Abstract
Non-small cell lung cancer (NSCLC) is a common malignant subtype of lung cancer with high mortality. Resveratrol (RSV) is a natural molecule that regulates mitochondrial metabolism. Here, we explored the effect of RSV on NSCLC cell mitophagy and paclitaxel (PTX) resistance. LncRNA ZFAS1, miR-150-5p, and PTEN-induced putative kinase 1 (PINK1) expressions in NSCLC cells were analyzed by RT-qPCR. Levels of PINK1, Parkin and autophagy related molecules LC3I and LC3II were assessed by western blot. Mitophagy was demonstrated by transmission electron microscopy. Luciferase reporter assay revealed that miR-150-5p directly interacted with ZFAS1 or PINK1. MTT was performed to test the IC50 of NSCLC cells. Cell proliferation and apoptosis were measured with CCK-8, EdU, and TUNEL assays. A549/PTX cells exhibited a higher mitophagy activity, and chemoresistance, whereas RSV suppressed PTX resistance and mitophagy in NSCLC cells. Furthermore, ZFAS1 was found to be a downstream effector of RSV in NSCLC cells. We next found ZFAS1 directly interacted with miR-150-5p and regulated the expression of a key mitophagy regulator PINK1. In addition, RSV modulated PTX resistance and mitophagy in NSCLC via ZFAS1/miR-150-5p/PINK1 axis. We validate that RSV influences mitophagy and PTX resistance in NSCLC via ZFAS1/miR-150-5p mediated PINK1/Parkin pathway. Combining these 2 drugs may be a new option of NSCLC therapy.
Keywords: lncRNA ZFAS1, miR-150-5p, mitophagy, non-small cell lung cancer, resveratrol
Introduction
Non-small cell lung cancer (NSCLC) accounts for ~80% of all lung cancers and is the malignant tumor with highest morbidity and mortality.1 Although the improvement of surgical protocols and the development of targeted drugs have greatly improved the treatment status of NSCLC, ~75% of patients are found in the middle and late stages, and their 5-year survival rate is very low.2 Currently, paclitaxel (PTX) has become a vital chemotherapeutic agent of advanced NSCLC.3 However, the therapeutic resistance to PTX presents a substantial clinical problem for NSCLC patients.4 Therefore, improving chemotherapeutic efficacy of PTX is of great importance to overcome PTX resistance of NSCLC. Resveratrol (RSV)—a non-flavonoid polyphenolic organic compound—is an antitoxin extracted from Polygonum cuspidatum.5 RSV has been shown to have antitumor and anti-inflammatory biological activities.6 Previous study has shown that combination of resveratrol-cyclodextrin enhanced cellular uptake, cytotoxicity, and apoptosis in NSCLC.7 However, the research on the related mechanism of RSV against NSCLC is still relatively few and incomplete.
Mitophagy is a type of cell-selective autophagy, mediating cell homeostasis.8 During mitophagy, cells can recognize damaged mitochondria and specifically encapsulate them into autophagosomes, which interact with lysosomes to complete the degradation of damaged mitochondria.9 The dysfunction of mitophagy is crucial for the pathogenesis of NSCLC.10 For example, erastin and celastrol activated PINK1/Parkin-dependent mitophagy in NSCLC cells.11 PTEN-induced putative kinase 1 (PINK1) and Parkin are recognized as the main regulatory proteins for mitophagy.12 Studies have confirmed that abnormally increased PINK1 in lung cancer indicated poor prognosis of patients. Meanwhile, PINK1-mediated mitophagy signaling promoted the progression of lung cancer.13,14 Herein, we predicted that RSV might mediate PINK1/Parkin-mediated mitophagy and enhance the anti-NSCLC effect of PTX.
Long noncoding RNAs (lncRNAs) are a class of endogenous noncoding RNAs longer than 200 nucleotides.15 Early studies showed that lncRNAs had significant functions in various cancers, including NSCLC. For example, lncRNA WTAPP1 induced cell invasion and migration in NSCLC cells.16 LncRNA TMPO-AS1 facilitated cell proliferation and metastasis in NSCLC.17 LncRNA zinc finger antisense 1 (ZFAS1)—located on chromosome 20q13—is a transcript antisense to the 5′ end of the gene Znfx1.18 It has been reported that ZFAS1 was increased in NSCLC tissues, contributing to the decreased survival in NSCLC patients.19 RSV can mediate disease progression by regulating its downstream noncoding RNAs. For instance, lncRNA DIO3OS facilitates the proliferation of prostate stromal cells and was downregulated by RSV.20 Our preliminary experiments found that there was differential expression of ZFAS1 in NSCLC cells induced by RSV. Therefore, we speculated that RSV might regulate the anti-lung cancer effect of PTX by affecting the expression of ZFAS1 and mediating mitophagy signaling.
Here, we investigated the regulatory function of RSV in NSCLC. We demonstrated that RSV was a sensitizer of PTX resistance in NSCLC. In addition, RSV suppressed cell viability and mitophagy in PTX-resistant NSCLC cells via inhibiting ZFAS1/miR-150-5p/PINK1 signaling pathway. Our findings may provide a new perspective for enhancing NSCLC sensitivity to PTX.
Materials and methods
Cell culture and treatment
Human NSCLC cell line (A549) was obtained from Shanghai Institute of Country Cell Bank (Shanghai, China). These cells were grown in RPMI 1640 medium enriched with 10% FBS (Invitrogen, Carlsbad, CA, United States), 100-U/mL penicillin, and 100-mg/mL streptomycin (Sigma, Shanghai, China) at 37 °C with CO2. The PTX-resistant cells (A549/PTX) were established as previously described.21 Briefly, parental A549 cells were stepwise screened by increasing the concentration of PTX (Sigma) in culture by a range of 0.1–0.5 mM over 6 months. Then, PTX resistance was maintained with 0.5-mM PTX in the culture. Cell assays were performed when cells were in logarithmic growth phase. For RSV treatment, cells were incubated with 25-μM RSV (R5010, Sigma) for 30 min at 37 °C. DMSO solution (0.02%) was used as a control.
