Abstract
Filamentous fungus biomass is a protein-rich food, which can serve as an alternative to animal, plant, and legume protein sources. Neurospora crassa is a filamentous fungus that typically grows in tropical and sub-tropical regions. Traditionally, N. crassa has served as a model eukaryotic organism due to its ease of growth and propagation and suitability for genetic manipulation. However, filamentous fungi, such as Neurospora, have also been consumed or used to produce fermented foods for centuries and have been developed into protein-rich biomass ingredients to be used in conventional foods and meat substitutes. A panel of toxicological tests including genotoxic, acute, and subchronic studies were conducted on dried N. crassa biomass to support its safe use in food. The dried N. crassa biomass was found to be not genotoxic in a bacterial reverse mutation (Ames) assay, an in vitro chromosomal aberration test, and an in vivo micronucleus test. In the acute and subchronic toxicity studies, rats were orally gavaged with N. crassa biomass at concentrations of 0, 1,000, 2,500, and 5,000 mg/kg body weight/day for 14 and 90 days, respectively. At the conclusion of the studies, there were no test article-related toxicity results observed in clinical observations, body weight, food consumption, ophthalmology, hematology, clinical chemistry, coagulation, thyroid hormone, urinalysis, and macroscopic and microscopic findings. The no-observed-adverse-effect level for the dried N. crassa biomass ingredient was determined to be 5,000 mg/kg body weight/day, the highest dose tested.
Keywords: Neurospora, protein rich, rat, toxicity
Introduction
Economic, environmental, and ethical concerns have gradually been raised about the global food supply as the world deals with pressing issues such as population growth, climate change, and food security. As the world population grows and natural resources get scarcer, sourcing for the human food supply becomes an important topic of discussion.1 High-quality protein, an essential part of the human diet, has traditionally been fulfilled by protein from animal sources due to its amino acid composition and digestibility.2 However, meat consumption from animal sources exacerbates the issues we are dealing with today as the livestock sector represents a large share of greenhouse gas emissions and necessitate a substantial amount of natural resources.1,3 These concerns have led to an increased demand for meat substitutes currently led by legume and other plant-based protein products.2,3 Unfortunately, many plant sources have low protein content, lack some essential amino acids, and may contain anti-nutritive compounds.2,4 Filamentous fungus biomass is a protein-rich food, which can serve as an alternative to plant and legume protein sources. Filamentous fungi, such as Neurospora and Aspergillus, have been consumed or used to produce fermented foods like oncom, a fermented soybean cake, and soy sauce for centuries.5 One company on the market, Marlow Foods (UK), produces a mycoprotein product (Quorn®) from the filamentous fungus Fusarium venenatum mixed with additional ingredients to mimic meat. Emergy Inc. has developed a protein-rich biomass (55–60% protein) ingredient derived from the submerged fermentation of Neurospora crassa intended for use in conventional foods including alternative meat products.
Neurospora crassa is a filamentous fungus, of the phylum Ascomycota, first identified in 1843 as “Monilia sitophila.”6 It typically grows in tropical and sub-tropical regions and is commonly found on burnt wood after forest fires.7 For years, N. crassa has served as a model eukaryotic organism due to its ease of growth and propagation and suitability for genetic manipulation.6Neurospora was selected by Beadle and Tatum to develop biochemical mutants which supported the emerging “1 gene—1 enzyme” hypothesis and later won them a Nobel Prize in Physiology or Medicine in 1958.8 Since then, its genome has been sequenced in 2003 and N. crassa has been used in many hallmark studies involving circadian rhythms, gene silencing and DNA methylation, and cellular development.6,9,10
This manuscript details the studies evaluating the genotoxicity and oral toxicity of Emergy’s N. crassa protein-rich biomass ingredient. Genotoxicity was assessed using the bacterial reverse mutation (Ames) assay, an in vitro chromosomal aberration test, and an in vivo micronucleus test. A 14-day repeat dose range-finding and 90-day repeat dose oral toxicity studies were conducted in male and female Sprague–Dawley rats administered dried N. crassa biomass by oral gavage to evaluate acute and subchronic toxicity.
Materials and methods
Studies were conducted at Product Safety Labs (Dayton, New Jersey) in compliance with Good Laboratory Practice standards as described under 40 CFR Part 160, 40 CFR Part 792, 21 CFR Part 58,11–13 and OECD Principles of Good Laboratory Practice (as revised in 1997) published in ENV/MC/CHEM (98)1714 (genotoxicity studies), and 21 CFR Part 58 and OECD Principles of Good Laboratory Practice (as revised in 1997) published in ENV/MC/CHEM (98)1714 (90-day repeat dose toxicity study). All animal investigations were conducted in accordance with the most recent “Guide for the Care and Use of Laboratory Animals.”15
Materials
The test article used in the genotoxicity studies, dose range-finding study, and repeat dose toxicity studies was dried fungal biomass of N. crassa (wild-type strain). The dried fungal biomass is a white/tan powder consisting of 100% N. crassa with a 55–60% protein content, 2–5% fat content, 24–32% carbohydrate content, 5.5–8% ash, and less than 10% moisture. The test substances used in these studies were supplied by Emergy Inc. (Boulder, CO) and stored under refrigerated conditions. Two lots (EM0822-1 and 012121-730) meeting product specifications as described above were used in the genotoxicity and rodent toxicity studies, respectively. The N. crassa biomass is stable up to 1 year when stored at 4 °C.
Genotoxicity studies
Bacterial reverse mutation (Ames) test
The bacterial reverse mutation test was conducted based on the test guidelines as described in the US FDA Toxicological Principles for the Safety Assessment of Food Ingredients, Redbook 2000, IV.C.1.a,16 OECD Guidelines for Testing of Chemicals, Section 4 (Test No. 471),17 and ICH S2(R1) “Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use.”18
The main experiments were conducted using the plate incorporation method with Salmonella Typhimurium TA98, TA100, TA1535, and TA1537, and Escherichia coli WP2uvrA, while a confirmatory test was conducted using the pre-incubation method. All test strains were obtained from Molecular Toxicology Inc. (Boone, NC). The S9 microsomal fraction (5% v/v; Molecular Toxicology Inc. Boone, NC) was freshly prepared on the day of use from liver homogenates from male Sprague–Dawley rats induced with phenobarbital and 5,6-benzoflavone. Sodium phosphate buffer was used as the substitution buffer for plates treated in the absence of S9.
A total of 8 concentrations (1.58, 5.0, 15.8, 50, 158, 500, 1,580, and 5,000 μg/plate) of the test substance were tested in the presence and absence of metabolic activation. The vehicle, sterile water, was used as the negative control. The following compounds were used as positive controls without metabolic activation: sodium azide (NaN3); ICR 191 acridine; daunomycin; and methylmethanesulfonate (MMS). In the presence of metabolic activation, 2-aminoanthracene (2-AA) was used as a positive control. Each tester strain was tested in triplicate for the negative and positive controls, and the test compound.
The plate incorporation method of the main test included mixing 1) 100 μL of the prepared test substance solutions, negative (vehicle) control, or prepared positive control substance; 2) 500 μL S9 mix or substitution buffer; 3) 100 μL bacteria suspension; and 4) 2,000 μL overlay agar maintained at approximately 45 °C. The mixture was then poured over the surface of a minimal agar plate. Once gelled, the plates were inverted and incubated at 37 °C until growth was adequate for enumeration (~65 h).
In the pre-incubation method of the confirmatory test, the test or control substances, bacteria suspension, and S9/substitution buffer were incubated under agitation for ~30 min at ~37 °C prior to mixing with the overlay agar and pouring onto the minimal agar plates before proceeding as previously described in the main test.
After incubation, the number of colonies per plate was counted manually or with a plate counter (Colony Plate Reader, Model Colony-Doc-It™; Upland, CA). The mean and standard deviation were calculated for each set of triplicate plates.
For each experimental point, the mutation factor (MF) was calculated by dividing the mean revertant colony count by the mean revertant colony count for the corresponding concurrent vehicle control group. The results were considered positive if the results for the test item showed a substantial increase in revertant colony counts, i.e. response MF ≥ 2 for strains TA98, TA100, and WP2 uvrA or MF ≥ 3 for strains TA1535 and TA1537, with mean value(s) outside the laboratory historical control range. The increase must be dose-related and/or reproducible, i.e. increases must be obtained at more than 1 experimental point (at least 1 strain, more than 1 dose level, more than 1 occasion, or with different methodologies). Otherwise, results were considered negative.
In vitro chromosome aberration test
The in vitro mammalian chromosome aberration test was conducted based on the test guidelines as described in the US FDA Toxicological Principles for the Safety Assessment of Food Ingredients, Redbook 2000, IV.C.1.a,16 Ninth Addendum to OECD Guidelines for Testing of Chemicals, Section 4 (Test No. 473),19 and Commission Regulation (EU) No 2017/735 B.10.20
Chinese hamster V79 cells were obtained from the American Type Tissue Collection (ATCC, CCL-93) and were grown in Modified Eagle’s medium (MEM) supplemented with 2 mM L-glutamine, 100 U/100 μg/mL penicillin/streptomycin solution, 2.5 μg/mL amphotericin, 25 mM N-2-hydroxyethylpiperazine-N-2-ethane sulfonic acid (HEPES), and 10% fetal bovine serum (FBS).
The main experiments consisted of a 4-h test article exposure with and without metabolic activation and a 21-h test article exposure without metabolic activation. Cells were treated for 4 hours with test article at concentrations of 0, .5, 1.0, 2.5, 5.0, 7.5, and 15 μg/mL with metabolic activation and without metabolic activation. A second experiment was conducted with a 21-h treatment time at concentrations of 0, .25, .50, .96, 2.4, 4.8, and 7.5 μg/mL. Metabolic activation was achieved similar to the bacterial reverse mutation test. The test article was prepared to a concentration of 100 mg/mL with dimethyl sulfoxide (DMSO) prior to treatment of the cells. To reach a final concentration of 1% DMSO (v/v) in cell culture medium, the test item stock solution was further diluted in MEM + 0% FBS for short-term exposure or MEM + 10% FBS for long-term exposure. In both treatment periods, MEM or DMSO was used as the negative control. In the absence of metabolic activation, the positive control, ethylmethane sulphonate (EMS), was dissolved in MEM and tested at final concentrations of 400 and 600 μg/mL. In the presence of metabolic activation, the positive control, cyclophosphamide (CPA), was dissolved in MEM and tested at a final concentration of .83 μg/mL. The positive controls were obtained from Sigma-Aldrich (Taufkirchen, Germany).
In the first experiment, 3- to 4-day-old cultures were treated with trypsin and suspensions were prepared in MEM. Next, 1 x 104 cells/mL were seeded into culture flasks and incubated in culture medium. After 48 h, cells were treated with concentrations of test article, negative control, or positive control, in the absence or presence of metabolic activation, and incubated at 37 °C for 4 h. After the treatment, cells were washed and incubated in fresh MEM for 21 h until preparation of the cells. In the second experiment, 48 h after seeding, the cells were treated with the different concentrations of test article without metabolic activation for 21 h until preparation of the cells. Preparation of the cells included adding colcemid (0.2 μg/mL MEM) to the cultures around 17.5 h after start of treatment. Cell cultures were washed, trypsinized, and resuspended in ~9 mL MEM. An aliquot was removed for cell counting using a cell counter (AL-Systems). Resuspended cells were then incubated with a hypotonic solution (0.4% potassium chloride) for 15–20 min. After hypotonic treatment, the cells were fixed at least 2 times with 3:1 methanol:glacial acetic acid and spread onto the slides. After the fixation steps, the slides were dried and stained with Giemsa, coverslipped, and air-dried.
All slides were microscopically analyzed for structural chromosomal aberrations and number of polyploid cells (near tetraploid karyotype). The cytotoxic effect was recorded as the relative increase in cell count (RICC) and calculated as follows: RICC (%) = N – N0 (treated) / N – N0 (untreated), where N0 is the initial cell number and N is the cell number at the end of treatment. The test was considered acceptable if the number of chromosomal aberrations in the negative control was consistent with historical control data, the positive controls induced a statistically significant increase in responses compared to the negative control, and the proliferation criteria in the solvent control was similar to the corresponding negative control. The test article was considered clastogenic if at least 1 of the test concentrations exhibited a statistically significant increase compared with the concurrent negative control, the increase was dose-related when evaluated with an appropriate trend test, and any of the results were outside the 95% control limits of the historical negative control data. Statistical significance was evaluated using Fischer’s exact test.
