bioanalyzer (Agilent Technologies, USA) to confirm the insert size and calculate the mole concentration.
Cell culture and treatment
Exosomes for uptake experiments
To determine the efficiency of exosome uptake, exosomes were labeled with PKH26 (Invitrogen, USA). Briefly, 400 μg of exosomes was resuspended in 0.5 mL of Diluent C, and 2.5 μL of PKH26 was then mixed with Diluent C prior to staining. The PKH26 suspension was mixed with the exosome suspension, incubated for 5 min, and protected from light. The staining reaction was stopped by the addition of DMEM/F12 and centrifuged at 100,000 × g and 4°C for 70 min. The pelleted exosomes were then washed with 200 μL of PBS and separately cultured with mesenchymal and epithelial cells. Following coincubation for 24 h, the cells were washed with PBS, stained with DAPI for 5 min, washed with PBS, and fixed with 4% formaldehyde. Images of exosome uptake by cells were acquired with a C2+ confocal 60× oil objective.
Cells induced with different exosomes
The MEPM cells and epithelial cells were incubated medium in a humidified incubator at 37°C with a 5% CO2 atmosphere. The cells belonging to the experimental group were cultured separately in flasks with 100 μg/mL exosomes obtained from the T and C groups.
Cell proliferative viability
CCK-8 assay
MEPM cells (1 × 104 cells per well) were seeded in 96-well plates (Corning, USA). The cells in the experimental groups were treated with 100 μg/mL exosomes of different types from the T and C groups. The cells in all the groups were cultured for 72 h. Cell proliferation was detected by CCK-8 (CK-04, Dojindo, Japan) assays according to the manufacturer’s protocol.
EdU assay
MEPM cells (5 × 104 cells per well) were cultured in 24-well plates with different exosomes for 72 h. After the addition of 200 μL of 50 μM EdU (RiboBio, Guangzhou, China), the cells were incubated at 37°C for 2 h and then fixed in 4% paraformaldehyde for 30 min. After permeabilization with 0.5% Triton X-100 for 10 min, 200 μL of Apollo reaction liquid was added to the reaction, and the reaction proceeded for 30 min at room temperature. The cells in each well were stained with Hoechst 33342 (200 μL) for 30 min for nucleus staining. The images were then obtained under a laser-scanning confocal microscope (Nikon A1R, Japan) at 200× magnification, and the number of proliferative cells in 5 random fields in each sample was calculated and analyzed. Each sample was assayed in duplicate, and the experiment was repeated 3 times.
Cell migration assay
Wound healing assay
Wound healing assays were carried out using migration culture dish inserts from Ibidi (Martinsried, Germany) with a straight scratch. Cells were seeded in the chambers of 24-well plates and induced with the appropriate exosomes. After culture for 24 h, the cells were fully confluent, the insert was removed, and fresh culture medium was added to start the migration process. Images were acquired after 24 h using a microscope with a 40× objective (TE2000-U, Nikon). Image analysis was performed using ImageJ (http//imagej.nih.gov.ij).
Transwell assay
MEPM cells were seeded in 6-well plates and incubated with different exosomal media for 24 h. The cells were harvested and then seeded in the upper chambers (1 × 104 cells per chamber) with 0% FBS medium, whereas normal FBS medium was added to the lower chambers. After incubation under standard conditions for 24 h and staining with crystal violet, the cells were observed with an optical microscope at 100× magnification. The migrated cells were counted using ImageJ software (http//imagej.nih.gov.ij).
Cell apoptosis assay
Cell apoptosis assay via FCM (flow cytometry)
After exposure of the MEPM cells to different exosomes for 72 h, the cells were harvested by 0.25% trypsin without EDTA, washed twice with ice-cold PBS, and then resuspended in 200 μL of binding buffer. For the Annexin V-fluorescein isothiocyanate (FITC) binding assay, the cells were stained with 10 μL of Annexin V-FITC and 5 μL of propidium iodide (PI) for 10 min in a dark room at room temperature and then analyzed by flow cytometry (BD FACS, USA). Cell apoptosis was evaluated with an Annexin V/FITC/PI apoptosis kit (NeoBioscience, China). The results of the Annexin V/FITC total binding assay were calculated as the percentage of apoptotic cells among the total number of cells after immediate analysis by flow cytometry with fluorescence-activated cell sorting.