Cell transfection
We purchased short hairpin RNA against ZFAS1 (sh-ZFAS1) from Guangzhou RiboBio (RiboBio, Guangzhou, China). The pcDNA3.1 vector (GenePharma, Shanghai, China) containing the full-length cDNA sequence of ZFAS1 was used as OE-ZFAS1. The miR-150-5p mimics and inhibitor were synthesized by GenePharma (Shanghai, China). The cell transfection was carried out using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instruction.
MTT assay for IC50
The current work inoculated cells (1 × 104/well) to 96-well plates, followed by 2-h incubation. After removing the medium, cells were further exposed to different drugs. At 24-h post-incubation, all wells were supplemented with 20 μL of 5 mg/mL MTT (contained within PBS, Sigma), followed by 4-h incubation under 37 °C. DMSO (150 μL) was then added to each well for formazan dissolving. Absorbance (OD) values were recorded with the microplate reader (Bio-Rad, Hercules, CA, United States) at 490 nm. In addition, the half-maximal inhibitory concentration (IC50) value was determined.
CCK-8 assay
This work inoculated cells (2 × 103/well) in the 96-well plates, followed by 24/48/72/96-h incubation. All wells were added with CCK-8 solution (10 μL, Beyotime, Shanghai, China) and incubated under 37 °C for another 2-h period. Then, OD values were measured with the microplate reader (Bio-Rad) at 450 nm.
Transmission electron microscopy
NSCLC cells were trypsinized and fixed in 2% glutaraldehyde. Samples were then dehydrated in ethanol containing 3% uranyl acetate. Cells were embedded, sectioned after solidifying, and stained with lead citrate. Mitochondrial dysfunction was then assessed using transmission electron microscopy (H-600, Hitachi, Japan).
EdU assay
Cell proliferation assay was conducted using EdU Kit (RiboBio, Guangzhou, China). The present work incubated cells (5 × 103/well) with 50 μmol/L EdU medium for 2 h, followed by 4% paraformaldehyde (PFA) fixation as well as DAPI (Invitrogen) staining. After PBS washing, images were captured with a fluorescence microscopy (Olympus, Tokyo, Japan).
TUNEL assay
Cells were plated onto coverslips in 6-well plates (1 × 105/well). After seeding, cells were fixed with 4% paraformaldehyde for 30 min. Apoptotic cells were determined with One Step TUNEL Apoptosis Assay Kit (Beyotime) according to the manufacturer’s instruction. Coverslips were then stained with DAPI (Beyotime). Finally, the fluorescence staining was observed with a fluorescence microscope (Olympus). The number of apoptotic cells was counted and expressed as a percentage of the total cell population.
RNA extraction and real-time quantitative PCR
Total mRNA was obtained from NSCLC cells with TRIzol (Invitrogen). For mRNA and lncRNA, complementary DNA (cDNA) was synthesized using PrimeScriptTM reagent kit with gDNA Eraser (Takara, Dalian, China). For miRNA, reverse transcription was performed with a Mir-X™ miRNA First Strand Synthesis Kit (Takara). PCR reactions was conducted using SYBR Premix Ex Taq kit (Takara) in Applied Biosystems 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, United States). Primer sequences are listed below: ZFAS1 F: 5′-ACGTGCAGACATCTACAACCT-3′, R: 5′-TACTTCCAACACCCGCAT-3′; miR-150-5p F: 5′-AACCCTTGTACCAGTGGTCG-3′, R: 5′-GTATCC AGTGCGTGTCGTGG-3′; PINK1 F: 5′-CAAGAGAGGTCCCAAGCAAC-3′, R: 5′-GGCAGCACATCAGGGTAGTC-3′. GAPDH or U6 was used as an internal control. The standard 2−∆∆Ct method was used to analyze gene expression.
Western blot
NSCLC cells were lysed in RIPA lysis buffer (Beyotime). Total protein concentration was quantified using a BCA reagent (Beyotime). Protein was separated using 10% SDS-PAGE gel and transferred to PVDF membranes (Millipore, Boston, MA, United States). After blocked with 5% skim milk, membranes were incubated with primary antibodies at 4 °C overnight: PINK1 (ab23707, 1:1,000, Abcam, Cambridge, MA, Unites States), Parkin (#2132, 1:1,000, CST, Danvers, United States), LC3 I/II (abc929, 1:1,000, Sigma), p62 (ab91526, 1:1,000, Abcam). Next, membranes were incubated with HRP-coupled secondary antibody (ab205718, 1:1,000, Abcam), exposed using an ECL reagent (Pharmacia, Piscataway, United States). GAPDH served as an internal loading control. Protein bands were analyzed using Image J software (NIH, Bethesda, MD, United States).
Luciferase reporter assay
The wild-type (WT) or mutant (MUT) sequences of ZFAS1 and PINK1 3′UTR containing binding sites for miR-150-5p were subcloned into pGL3-basic vectors (Promega, Madison, WI, United States). Cells were co-transfected with a recombinant plasmid containing ZFAS1/PINK1-WT vector or ZFAS1/PINK1-MUT vector with miR-150-5p mimics or NC mimics using Lipofectamine 2000 (Invitrogen). After 48-h incubation, the dual luciferase assay system (Promega) was used to determine the luciferase activity.
Immunofluorescence staining
Transfected cells on glass slides were fixed using 4% paraformaldehyde (Sigma) and permeabilized using 0.2% Triton X-100 in PBS (Sigma) for 5 min, and blocked with 5% goat serum (Gibco, Grand Island, NY, United States) for 1 h. Cells were added with primary antibodies against PINK1 (PA5-86941, 1:200, Invitrogen) and Parkin (PA5-13399, 1:50, Invitrogen) at 4 °C overnight, followed by incubation with Cy3 or FITC labeled secondary antibodies (1:200, Beyotime) for 1 h at room temperature. DAPI (1:5,000, Beyotime) staining was performed for 5 min to stain the nuclei. Image acquisition was performed using a fluorescence microscope (Olympus).