Micronucleus assay
The micronucleus test was conducted based on the test guidelines as described in the OECD Guidelines for the Testing of Chemicals, Test No. 474,21 and US FDA Toxicological Principles for the Safety Assessment of Food Ingredients, Redbook 2000, IV.C.1.d.22
Two experiments of the in vivo mammalian erythrocyte micronucleus test were performed using male and female Swiss albino (ICR) mice (Charles River Laboratories, Inc.) housed in plastic solid bottom cages, and acclimatized for 5 days to the laboratory conditions (19–22 °C; 35–70% relative humidity; 12 air changes per hour; 12-hour light/dark cycle). Animals were provided Envigo Teklad Global 16% Protein Rodent Diet® 2016C and filtered tap water ad libitum. Dosing solutions were prepared on the day of administration. The dose was calculated based on the initial body weight to achieve the target dose level for all animals in units of mg/kg body weight/day. The dose volume was maintained constant at 10 mL/kg. The negative control group received vehicle only. The positive control group was treated at 5 mL/kg. The negative (vehicle) control used was mineral oil and the positive control used was cyclophosphamide monohydrate (Sigma-Aldrich, St. Louis, MO).
In the preliminary test conducted to determine the maximum tolerated dose (MTD) for the test article, 7–8 week-old male and female mice (3 animals/sex/group) were administered vehicle control or test article at 2,000 mg/kg body weight/day by oral gavage. The test article was administered twice over a period of 24 ± 2 h. The toxic effects of the test substance were evaluated, including daily clinical observations and mortality. The highest dose tested, 2,000 mg/kg body weight/day, was determined to be the MTD to be used in the main test.
The main test was performed using 7–8 week-old male and female mice (5/sex/group). Animals were administered test article by oral gavage at doses of 0, 500, 1,000, or 2,000 mg/kg body weight/day. The positive control, cyclophosphamide monohydrate, was dissolved in water for injection and was administered at a dose of 40 mg/kg body weight/day to a group of 5 male and female mice. Animals were observed twice daily for clinical signs and mortality, and cage-side observations of all animals were performed daily throughout the study. Animals were euthanized 44–48 h following the second dose administration by exsanguination. All test animals survived and appeared active and healthy during the study period. Blood samples were collected by cardiac puncture and prepared in duplicate according to the Litron In Vivo Micronucleus Kit (MicroFlow Rodent Fixed Blood, Rochester, New York). An additional blood sample was collected for potential toxicokinetic analysis. One set of samples was shipped to Litron Laboratories (Rochester, New York) for flow cytometry analysis. The analysis target was a minimum of 4,000 immature erythrocytes per animal for the presence of micronucleus. The proportions of immature erythrocytes and total erythrocytes and micronucleated erythrocytes were analyzed using paired and unpaired t-tests using GraphPad Prism (v5.01, San Diego, CA).
Rodent toxicity studies
14-day repeat dose range-finding study
The 14-day repeated dose range-finding study was conducted based on the test guidelines as described in the US FDA Toxicological Principles for the Safety Assessment of Food Ingredients, Redbook 2000, IV.C.4.a23 and OECD Guidelines for Testing of Chemicals, Section 4 (Test No. 407).24
A total of 40 6–7-week-old CRL Sprague–Dawley CD IGS rats were obtained from Charles River Laboratories Inc. and acclimatized (room temperature: 20–22 °C; relative humidity: 46–62%; 12-h light/dark cycle) for 6 days prior to testing. Animals were distributed randomly to 4 groups (3 dose levels plus 1 control group per sex) of 10 male and 10 female rats per group according to body weight. The animals were group housed in suspended stainless steel caging. Animals were provided a pellet diet (2016 Certified Envigo Teklad Global Rodent Diet®, Envigo Teklad, Madison, Wisconsin) and filtered tap water ad libitum. Male (132–203 g) and female (144–224 g) animals were approximately 7–8 weeks of age at the start of the dosing period. The test article was provided by oral intubation using a stainless steel ball-tipped gavage needle attached to an appropriate syringe at levels of 0, 1,000, 2,500, or 5,000 mg/kg body weight/day for 15 days. Individual doses were prepared fresh daily and calculated based on the most recent weekly body weights and were adjusted each week to maintain the targeted dose level for all rats (mg/kg/day). The control group received vehicle only, at the same dose volume as the test animals. Dose administration was twice daily at half of the target dose concentration due to viscosity of the test article (7 days/week) with the second administration coming 4 h after the first for a period of 15 days. Between dose administrations, the dose preparations were stored at 4 °C until the second administration 4 h later.
All animals were observed for mortality, signs of gross toxicity, and behavioral changes immediately after dose administration, during the first several hours after dosing, and at least twice daily for viability. A detailed clinical observation was conducted prior to the first dose administration and weekly thereafter. Observations included gross evaluation of skin and fur, eyes and mucous membranes, occurrence of secretions and excretions and autonomic activity, gait, posture, response to handling, clonic or tonic movements, stereotypies, or bizarre behavior. Individual body weights and food consumption were also measured during the duration of the study. At the end of the observation period, all animals were euthanized by carbon dioxide asphyxiation. Gross necropsies were performed on all animals and included examinations of the external surface of the body, orifices, musculoskeletal system, and the cranial, thoracic, abdominal, and pelvic cavities and their associated organs and tissues.
90-day repeated dose toxicity study
The 90-day repeated dose toxicity study was conducted based on the test guidelines as described in the US FDA Toxicological Principles for the Safety Assessment of Food Ingredients, Redbook 2000, IV.C.4.a23 and OECD Guidelines for Testing of Chemicals, Section 4 (Test No. 408).25
Preparation method of the test substance
Prior to preparation, the test substance was ground in a mechanical grinder (Nutribullet). The test substance was mixed weight to volume (w/v) in corn oil (vehicle control). Each day, a single mixture, at a sufficient volume for twice-a-day dosing, was prepared for each dose group. After the first administration, the remaining preparation was stored refrigerated until 1 h prior to the second dosing. The formulations contained 0, 50, 125, and 250 mg test substance/mL of corn oil. The formulations were mixed with a homogenizer, and then stirred at ambient temperature until a visually homogeneous mixture was achieved.
The neat test substance and prepared dosing mixtures were sampled in duplicate according to standard operating procedures. Neat test samples were collected as additional samples and analyzed for concentration verification. To assess for homogeneity, prior to initial dosing on Day 1, samples from the dose preparations were collected from the top, middle, and bottom of each concentration. The vehicle control mixture was sampled from the middle of the dose preparation only. The dose preparations were sampled at the beginning (as part of the homogeneity assessment), near the middle, and again at the end of the study for verification of dose concentration. Given that the dose preparations were prepared daily, and used within approximately 6 h of each preparation, the test substance as administered was considered stable. Samples were collected from preparations of each concentration of test substance and 1 sample from the control.
Test animals
A total of 80 8-week-old CRL Sprague–Dawley CD IGS rats were obtained from Charles River Laboratories Inc. and acclimatized for 7 days prior to testing. Animals were distributed randomly to 4 groups (3 dose levels plus 1 control group per sex) of 10 male and 10 female rats per group according to body weight. Male and female animals were approximately 9 weeks of age at the start of the dosing period.
Animals were individually housed in stainless steel caging, which conforms to the size recommendations in the most recent Guide for the Care and Use of Laboratory Animals.15 The temperature and humidity were maintained at 16–25 °C and 6–62%, respectively. Humidity was below the targeted lower limit for 43 days during the study. A portable humidifier was used to raise the humidity levels during this time. This excursion was considered minor and had no impact on this study. Animals were provided a pellet diet (2016 Certified Envigo Teklad Global Rodent Diet®, Envigo Teklad, Madison, Wisconsin) and filtered tap water ad libitum.
Test article administration
The test article was provided by oral intubation using a stainless steel ball-tipped gavage needle attached to an appropriate syringe at levels of 0, 1,000, 2,500, or 5,000 mg/kg body weight/day (0, 50, 125, and 250 mg/mL, respectively) for 90 days. Individual doses were calculated based on the most recent weekly body weights and were adjusted each week to maintain the targeted dose level for all rats (mg/kg/day). All doses were administered volumetrically at 10 mL/kg twice daily. The control group received vehicle only, at the same dose volume as the test animals. Dose administration was twice daily at half of the target dose concentration due to viscosity of the test article (7 days/week) with the second administration coming 4–6 h after the first for a period of 96 days for males and 97 days for females. Between dose administrations, the dose preparations were stored in the refrigerator and taken out about 1 h prior to the second dose. The concentration levels were selected based on the earlier 14-day repeat dose oral toxicity study.
Observation, measurement, and examination
Clinical observations: All animals were observed twice daily for viability, and cage-side observations of all animals were performed daily during the study.
Body weight and food consumption: Individual body weights were measured at least twice during the acclimation period and weekly thereafter. Food consumption measurements coincided with all body weight measurements. Body weight gain was calculated for selected intervals and for the entire study period.
Ophthalmology: Ophthalmologic examinations were conducted on all animals prior to the study initiation and on all animals at the end of the study period.
Hematology, coagulation, and blood chemistry: Blood samples for hematology and blood chemistry were collected from all animals prior to the scheduled necropsy at the end of the dosing period. All animals were fasted overnight prior to blood collection. Blood samples were collected from the sublingual vein or vena cava/abdominal aorta under isoflurane anesthesia.
For the hematology assessment, blood samples were collected in tubes containing potassium ethylenediaminetetraacetic acid (EDTA) and stored under refrigeration and transferred on cold packs to the clinical pathology department. The following parameters were evaluated: hematocrit, platelet count, reticulocyte count, hemoglobin concentration, red blood cell count, red cell distribution width, white blood cell and differential leukocyte count, mean corpuscular hemoglobin, mean corpuscular volume, and mean corpuscular hemoglobin concentration (calculated). Due to supply chain difficulties arising from the Coronavirus pandemic, the blood slides were not stained, but stored in a climate-controlled environment in the event that Wright-Giemsa staining is required in the future to substantiate or refute any of the hematology findings.
For coagulation, blood samples were collected in a pre-calibrated tube containing 3.2% sodium citrate. These samples were centrifuged in a refrigerated centrifuge and the plasma was transferred to labeled tubes. Plasma samples were stored in a −80 °C freezer until analysis. The following parameters were evaluated: activated partial thromboplastin time and prothrombin time.
For blood chemistry analysis, blood was collected into tubes containing no preservative and centrifuged in a refrigerated centrifuge. The serum was stored in a −80 °C freezer until analysis. The following parameters were evaluated: albumin, alkaline phosphatase, bilirubin (total), blood creatinine, calcium, chloride, cholesterol (total), fasting glucose, globulin, inorganic phosphorous, lipoprotein (high density), lipoprotein (low density), potassium, serum alanine aminotransferase, serum aspartate aminotransferase, serum protein (total), sodium, sorbitol dehydrogenase, triglycerides, and urea nitrogen.
Urinalysis: Urine samples were taken from animals the day prior to blood sample collection for clinical pathology evaluation. Animals were placed in metabolism cages and fasted for at least 15 h. Urine was collected from each animal and samples were stored under refrigeration or ice until analysis. The following urinalysis parameters were evaluated: bilirubin, blood, color, clarity, glucose, ketone, quality, microscopic urine sediment, pH, protein (total), quality, specific gravity, volume, and urobilinogen.
Thyroid hormone assessment: Blood samples were collected sublingually for assessment of effect on the pituitary–thyroid axis from all animals. Samples were analyzed for triiodothyronine (T3), thyroxine (T4), and thyroid-stimulating hormone (TSH) using validated enzyme-linked immunosorbent assay (ELISA)-based assays. Appropriate rat quality controls were used when applicable.
Necropsy: All animals were euthanized by exsanguination under isoflurane anesthesia at the end of the study period and subjected to a gross necropsy of the following tissues and organs which were extracted and weighed: adrenal glands, brain, epididymides, heart, kidneys, liver, ovaries with oviducts, spleen, testes, thymus, and uterus. The prostate and seminal vesicles with coagulating gland, thyroid/parathyroid, and pituitary were weighed at least 24 h after preservation in 10% neutral buffered formalin. Bilateral organs were measured together.