Western blot assay
After the cells were incubated on 60-mm Petri dishes with different exosomes for 72 h, each sample was scratched with a cell scraper and then placed in ice-cold lysis buffer (KeyGEN BioTECH, China) for 30 min. The sample was homogenized on ice and centrifuged at 12,000 × g and 4°C for 30 min. The supernatant was mixed with protein loading buffer (Beyotime Biotech, Inc., China) at a 1:4 ratio and denatured at 95°C. The protein contents in the processed samples were determined using a BCA analysis kit (Solarbio, China) while ensuring that the protein content in each sample was consistent. Twenty micrograms of protein was placed in each well, electrophoresed on a 12.5% sodium dodecyl sulfate polyacrylamide gel, and then transferred onto a polyvinylidene difluoride membrane. The membrane was blocked with blocking buffer (NCM Biotech, China) for 10 min, probed with polyclonal rabbit anti-rat Bax antibodies (1:500; Affinity Bioscience, China), caspase-3 antibodies (1:500; Wanlei Bio, China), and GAPDH antibodies (1:5000; loading control) for 16 h at 4°C and then incubated with the appropriate horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000; ZenBio, China) at room temperature for 1 h. Immunoreactive traces were detected using a chemiluminescent ECL kit (4 Biotech Co, China). Each protein band on the membrane was scanned using a GeneGnome 5 No. 75000 system (Synoptics, UK) and is presented between appropriate marker bands.
EMT assay
EMT was verified via immunofluorescence double staining and laser-scanning confocal microscopy. The primary antibodies used in our study were mouse anti-vimentin monoclonal antibody (Protein 60330-1-igG, USA), rabbit anti-Zo-1 polyclonal antibody (Protein 21773-1-ap, USA), TRITC-labeled goat anti-rabbit IgG (ZenBio 511202, China), and FITC-labeled goat anti-mouse IgG (ZenBio 511101, China). The epithelial cells were seeded separately on slides with different exosomal media for 72 h. The cells were then rinsed with PBS, fixed with 4% paraformaldehyde, and permeabilized in PBS containing 0.1% Triton. The cells were blocked with 3% BSA in PBS and incubated with ZO-1 antibody and vimentin antibody for 12 h.
Statistical analysis
Each experiment was performed at least in triplicate. The results are shown as the mean values ± SDs. Statistical comparisons were performed with Student’s t test. All statistical analyses were performed using IBM SPSS Statistics 25 software (SPSS, Chicago, IL, USA), and differences with P-values <0.05 were considered statistically significant. All schematic diagrams were drawn with GraphPad Prism 8.0 software.
Results
Mesenchymal and epithelial cells showed different morphologies
An optical microscope was used for 100× magnification of the cell morphology. The primary epithelial cells showed a pebble-like morphology without pseudopodia, whereas the mesenchymal cells were star shaped with antennas, as illustrated in Fig. 1.
Fig. 1.
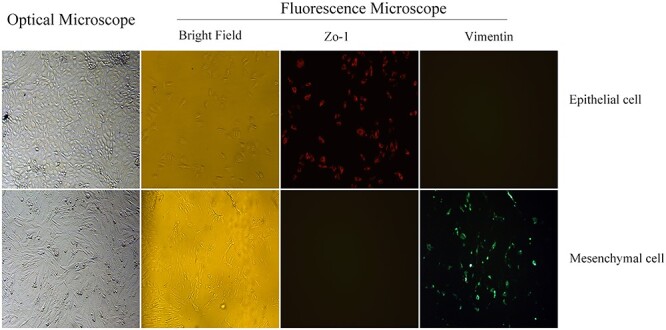
The primary cells were homogeneous in their cobblestone morphology, and the marker Zo-1 of the epithelial cells exhibited red fluorescence. In contrast, the mesenchymal cells were fusiform or star-shaped and expressed the marker vimentin with green fluorescence.