Subcellular fractionation assay
The PARIS Kit (Life Technologies, United States) was utilized for detecting the distribution of ZFAS1 in the cytoplasmic and nuclear fractions of NSCLC cells according to manufacturer's protocol. Briefly, cells were resuspended using cell fractionation buffer, following by centrifugation. Cell disruption buffer preserved nuclear precipitation and supernatant. ZFAS1 expression was measured by real-time PCR. GAPDH and U6 transcripts were utilized as internal markers.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). Data analyses were conducted with Student’s t-test (2 groups) or 1-way analysis of variance (multiple groups) using Graphpad Prism 7 (Graphpad, La Jolla, CA, United States). P value <0.05 was regarded as a significant. Experiments were repeated 3 times.
Results
A549/PTX cells presented increased chemoresistance and enhanced mitophagy
We first measured the IC50 of PTX, DDP, and VCR treatment of A549 and A549/PTX cells using MTT assay. IC50 values of A549/PTX cells treated with these drugs were greatly higher compared with A549 cells stimulated with the respective drugs, suggesting PTX-resistant (Fig. 1A). Nevertheless, there were no significant differences in proliferative ability of these 2 cells (Fig. 1B). Next, we evaluated mitophagy function by transmission electron microscopy (TEM) and found that the activity of mitophagy was remarkably increased in A549/PTX cells compared with A549 cells (Fig. 1C). Meanwhile, Immunofluorescence assay further confirmed that the number of mitophagy in A549/PTX cells was much higher than that in A549 cells (Fig. 1D). In addition, compared with A549 cells, protein levels of mitophagy-related molecules PINK1, Parkin, and LC3II in A549/PTX cells was induced, whereas the expression of LC3I and p62 was suppressed (Fig. 1E). Thus, these results suggested that mitophagy was accelerated in PTX-resistant A549 cells.
Fig. 1.
A549/PTX cells presented increased chemoresistance and enhanced mitophagy. A) MTT tested the IC50 of PTX, DDP, and VCR treatment of A549 and A549/PTX cells. B) CCK-8 assay evaluated the proliferative ability of these 2 cells. C) Assessment of mitophagy by TEM. D) Immunofluorescence detection of mitophagy. E) Western blot analysis of mitophagy-related proteins (PINK1, Parkin, LC3I, LC3II, and p62). Results expressed as mean ± SD for 3 independent experiments. *P < 0.05.
RSV decreased PTX-resistant NSCLC cell viability and mitophagy
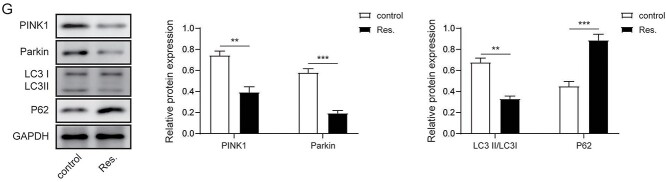
We next explored the function of RSV in PTX resistance of NSCLC cells. IC50 of cells treated with PTX, DDP, and VCR was significantly reduced after treatment with RSV (Fig. 2A). Compared with A549/PTX cells, the cell proliferation ability in RSV-treated A549/PTX cells was extremely suppressed (Fig. 2B). Moreover, TUNEL assay revealed that after RSV stimulation, the proportion of positive cells increased dramatically (Fig. 2C). Notably, TEM showed that RSV obviously inhibited the activity of mitophagy in A549/PTX cells (Fig. 2D). In addition, the fluorescence activity of mitophagy, PINK1 and Parkin in RSV-treated A549/PTX cells was markedly reduced compared with untreated cells (Fig. 2E and F). Furthermore, RSV decreased the expression of PINK1, Parkin and LC3II in A549/PTX cells but increased p62 levels (Fig. 2G).
Fig. 2.
RSV decreased PTX-resistant NSCLC cell viability and mitophagy. A) MTT tested the IC50 of A549/PTX cells treated with/without RSV. B) EdU assay evaluated the proliferation ability of cells. C) TUNEL assay determined cell apoptosis. D) Assessment of mitophagy by TEM. E) Immunofluorescence detection of mitophagy. F) Immunofluorescence detection of PINK1 and Parkin. G) Western blot analysis of mitophagy related proteins. Values were expressed as mean ± SD of 3 separate determinations. *P < 0.05.