In addition, the following tissues and organs were preserved in 10% neutral buffered formalin solution for histopathological examination: accessory genital organs (prostate and seminal vesicles), adrenals, aorta, bone (femur), bone marrow (from femur and sternum), brain (medulla/pons, cerebellar, and cerebral cortex), cecum, cervix, colon, duodenum, esophagus, Harderian gland, heart, ileum with Peyer’s patches, jejunum, kidneys, larynx, liver, lungs, lymph node (mandibular and mesenteric), mammary gland, nasal turbinates, nose, ovaries, oviducts, pancreas, parathyroid, peripheral nerve (sciatic), pharynx, pituitary gland, rectum, salivary glands (sublingual, submandibular, and parotid), skeletal muscle, skin, spinal cord (cervical, mid-thoracic, and lumbar), spleen, sternum, stomach, thymus, thyroid, trachea, urinary bladder, uterus, vagina, and all gross lesions. The eyes, epididymides, optic nerve, and testes were preserved in modified Davidson’s fixative and then stored in ethanol for possible future histopathological examination.
Histopathology: Histological examination was performed on the preserved organs and tissues of all surviving animals in Groups 1 and 4 and the found-dead or humanely euthanized animals, and any gross lesions from all animals were processed. Slide preparation and histological examination was performed by a board-certified veterinary pathology at StageBio (Mount Jackson, VA).
Statistical analyses
Mean and standard deviations were calculated for all quantitative data. Male and female rats were evaluated separately. Statistical analyses were performed on all quantitative data for in-life and organ weight parameters using Provantis™ Version 10 (Instem LSS, Staffordshire, UK).
For all in-life endpoints identified as multiple measurements of continuous data over time (e.g. body weight, food consumption, etc.), treatment and control groups were compared using a 2-way analysis of variance (ANOVA), testing the effects of both time and treatment, with methods accounting for repeated measures in 1 independent variable (time). Significant interactions observed between treatment and time as well as main effects were further analyzed by a post hoc multiple comparisons test (e.g. Dunnett’s test) of the individual treated groups to control.
If warranted by sufficient group sizes, endpoints with single measurements of continuous data within groups (e.g. organ weight, relative organ weight, etc.) were evaluated for homogeneity of variances (Bartlett) and normality (Shapiro). Where homogeneous variances and normal distributions were observed, treatment and control groups were compared using a one-way ANOVA. When one-way ANOVA indicated a significant difference, a comparison of the treated groups to control was performed with a multiple comparisons test (e.g. Dunnett’s test). Where variances were considered significantly different, groups were compared using a non-parametric method (e.g. Kruskal-Wallis non-parametric ANOVA) and where significant, a comparison of treated groups to control was performed (e.g. Dunn’s test).
Clinical pathology data were analyzed by Bartlett’s test for homogeneity and Shapiro–Wilk test for normality. One-way ANOVA followed with Dunnett’s test was used if the preliminary test was not significant. If the preliminary test was significant, the data were transformed to achieve normality and variance homogeneity. The order of transforms attempted was log, square-root, and rank-order. If the log and square root transformations failed, rank-order was used. Statistical analyses of clinical pathology data was performed using Provantis™ Version 10 (Instem LSS, Staffordshire, UK).
Results
Genotoxicity studies
Bacterial reverse mutation (Ames) test
In both the main and confirmatory tests, the presence of test article at concentrations up to 5,000 μg/plate did not increase the number of revertant colonies in any test strain in the presence or absence of metabolic activation compared to the negative control (Tables 1 and 2). There was no significant effect on calculated MF. The number of revertant colonies in test strains treated with the negative control was within the laboratory’s historical control values and published values by Mortelmans and Zeiger26 and Gatehouse.27 Conversely, the positive controls caused an expected increase in the number of revertant colonies in both the presence and absence of metabolic activation, thus confirming the validity of the test. Precipitation was seen in both the main and confirmatory tests in all strains at doses ≥1,580 μg/plate with and without metabolic activation that obscured lawn evaluation. Individual plate contamination which did not obscure counts was observed sporadically for all strains. Contamination did not impact mutagenic evaluation. For all strains, at least 6 nontoxic dose levels were evaluated; therefore, bacterial mutagenicity was adequately assessed.
Table 1.
Results of the main bacterial reverse mutation test conducted using the plate incorporation method with N. crassa biomass.
| Concentration (μg/plate) |
Revertant colonies per plate (mean ± standard deviation) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA98 | TA100 | TA1535 | TA1537 | WP2uvrA | ||||||
| –S9 | +S9 | –S9 | +S9 | –S9 | +S9 | –S9 | +S9 | –S9 | +S9 | |
| Negative controla | 20 ± 1.0 | 22 ± 3.5 | 96 ± 5.5 | 97 ± 3.5 | 15 ± 2.0 | 14 ± 2.0 | 10 ± 3.5 | 11 ± 1.0 | 35 ± 3.6 | 37 ± 1.0 |
| 1.58 | 20 ± 1.0 | 18 ± 2.1 | 95 ± 5.5 | 94 ± 2.0 | 16 ± 4.7 | 13 ± 3.8 | 10 ± 4.7 | 8 ± 2.1 | 40 ± 3.5 | 36 ± 3.6 |
| 5.0 | 22 ± 4.0 | 22 ± 2.5 | 96 ± 6.1 | 95 ± 2.6 | 15 ± 1.5 | 14 ± 3.2 | 8 ± .6 | 8 ± 3.1 | 34 ± 1.5 | 38 ± 1.5 |
| 15.8 | 23 ± 3.1 | 24 ± 5.3 | 93 ± 1.5 | 97 ± 6.1 | 17 ± 6.7 | 14 ± 1.5 | 7 ± 2.1 | 8 ± 1.5 | 38 ± 5.5 | 39 ± 2.1 |
| 50 | 22 ± 3.0 | 24 ± 1.7 | 92 ± 1.5 | 93 ± 4.9 | 15 ± 1.0 | 15 ± 3.5 | 9 ± .6 | 7 ± 1.7 | 36 ± 3.8 | 34 ± 4.5 |
| 158 | 20 ± 1.2 | 21 ± 1.0 | 92 ± 3.2 | 94 ± 2.5 | 19 ± 1.0 | 14 ± 1.5 | 9 ± 1.2 | 10 ± .6 | 36 ± 7.2 | 35 ± 1.5 |
| 500 | 19 ± 1.5 | 21 ± 2.5 | 92 ± 2.1 | 93 ± 5.1 | 13 ± 2.5 | 16 ± 6.7 | 8 ± 1.0 | 7 ± 1.5 | 36 ± 4.0 | 36 ± 4.0 |
| 1,580b | – | – | – | – | – | – | – | – | – | – |
| 5,000b | – | – | – | – | – | – | – | – | – | – |
| Positive controlc,d | 850 ± 48.2 | 4067 ± 66.7 | 622 ± 52.0 | 3379 ± 321.1 | 514 ± 14.6 | 258 ± 33.9 | 195 ± 17.6 | 507 ± 26.4 | 430 ± 18.8 | 122 ± 5.0 |
+S9 = with metabolic activation; –S9 = without metabolic activation; 2-AA = 2-aminoanthracene; MMS = methylmethanesulfonate; NaN3 = sodium azide.
a100 μL/plate sterile water.
bThe assessment of background lawn was obscured by precipitate.
cPositive controls, –S9: WP2uvrA = 2.5 μL/plate MMS; TA1537 = 8.0 μg/plate IC 191 acridine; TA98 = 6.0 μg/plate daunomycin; TA100 and TA1535 = 1.5 μg/plate NaN3.
dPositive controls, +S9: All = 10 μg/plate 2-AA.
Table 2.
Results of the confirmatory bacterial reverse mutation test conducted using the pre-incubation method with N. crassa biomass.
| Concentration (μg/plate) |
Revertant colonies per plate (mean ± standard deviation) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TA98 | TA100 | TA1535 | TA1537 | WP2uvrA | ||||||
| –S9 | +S9 | –S9 | +S9 | –S9 | +S9 | –S9 | +S9 | –S9 | +S9 | |
| Negative controla | 25 ± 3.1 | 23 ± 2.5 | 97 ± 4.7 | 98 ± 2.1 | 12 ± 2.6 | 12 ± .6 | 9 ± 1.0 | 10 ± 1.2 | 32 ± 1.0 | 35 ± 1.2 |
| 1.58 | 24 ± 2.1 | 21 ± 2.5 | 94 ± 2.0 | 97 ± 6.4 | 16 ± 2.0 | 12 ± 2.1 | 9 ± 1.0 | 9 ± 1.0 | 33 ± 3.2 | 36 ± 4.0 |
| 5.0 | 19 ± 2.6 | 24 ± 3.5 | 94 ± 4.9 | 96 ± 2.5 | 16 ± 2.3 | 16 ± 2.1 | 8 ± 1.7 | 8 ± 1.5 | 30 ± 1.2 | 35 ± 3.1 |
| 15.8 | 23 ± 4.7 | 21 ± 4.6 | 92 ± 1.5 | 99 ± 2.5 | 12 ± 3.0 | 12 ± 3.5 | 8 ± 2.0 | 9 ± .6 | 35 ± 2.6 | 38 ± 3.8 |
| 50 | 19 ± 3.5 | 23 ± 1.7 | 91 ± 1.5 | 94 ± 1.0 | 13 ± 2.0 | 11 ± 1.2 | 9 ± 0.6 | 9 ± 1.5 | 33 ± 7.2 | 39 ± 4.9 |
| 158 | 22 ± 0.6 | 19 ± 3.2 | 92 ± 1.5 | 97 ± 6.1 | 15 ± 1.5 | 10 ± 1.5 | 8 ± 1.2 | 8 ± 1.5 | 38 ± 4.0 | 38 ± 7.1 |
| 500 | 26 ± 3.0 | 25 ± 7.4 | 95 ± 2.6 | 93 ± 2.1 | 15 ± 2.5 | 10 ± 3.5 | 6 ± 1.0 | 8 ± 1.2 | 39 ± 6.6 | 32 ± 4.7 |
| 1,580b | – | – | – | – | – | – | – | – | – | – |
| 5,000b | – | – | – | – | – | – | – | – | – | – |
| Positive controlc,d | 1,738 ± 34.6 | 3,862 ± 458.8 | 574 ± 56.2 | 2,549 ± 21.4 | 506 ± 11.4 | 333 ± 16.4 | 3,701 ± 81.8 | 356 ± 78.5 | 398 ± 37.5 | 114 ± 18.5 |
+S9 = with metabolic activation; –S9 = without metabolic activation; 2-AA = 2-aminoanthracene; MMS = methylmethanesulfonate; NaN3 = sodium azide.
a100 μL/plate sterile water.
bThe assessment of background lawn was obscured by precipitate.
cPositive controls, –S9: WP2uvrA = 2.5 μL/plate MMS; TA1537 = 8.0 μg/plate IC 191 acridine; TA98 = 6.0 μg/plate daunomycin; TA100 and TA1535 = 1.5 μg/plate NaN3.
dPositive controls, +S9: All = 10 μg/plate 2-AA.
On the basis of the above results, it was concluded that the test article was not mutagenic to the tester strains under the conditions of these tests.
In vitro chromosome aberration test
In the first experiment, test article concentrations of 1.0, 2.5, 5.0, and 7.5 μg/mL (without metabolic activation) and 1.0, 2.5, and 7.5 μg/mL (with metabolic activation) were selected for microscopic analysis of chromosomal aberrations. In the second experiment without metabolic activation, test article concentrations of 0.96, 2.4, and 4.8 μg/mL were selected for microscopic analysis of chromosomal aberrations. Precipitate of the test article was observed at a concentration of 7.5 μg/mL in the first experiment with and without metabolic activation, and at a concentration of 4.8 μg/mL in the second experiment. In both experiments, the test article was not cytotoxic as it resulted in no biologically relevant decrease in cell count (decrease below 70% RICC) in all dose groups (Table 3).
Table 3.