Presence of exosomes derived from the different parental MEPM cells
First, we identified exosomes derived from MEPM cells. Transmission electron microscopy (TEM) revealed MEPM cell-derived vesicles with a cup-shape morphology (Fig. 2a). Nanoparticle Tracking Analysis (NTA) revealed sizes between 50 and 150 nm, which was consistent with the known exosomal size in cultured mesenchymal cell supernatant (Fig. 2b). CD63, Alix, and TSG101, which are markers of EVs, were highly expressed in mesenchymal exosomes (Fig. 2c). Thus, exosomes are present in mesenchymal cells, diffuse through the basement membrane, and can be readily isolated.
Fig. 2.
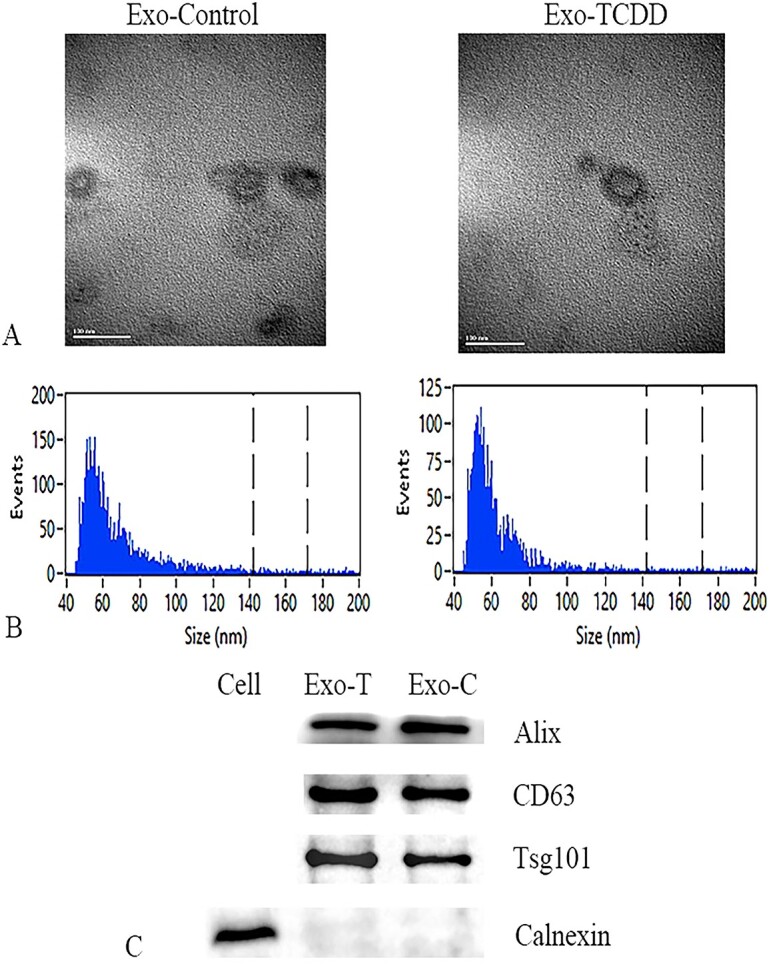
Identification of TCDD-induced and control exosomes. A) TEM scanning images. B) Size distribution of the exosomes determined by NTA. C) Western blots of exosomal marker proteins.
Both mesenchymal epithelial cells take up exosomes derived from MEPM cells
To confirm that mesenchymal and epithelial cells can engulf exosomes, we added exosomes labeled with red fluorescence to the medium, which was used to culture palatal cells. We found that the exosomes entered the cells and were distributed in the cytoplasm (Fig. 3).
Fig. 3.

Verification of phagocytic exosomes. Exosomes were labeled with red fluorescence, and mesenchymal cell and epithelial cell nuclei were marked with blue fluorescence. After 24 h of incubation, the cells phagocytosed exosomes.
MiRNA microarray analysis
To determine the differences in miRNAs between TCDD-induced exosomes and normal exosomes, we analyzed 6 samples via a comprehensive miRNA microarray analysis: 3 TCDD-induced exosomal samples and 3 normal exosomal samples. Hierarchical clustering showed 20 major differentially expressed miRNAs between the 2 groups (Fig. 4).
Fig. 4.

Heatmap of the differentially expressed miRNAs in the 2 groups of exosomes.