ZFAS1 was identified as a downstream effector of RSV-mediated suppressive effects on NSCLC cells
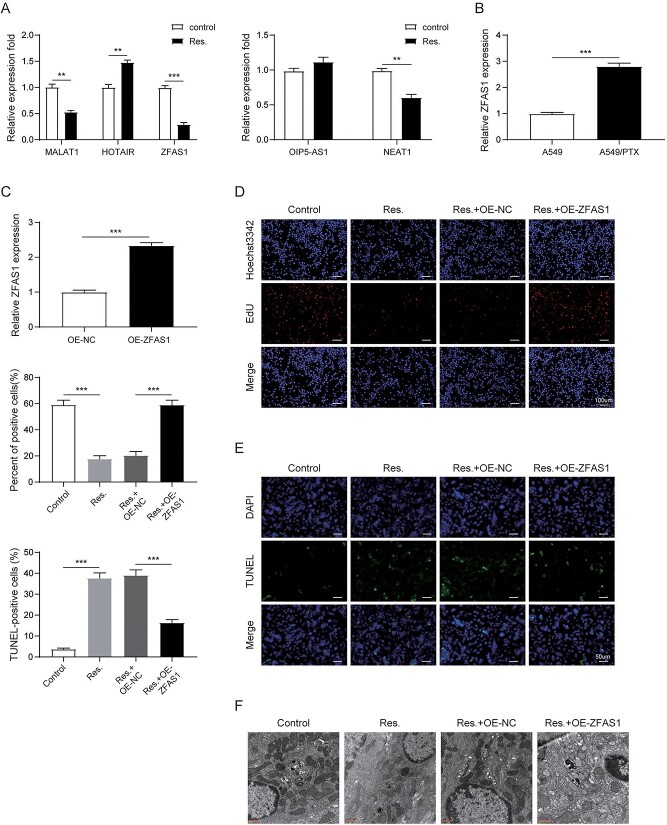
Furthermore, we screened for lncRNAs that may be related to PTX resistance in NSCLC and may be a downstream regulator of RSV. RT-qPCR detected the differential expression of lncRNAs (MALAT1, HOTAIR, ZFAS1, OIP5-AS1, NEAT1, and H19) in A549/PTX cells with or without RSV treatment, and finally we selected lncRNAs ZFAS1 for further verification (Fig. 3A). Furthermore, we found that ZFAS1 was increased in A549/PTX cells than that in A549 cells (Fig. 3B). In ZFAS1 overexpression A549/PTX cells, ZFAS1 was also obviously upregulated compared with control group (Fig. 3C). To confirm ZFAS1 that might mediate the crosstalk between RSV and PTX resistance in NSCLC, A549/PTX cells were transfected with OE-ZFAS1 and treated with RSV. Compared with untreated cells, RSV dramatically suppressed proliferation and enhanced apoptosis in A549/PTX cells, but overexpressed ZFAS1 reversed these effects in RSV-treated cells (Fig. 3D and E). Mitophagy was greatly repressed in RSV-treated cells compared with control cells, whereas overexpression of ZFAS1 overturned the inhibitory effect (Fig. 3F and G). In addition, compared with control cells, the fluorescence intensity of PINK1 and Parkin was extremely reduced in RSV-treated cells, but this effect could be partially abrogated by ZFAS1 upregulation (Fig. 3H). Meanwhile, RSV reduced the levels of PINK1, Parkin and LC3II in A549/PTX cells, and induced p62 expression. However, overexpressed ZFAS1 had the opposite effects (Fig. 3I). These results implied that RSV participated in PTX resistance in NSCLC cells by regulating ZFAS1.
Fig. 3.
ZFAS1 was identified as a downstream effector of RSV-mediated suppressive effects on NSCLC cells. A) RT-qPCR detection of different lncRNAs in A549/PTX cells with or without RSV treatment. B, C) RT-qPCR detection of ZFAS1 expression. D) EdU assay evaluated the proliferation ability of cells. E) TUNEL assay determined cell apoptosis. F) Assessment of mitophagy by TEM. G) Immunofluorescence detection of mitophagy. H) Immunofluorescence detection of PINK1 and Parkin. I) Western blot measurement of mitophagy related proteins. All data are represented as the mean ± SD of 3 independent experiments. *P < 0.05, **P < 0.01,***P < 0.001.
LncRNA ZFAS1 targeted binding miR-150-5p
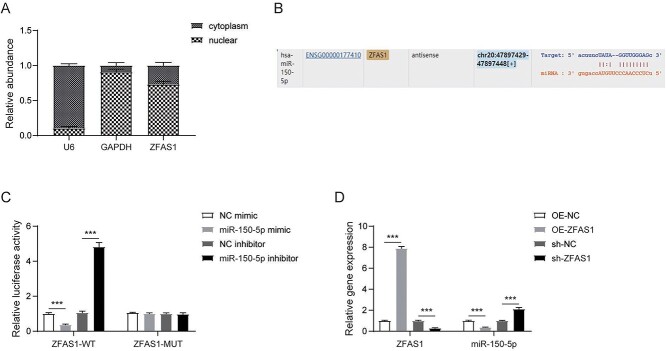
We next explored molecular mechanism of ZFAS1 regulating PTX resistance in NSCLC cells and found that ZFAS1 was distributed in both cytoplasm and nucleus, and was mainly distributed in the cytoplasm of A549/PTX cells (Fig. 4A). Notably, we found the predicted binding site between ZFAS1 and miR-150-5p by bioinformatics tool StarBase (Fig. 4B). In addition, dual luciferase reporter assay verified the interaction between these 2 molecules (Fig. 4C). Furthermore, RT-qPCR results demonstrated that compared with control cells, overexpressed ZFAS1 greatly increased ZFAS1 and miR-150-5p levels, whereas ZFAS1 silencing remarkably decreased the expression of ZFAS1 and miR-150-5p (Fig. 4D). Thus, ZFAS1 could bind with miR-150-5p and induce its expression in A549/PTX cells.
Fig. 4.
LncRNA ZFAS1 targeted binding miR-150-5p. A) Nucleocytoplasmic separation assay detected the subcellular localization of ZFAS1. B) Binding site of ZFAS1 to miR-150-5p by starbase. C) Luciferase reporter analysis confirmed that miR-150-5p bound to ZFAS1. D) RT-qPCR assessed ZFAS1 and miR-150-5p levels. Bars represent mean ± SD of 3 independent experiments. *P < 0.05.
MiR-150-5p directly targeted the 3′-UTR of PINK1
Next, bioinformatics predicted that miR-150-5p could bind to PINK1 3′-UTR (Fig. 5A). Luciferase activity of wt-PINK1 was remarkably reduced by miR-150-5p overexpression, and it was induced by miR-150-5p inhibitor, whereas no effects were observed when the binding site was mutated (Fig. 5B). Furthermore, miR-150-5p and PINK1 levels were increased in A549/PTX cells with overexpressed miR-150-5p, but downregulated in A549/PTX cells with miR-150-5p inhibitor (Fig. 5C and D). Therefore, miR-150-5p directly bond to PINK1 and regulated its expression.
Fig. 5.
MiR-150-5p bond to 3′-UTR of PINK1. A) The predicted binding site between miR-150-5p and PINK1 by starbase. B) Dual luciferase reporter assay verified the targeting relationship between miR-150-5p and PINK1. C) RT-qPCR measured miR-150-5p and PINK1 expressions. D) Western blot detected PINK1 expression. Error bars stand for the mean ± SD of at least triplicate experiments. *P < 0.05, **P < 0.01,***P < 0.001.