Results of the in vitro mammalian chromosome aberration test with N. crassa biomass.
| Concentration (μg/mL) |
Cell counta (mean) |
Relative increase in cell countb (%) | Mean aberrant cells including gapsc (%) | Mean aberrant cells excluding gapsc (%) | Precipitate (+/−) |
|---|---|---|---|---|---|
| 4-h short-term treatment: –S9 | |||||
| Negative control (culture medium) | 114.20 | 102 | 3.3 | 2.0 | − |
| Solvent control (1% DMSO; v/v) | 111.83 | 100 | 5.0 | 3.0 | − |
| 0.5 | 114.20 | 102 | N/A | N/A | − |
| 1 | 12.79 | 109 | 4.0 | 3.3 | − |
| 2.5 | 109.90 | 98 | 3.0 | 1.7 | − |
| 5.0 | 117.04 | 105 | 3.0 | 1.3 | − |
| 7.5 | 103.27 | 92 | 2.3 | 0.3 | + |
| 15 | 103.27 | 92 | N/A | N/A | + |
| Positive control (600 μg/mL EMS) |
97.59 | 86 | 1.0 | 8.0* | − |
| 4-h short-term treatment: +S9 | |||||
| Negative control (culture medium) |
102.79 | 92 | 2.7 | 2.0 | − |
| Solvent control (1% DMSO; v/v) |
111.36 | 100 | 2.3 | 1.0 | − |
| 0.5 | 83.81 | 73 | N/A | N/A | − |
| 1 | 101.37 | 90 | 5.3 | 3.0 | − |
| 2.5 | 93.80 | 83 | 3.3 | 1.3 | − |
| 5.0 | 96.59 | 85 | N/A | N/A | − |
| 7.5 | 98.06 | 87 | 4.7 | 2.0 | + |
| 15 | 102.32 | 91 | N/A | N/A | + |
| Positive control (0.83 μg/mL CPA) |
71.03 | 60 | 15.2 | 11.6* | − |
| 21-h continuous treatment: –S9 | |||||
| Negative control (culture medium) |
224.52 | 115 | 1.7 | 1.0 | − |
| Solvent control (1% DMSO; v/v) |
196.58 | 100 | 2.3 | 0.7 | − |
| 0.24 | 207.95 | 106 | N/A | N/A | − |
| 0.48 | 195.16 | 99 | N/A | N/A | − |
| 0.96 | 210.31 | 107 | 2.0 | 1.0 | − |
| 2.4 | 198.48 | 101 | 1.7 | 0.7 | − |
| 4.8 | 202.26 | 103 | 2.0 | 1.0 | + |
| 7.2 | 179.07 | 91 | N/A | N/A | + |
| Positive control (400 μg/mL EMS) | 161.07 | 81 | 7.8 | 6.0* | − |
+S9 = with metabolic activation; –S9 = without metabolic activation; CPA = cyclophosphamide; DMSO = dimethyl sulfoxide; EMS = ethylmethane sulfonate; N/A = not analyzed.
*Statistically significant (P < 0.05) from solvent control using Fischer’s exact test (for aberrant cells without gaps).
aThe cell count was determined with a cell counter per culture for each test group.
bCalculated as the increase in cell number of the test groups compared to the solvent control.
c300 cells evaluated for each concentration except for the positive controls (CPA: 250 cells due to a clearly positive increase in chromosomal aberrations; EMS: 450 cells due to less metaphases with chromosomal aberrations observed on the first slide of the second culture).
In the first experiment with and without metabolic activation and the second experiment without metabolic activation, there were no statistically significant increases or concentration-related increases in chromosomal aberrations in the dose groups of the test article as compared to the negative controls and the chromosomal aberration rate was within the laboratory’s historical control values for all doses tested (Table 3). No biologically relevant increase in the frequencies of polyploid cells was found after treatment with the test article. The positive control induced distinct and biologically relevant increases in cells with chromosomal aberrations, therefore confirming the validity of the test.
Taken together, the test article was concluded to be non-clastogenic under the conditions of these tests.
Micronucleus assay
In the preliminary test, all animals survived exposure and appeared active during the study. The percentages of immature erythrocytes for the 2,000 mg/kg body weight/day dose were not statistically different from the control values in both male and female mice; therefore, the limit dose of 2,000 mg/kg body weight/day was designated as the MTD for the main test.
In the main test, there were no test article-related clinical observations in any of the animals at any dose group. The test article administered at doses up to 2,000 mg/kg body weight/day to male and female mice did not result in a statistically significant increase in the frequency of micronucleated immature erythrocytes (%MN-RET) (Table 4). Additionally, there were no test article-related changes in the frequency of reticulocytes (%RET) or micronucleated normochromatic erythrocytes (%MN-NCE) observed in male or female mice. The negative control group showed micronucleated immature erythroycyte (MIE) values consistent with the laboratory’s historical control data and the positive control resulted in a statistically significant increase in MIE and decrease in %RET, confirming the validity of the experiment.
Table 4.
In vivo mammalian erythrocyte micronucleus test in male and female mice orally administered N. crassa biomass once daily for 2 days.
| Dose (mg/kg bw/day) |
% MN-RETa (mean ± SEM) |
% RETb (mean ± SEM) |
% MN-NCEc (mean ± SEM) |
|||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | |
| Negative control | 0.20 ± 0.02 | 0.17 ± 0.01 | 1.63 ± 0.15 | 1.53 ± 0.21 | 0.15 ± 0.02 | 0.12 ± 0.01 |
| 500 | 0.14 ± 0.01 | 0.14 ± 0.02 | 1.45 ± 0.20 | 1.54 ± 0.12 | 0.014 ± 0.01 | 0.10 ± 0.01 |
| 1,000 | 0.15 ± 0.02 | 0.18 ± 0.04 | 1.63 ± 0.07 | 1.21 ± 0.18 | 0.014 ± 0.01 | 0.13 ± 0.01 |
| 2,000 | 0.19 ± 0.02 | 0.13 ± 0.01 | 1.63 ± 0.18 | 1.64 ± 0.15 | 0.014 ± 0.01 | 0.10 ± 0.01 |
| Positive control (40 mg/kg bw cyclophosphamide monohydrate) | 1.64 ± 0.20* | 1.24 ± 0.29d,* | 0.48 ± 0.10* | 0.64 ± 0.17d,* | 0.014 ± 0.01 | 0.12 ± 0.01d |
bw = body weight; MN-NCE = micronucleated normochromatic erythrocytes; MN-RET = micronucleated reticulocytes; RET = reticulocytes; SEM = standard error of the mean.
*Statistically significant (P < 0.05) from negative control or maximum tolerated dose (2,000 mg/kg) using paired or unpaired t-tests.
aFrequency of positive CD71 micronucleated reticulocytes.
bFrequency of CD71 positive reticulocytes.
cFrequency of micronucleated normochromatic erythrocytes.
d1 Female in the positive control group had dosing recorded in the raw data. However, results of flow cytometry demonstrate that dosing for this animal was inadvertently missed; the animal was therefore removed from the data analyses.
In conclusion, the test article was considered non-genotoxic under the conditions of the in vivo micronucleus test.
Rodent toxicity studies
14-day repeat dose range-finding study
There were no mortalities during the duration of the study and no clinical observations in any of the animals attributed to the test article. The mean body weights, weight gain, and daily food consumption of the test article groups were comparable to the control group. Lastly, there were no macroscopic observations at necropsy attributable to the administration of test article. In conclusion, the expected tolerable dose of the test article was 5,000 mg/kg body weight/day for the 90-day subchronic repeat dose study.
90-day repeated dose toxicity study
Clinical observations: There were no deaths of the animals related to the toxicity of the test article. There were 6 unplanned mortalities in the test article groups: 1 female was found dead on Day 8 in the 1,000 mg/kg/day group, 1 male was found dead on Day 92 in the 2,500 mg/kg/day group, and 2 males (Days 14 and 23) and 1 female (Day 8) were found dead in the 5,000 mg/kg/day group. Prior to death, 4 of the 5 dead animals exhibited slight to moderate moist rales, while 1 animal exhibited no symptoms. One male in the 2,500 mg/kg/day group was humanely sacrificed on Day 42. Prior to sacrifice, the animal exhibited yellow anogenital staining, slight alopecia on the back, damaged right forelimb, and visible swelling on the right forelimb. Corresponding detailed observations for this animal included hair loss and impaired locomotion. The thickness of the test preparations resulted in difficulties dosing some animals. Upon gross necropsy evaluation of the deceased animals, clear dosing injuries were caused, and none were due to toxicity of the test article. All other observed clinical signs were typical of the dose route and not considered to be test article-related.
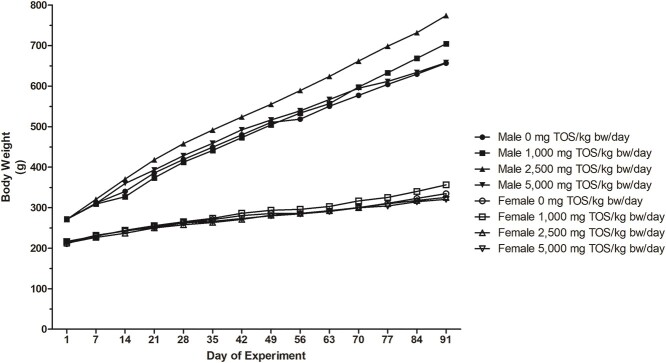
Body weight and food consumption: There were no statistical differences observed for weekly body weights in either male or female rats in the treated groups compared to the control groups (Fig. 1). Mean daily body weight gain for male rats in test groups were generally comparable to control animals, with the exception of statistically significant increases (P < 0.05–0.001) in the 1,000 mg/kg/day group on Days 49–56 and 63–70, and in the 2,500 mg/kg/day group on Days 7–14, 49–56, and 84–91, as well a decrease (P < 0.05) in the 5,000 mg/kg/day group on Days 14–21. Mean daily body weight gain for female rats in test groups was generally comparable to control animals, with the exception of a statistically significant decrease (P < 0.05) in the 1,000 mg/kg/day group on Days 1–7 and in the 5,000 mg/kg/day group on Days 84–91. While the mean body weight gains did show some significant differences between test groups and the control group for both sexes, these were erratic, not dose-dependent, and not considered to be a toxic response.
Fig. 1.
Mean weekly body weights in male and female rats provided dried N. crassa biomass by oral gavage for 90 days.
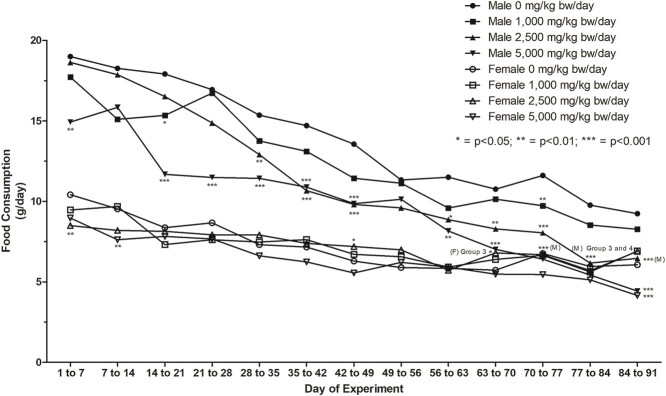
Food consumption was generally comparable between control and test groups throughout the study for both sexes except at occasional time intervals (Fig. 2). Mean daily food consumption in males was decreased (P < 0.05–0.001) in the 1,000 mg/kg/day group on Days 14–21 and 70–71, in the 2,500 mg/kg/day group on Days 28–49 and 56–91, and in the 5,000 mg/kg/day group on Days 1–7, 14–49, and 56–91. Mean daily food consumption in females was decreased (P < 0.05–0.001) in the 2,500 mg/kg/day group on Days 1–7, 42–49, and 63–70, and in the 5,000 mg/kg/day group on Days 7–14 and 85–91. Consumption at all other reported time points was comparable to control group values. When food consumption did differ, it was likely a result of increasing dose volume without a commensurate increase in gastric volume resulting in more prolonged satiety later in the study, particularly in the 5,000 mg/kg/day group.
Fig. 2.
Food consumption of male and female rats provided dried N. crassa biomass by oral gavage for 90 days.
Ophthalmology: During the final observation, 1 male in the control group had mild chorioretinal scarring in the left eye. This finding was not attributed to exposure to the test article, and thus the test article was not considered an ocular toxicant. All remaining animals were normal upon ophthalmic exam.