The proliferation of MEPM cells was inhibited by TCDD-induced exosomes
In this experiment, we evaluated the effect of exosomes on the proliferation of MEPM cells. After treatment of the experimental and control groups with 100 μg/mL exosomes, we found that cell proliferation was more significantly inhibited by the TCDD-treated exosomes compared with the control exosomes. As shown in Fig. 5, cell proliferation in the TCDD group was significantly lower than that in the control group after 72 h.
Fig. 5.

Effects on the proliferation of mesenchymal cells incubated with 2 types of exosomes. A) CCK-8 assay. B) EdU assays. **P < 0.01 vs. the corresponding control values.
TCDD-induced exosomes influence the motility of MEPM cells
To determine whether TCDD-induced exosomes affect the motility of MEPM cells, we detected the migration of the cells by transwell and wound healing assays. After treatment with TCDD-induced exosomes and normal exosomes for 24 h, the mobility of the cells was inhibited in the TCDD-treated exosome group (Fig. 6).
Fig. 6.

Effects of different types of exosome-induced mesenchymal cells on cell migration. After 24 h of treatment, the cell migration level was determined by wound healing (A) and transwell (B) assays according to the manufacturer’s protocols. **P < 0.01 vs. the corresponding control values.
TCDD-induced exosomes induced the apoptosis of MEPM cells
Bax, a member of the Bcl-2 family, is a proapoptotic effector that induces mitochondrial outer membrane permeabilization and cytochrome c release. Caspase-3 is a frequently activated death-associated protease that catalyzes the specific cleavage of many key cellular proteins and is essential for certain processes associated with cellular degradation and the formation of apoptotic bodies. As illustrated in Fig. 7, MEPM cells that were cultivated with TCDD-induced exosomes had lower levels of Bax and caspase-3 than normal exosome-treated cells. Additionally, as shown by flow cytometric and EdU assays, the apoptosis rate of the TCDD-treated exosome group was higher than that of the control group.
Fig. 7.

Effects of the 2 types of exosomes on mesenchymal cell apoptosis. A) As demonstrated by western blotting, the expression of apoptosis-related proteins (Bax and Caspase 3) in the T group was higher than that in the C group. B) Flow cytometry assays showed more apoptotic cells in the T group. **P < 0.01 vs. the corresponding control values.
TCDD-induced exosomes inhibited epithelium EMT
Vimentin, as an intermediate filament protein, is generally found in mesenchyme-originating or migrating epithelial cells. As illustrated in Fig. 8, the vimentin expression level of the cells in the control group was significantly higher than that of the cells in the TCDD group, whereas the ZO-1 expression level of the cells in the control group was lower than that of those in the TCDD group, which suggested that the EMT process in the TCDD group was inhibited.
Fig. 8.
Effects on the EMT of epithelial cells induced by 2 types of exosomes. After 72 h of treatment, the expression of the epithelial marker Zo-1 and the mesenchymal marker vimentin was assessed by immunofluorescence. **P < 0.01 vs. the corresponding control values.
Discussion
TCDD, a highly toxic, widely distributed, and persistent chlorinated contaminant, shows strong toxicity and is poorly metabolized in organisms.14 Previous studies have proven that TCDD can induce embryonic developmental disorders and is highly linked with CP.15–17 TCDD can inhibit the growth and migration of mesenchymal cells while increasing their apoptosis.18 The transformation of epithelial cells to mesenchymal cells could be induced by TCDD.11 Both the epithelium and mesenchyme participate in the development of the palate and are influenced by TCDD, and communication between these two types of cells can occur during palate formation.