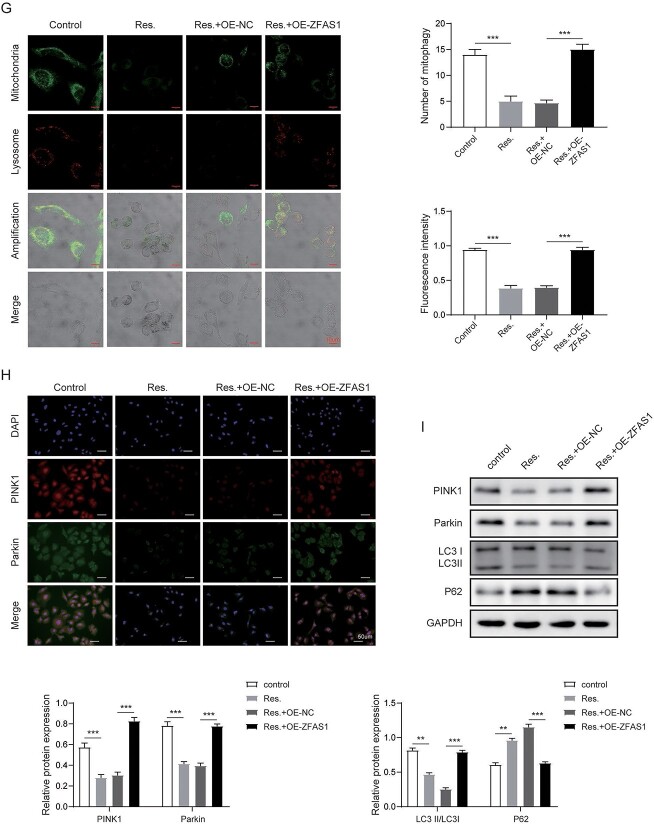
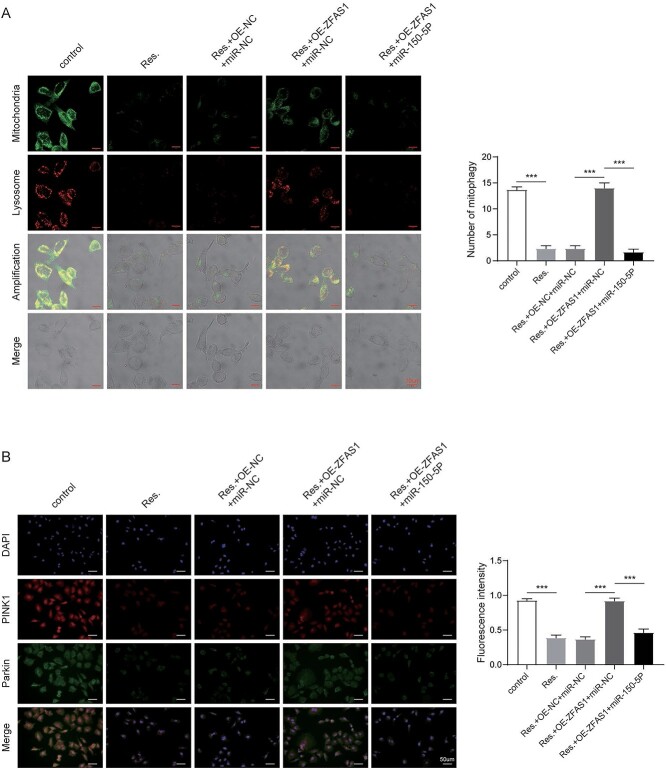
RSV regulated PTX-resistant NSCLC through ZFAS1/miR-150-5p/PINK1 axis
We next investigated contribution of miR-150-5p suppression to mitophagy in A549/PTX cells with RSV treatment and ZFAS1 overexpression. TEM results demonstrated that overexpression of ZFAS1 promoted mitophagy suppressed by RSV, which was further suppressed by co-transfection of miR-150-5p inhibitor in BVZ cells (Fig. 6A). Compared with untreated cells, the fluorescence intensities of PINK1 and Parkin in the RSV treatment group were greatly reduced. Nevertheless, we found that these indicators that were overturned by ZFAS1 upregulation were reduced with miR-150-5p inhibition (Fig. 6B). Moreover, ZFAS1 overexpression induced the expression of PINK1, Parkin and LC3II suppressed by RSV, and reduced the level of p62 increased by RSV. However, these effects were abolished by co-transfection of miR-150-5p inhibitor (Fig. 6C). Thus, loss of miR-150-5p expression reversed the effect of ZFAS1 overexpression on RSV-mediated PTX resistance in NSCLC cells.
Fig. 6.
RSV regulated PTX-resistant NSCLC through ZFAS1/miR-150-5p/PINK1 axis. A) Immunofluorescence detection of mitophagy. B) Immunofluorescence detection of PINK1 and Parkin. C) Western blot analysis of mitophagy related proteins. Error bars represent SD of the mean of 3 separate determinations. *P < 0.05.
Discussion
NSCLC is a type of lung cancer that is difficult to treat and has a high mortality rate.22 At present, the incidence of NSCLC patients is increasing at a rapid rate.23 However, it can be found that many NSCLC patients are prone to drug resistance, such as PTX resistance, which further increases the difficulty of chemotherapy.24 RSV was reported to play a crucial role in NSCLC.25 As reported, RSV promoted cell apoptosis through activating p38-MAPK pathway in NSCLC cells.26 Besides, combination therapy using co-encapsulated RSV and PTX improved the treatment of breast cancer.27 Here, we explored the function of RSV in PTX resistance in NSCLC. RSV could sensitize NSCLC cells to PTX treatment, mainly by reducing mitophagy in NSCLC cells. Meanwhile, lncRNA ZFAS1 was identified as an important molecule in this process.