Hematology, coagulation, and blood chemistry: There were no test article-related changes in hematology parameters (Table 5). An increase in eosinophil counts in the 5,000 mg/kg/day male group was observed but considered incidental as it was not associated with an infiltration of eosinophils/eosinophilic inflammation in tissues examined. All other the changes in hematology parameters were considered unrelated to test article administration, including those that attained statistical significance, because they occurred sporadically and were considered a result of biological variance among rats given that the magnitude of variation (less than 1.5x in all parameters) was minimal.
Table 5.
Hematology values for male and female rats administered N. crassa biomass via gavage twice daily for 13 weeks.
| Parameters measureda | XX dose group (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| 0 (control) (n = 10) | 1000 (n = 10) | 2500 (n = 8) | 5000 (n = 8) | 0 (control) (n = 10) | 1000 (n = 9) | 2500 (n = 10) | 5000 (n = 9) | |
| Hematology | ||||||||
| Basophils[a] (x103/μL) | 0.112 ± .053 | 0.126 ± .054 | 0.146 ± .080 | 0.223 ± .089* | 0.107 ± .034 | 0.103 ± .062 | 0.147 ± .063 | 0.099 ± .021 |
| Eosinophils[a1] (x103/μL) | 0.044 ± .036 | 0.055 ± .025 | 0.081 ± .039 | 0.099 ± .042** | 0.064 ± .039 | 0.061 ± .028 | 0.061 ± .031 | 0.068 ± .014 |
| LUC (x103/μL) | 0.084 ± .048 | 0.057 ± .036 | 0.060 ± .028 | 0.058 ± .035 | 0.032 ± .018 | 0.026 ± .010 | 0.052 ± .028 | 0.038 ± .012 |
| Lymphocytes (x103/μL) | 7.705 ± 2.287 | 6.318 ± 2.353 | 7.066 ± 1.813 | 8.375 ± 2.599 | 4.086 ± 1.697 | 3.744 ± .804 | 4.736 ± 1.021 | 4.341 ± 1.457 |
| Monocytes (x103/μL) | 0.459 ± .165 | 0.301 ± .126 | 0.351 ± .150 | 0.330 ± .096 | 0.183 ± .093 | 0.213 ± .129 | 0.231 ± .094 | 0.158 ± .047 |
| Neutrophils (x103/μL) | 2.129 ± .888 | 2.008 ± .665 | 2.714 ± 1.455 | 2.153 ± .373 | 1.235 ± .323 | 1.474 ± .656 | 1.521 ± .499 | 1.234 ± .629 |
| Reticulocyte (x103/μL) | 162.440 ± 22.742 | 178.450 ± 33.182 | 179.538 ± 37.577 | 151.138 ± 32.826 | 157.410 ± 36.434 | 167.389 ± 21.713 | 159.180 ± 23.647 | 159.922 ± 2.819 |
| Hematocrit[a2], [a1] (%) | 44.94 ± 1.63 | 45.82 ± 3.40 | 47.51 ± 1.02** | 48.14 ± .93*** | 43.05 ± 1.31 | 44.48 ± 1.50 | 45.79 ± 1.00 | 45.17 ± 1.52 |
| Hemoglobin[a2],[a1] (g/dL) | 14.09 ± .46 | 14.36 ± 1.17* | 15.11 ± .25*** | 15.50 ± .30*** | 13.60 ± .50 | 14.11 ± .38* | 14.53 ± .30*** | 14.44 ± .50*** |
| MCV (fL) | 55.35 ± 1.59 | 56.21 ± 2.26 | 56.89 ± 2.33 | 55.30 ± 1.11 | 54.67 ± 1.90 | 55.90 ± .77 | 56.41 ± 1.61 | 56.13 ± 1.87 |
| MCH (pg) | 17.40 ± .49 | 17.62 ± .81 | 18.11 ± .99 | 17.80 ± .40 | 17.32 ± .66 | 17.74 ± .30 | 17.90 ± .47 | 17.93 ± .58 |
| MCHC[a1] (g/dL) | 31.43 ± .36 | 31.31 ± .48 | 31.80 ± .58 | 32.20 ± .44** | 31.59 ± .44.35 | 31.74 ± .58 | 31.72 ± .35 | 32.01 ± .47 |
| Platelets (x103/μL) | 76.90 ± 131.32 | 516.10 ± 326.38 | 69.63 ± 269.35 | 814.25 ± 283.42 | 923.80 ± 54.75 | 882.00 ± 203.14 | 885.70 ± 16.31 | 904.11 ± 127.46 |
| RBC[a2] (x106/μL) | 8.110 ± .287 | 8.156 ± .615 | 8.360 ± .396 | 8.704 ± .211*** | 7.878 ± .325 | 7.958 ± .236 | 8.128 ± .333 | 8.054 ± .307 |
| RDW[a1] (%) | 14.93 ± .38 | 14.77 ± .34 | 14.79 ± .34 | 14.43 ± .41* | 11.90 ± .39 | 11.54 ± .25* | 11.49 ± .34* | 11.47 ± .22* |
| WBC (x103/μL) | 1.535 ± 3.115 | 8.867 ± 2.726 | 1.419 ± 3.197 | 11.238 ± 2.602 | 5.686 ± 1.920 | 5.643 ± 1.334 | 6.749 ± 1.438 | 5.939 ± 1.391 |
ANOVA = analysis of variance; LUC = large unstained cells; MCH = mean corpuscular hemoglobin; MCHC = mean corpuscular hemoglobin concentration; MCV = mean corpuscular volume; RBC = red blood cells; RDW = red cell distribution width; WBC = white blood cells.
Values represent the mean ± standard deviation.
aStatistical tests are indicated in square brackets for each parameter. If two different tests were used for males and females, the footnotes are separated by a comma.
[a]Statistically significant (* = p < .05; ** = p < .01) using ANOVA and Dunnett’s (log) two-sided test.
[a1]Statistically significant (* = p < .05; ** = p < .01; *** = p < .001) using ANOVA and Dunnett’s two-sided test.
[a2]Statistically significant (* = p < .05; ** = p < .01; *** = p < .001) using ANOVA and Dunnett’s (rank) two-sided test.
There were no test article-related changes in coagulation parameters.
There were no test article-related changes in clinical chemistry parameters (Table 6). In males, statistically significant increased low density lipoprotein cholesterol and decreased total cholesterol and high-density lipoprotein cholesterol in all treated groups was considered toxicologically insignificant as there were no alterations in pathology parameters indicative of impaired lipoprotein metabolism. Increased blood urea nitrogen in the 5,000 mg/kg/day female group without any changes in creatinine concentration and renal histology was observed and considered incidental. All other changes in clinical chemistry were considered unrelated to test article administration, including those that attained statistical significance, and were likely due to biological variance among rats given that the magnitude of variation was minimal.
Table 6.
Clinical chemistry values for male and female rats administered N. crassa biomass via gavage twice daily for 13 weeks.
| Parameters measured | XX dose group (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| 0 (control) (n = 10) | 1,000 (n = 10) | 2,500 (n = 8) | 5,000 (n = 8) | 0 (control) (n = 10) | 1,000 (n = 9) | 2,500 (n = 10) | 5,000 (n = 9) | |
| Parameters measured | XX dose group (mg/kg) | |||||||
| Males | Females | |||||||
| 0 (control) (n = 10) | 1000 (n = 10) | 2500 (n = 8) | 5000 (n = 8) | 0 (control) (n = 10) | 1000 (n = 9) | 2500 (n = 10) | 5000 (n = 9) | |
| ALT (U/L) | 56.2 ± 23.3 | 89.1 ± 82.0 | 94.5 ± 125.2 | 44.9 ± 9.8 | 28.3 ± 5.5 | 32.2 ± 12.7 | 32.1 ± 22.8 | 24.6 ± 1.0 |
| Albumin (g/dL) | 4.12 ± .30 | 4.10 ± .34 | 3.85 ± .20 | 3.85 ± .35 | 5.21 ± .66 | 5.16 ± .53 | 5.28 ± .39 | 5.38 ± .40 |
| ALP[a] (U/L) | 157.7 ± 45.1 | 135.9 ± 52.2 | 103.5 ± 26.7* | 1.3 ± 32.1** | 48.5 ± 14.0 | 64.8 ± 43.7 | 46.5 ± 14.5 | 38.3 ± 8.2 |
| AST (U/L) | 109.7 ± 33.5 | 141.2 ± 87.3 | 146.5 ± 183.9 | 87.9 ± 21.5 | 81.3 ± 17.1 | 72.6 ± 1.2 | 87.2 ± 45.6 | 85.9 ± 32.9 |
| Calcium (mg/dL) | 11.25 ± .97 | 11.06 ± .64 | 11.29 ± .87 | 11.45 ± 1.21 | 11.38 ± .46 | 11.79 ± .95 | 11.80 ± .28 | 11.62 ± .67 |
| Chloride (mmol/L) | 97.28 ± 1.91 | 97.92 ± 3.41 | 95.64 ± 1.56 | 96.29 ± 1.26 | 99.70 ± 2.02 | 98.30 ± 1.85 | 98.72 ± 2.02 | 98.93 ± 1.60 |
| Cholesterol[a1] (mg/dL) | 55.0 ± 1.2 | 43.9 ± 7.7* | 46.0 ± 6.1 | 39.6 ± 8.5** | 49.1 ± 12.9 | 46.6 ± 16.6 | 57.4 ± 13.3 | 52.1 ± 9.4 |
| Creatinine (mg/dL) | 0.193 ± .025 | 0.210 ± .030 | 0.218 ± .015 | 0.206 ± .039 | 0.247 ± .041 | 0.274 ± .038 | 0.302 ± .063 | 0.288 ± .057 |
| Globulin[a1] (g/dL) | 1.43 ± .18 | 1.59 ± .31 | 1.81 ± .19** | 1.94 ± .18** | 1.32 ± .17 | 1.58 ± .37 | 1.56 ± .39 | 1.44 ± .11 |
| Glucose (mg/dL) | 254.0 ± 7.6 | 28.1 ± 6.4 | 296.9 ± 114.5 | 318.9 ± 104.9 | 21.7 ± 41.6 | 24.6 ± 37.0 | 229.7 ± 65.9 | 241.8 ± 41.1 |
| HDL[a1] (mmol/L) | 1.289 ± .272 | 0.902 ± .166*** | 0.876 ± .176*** | 0.688 ± .192*** | 1.246 ± .345 | 1.167 ± .380 | 1.386 ± .360 | 1.309 ± .243 |
| Inorganic phosphorus (mg/dL) | 8.92 ± 1.68 | 9.18 ± .96 | 9.19 ± 1.00 | 8.70 ± 1.24 | 7.37 ± .80 | 7.58 ± 1.16 | 7.38 ± .79 | 7.31 ± 1.00 |
| LDL[a1],[a] (mmol/L) | 0.113 ± .025 | 0.146 ± .040 | 0.205 ± .054*** | 0.174 ± .047* | 0.102 ± .004 | 0.127 ± .053 | 0.135 ± .025* | 0.124 ± .035 |
| Potassium (mmol/L) | 8.039 ± 2.058 | 8.167 ± 1.258 | 8.361 ± 1.216 | 8.139 ± 1.491 | 7.022 ± 1.215 | 7.251 ± 1.291 | 7.020 ± 1.428 | 6.979 ± .813 |
| Sodium (mmol/L) | 136.50 ± 3.69 | 136.50 ± 3.81 | 135.50 ± 2.07 | 136.75 ± 3.11 | 141.80 ± 2.62 | 141.67 ± 2.35 | 141.80 ± 1.69 | 142.22 ± 2.22 |
| SDH | 37.86 ± 24.72 | 71.99 ± 5.91 | 5.41 ± 34.39 | 39.33 ± 14.82 | 17.81 ± 11.77 | 17.60 ± 6.81 | 23.70 ± 29.05 | 15.11 ± 3.80 |
| Bilirubin (mg/dL) | 0.088 ± .023 | 0.078 ± .023 | 0.066 ± .030 | 0.056 ± .023 | 0.066 ± .028 | 0.072 ± .014 | 0.079 ± .017 | 0.079 ± .016 |
| Total protein (g/dL) | 5.55 ± .36 | 5.69 ± .23 | 5.66 ± .29 | 5.79 ± .45 | 6.53 ± .67 | 6.73 ± .63 | 6.84 ± .34 | 6.82 ± .41 |
| Triglycerides (mg/dL) | 106.1 ± 26.0 | 106.2 ± 35.9 | 92.8 ± 23.7 | 11.5 ± 38.3 | 61.3 ± 22.1 | 64.6 ± 23.0 | 6.5 ± 13.9 | 55.7 ± 23.8 |
| BUN[a1] (mg/dL) | 7.1 ± 2.2 | 6.8 ± 1.2 | 7.3 ± 1.3 | 7.0 ± .9 | 1.6 ± 2.0 | 12.8 ± 2.8 | 12.3 ± 3.7 | 14.4 ± 2.6* |
ALP = alkaline phosphatase; ANOVA = analysis of variance; ALT = alanine aminotransferase; AST = aspartate aminotransferase; BUN = blood urea nitrogen; HDL = high-density lipoprotein; LDL = low-density lipoprotein; SDH = sorbitol dehydrogenase.