Exosomes, vesicles with a cup-shaped morphology, a diameter of 40–200 nm and a density of 1.13–1.18 g/mL, contain a cargo of curated nucleic acids, proteins, and lipids that can alter gene expression in recipient cells.19 Exosomes can specifically encapsulate biologically active molecules according to the maternal cell and the pathophysiological environment and are then secreted and transferred between cells.20 These vesicles transmit signals by contacting the surface of the recipient cell or transferring their contents into the cell to stimulate the functional response of the recipient cell. According to related studies, exosomes are involved in various physiological processes, including pregnancy, stem cell differentiation, organ development, tissue repair, and blood coagulation.21,22 Toxic agents can affect exosomal synthesis and the bioactive cargo composition and thus allow exosomes to serve as biomarkers of exposure and response and participate in the related signaling pathway.23 Jiang et al.24 found that exosomes derived from epithelial cells are more likely to be endocytosed into mesenchymal cells and vice versa; these researchers also found that exosomes derived from epithelial and mesenchymal cells can mutually induce their matrix synthesis and that a reduction of exosome secretion can induce epithelial–mesenchymal abnormalities. During the palate development process, MEE cells transform into MEPM cells through EMT, migrate, undergo apoptosis, and ultimately disappear. As demonstrated in our previous study, TCDD inhibits EMT of MEE cells and the proliferation and migration of MEPM cells to cause palatal fusion and/or contact disorder; thus, we proposed that TCDD passes through exosomes derived from mesenchymal cells to affect the biological behavior of MEE and MEPM cells and participate in the development of the palate.
In our experiments, we isolated vesicles from the supernatant of MEPM cells and identified these microvesicles based on their morphology, size, and membrane marker proteins to prove that palatal mesenchymal cells can produce exosomes. We also verified that exosomes derived from MEPM cells can be endocytosed by MEE and MEPM cells. We then treated MEPM and MEE cells in vitro with exosomes instead of TCDD to repeat these processes in TCDD experiments. Surprisingly, exosomes from parental cells induced by TCDD also inhibited proliferation and migration and promoted the apoptosis of MEPM cells. The effect on the EMT program of MEE cells was consistent with the direct effect of TCDD on cells. Toxic substances can affect the selection, production, and transportation of the exosomal contents of parental cells. This feature allows exosomal markers to predict the state of parental cells. Therefore, what exactly does TCDD change in the contents of the parent cell? Exosomes contain a variety of biologically active substances, including RNA, DNA, and proteins. Studies have found that changes in ncRNAs can change gene expression during embryonic development. For example, miRNA140 can intervene during mouse palatal process development and fusion. Positive expression of the mesenchymal region can negatively regulate the protein expression level of PDGF receptor alpha (PDGFR-α), mutation of the PDGFR-α gene, or an exogenous increase in miRNA140 can lead to the occurrence of cleft lip and palate, and miRNA140 deletion leads to increased expression of PDGFR-α protein, which interferes with the normal morphology of the palate.25 A previous report revealed the presence of approximately 40% miRNAs, 40% piwiRNAs, 3.7% pseudogenes, 2.4% lncRNAs, 2.1% tRNAs, and 2.1% mRNAs in exosomes.26 Because miRNAs are highly contained inside exosomes, if exosomal miRNAs could be involved in the development of the palate? In addition, if the effects of exosomes on cell phenotype are the consequence of the effects of TCDD on the loading and transport of miRNAs in parental cell exosomes?
MiRNAs are small ncRNAs with a length of 21–22 nucleotides that regulate more than 30% of protein-coding genes through posttranscriptional silencing in mammals.27 Extensive evidence has shown that miRNAs have important functions in the regulation of vertebrates and mammalian orofacial clefts.28 To further understand the difference between the exosomes induced by TCDD and the contents of normal exosomes, we sequenced the most common miRNAs in exosomes and found a total of 20 miRNAs with significant differences. MiR-92 is encoded by the miR17-92 cluster, which regulates the expression of T-box transcription regulators during facial development. This molecule is the target of Bmp signaling and the craniofacial pioneer transcription factor AP-2α and is highly related to cleft lip and palate.29 Li et al.30 also concluded that the miR-17-92 cluster can directly target TGFBR2, SMAD2, and SMAD4 to prevent the inhibition of proliferation and collagen synthesis of palatal mesenchymal cells induced by the TGFβ pathway. All the evidence supports the hypothesis that exosomal miRNAs may participate in the phenotypic change of mesenchymal and epithelial cells through CP-related signaling pathways.