Autophagy is a cellular pathway through which the endoplasmic reticulum membrane wraps and degrades abnormal proteins and organelles, and is essential for maintaining the normal physiological functions of cells.28 Mitophagy is an autophagic process that removes damaged mitochondria.29 Moreover, mitophagy serves a key role in development and drug resistance of cancers.30 In NSCLC, Norcantharidin triggered cell apoptosis through a mitophagy-mediated autophagy pathway.31 PINK1/Parkin pathway is crucial for maintaining the functional integrity of mitochondria.32 High level of PINK1 promoted proliferation and chemoresistance in NSCLC cells.33 PHB2 depletion attenuated Parkin-mediated mitophagy in NSCLC cells.34 Our data showed that mitophagy was remarkably enhanced and the expression of PINK1, Parkin and LC3II was increased in PTX-resistant NSCLC cells, whereas RSV treatment reversed these effects. Therefore, RSV protected mitochondrial dysfunction in A549/PTX cells. Similarly, RSV improved Bnip3-related mitophagy and preserved mitochondrial homeostasis.35 In addition, RSV alleviated mitochondrial dysfunction and mitophagy in a mouse model of sepsis.36
ZFAS1 is a newly discovered lncRNA, which was increased in colorectal cancer and hepatocellular carcinoma.37,38 We discovered that ZFAS1 was increased in PTX-resistant NSCLC cells. Literature has revealed that ZFAS1 contributed to the progression of NSCLC. For instance, lncRNA ZFAS1 promoted NSCLC proliferation via regulating miR-590-3p.39 In addition, we also found that overexpressed of ZFAS1 enhanced PTX-treated NSCLC cell proliferation and mitophagy, which was inhibited by RSV. LncRNAs can competitively bind to miRNAs and act as a competitive endogenous RNAs (ceRNAs).40 Next, we looked for the downstream molecular mechanism of ZFAS1 regulation in NSCLC and discovered that miR-150-5p could directly target ZFAS1. Previous reports suggested that ZFAS1 silencing inhibited NSCLC progression by targeting miR-150-5p.41 Moreover, ZFAS1 induced lung fibroblast-to-myofibroblast transition and ferroptosis via sponging miR-150-5p.42
It is well recognized that miR-150-5p was essential for various cancers by regulating lncRNAs. As reported, lncRNA MIAT promoted ovarian cancer via targeting miR-150-5p.43 MiR-150-5p was upregulated in NSCLC tissues and cell lines, and induced proliferation and migration.44 Here, miR-150-5p targeted PINK1 and positively regulated its expression. Of note, depletion of miR-150-5p overturned the effects of ZFAS1 upregulation on RSV-mediated PTX resistance in NSCLC cells. These finding demonstrated that miR-150-5p inhibition could alleviate NSCLC cell mitophagy.
In summary, we showed that RSV reduced NSCLC mitophagy and PTX resistance via modulating ZFAS1 expression and suppressing miR-150-5p/PINK-1/Parkin signaling pathway (Fig. 7). Therefore, combination therapy of RSV and PTX can be exploited as a promising approach in NSCLC treatment. However, clinical data and animal studies are needed to support our findings in further research.
Fig. 7.

The molecular mechanism by which RSV regulates PTX resistance in NSCLC cells. LncRNA ZFAS1 acts as a downstream effector of RSV-mediated suppressive effect on mitophagy in PTX-resistant NSCLC cells via the miR-150-5p/PINK1 axis.
Availability of data and material
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest: The authors declare that they have no conflict of interest.
Authors' contributions
Shengjun Ma and Hui Tian are guarantor of integrity of the entire study. Fanhua Kong conceptualized the study concepts. Fanhua Kong and Chuan Xie were involved in study design. Shengjun Ma and Hui Tian were involved in defining the definition of intellectual content. Xudong Zhao and Xiang Zong did literature research. Fanhua Kong and Lingguo Bu were involved in experimental studies. Chuan Xie and Bo Zhang took the responsibility of data acquisition. Fanhua Kong was involved in data analysis. Xudong Zhao did statistical analysis. Fanhua Kong led the drafting of the manuscript. Shengjun Ma and Hui Tian took the responsibility of manuscript editing for its final content.
Abbreviations
MTT: 3-(4,5-dimethyithiazol-2-yl)-2,5-diphenyl-tetrazolium bromide; EdU: 5-Ethynyl-2′-deoxyuridine; DAPI: 40,6-diamidino-2-phenylindole; BCA: bicinchoninic acid; CS: Cell Signaling Technology; DDP: cisplatin; ceRNAs: competitive endogenous RNAs; cDNA: complementary DNA; DMSO: dimethyl sulfoxide; ECL: enhanced chemiluminescence; FBS: fetal bovine serum; FITC: fluorescein isothiocyanate; GAPDH: glyceraldehyde-3-phosphate dehydrogenase; MIAT: lncRNA myocardial infarction-associated transcript; ZFAS1: lncRNA zinc finger antisense 1; lncRNAs: long noncoding RNAs; NSCLC: non-small cell lung cancer; PTX: paclitaxel; PBS: phosphate buffered saline; PINK1: PTEN-induced putative kinase 1; RIPA: radioimmunoprecipitation; RSV: resveratrol; SDS-PAGE: sodium dodecyl sulphate-polyacrylamide gel electrophoresis ; TEM: transmission electron microscopy; VCR: vincristine
Contributor Information
Fanhua Kong, Departments of Thoracic Surgery, Liao Cheng People’s Hospital, Liaocheng 252000, P. R. China; Departments of Thoracic Surgery, Qi Lu Hospital Affiliated to Shandong University, Jinan 250021, P. R. China; Departments of Thoracic Surgery, The Affiliated Taian City Centeral Hospital of Qingdao University, Taian 271000, P. R. China.
Chuan Xie, Departments of Thoracic Surgery, The Affiliated Taian City Centeral Hospital of Qingdao University, Taian 271000, P. R. China.
Xudong Zhao, Departments of Thoracic Surgery, The Affiliated Taian City Centeral Hospital of Qingdao University, Taian 271000, P. R. China.