Values represent the mean ± standard deviation.
aStatistical tests are indicated in square brackets for each parameter. If two different tests were used for males and females, the footnotes are separated by a comma.
[a]Statistically significant (* = p < .05; ** = p < .01) using ANOVA and Dunnett’s (rank) two-sided test.
[a1]Statistically significant (* = p < .05; ** = p < .01; *** = p < .001) using ANOVA and Dunnett’s two-sided test.
[a2]Statistically significant (* = p < .05; ** = p < .01) using ANOVA and Dunnett’s (log) two-sided test.
Urinalysis: There were no test substance-related changes in urinalysis parameters (Table 7).
Table 7.
Urinalysis values for male and female rats administered N. crassa biomass via gavage twice daily for 13 weeks.
| Parameters measured | XX dose group (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| 0 (control) (n = 10) | 1,000 (n = 10) | 2,500 (n = 8) | 5,000 (n = 8) | 0 (control) (n = 7) | 1,000 (n = 9) | 2,500 (n = 9) | 5,000 (n = 9) | |
| Volume (mL) | 1.15 ± 0.97 | 1.40 ± 0.81 | 1.31 ± 0.75 | 4.00 ± 4.43* | 1.50 ± 0.87 | 0.78 ± 0.51 | 0.83 ± 0.50 | 1.50 ± 1.17 |
| pH | 5.60 ± 0.61 | 5.30 ± 0.54 | 5.19 ± 0.26 | 5.81 ± 0.37 | 5.50 ± 0.50 | 5.33 ± 0.66 | 5.33 ± 0.43 | 5.61 ± 0.49 |
| Glucose (mg/dL) | 6.0 ± 51.6 | 2.0 ± 42.2 | 12.5 ± 35.4 | 12.5 ± 35.4 | 14.3 ± 37.8 | 5.0 ± 86.6 | 22.2 ± 44.1 | 11.1 ± 33.3 |
| Ketone (mg/dL) | 4.0 ± 2.1 | 3.5 ± 2.4 | 1.3 ± 2.3** | 1.3 ± 2.3** | 2.9 ± 2.7 | 2.2 ± 5.1 | 1.7 ± 2.5 | 0.0 ± 0.0 |
| Protein (mg/dL) | 163.0 ± 122.6 | 183.0 ± 127.4 | 225.0 ± 103.5 | 121.9 ± 115.4 | 53.6 ± 43.8 | 98.9 ± 82.7 | 151.1 ± 115.3 | 125.6 ± 105.2 |
| Specific gravity | 1.0300 ± 0.0000 | 1.0300 ± 0.0000 | 1.0300 ± 0.0000 | 1.0294 ± 0.0018 | 1.0300 ± 0.0000 | 1.0300 ± 0.0000a | 1.0300 ± 0.0000a | 1.0300 ± 0.0000a |
| URO (EU/dL) | 0.84 ± 0.34 | 0.76 ± 0.39 | 0.80 ± 0.37 | 0.40 ± 0.37 | 0.54 ± 0.43 | 0.64 ± 0.42 | 0.64 ± 0.42 | 0.56 ± 0.42 |
ANOVA = analysis of variance; URO = urobilinogen.
Values represent the mean ± standard deviation.
*Statistically significant (P < 0.05) using ANOVA and Dunnett’s (log) 2-sided test.
* *Statistically significant (P < 0.05) using ANOVA and Dunnett’s (rank) 2-sided test.
aInappropriate for statistical analysis.
Thyroid hormone assessment: There were no test substance-related changes in T3, T4, and thyroid-stimulating hormone levels at all the dose levels tested (Table 8). In the 2,500 and 5,000 mg/kg/day groups, increased T4 levels in males (P < 0.01) and increased T3 levels in males and females (P < 0.01) were considered incidental changes of no toxicological significance as these changes were not associated with any microscopic changes in the thyroid glands of high-dose animals.
Table 8.
Summary of the thyroid hormone profile in male and female rats administered N. crassa biomass via gavage twice daily for 13 weeks.
| Parameters measured | XX dose group (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| 0 (control) (n = 10) | 1,000 (n = 10) | 2,500 (n = 8) | 5,000 (n = 8) | 0 (control) (n = 10) | 1,000 (n = 9) | 2,500 (n = 10) | 5,000 (n = 9) | |
| TSH[a],[a1] (ng/mL) | 5.7535 ± .2894 | 5.6641 ± .3722 | 6.2984 ± .4667* | 6.4399 ± .4447** | 5.7648 ± .4589 | 6.1919 ± .7996 | 6.0693 ± .3354 | 6.2688 ± .3461** |
| T4[a] (ng/mL) | 37.2386 ± 2.8991 | 39.1659 ± 3.4391 | 45.0421 ± 4.2779*** | 44.5695 ± 4.9611** | 46.6981 ± 7.8198 | 43.3192 ± 6.4246 | 47.2002 ± 4.3941 | 54.3062 ± 27.1692 |
| T3[a1] (ng/mL) | 0.9924 ± .1385 | 1.1076 ± .0592 | 1.4488 ± .4466*** | 1.2721 ± .1729*** | 1.2670 ± .1564 | 1.2426 ± .1408 | 1.7991 ± .3596*** | 1.8036 ± .5294** |
ANOVA = analysis of variance; T3 = triiodothyronine; T4 = thyroxine; TSH = thyroid-stimulating hormone.
Values represent the mean ± standard deviation.
Statistically significant (* = p < .05; ** = p < .01; *** = p < .001) using ANOVA and Dunnett’s two-sided test.
Statistically significant (** = p < .01; *** = p < .001) using ANOVA and Dunnett’s (rank) two-sided test.
Necropsy: A high incidence of gavage procedure-related changes and injuries, including esophageal rupture in 5 test substance-administered rats found dead during the study and 2 additional rats surviving to scheduled termination, were attributed to the thickness of the dose preparations of the test article. Findings of test article in the larynx or nasal passages of several rats were attributed to volume-related gastric reflux. There were 6 unscheduled deaths among the animals submitted for histopathological evaluation. Five animals were found dead during the study period as a result of gavage-related complications. Notable macroscopic observations recorded by the testing facility included holes in the esophagus near the diaphragm, oil/test substance visible in the thoracic cavity, and distension of the stomach. Additionally, a mid-dose group male was humanely sacrificed on Day 42. Macroscopic observations were not recorded at necropsy but a clinical observation of damaged right forelimb with visible swelling was noted. No macroscopic observations recorded during scheduled necropsies were attributed to toxicity of the test article. Two animals in the dosage groups exhibited non-fatal gavage-related trauma and a third animal in the control group exhibited 2 raised white areas in the lungs consistent with minor aspiration of the corn oil vehicle.
In male animals, a statistically significant decrease in liver-to-body weights was observed in the 2,500 and 5,000 mg/kg/day groups. A statistically significant increase occurred in the 2,500 mg/kg/day group brain weights, 2,500 and 5,000 mg/kg/day group epididymides, and in the 1,000 mg/kg/day group testes. In addition, statistically significant increases occurred in the 2,500 mg/kg/day group and/or 5,000 mg/kg/day group kidneys, kidney-to-body, and kidney-to-brain weights. All other absolute and relative organ weights for male animals were comparable to the control group males (Tables 9 and 10).
Table 9.
Mean terminal body and organ weights of male and female rats administered N. crassa biomass via gavage twice daily for 13 weeks.
| Parameters measured (g) | XX dose group (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||
| 0 (control) (n = 10) | 1,000 (n = 10) | 2,500 (n = 8) | 5,000 (n = 8) | 0 (control) (n = 10) | 1,000 (n = 9) | 2,500 (n = 10) | 5,000 (n = 9) | |
| Body weight | 654.9 ± 108.7 | 716.2 ± 99.0 | 758.9 ± 147.8a | 666.5 ± 1.7 | 332.2 ± 33.3 | 352.6 ± 26.6 | 319.8 ± 36.1 | 315.9 ± 41.3 |
| Adrenal glands | 0.0443 ± .0096 | 0.0576 ± .0380 | 0.0579 ± .0126 | 0.0528 ± .0116 | 0.0605 ± .0162 | 0.0644 ± .0128 | 0.0611 ± .0162 | 0.0678 ± .012 |
| Brain[a1] | 2.174 ± .096 | 2.242 ± .090 | 2.313 ± .094* | 2.288 ± .125 | 2.006 ± .121 | 2.064 ± .103 | 1.970 ± .106 | 2.037 ± .103 |
| Epididymides[a1] | 1.3984 ± .0937 | 1.5002 ± .1016 | 1.5745 ± .1293** | 1.5578 ± .1356* | - | - | - | - |
| Heart | 1.649 ± .185 | 1.706 ± .184 | 1.829 ± .220 | 1.676 ± .235 | 1.040 ± .086 | 1.104 ± .133b | 1.074 ± .077 | 1.008 ± .095 |
| Kidneys[a1] | 2.945 ± .323 | 3.364 ± .371* | 3.755 ± .451*** | 3.568 ± .318** | 1.736 ± .153 | 1.819 ± .143 | 1.834 ± .213 | 1.826 ± .221 |
| Liver | 21.325 ± 6.399 | 2.523 ± 3.020 | 21.198 ± 2.985 | 18.539 ± 3.138 | 9.680 ± 1.411 | 9.586 ± 1.498 | 9.207 ± .789 | 8.556 ± 1.021 |
| Ovaries with oviducts | - | - | - | - | 0.1107 ± .0362 | 0.1181 ± .0221 | 0.1296 ± .0235 | 0.1213 ± .0239 |
| Pituitary gland | 0.0137 ± .0026 | 0.0133 ± .0011 | 0.0156 ± .0016a | 0.0154 ± .0025 | 0.0197 ± .0045 | 0.0196 ± .0032 | 0.0215 ± .0024 | 0.0236 ± .0031 |
| Prostate, SV, and CG (combined) | 3.100 ± .484 | 3.234 ± .488 | 3.177 ± .246a | 3.348 ± .324 | - | - | - | - |
| Spleen[a2] | 0.649 ± .121 | 0.699 ± .126 | 0.743 ± .173 | 0.694 ± .118 | 0.430 ± .061 | 0.453 ± .061 | 0.511 ± .088* | 0.440 ± .047 |
| Testes[a1] | 3.605 ± .264 | 3.685 ± .273 | 4.014 ± .217** | 3.680 ± .253 | - | - | - | - |
| Thymus gland | 0.1600 ± .0709 | 0.1830 ± .1057 | 0.1800 ± .0709 | 0.1915 ± .1095 | 0.1384 ± .0344 | 0.1694 ± .0371b | 0.1715 ± .0777 | 0.1727 ± .0604 |
| Thyroid/ parathyroid (combined)[a1] |
0.0245 ± .0066 | 0.0283 ± .0093 | 0.0258 ± .0051a | 0.0273 ± .0071 | 0.0254 ± .0042 | 0.0199 ± .0044* | 0.0231 ± .0034 | 0.0262 ± .0050 |
| Uterus | - | - | - | - | 0.795 ± .277 | 0.786 ± .299 | 0.664 ± .117 | 0.793 ± .375 |
- = not applicable; ANOVA = analysis of variance; CG = coagulating gland; SV = seminal vesicles.
Values represent the mean ± standard deviation.
a n = 9.
b n = 8.
[a]Statistically significant (p < .05) using ANOVA and Dunnett’s (rank) two-sided test.
[a1]Statistically significant (* = p < .05; ** = p < .01; *** = p < .001) using ANOVA and Dunnett’s two-sided test.
[a2]Statistically significant (p < .001) using ANOVA and Dunnett’s (log) two-sided test.
Table 10.