EMT is a special cell transformation process in which epithelial cells are polarized to obtain the appearance, migration, and invasive capabilities of mesenchymal cells. Epithelial cells transform into mesenchymal cells that can migrate and then follow the conventional migratory pathway to produce different embryonic layers, which in turn form the body structure and are accompanied by embryonic morphological changes.31 Various signaling pathways are involved in the process of palatal process fusion, including TGFβ, BMP, and Wnt, which play a role at different stages of palate formation.32,33 The TGFβ superfamily plays an important role in cell differentiation, embryogenesis, and embryo development.34 Early studies showed that TGFβ3 knockout in mice causes CP due to epithelial interlobular transformation disorders.35 Other studies have further proven that TGFβ3 is closely related to congenital cleft lip and palate by analyzing genetic polymorphisms in different ethnic groups.36,37 Exosomes have been confirmed to mediate epithelial–mesenchymal crosstalk during organ development through the Wnt signaling pathway.24 During tumor invasion, EMT mediated by exosomes through the TGFβ/Smad signaling pathway has also been revealed.38 In our experiments, epithelial cells phagocytosed exosomes produced by mesenchymal cells and inhibited EMT. Therefore, we considered that the miRNAs encapsulated in exosomes derived from mesenchymal cells induced by TCDD may pass through CP-related signaling pathways and induce abnormal behaviors of palatal epithelial cells (Fig. 9).
Fig. 9.
Utilization of exosomes in the clinic for the diagnosis and treatment of CP.
In summary, we speculate that TCDD may affect the status of parental cells to cause changes in the contents of their secreted exosomes and then transmit signals to regulate the phenotype of target cells and participate in the formation of CP. However, the specific molecular pathways and regulatory mechanisms still need to be further studied. In addition, exosomes carrying a variety of biologically active substances can affect recipient cells through their contents and participate in developmental and regenerative processes. Toxic substances can interfere with the composition of biologically active substances in exosomes, and this change allows exosomes to be used as biomarkers of exposure to toxic substances. Given the research on exosomes as biomarkers of toxicant exposure and disease, exosomes may become promising and valuable factors for the diagnosis and treatment of CP in the near future.
Author contributions
(I) Conception and design: LQ; (II) Administrative support: All authors; (III) Provision of study materials and figures: QC, YX, XD, and XZ; (IV) Collection and assembly of data: XY, XD, YZ, and XD; (V) Data analysis and interpretation: All authors; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Institutional Review Board Statement
The Ethics Committee of Children’s Hospital of Chongqing Medical University approved our study involving animals.
Supplementary Material
Acknowledgments
We thank the people who helped us with the experiments.
Contributor Information
Qiang Chen, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China; Department of Pediatrics Surgery, Chongqing University Three Gorges Hospital, Chongqing 400000 P.R. China.
Yue Xie, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China.
Xiaobo Dong, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China.
Xiao Zhang, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China.
Yunxuan Zhang, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China.
Xingang Yuan, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China.
Xionghui Ding, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China.
Lin Qiu, Department of Burn and Plastic Surgery, Children's Hospital of Chongqing Medical University, National Clinical Research Centre for Children Health and Disorders, Ministry of Education Key Laboratory of Child Development and Disorders, Chongqing Key Laboratory of Pediatrics, Chongqing 400000 P.R. China.
Funding
The Chongqing Returned Overseas Students Innovation Plan Fund (cx2019134) and the Chongqing Natural Science Foundation (cstc2020jcyj-msxmX0228).
Conflict of interest statement. The authors declare no conflict of interest.
Data Availability Statement
The transparency document associated with this article can be found in the online version.