Xiang Zong, The Affiliated Taian City Centeral Hospital of Qingdao University, Taian 271000, P. R. China.
Lingguo Bu, Departments of Thoracic Surgery, The Affiliated Taian City Centeral Hospital of Qingdao University, Taian 271000, P. R. China.
Bo Zhang, Departments of Thoracic Surgery, The Affiliated Taian City Centeral Hospital of Qingdao University, Taian 271000, P. R. China.
Hui Tian, Departments of Thoracic Surgery, Qi Lu Hospital Affiliated to Shandong University, Jinan 250021, P. R. China.
Shengjun Ma, Departments of Cardiac Surgery, Liao Cheng People’s Hospital, Liaocheng 252000, P. R. China.
References
- 1. Valentino F, Borra G, Allione P, Rossi L. Emerging targets in advanced non-small-cell lung cancer. Future Oncol. 2018:14(13s):61–72. [DOI] [PubMed] [Google Scholar]
- 2. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018:553(7689):446–454. [DOI] [PubMed] [Google Scholar]
- 3. Zhu L, Chen L. Progress in research on paclitaxel and tumor immunotherapy. Cell Mol Biol Lett. 2019:24:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cui H, Arnst K, Miller DD, Li W. Recent Advances in Elucidating Paclitaxel Resistance Mechanisms in Non-small Cell Lung Cancer and Strategies to Overcome Drug Resistance. Curr Med Chem. 2020:27(39):6573–6595. [DOI] [PubMed] [Google Scholar]
- 5. Vervandier-Fasseur D, Latruffe N. The Potential Use of Resveratrol for Cancer Prevention. Molecules. 2019:24(24):4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules. 2021:26(1):229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang X, Parvathaneni V, Shukla SK, Kulkarni NS, Muth A, Kunda NK, Gupta V. Inhalable resveratrol-cyclodextrin complex loaded biodegradable nanoparticles for enhanced efficacy against non-small cell lung cancer. Int J Biol Macromol. 2020:164:638–650. [DOI] [PubMed] [Google Scholar]
- 8. Killackey SA, Philpott DJ, Girardin SE. Mitophagy pathways in health and disease. J Cell Biol. 2020:219(11):e202004029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Onishi M, Yamano K, Sato M, Matsuda N, Okamoto K. Molecular mechanisms and physiological functions of mitophagy. EMBO J. 2021:40(3):e104705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dai K, Radin DP, Leonardi D. PINK1 depletion sensitizes non-small cell lung cancer to glycolytic inhibitor 3-bromopyruvate: Involvement of ROS and mitophagy. Pharmacol Rep. 2019:71(6):1184–1189. [DOI] [PubMed] [Google Scholar]
- 11. Liu M, Fan Y, Li D, Han B, Meng Y, Chen F, Liu T, Song Z, Han Y, Huang Let al. Ferroptosis inducer erastin sensitizes NSCLC cells to celastrol through activation of the ROS-mitochondrial fission-mitophagy axis. Mol Oncol. 2021:15(8):2084–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin Q, Li S, Jiang N, Shao X, Zhang M, Jin H, Zhang Z, Shen J, Zhou Y, Zhou Wet al. PINK1-parkin pathway of mitophagy protects against contrast-induced acute kidney injury via decreasing mitochondrial ROS and NLRP3 inflammasome activation. Redox Biol. 2019:26:101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chang G, Zhang W, Ma Y, Wen Q. PINK1 Expression Is Associated with Poor Prognosis in Lung Adenocarcinoma. Tohoku J Exp Med. 2018:245(2):115–121. [DOI] [PubMed] [Google Scholar]
- 14. Lu X, Liu QX, Zhang J, Zhou D, Yang GX, Li MY, Qiu Y, Chen Q, Zheng H, Dai JG. PINK1 Overexpression Promotes Cell Migration and Proliferation via Regulation of Autophagy and Predicts a Poor Prognosis in Lung Cancer Cases. Cancer Manag Res. 2020:12:7703–7714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Napoli S. LncRNAs and Available Databases. Methods Mol Biol. 2021:2348:3–26. [DOI] [PubMed] [Google Scholar]
- 16. Zhang L, Jin C, Yang G, Wang B, Hua P, Zhang Y. LncRNA WTAPP1 promotes cancer cell invasion and migration in NSCLC by downregulating lncRNA HAND2-AS1. BMC Pulm Med. 2020:20(1):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yu X, Lin Q, Liu F, Yang F, Mao J, Chen X. LncRNA TMPO-AS1 facilitates the proliferation and metastasis of NSCLC cells by up-regulating ERBB2 via sponging miR-204-3p. Int J Immunopathol Pharmacol. 2020:34:2058738420958947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ghafouri-Fard S, Kamali MJ, Abak A, Shoorei H, Taheri M. LncRNA ZFAS1: Role in tumorigenesis and other diseases. Biomed Pharmacother. 2021:142:111999. [DOI] [PubMed] [Google Scholar]
- 19. Tian FM, Meng FQ, Wang XB. Overexpression of long-noncoding RNA ZFAS1 decreases survival in human NSCLC patients. Eur Rev Med Pharmacol Sci. 2016:20(24):5126–5131. [PubMed] [Google Scholar]
- 20. Chen Y, Xu H, Liu C, Gu M, Zhan M, Chen Q, Wang Z. LncRNA DIO3OS regulated by TGF-β1 and resveratrol enhances epithelial mesenchymal transition of benign prostatic hyperplasia epithelial cells and proliferation of prostate stromal cells. Transl Androl Urol. 2021:10(2):643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sun H, Zhou X, Bao Y, Xiong G, Cui Y, Zhou H. Involvement of miR-4262 in paclitaxel resistance through the regulation of PTEN in non-small cell lung cancer. Open Biol. 2019:9(7):180227. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Dundr P, Matěj R, Němejcová K, Bártů M, Stružinská I. Predictive testing in non-small cell lung carcinoma. Klin Onkol. 2021:34(Supplementum 1):29–34. [DOI] [PubMed] [Google Scholar]