Relative mean organ-to-body weight of male and female rats administered N. crassa biomass via gavage twice daily for 13 weeks.
| Parameters measured (ratio) | XX dose group (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Males (n = 10) | Females (n = 10) | |||||||
| 0 (control) (n = 10) | 1,000 (n = 10) | 2,500 (n = 8) | 5,000 (n = 8) | 0 (control) (n = 10) | 1,000 (n = 9) | 2,500 (n = 10) | 5,000 (n = 9) | |
| Adrenal glands | 0.0701 ± .0218 | 0.0799 ± .0454 | 0.0736 ± .0130 | 0.0801 ± .0169 | 0.1865 ± .0614 | 0.1840 ± .0409 | 0.1914 ± .0504 | 0.2199 ± .0557 |
| Brain | 3.387 ± .476 | 3.188 ± .479 | 2.995 ± .464 | 3.507 ± .583 | 6.085 ± .600 | 5.880 ± .465 | 6.241 ± .842 | 6.520 ± .685 |
| Epididymides | 2.1727 ± .2703 | 2.1373 ± .3643 | 2.0287 ± .2618 | 2.3635 ± .2273 | - | - | - | - |
| Heart | 2.542 ± .207 | 2.409 ± .295 | 2.340 ± .222 | 2.532 ± .285 | 3.140 ± .180 | 3.090 ± .212a | 3.383 ± .335 | 3.210 ± .255 |
| Kidneys[a1] | 4.543 ± .418 | 4.737 ± .475 | 4.797 ± .358 | 5.414 ± .553*** | 5.239 ± .294 | 5.171 ± .392 | 5.769 ± .716 | 5.806 ± .531 |
| Liver[a2] | 32.125 ± 5.009 | 28.738 ± 2.419 | 27.035 ± 2.244** | 27.782 ± 1.824* | 29.139 ± 3.142 | 27.093 ± 2.524 | 29.022 ± 3.351 | 27.136 ± 1.088 |
| Ovaries with oviducts | - | - | - | - | 0.3421 ± .1278 | 0.3353 ± .0608 | 0.4063 ± .0646 | 0.3896 ± .0896 |
| Pituitary gland[a1] | 0.0021 ± .0005 | 0.0019 ± .0002 | 0.0021 ± .0005b | 0.0023 ± .0002 | 0.0059 ± .0010 | 0.0055 ± .0008 | 0.0067 ± .0006 | 0.0075 ± .0012** |
| Prostate, SV, and CG (combined) | 0.005 ± .001 | 0.005 ± .001 | 0.004 ± .001b | 0.005 ± .001 | - | - | - | - |
| Spleen[a2] | 0.994 ± .120 | 0.983 ± .155 | 0.942 ± .173 | 1.045 ± .138 | 1.297 ± .162 | 1.289 ± .155 | 1.606 ± .282** | 1.412 ± .217 |
| Testes | 5.590 ± .637 | 5.228 ± .756 | 5.216 ± .942 | 5.603 ± .669 | - | - | - | - |
| Thymus gland | 0.2487 ± .1101 | 0.2539 ± .1419 | 0.2363 ± .1011 | 0.2883 ± .1489 | 0.4155 ± .0882 | 0.4777 ± .1130a | 0.5318 ± .2145 | 0.5559 ± .2091 |
| Thyroid/parathyroid (combined)[a1] | 0.37683 ± .09138 | 0.40017 ± .13540 | 0.35074 ± .09444b | 0.41127 ± .10209 | 0.76285 ± .08506 | 0.56866 ± .13476* | 0.73371 ± .15738 | 0.83874 ± .17418 |
| Uterus | - | - | - | - | 2.429 ± .922 | 2.240 ± .906 | 2.098 ± .430 | 2.630 ± 1.529 |
- = not applicable; ANOVA = analysis of variance; CG = coagulating gland; SV = seminal vesicles.
Values represent the mean ± standard deviation.
a n = 8.
b n = 9.
[a]Statistically significant (p < .05) using ANOVA and Dunnett’s (rank) two-sided test.
[a1]Statistically significant (p < .01) using ANOVA and Dunnett’s two-sided test.
[a2]Statistically significant (p < .001) using ANOVA and Dunnett’s (log) two-sided test.
In female animals, a statistically significant decrease was observed in the 1,000 mg/kg/day group thyroid–parathyroid-to-body weights. A statistically significant increase occurred in the 2,500 mg/kg/day group spleen, spleen-to-body, and spleen-to-brain weights. In addition, statistically significant increases occurred in the 5,000 mg/kg/day group pituitary-to-body weights. All other absolute and relative organ weights for male animals were comparable to the control group females (Tables 9 and 10).
Histopathology: No microscopic observations in animals terminated on Day 97/98 were attributed to test article toxicity (Tables 11). Several findings were attributed to the gavage procedure and related gastric reflux or to the corn oil vehicle. Periesophageal abscessation and granuloma formation was observed in 3 animals attributed to gavage-related non-fatal esophageal rupture. Minimal degeneration/regeneration of esophageal myofibers was seen in a couple of animals consistent with the stretching effect of gavage. Foamy alveolar macrophages, Type II pneumocyte hypertrophy, and minimal to mild larynx inflammation were found in a few animals consistent with reflux caused by gavage or high gastric volume. Minimal to moderate hepatocellular microvesicular and macrovesicular vacuolization was present in nearly all examined males from all groups, including the control group, as well as in some females, often with lower severity than in males. This finding, consistent with fatty change, was attributed to high dietary lipid from corn oil ingestion. Histopathological examination of organs with significant differences in organ weight and organ-to-body weights in males (e.g. liver and kidney) and females (e.g. spleen) were unremarkable. All other microscopic findings were considered spontaneous, incidental changes unrelated to test substance or control vehicle administration.
Table 11.
Histopathological examination of male and female rats administered N. crassa biomass via gavage twice daily for 13 weeksa.
| Organ/muscle | Lesion | XX dose group (mg/kg) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Males | Females | ||||||||
| 0 (control) (n = 10) | 1,000 (n = 10) | 2,500 (n = 8) | 5,000 (n = 8) | 0 (control) (n = 10) | 1,000 (n = 9) | 2,500 (n = 10) | 5,000 (n = 9) | ||
| Lungs | Raised white areas, accumulation of alveolar macrophages consistent with minor corn oil aspiration | – | – | – | – | 1/10 | – | – | – |
| Heart | Thoracic massb | – | – | – | – | – | 1/9 | – | |
| Esophagus | Adhesion between trachea and thymus | – | – | – | – | – | 1/9 | – | |
| Gavage-related rupture (abscessation and granuloma formation) | – | – | – | – | – | 1/9 | 1/10 | – | |
| Minimal change in esophageal myofibers (stretching effect of gavage) | 1/10 | – | – | 2/8 | 1/10 | – | – | – | |
| Gavage-related inflammation of caudal nares or larynx | – | – | – | 1/8 | – | – | – | 4/9 | |
| Liver | Pale color, minimal to moderate hepatocellular cytoplasmic vacuolization attributed to corn oil intake | 10/10 | 8/10 | 8/10 | 8/10 | – | 1/9 | – | |
| Nodulec | – | – | – | – | – | – | 1/10 | – | |
| Uterus | Fluid-filled, corresponding to estrous cycle-related dilation of lumenc | – | – | – | – | 1/10 | – | 2/10 | 1/9 |
– = no effect.
aAll histopathological lesions identified during the examination are included in this table. The following organs/tissues were examined: accessory genital organs (prostate and seminal vesicles), adrenals, aorta, bone (femur), bone marrow (from femur and sternum), brain (medulla/pons, cerebellar, and cerebral cortex), cecum, cervix, colon, duodenum, esophagus, Harderian gland, heart, ileum with Peyer’s patches, jejunum, kidneys, larynx, liver, lungs, lymph node (mandibular and mesenteric), mammary gland, nasal turbinates, nose, ovaries, oviducts, pancreas, parathyroid, peripheral nerve (sciatic), pharynx, pituitary gland, rectum, salivary glands (sublingual, submandibular, and parotid), skeletal muscle, skin, spinal cord (cervical, mid-thoracic, and lumbar), spleen, sternum, stomach, thymus, thyroid, trachea, urinary bladder, uterus, vagina, eyes, epididymides, optic nerve, testes, and all gross lesions.
bThoracic cavity mass incorporating the heart, thymus, lungs, and esophagus with adhesions to the parietal pleura.
cConcluded to be spontaneous, incidental background changes.
Under the conditions of the study, and based on the toxicological endpoints evaluated, the no-observed-adverse-effect level (NOAEL) for the test article when administered orally over 96/97 days (males/females) was determined to be 5,000 mg/kg/day for both male and female Sprague–Dawley rats.
Discussion
The safety of the consumption of Emergy’s protein-rich N. crassa fungal biomass was evaluated by the standard battery of toxicological studies, conducted under proper OECD guidelines, to assess its genotoxicity, clastogenic, and mutagenic potential, as well as its acute and subchronic toxicity by oral administration, the most common route of exposure. While there was some precipitation of the test article at the higher concentration levels in both the Ames and in vitro chromosomal aberration test, enough doses were evaluated per the evaluation protocols to accurately assess the mutagenic and clastogenic potential of the article. The results of the bacterial reverse mutation (Ames), an in vitro chromosomal aberration test, and an in vivo micronucleus test showed that Emergy’s protein-rich fungal biomass was not mutagenic, clastogenic, or genotoxic under the test conditions.
The 14-day repeat dose range-finder study in Sprague–Dawley rats established an expected maximum tolerable dose of 5,000 mg/kg body weight/day of the test article for the 90-day subchronic oral toxicity study. In the 90-day subchronic oral toxicity study in Sprague–Dawley rats, the test article was provided at concentrations of 0, 1,000, 2,500, or 5,000 mg/kg body weight/day (0, 50, 125, and 250 mg/mL, respectively) by oral gavage. While there were 6 unplanned mortalities during the course of the study, necropsy evaluation revealed that 5 of the deceased animals showed visible signs of injuries consistent with dose route. These results are also consistent with macroscopic and histopathological findings in the necropsy evaluation of the surviving animals, which showed non-fatal gavage-related injuries and gastric reflux due to route of administration.28 No gross findings were attributed to the test article and the surviving animals were in good general health. While there was a significant decrease in food consumption among both male and female animals in the higher dose groups as compared to the controls, it was likely due to prolonged satiety in the animals from the higher dose volumes. This conclusion was supported by the fact that larger gastric volumes were not observed in the higher dose groups. In animal toxicity studies, larger gastric volumes could be a sign of delayed gastric emptying due to specific and non-specific effects of the test article.28 Additionally, at necropsy there were several statistically significant increases in organ weights and organ-to-body weight in male brains, epididymides, and kidneys, and female spleens and pituitary glands. However, all of these findings were not considered test article-related as the magnitude of the changes was small, they were not accompanied by changes in related hematological or urinalysis biomarkers, and they were within historical control data from the lab. The increase in kidney weight is also a normal finding in animal studies fed high-protein diets as the body deals with the excess protein and dietary nitrogen.29–31 Under the conditions of the study, the N. crassa biomass exhibited a NOAEL at the highest dose tested, 5,000 mg/kg/day, in Sprague–Dawley rats.
As the market searches for animal-based meat alternatives, filamentous fungal biomass becomes an increasingly viable option as a protein base for the food industry. Depending on fungal species and cultivation techniques, protein concentrations ranging from 21.1 to 62.2% have been analyzed in Aspergillus oryzae, Neurospora intermedia, and Rhizopus oryzae biomasses.4,32,33 Additionally, studies using the protein digestibility-corrected amino acid score (PDCAAS), which quantifies protein quality for human consumption, has assigned an average value of around 0.91 to fungal protein products which is similar to soy (0.91) and beef (0.92) sources.2,34 Not only is Emergy’s N. crassa biomass product protein-rich (55–60%) but it is also highly digestible and contains a good amino acid profile, consisting of both dispensable and indispensable amino acids (PDCAAS = 1.0). Plant sources of protein such as legumes, by comparison, are lower in protein concentration, are less digestible, and contain several anti-nutritional compounds such as phytic acid, tannins, and alkaloids.2,35,36 These compounds can inhibit human or animal growth, alter the intake or utilization of food or nutritive components in food, or cause discomfort in humans or animals.
