Abstract
Haemophilus ducreyi expresses a peptidoglycan-associated lipoprotein (PAL) that exhibits extensive homology to Haemophilus influenzae protein 6. We constructed an isogenic PAL mutant (35000HP-SMS4) by the use of a suicide vector that contains lacZ as a counterselectable marker. H. ducreyi 35000HP-SMS4 and its parent, 35000HP, had similar growth rates in broth and similar lipooligosaccharide profiles. 35000HP-SMS4 formed smaller, more transparent colonies than 35000HP and, unlike its parent, was hypersensitive to antibiotics. Complementation of the mutant in trans restored the parental phenotypes. To test whether expression of PAL is required for virulence, nine human volunteers were experimentally infected. Each subject was inoculated with two doses (41 to 89 CFU) of live 35000HP and one dose of heat-killed bacteria on one arm and with three doses (ranging from 28 to 800 CFU) of live 35000HP-SMS4 on the other arm. Papules developed at similar rates at sites inoculated with the mutant or parent but were significantly smaller at mutant-inoculated sites than at parent-inoculated sites. The pustule formation rate was 72% (95% confidence interval [CI], 46.5 to 90.3%) at 18 parent sites and 11% (95% CI, 2.4 to 29.2%) at 27 mutant sites (P < 0.0001). The rates of recovery of H. ducreyi from surface cultures were 8% (n = 130; 95% CI, 4.3 to 14.6%) for parent-inoculated sites and 0% (n = 120; 95% CI, 0.0 to 2.5%) for mutant-inoculated sites (P < 0.001). H. ducreyi was recovered from six of seven biopsied parent-inoculated sites and from one of three biopsied mutant-inoculated sites. Confocal microscopy confirmed that the bacteria present in a mutant inoculation site pustule lacked a PAL-specific epitope. Although biosafety regulations precluded our testing the complemented mutant in humans, these results suggest that expression of PAL facilitates the ability of H. ducreyi to progress to the pustular stage of disease.
Haemophilus ducreyi is the etiologic agent of chancroid, a genital ulcer disease (GUD) that is still common in many developing countries (7, 8, 17, 34, 43, 53). Although now rare in the United States (16), chancroid persists in some urban areas and is frequently not recognized (31). Like other agents of GUD, H. ducreyi and the human immunodeficiency virus (HIV) facilitate the transmission of each other in a process coined “epidemiologic synergy” (21, 56). The impact of chancroid on heterosexually acquired HIV infection has led to renewed interest in H. ducreyi pathogenesis (33, 55).
Bacterial cell wall lipoproteins are proinflammatory and are important in the pathogenesis of several gram-negative infections. Escherichia coli expresses 10 to 20 outer membrane lipoproteins, and murein lipoprotein is one of the most abundant of its outer membrane proteins (OMPs) (59). E. coli lipoprotein is an extremely potent mitogen of B lymphocytes, induces tumor necrosis factor alpha and interleukin-6 production by macrophages, and induces lethal shock in lipopolysaccharide-nonresponsive mice (22, 62–64). Treponema pallidum lipoproteins, or synthetic lipopeptides corresponding to the N-terminal region of the lipoproteins, are potent activators of monocytes and macrophages and induce expression of intercellular adhesion molecule 1 in human umbilical vein endothelial cells (29, 42, 45). T. pallidum lipoproteins and their surrogates also induce HIV gene expression in and CCR5 expression on human monocytes, providing a possible mechanism for enhancement of the sexual transmission of macrophage-tropic HIV by this agent of GUD (45, 51). The proinflammatory effects of lipoproteins, including the ability to initiate innate and adaptive immune responses, appear to be mediated through activation of toll-like receptors on macrophages (14, 30, 32).