References
- 1. Baldridge MG, Marks GT, Rawlins RG, Hutz RJ. Very low-dose (femtomolar) 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) disrupts steroidogenic enzyme mRNAs and steroid secretion by human luteinizing granulosa cells. Reprod Toxicol. 2015:52:57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pausch NC, Herzberg PY, Wirtz C, Hemprich A, Dhanuthai K, Hierl T, Pitak-Arnnop P. German animal terms for oral cleft deformity: a Leipzig survey. J Craniomaxillofac Surg. 2012:40:e236–e242. [DOI] [PubMed] [Google Scholar]
- 3. Yan F, Dai Y, Iwata J, Zhao Z, Jia P. An integrative, genomic, transcriptomic and network-assisted study to identify genes associated with human cleft lip with or without cleft palate. BMC Med Genet. 2020:13:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stuppia L, Capogreco M, Marzo G, La Rovere D, Antonucci I, Gatta V, Palka G, Mortellaro C, Tetè S. Genetics of syndromic and nonsyndromic cleft lip and palate. J Craniofac Surg. 2011:22:1722–1726. [DOI] [PubMed] [Google Scholar]
- 5. Shin JO, Lee JM, Cho KW, Kwak S, Kwon HJ, Lee MJ, Cho SW, Kim KS, Jung HS. MiR-200b is involved in Tgf-β signaling to regulate mammalian palate development. Histochem Cell Biol. 2012:137:67–78. [DOI] [PubMed] [Google Scholar]
- 6. Zhang W, Shen Z, Xing Y, Zhao H, Liang Y, Chen J, Zhong X, Shi L, Wan X, Zhou J, et al. MiR-106a-5p modulates apoptosis and metabonomics changes by TGF-β/Smad signaling pathway in cleft palate. Exp Cell Res. 2020:386:111734. [DOI] [PubMed] [Google Scholar]
- 7. Manolio TA, Collins FS, Cox NJ, Goldstein DB, Hindorff LA, Hunter DJ, McCarthy MI, Ramos EM, Cardon LR, Chakravarti A, et al. Finding the missing heritability of complex diseases. Nature. 2009:461:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. 2018:118:1917–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cell. 20198, 307.:2021(10):462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cheng J, Meng J, Zhu L, Peng Y. Exosomal noncoding RNAs in glioma: biological functions and potential clinical applications. Mol Cancer. 2020:19:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Chen Q, Ding X, Lei J, Qiu L. Comparison of the biological behaviors of palatal mesenchymal and epithelial cells induced by 2,3,7,8-tetrachlorodibenzo-p-dioxin in vitro. Toxicol Lett. 2020:333:90–96. [DOI] [PubMed] [Google Scholar]
- 12. Feng C, Xu Z, Li Z, Zhang D, Liu Q, Lu L. Down-regulation of Wnt10a by RNA interference inhibits proliferation and promotes apoptosis in mouse embryonic palatal mesenchymal cells through Wnt/beta-catenin signaling pathway. J Physiol Biochem. 2013:69:855–863. [DOI] [PubMed] [Google Scholar]
- 13. Liyun G, Xu J, Li X, Wang T, Wu W, Cao J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and TGF-beta3 mediated-mouse embryonic palatal mesenchymal cells. Dose Response. 2018:16:1559325818810637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armitage JM, Ginevan ME, Hewitt A, Ross JH, Watkins DK, Solomon KR. Environmental fate and dietary exposures of humans to TCDD as a result of the spraying of agent Orange in upland forests of Vietnam. Sci Total Environ. 2015:506–507:621–630. [DOI] [PubMed] [Google Scholar]
- 15. Abbott BD, Birnbaum LS. Rat embryonic palatal shelves respond to TCDD in organ culture. Toxicol Appl Pharmacol. 1990:103:441–451. [DOI] [PubMed] [Google Scholar]
- 16. Imura H, Yamada T, Mishima K, Fujiwara K, Kawaki H, Hirata A, Sogawa N, Ueno T, Sugahara T. Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin suggests abnormal palate development after palatal fusion. Congenit Anom (Kyoto). 2010:50:77–84. [DOI] [PubMed] [Google Scholar]
- 17. Yamada T, Mishima K, Fujiwara K, Imura H, Sugahara T. Cleft lip and palate in mice treated with 2,3,7,8-tetrachlorodibenzo-p-dioxin: a morphological in vivo study. Congenit Anom (Kyoto). 2006:46:21–25. [DOI] [PubMed] [Google Scholar]
- 18. Gao L, Wang Y, Yao Y, Zhang G, Han G, Wu W. 2,3,7,8-tetrachlorodibenzo-p-dioxin mediated cleft palate by mouse embryonic palate mesenchymal cells. Arch Oral Biol. 2016:71:150–154. [DOI] [PubMed] [Google Scholar]