Neurospora are obligate aerobes, with a reduced ability to colonize the human gut and bladder, in tissues, or systematically. While the genome of N. crassa contains homologs of enzymes involved in the production of toxins, they are small in number and non-clustered, opposite of what is typically found in pathogenic fungi.37,38 As discussed above, Neurospora has been consumed for centuries as part of fermented foods in all parts of the world and little evidence of pathogenesis has been reported. A current review of the literature does not uncover any reports of pathogenicity by N. crassa and Neurospora is considered unable to cause disease in humans.39 Taken along with the results of the safety studies, this supports the safe use of Emergy’s N. crassa protein-rich biomass ingredient for use in conventional foods including alternative meat products.
Authors’ contribution
PC and JW conceptualized, supervised, and provided funding for the study. FL and JM were responsible for analysis of the study, and drafting and reviewing the manuscript. RS was involved in designing the methodology of the study and reviewing the manuscript.
Acknowledgements
The authors would like to acknowledge the contributions of James Akingbasote of Intertek for his insightful discussions about the toxicological revelance of certain findings.
Contributor Information
Fred Lozy, Intertek Health Sciences Inc., 2233 Argentia Road, Suite 201, Mississauga, ON L5N 2X7, Canada.
Jwar Meetro, Intertek Health Sciences Inc., 2233 Argentia Road, Suite 201, Mississauga, ON L5N 2X7, Canada.
Ryan Simon, Intertek Health Sciences Inc., 2233 Argentia Road, Suite 201, Mississauga, ON L5N 2X7, Canada.
Philip Calabrese, Emergy Inc, 6880 Winchester Circle, Boulder, CO 80301, USA.
Justin M Whiteley, Emergy Inc, 6880 Winchester Circle, Boulder, CO 80301, USA.
Funding sources statement
Emergy Inc. provided the funding for this study.
Conflict of interest statement: PC and JW are employees of Emergy Inc. FL, JM, and RS are employees of Intertek Health Sciences Inc. Intertek has provided consulting services to Emergy Inc.
References
- 1. Godfray HCJ, Aveyard P, Garnett T, et al. Meat consumption, health, and the environment. Science. 2018:361:eaam5324.8pp, plus summary. [DOI] [PubMed] [Google Scholar]
- 2. Schweiggert-Weisz U, Eisner P, Bader-Mittermaier S, Osen R. Food proteins from plants and fungi. Curr Opin Food Sci. 2020:32:156–162. [Google Scholar]
- 3. Aiking H, De Boer J. The next protein transition. Trends Food Sci Technol. 2020:105:515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gmoser R, Fristedt R, Larsson K, et al. From stale bread and brewers spent grain to a new food source using edible filamentous fungi. Bioengineered. 2020:11:582–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hüttner S, Johansson A, Gonçalves Teixeira P, et al. Recent advances in the intellectual property landscape of filamentous fungi. Fungal Biol Biotechnol. 2020:7:16.[17pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Roche CM, Loros JJ, McCluskey K, Glass NL. Neurospora crassa: looking back and looking forward at a model microbe. Am J Bot. 2014:101:2022–2035. [DOI] [PubMed] [Google Scholar]
- 7. Aramayo R, Selker EU. Neurospora crassa, a model system for epigenetics research. Cold Spring Harb Perspect Biol. 2013:5:a017921.[17pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beadle GW, Tatum EL. Genetic control of biochemical reactions in Neurospora. Proc Natl Acad Sci U S A. 1941:27:499–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galagan JE, Calvo SE, Borkovich KA, et al. The genome sequence of the filamentous fungus Neurospora crassa. Nature. 2003:422:859–868. [DOI] [PubMed] [Google Scholar]
- 10. Havlik D, Brandt U, Bohle K, Fleißner A. Establishment of Neurospora crassa as a host for heterologous protein production using a human antibody fragment as a model product. Microb Cell Factories. 2017:16:128.[15pp]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. U.S. EPA . Part 160—Good laboratory practice standards. In: U.S. Code of Federal Regulations (CFR). Title 40: Protection of Environment (Environmental Protection Agency). Washington, DC: U.S. EPA, 2020. https://www.govinfo.gov/app/collection/cfr/2020/title40 (2022 March 15 date last accessed). [Google Scholar]
- 12. U.S. EPA . Part 792—Good laboratory practice standards. In: U.S. Code of Federal Regulations (CFR). Title 40: Protection of Environment. (U.S. Environmental Protection Agency). Washington, DC: U.S. EPA, 2020. https://www.govinfo.gov/app/collection/cfr/2021/title40 (2022 March 15 date last accessed). [Google Scholar]
- 13. U.S. FDA . Part 58—Good laboratory practice for nonclinical laboratory studies. In: U.S. Code of Federal Regulations (CFR). Title 21: Food and Drugs. (Food and Drug Administration). Silver Spring, MD: U.S. FDA, 2021. https://www.govinfo.gov/app/collection/cfr/2021/title21 (2022 March 15 date last accessed). [Google Scholar]
- 14. OECD . OECD principles of good laboratory practice. Series on principles of good laboratory practice and compliance monitoring, no. 1 [ENV/MC/CHEM(98)17]. Paris: OECD, 1998. https://www.oecd-ilibrary.org/environment/oecd-principles-on-good-laboratory-practice_9789264078536-en (revised 1997; published 26 January 1998, 2022 March 15 date last accessed). [Google Scholar]
- 15. NRC . Guide for the care and use of laboratory animals, 8th ed. Washington, DC: NRC, 2011. http://www.nap.edu/catalog/12910/guide-for-the-care-and-use-of-laboratory-animals-eighth(March 2022 date last accessed). [Google Scholar]
- 16. U.S. FDA . C. Guidelines for specific toxicity studies. IV.C.1.A. Bacterial reverse mutation test. In: Toxicological principles for the safety assessment of food ingredients: Redbook 2000. College Park, MD: U.S. FDA,2018. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/redbook-2000-ivc1a-bacterial-reverse-mutation-test (July 2018 final; content current as of: 2017 February 11, 2022 March 15 date last accessed). [Google Scholar]
- 17. OECD . Bacterial reverse mutation test (OECD test no. 471). In: OECD guidelines for the testing of chemicals. Paris: OECD, 1997. https://www.oecd-ilibrary.org/fr/environment/test-no-471-bacterial-reverse-mutation-test_9789264071247-en (adopted: 21 July 1997, 2022 March 15 date last accessed). [Google Scholar]
- 18. ICH . Guidance on genotoxicity testing and data interpretation for pharmaceuticals intended for human use: S2(R1). ICH harmonised tripartite guideline. Geneva: ICH, 2011. https://database.ich.org/sites/default/files/S2%28R1%29%20Guideline.pdf (step 4: 9 November 2011, 2022 March 15 date last accessed). [Google Scholar]
- 19. OECD . In vitro mammalian chromosomal aberration test (OECD test no. 473). In: OECD guidelines for the testing of chemicals. Paris: OECD, 2016. https://www.oecd-ilibrary.org/fr/environment/test-no-473-in-vitro-mammalian-chromosomal-aberration-test_9789264264649-en (adopted: 29 July 2016, 2022 March 15 date last accessed). [Google Scholar]
- 20. Commission regulation (EU) 2017/735 of 2017 February 14 amending, for the purpose of its adaptation to technical progress, the annex to regulation (EC) no 440/2008 laying down test methods pursuant to regulation (EC) no 1907/2006 of the European Parliament and of the council on the registration, evaluation, authorisation and restriction of chemicals (REACH). Off J Eur Union. 2017:L112:1–402. [Google Scholar]
- 21. OECD . Mammalian erythrocyte micronucleus test (OECD test no. 474). In: OECD guidelines for the testing of chemicals. Paris: OECD, 2016. https://www.oecd-ilibrary.org/environment/test-no-474-mammalian-erythrocyte-micronucleus-test_9789264264762-en (adopted: 29 July 2016, 2022 March 15 date last accessed). [Google Scholar]
- 22. U.S. FDA . C. Guidelines for specific toxicity studies. IV.C.1.D. Mammalian erythrocyte micronucleus test. In: Toxicological principles for the safety assessment of food ingredients: Redbook 2000. College Park, MD: U.S. FDA, 2000. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/redbook-2000-ivc1d-mammalian-erythrocyte-micronucleus-test (July 2000 final; content current as of: 2017 February 11, 2022 March 15 date last accessed). [Google Scholar]
- 23. U.S. FDA . C. Guidelines for specific toxicity studies. IV.C.4.A. Subchronic toxicity studies with rodents. In: Toxicological principles for the safety assessment of food ingredients: Redbook 2000. College Park, MD: U.S. FDA, 2003. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/redbook-2000-ivc4a-subchronic-toxicity-studies-rodents (November 2003 final; content current as of: 2017 February 11, 2022 March 15 date last accessed). [Google Scholar]
- 24. OECD . Repeated dose 28-day oral toxicity study in rodents (OECD test no. 407). In: OECD guidelines for the testing of chemicals. Paris: OECD, 2008. https://www.oecd-ilibrary.org/environment/test-no-407-repeated-dose-28-day-oral-toxicity-study-in-rodents_9789264070684-en (adopted: 16 October 2008, 2022 March 15 date last accessed). [Google Scholar]
- 25. OECD . Repeated dose 90-day oral toxicity study in rodents (OECD test no. 408). In: OECD guidelines for the testing of chemicals. Paris: OECD, 2018. https://www.oecd-ilibrary.org/environment/test-no-408-repeated-dose-90-day-oral-toxicity-study-in-rodents_9789264070707-en (adopted: 27 June 2018, 2022 March 15 date last accessed). [Google Scholar]
- 26. Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000:455:29–60. [DOI] [PubMed] [Google Scholar]
- 27. Gatehouse D. Bacterial mutagenicity assays: test methods. Methods Mol Biol. 2012:817:21–34. [DOI] [PubMed] [Google Scholar]
- 28. Damsch S, Eichenbaum G, Tonelli A, et al. Gavage-related reflux in rats: identification, pathogenesis, and toxicological implications (review). Toxicol Pathol. 2011:39:348–360. [DOI] [PubMed] [Google Scholar]
- 29. Hammond KA, Janes DN. The effects of increased protein intake on kidney size and function. J Exp Biol. 1998:201:2081–2090. [DOI] [PubMed] [Google Scholar]
- 30. Jean C, Rome S, Mathé V, et al. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001:131:91–98. [DOI] [PubMed] [Google Scholar]
- 31. Aparicio VA, Nebot E, García-Del Moral R, et al. High-protein diets and renal status in rats. Nutr Hosp. 2013:28:232–237. [DOI] [PubMed] [Google Scholar]
- 32. Karimi S, Mahboobi Soofiani N, Lundh T, et al. Evaluation of filamentous fungal biomass cultivated on vinasse as an alternative nutrient source of fish feed: protein, lipid, and mineral composition. Fermentation. 2019:5:99.[17pp]. [Google Scholar]
- 33. Karimi S, Mahboobi Soofiani N, Mahboubi A, et al. Evaluation of nutritional composition of pure filamentous fungal biomass as a novel ingredient for fish feed. Fermentation. 2021:7:152.[14pp]. [Google Scholar]
- 34. Linder T. Making the case for edible microorganisms as an integral part of a more sustainable and resilient food production system. Food Sec. 2019:11:265–278. [Google Scholar]
- 35. Muzquiz M, Varela A, Burbano C, et al. Bioactive compounds in legumes: pronutritive and antinutritive actions. Implications for nutrition and health. Phytochem Rev. 2012:11:227–244. [Google Scholar]
- 36. Mattila PH, Pihlava J-M, Hellström J, et al. Contents of phytochemicals and antinutritional factors in commercial protein-rich plant products. Food Qual Saf. 2018:2:213–219. [Google Scholar]
- 37. Yoder OC, Turgeon BG. Fungal genomics and pathogenicity. Curr Opin Plant Biol. 2001:4:315–321. [DOI] [PubMed] [Google Scholar]
- 38. Mannhaupt G, Montrone C, Haase D, et al. What's in the genome of a filamentous fungus? Analysis of the Neurospora genome sequence. Nucleic Acids Res. 2003:2003(31):1944–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Perkins DD, Davis RH. Evidence for safety of Neurospora species for academic and commercial uses. Appl Environ Microbiol. 2000:66:5107–5109. [DOI] [PMC free article] [PubMed] [Google Scholar]