Peptidoglycan-associated lipoproteins (PALs), along with other OMPs such as Lpp, OmpA, and porins, help link the outer membrane to peptidoglycan through covalent and noncovalent forces (10, 18, 28, 29, 41). In E. coli, PAL forms a complex with TolB near the outer membrane and may transiently interact with the cytoplasmic TolQ-TolR-TolA complex to bring the cytoplasmic and outer membranes together to facilitate transport of high-molecular-weight molecules across the cell wall (18, 29). Mutations in any of the tol-pal genes disrupt outer membrane integrity and have pleiotropic effects, including hypersensitivity to antibiotics and increased formation of outer membrane vesicles (10, 41). Interestingly, extragenic PAL suppressor mutations located in tolB correct mutant PAL function without restoring the association between PAL and peptidoglycan (41). Although the role of PAL in pathogenesis has not been studied, a Salmonella typhimurium tolB mutant is unable to survive within macrophages, resist the bactericidal activity of nonimmune serum, or cause fatal infection in mice (11).
We recently characterized several H. ducreyi lipoproteins (25, 46, 47), one of which is the 18-kDa PAL. H. ducreyi PAL contains a conserved, surface-exposed epitope defined by monoclonal antibody (MAb) 3B9 (46). MAb 3B9 cross-reacts with many proteins of similar molecular mass found in members of the family Pasteurellaceae and binds to the 16.6-kDa peptidoglycan-associated lipoprotein (P6) of Haemophilus influenzae (46). P6, a target of serum bactericidal antibodies, is being intensively studied as a candidate for a vaccine to prevent nontypeable H. influenzae infections, particularly otitis media (9, 24, 36). We made a recombinant nonlipidated form of H. ducreyi PAL and generated anti-PAL sera in rabbits (47). Low percentages of survival were obtained by incubation of H. ducreyi in 50% anti-PAL serum and active complement, but no killing occurred in higher dilutions of this serum (24).
We have shown that PAL is expressed by H. ducreyi during experimental infection of human volunteers (6). To test the hypothesis that expression of PAL is required for virulence, we constructed an isogenic mutant in H. ducreyi by insertion of a nonmobilizable chloramphenicol resistance cassette into pal. Here we describe the characteristics of the PAL mutant and the results of a trial in which human volunteers were experimentally infected with the PAL mutant and its isogenic parent.
MATERIALS AND METHODS
Bacteria.
H. ducreyi 35000HP (ATCC 700724) is a human-passaged variant of strain 35000 which was isolated from a volunteer's lesion 13 days after inoculation with H. ducreyi 35000 (3, 5, 48).
Antibodies.
MAb 3B9 was described previously (47). The preparation of polyclonal rabbit antisera to H. ducreyi whole cells and recombinant PAL is described elsewhere (24).
DNA sequence analysis.
The H. ducreyi pal gene, whose sequence was previously published, was identified in the completed but unannotated genome sequence by using the Blast algorithm. Sequences 5′ and 3′ of the pal gene were characterized with the GCG suite of programs (Genetics Computer Group, Madison, Wis.) and the Lasergene package (DNASTAR, Madison, Wis.).
Construction of an isogenic pal mutant in the H. ducreyi 35000HP background.
The plasmid pHD18pal:mTn3(Cm) contains a 4.3-kb insert, which includes a 1.5-kb chloramphenicol acetyltransferase gene (cat) cassette located in the pal open reading frame (ORF) (44, 46, 47). The plasmid pRSM1791 utilizes lacZ as a counterselectable marker to facilitate allele exchange (12). A 4.3-kb NotI fragment from pHD18pal:mTn3(Cm) was ligated into pRSM1791. The resulting plasmid, pRSM1791pal:mTn3(Cm), was electroporated into strain 35000HP. Selection was performed on plates containing chloramphenicol. Colonies were then picked and grown on plates containing X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) and chloramphenicol. Cointegrates appeared as small, blue colonies because the growth of H. ducreyi containing lacZ is suppressed in the presence of X-Gal (12). LacZ-deficient colonies in which a second crossover event had occurred appeared larger and white and were screened for loss of reactivity to MAb 3B9 by Western blot analysis. An isogenic pal mutant was recovered and designated 35000HP-SMS4.