- 19. Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, et al. Reassessment of exosome composition. Cell. 2019:177:428, e18–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pegtel DM, Gould SJ. Exosomes. Annu Rev Biochem. 2019:88:487–514. [DOI] [PubMed] [Google Scholar]
- 21. Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther. 2016:1:15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shang Q, Yang Z, Jia R, Ge S. The novel roles of circRNAs in human cancer. Mol Cancer. 2019:18:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bowers EC, Hassanin AAI, Ramos KS. In vitro models of exosome biology and toxicology: new frontiers in biomedical research. Toxicol in Vitro. 2020:64:104462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jiang N, Xiang L, He L, Yang G, Zheng J, Wang C, Zhang Y, Wang S, Zhou Y, Sheu TJ, et al. Exosomes mediate epithelium-mesenchyme crosstalk in organ development. ACS Nano. 2017:11:7736–7746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eberhart JK, He X, Swartz ME, Yan YL, Song H, Boling TC, Kunerth AK, Walker MB, Kimmel CB, Postlethwait JH. MicroRNA Mirn140 modulates Pdgf signaling during palatogenesis. Nat Genet. 2008:40:290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yuan T, Huang X, Woodcock M, Du M, Dittmar R, Wang Y, Tsai S, Kohli M, Boardman L, Patel T, et al. Plasma extracellular RNA profiles in healthy and cancer patients. Sci Rep. 2016:6:19413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li A, Jia P, Mallik S, Fei R, Yoshioka H, Suzuki A, Iwata J, Zhao Z. Critical microRNAs and regulatory motifs in cleft palate identified by a conserved miRNA-TF-gene network approach in humans and mice. Brief Bioinform. 2020:21:1465–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zou J, Li J, Li J, Ji C, Li Q, Guo X. Expression profile of plasma microRNAs in nonsyndromic cleft lip and their clinical significance as biomarkers. Biomed Pharmacother. 2016:82:459–466. [DOI] [PubMed] [Google Scholar]
- 29. Wang J, Bai Y, Li H, Greene SB, Klysik E, Yu W, Schwartz RJ, Williams TJ, Martin JF. MicroRNA-17-92, a direct Ap-2α transcriptional target, modulates T-box factor activity in orofacial clefting. PLoS Genet. 2013:9:e1003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li L, Shi JY, Zhu GQ, Shi B. MiR-17-92 cluster regulates cell proliferation and collagen synthesis by targeting TGFB pathway in mouse palatal mesenchymal cells. J Cell Biochem. 2012:113:1235–1244. [DOI] [PubMed] [Google Scholar]
- 31. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009:119:1420–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu W, Sun X, Braut A, Mishina Y, Behringer RR, Mina M, Martin JF. Distinct functions for Bmp signaling in lip and palate fusion in mice. Development. 2005:132:1453–1461. [DOI] [PubMed] [Google Scholar]
- 33. Nakajima A, Shuler CF, Gulka AOD, Hanai JI. TGF-β signaling and the epithelial-mesenchymal transition during palatal fusion. Int J Mol Sci. 2018:19:3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-beta 3 indicates defects of epithelial-mesenchymal interaction. Nat Genet. 1995:11:415–421. [DOI] [PubMed] [Google Scholar]
- 35. Ozturk F, Li Y, Zhu X, Guda C, Nawshad A. Systematic analysis of palatal transcriptome to identify cleft palate genes within TGFβ3-knockout mice alleles: RNA-Seq analysis of TGFβ3 mice. BMC Genomics. 2013:14:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ghazali N, Rahman NA, Kannan TP, Jaafar S. Screening of transforming growth factor beta 3 and jagged2 genes in the malay population with nonsyndromic cleft lip with or without cleft palate. Cleft Palate Craniofac J. 2015:52:e88–e94. [DOI] [PubMed] [Google Scholar]
- 37. Tang M, Wang Y, Han S, Guo S, Wang D. Transforming growth factor-beta3 gene polymorphisms and nonsyndromic cleft lip and palate risk: a meta-analysis. Genet Test Mol Biomarkers. 2013:17:881–889. [DOI] [PubMed] [Google Scholar]
- 38. Qu Z, Feng J, Pan H, Jiang Y, Duan Y, Fa Z. Exosomes derived from HCC cells with different invasion characteristics mediated EMT through TGF-β/Smad signaling pathway. OncoTargets Ther. 2019:12:6897–6905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The transparency document associated with this article can be found in the online version.