- 23. Akhurst T. Staging of Non-Small-Cell Lung Cancer. PET Clin. 2018:13(1):1–10. [DOI] [PubMed] [Google Scholar]
- 24. Liu WJ, du Y, Wen R, Yang M, Xu J. Drug resistance to targeted therapeutic strategies in non-small cell lung cancer. Pharmacol Ther. 2020:206:107438. [DOI] [PubMed] [Google Scholar]
- 25. Gao P, Ren G. Identification of potential target genes of non-small cell lung cancer in response to resveratrol treatment by bioinformatics analysis. Aging (Albany NY). 2021:13(19):23245–23261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang J, Li J, Cao N, Li Z, Han J, Li L. Resveratrol, an activator of SIRT1, induces protective autophagy in non-small-cell lung cancer via inhibiting Akt/mTOR and activating p38-MAPK. Onco Targets Ther. 2018:11:7777–7786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meng J, Guo F, Xu H, Liang W, Wang C, Yang XD. Combination Therapy using Co-encapsulated Resveratrol and Paclitaxel in Liposomes for Drug Resistance Reversal in Breast Cancer Cells in vivo. Sci Rep. 2016:6(1):22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu G, Pei F, Yang F, Li L, Amin A, Liu S, Buchan J, Cho W. Role of Autophagy and Apoptosis in Non-Small-Cell Lung Cancer. Int J Mol Sci. 2017:18(2):367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Poole LP, Macleod KF. Mitophagy in tumorigenesis and metastasis. Cell Mol Life Sci. 2021:78(8):3817–3851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guan Y, Wang Y, Li B, Shen K, Li Q, Ni Y, Huang L. Mitophagy in carcinogenesis, drug resistance and anticancer therapeutics. Cancer Cell Int. 2021:21(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liu Z, Li B, Cao M, Jiang J. Norcantharidin triggers apoptotic cell death in non-small cell lung cancer via a mitophagy-mediated autophagy pathway. Ann Transl Med. 2021:9(12):971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barazzuol L, Giamogante F, Brini M, Calì T. PINK1/Parkin Mediated Mitophagy, Ca(2+) Signalling, and ER-Mitochondria Contacts in Parkinson's Disease. Int J Mol Sci. 2020:21(5):1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang R, Gu J, Chen J, Ni J, Hung J, Wang Z, Zhang X, Feng J, Ji L. High expression of PINK1 promotes proliferation and chemoresistance of NSCLC. Oncol Rep. 2017:37(4):2137–2146. [DOI] [PubMed] [Google Scholar]
- 34. Zhang H, Yin C, Liu X, Bai X, Wang L, Xu H, Ju J, Zhang L. Prohibitin 2/PHB2 in Parkin-Mediated Mitophagy: A Potential Therapeutic Target for Non-Small Cell Lung Carcinoma. Med Sci Monit. 2020:26:e923227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li C, Tan Y, Wu J, Ma Q, Bai S, Xia Z, Wan X, Liang J. Resveratrol Improves Bnip3-Related Mitophagy and Attenuates High-Fat-Induced Endothelial Dysfunction. Front Cell Dev Biol. 2020:8:796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang C, Yuan J, Du J. Resveratrol alleviates acute lung injury through regulating PLSCR-3-mediated mitochondrial dysfunction and mitophagy in a cecal ligation and puncture model. Eur J Pharmacol. 2021:913:174643. [DOI] [PubMed] [Google Scholar]
- 37. Thorenoor N, Faltejskova-Vychytilova P, Hombach S, Mlcochova J, Kretz M, Svoboda M, Slaby O. Long non-coding RNA ZFAS1 interacts with CDK1 and is involved in p53-dependent cell cycle control and apoptosis in colorectal cancer. Oncotarget. 2016:7(1):622–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li T, Xie J, Shen C, Cheng D, Shi Y, Wu Z, Deng X, Chen H, Shen B, Peng Cet al. Amplification of Long Noncoding RNA ZFAS1 Promotes Metastasis in Hepatocellular Carcinoma. Cancer Res. 2015:75(15):3181–3191. [DOI] [PubMed] [Google Scholar]
- 39. Zhou Y, Si L, Liu Z, Shi Y, Agula B. Long Noncoding RNA ZFAS1 Promotes Progression of NSCLC via Regulating of miR-590-3p. Cell Transplant. 2020:29:963689720919435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wu X, Sui Z, Zhang H, Wang Y, Yu Z. Integrated Analysis of lncRNA-Mediated ceRNA Network in Lung Adenocarcinoma. Front Oncol. 2020:10:554759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeng Z, et al. Knockdown of lncRNA ZFAS1-suppressed non-small cell lung cancer progression via targeting the miR-150-5p/HMGA2 signaling. J Cell Biochem. 2019. [DOI] [PubMed] [Google Scholar]
- 42. Yang Y, Tai W, Lu N, Li T, Liu Y, Wu W, Li Z, Pu L, Zhao X, Zhang Tet al. lncRNA ZFAS1 promotes lung fibroblast-to-myofibroblast transition and ferroptosis via functioning as a ceRNA through miR-150-5p/SLC38A1 axis. Aging (Albany NY). 2020:12(10):9085–9102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhou S, Xu A, Song T, Gao F, Sun H, Kong X. lncRNA MIAT Regulates Cell Growth, Migration, and Invasion Through Sponging miR-150-5p in Ovarian Cancer. Cancer Biother Radiopharm. 2020:35(9):650–660. [DOI] [PubMed] [Google Scholar]
- 44. Wu Z, Li W, Li J, Zhang Y, Zhang X, Xu Y, Hu Y, Li Q, Sun Q, Ma Z. Higher expression of miR-150-5p promotes tumorigenesis by suppressing LKB1 in non-small cell lung cancer. Pathol Res Pract. 2020:216(11):153145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Conflicts of interest: The authors declare that they have no conflict of interest.