Bactericidal assays.
Bactericidal assays were done exactly as described previously (24). After informed consent was obtained, normal human serum (NHS) was obtained from a volunteer with no history of chancroid. Bacteria were grown to early log phase, and appropriate dilutions were made in Hanks' balanced salt solution containing 0.1% gelatin (HBSSG). Approximately 103 CFU of H. ducreyi were incubated in NHS (active or heat inactivated) diluted in HBSSG. Colony counts were done in triplicate after 3 h of incubation. Inoculum size was determined by plating a well containing HBSSG and the H. ducreyi inoculum at 0 h. Percent survival values, calculated as the geometric-mean CFU in a serum-containing well divided by the geometric-mean CFU in the inoculum well multiplied by 100, represent the means ± standard deviations (SD) of data from three independent experiments.
Complementation of H. ducreyi 35000HP-SMS4.
Sequences including the pal promoter region, ORF, and transcriptional terminator were amplified using the 5′ primer CCGTTTCAAAGCTAACTTGCCAG and the 3′ primer TTGGGCTTATCTAACCGCTTAGC, which correspond to bp 18 through 40 and bp 648 through 670 of the published pal sequence (47). A NotI linker was included in the 5′ primer. The resulting 660-bp amplicon was ligated into the pCR2.1-TOPO cloning vector (Invitrogen, Carlsbad, Calif.) and electroporated into Invitrogen One Shot TOP10F′ E. coli. Transformants were selected on Luria-Bertani plates supplemented with kanamycin (50 μg/ml) and screened for reactivity to MAb 3B9. Insertion of the 660-bp fragment was confirmed by restriction mapping. The insert was released from the vector by digestion with NotI, and a ∼690-bp fragment was isolated, ligated into the plasmid pLSKS (kindly supplied by Patricia Totten) (58), and electroporated into E. coli DH5α. Transformants were selected on chocolate agar supplemented with streptomycin (100 μg/ml). The transformants were screened for reactivity to MAb 3B9, and the plasmid bearing the ∼690-bp fragment was confirmed by restriction mapping and designated pLSKSpal. H. ducreyi 35000HP-SMS4 was electroporated with plasmid pLSKS or pLSKSpal, and transformants, selected on chocolate agar plates containing streptomycin (50 μg/ml), were designated 35000HP-SMS4(pLSKSpal) and 35000HP-SMS4(pLSKS).
Antibiotic susceptibilities.
35000HP, 35000HP-SMS4, 35000HP-SMS4 (pLSKSpal), and 35000HP-SMS4(pLSKS) were grown overnight on chocolate agar plates supplemented with streptomycin when appropriate. Bacteria were scraped from the plates and suspended to an optical density at 600 nm of 0.2. Clumps were allowed to settle for 15 min, and a dilution containing approximately 105 CFU was applied to 150- by 15-mm clear agar plates (54). Erythromycin-, ciprofloxacin-, and cefotaxime-impregnated disks (Remel, Lenexa, Kans.) were applied to the lawns, and the diameters of zones of inhibition were measured in centimeters 48 h later. Zones of inhibition were compared by two-way analysis of variance (ANOVA). The ANOVA model included strain, antibiotic, and their interaction. Pairwise comparisons among the strains were performed by Tukey's multiple comparison procedure.
Southern blotting and outer membrane and LOS analysis.
Genomic DNAs from strains 35000HP and 35000HP-SMS4 were digested with XbaI and electrophoresed on a 0.8% agarose gel. DNA was transferred to a nylon membrane by a standard capillary method as described previously (61). Southern blots were probed with either the pal coding sequence or the cat cassette. Lipooligosaccharides (LOS) and outer membranes were prepared from strains 35000HP, 35000HP-SMS4, 35000HP-SMS4(pLSKSpal), and 35000HP-SMS4(pLSKS) and subjected to sodium dodecyl sulfate-polyacrylamide electrophoresis (SDS-PAGE) analysis as described previously (15, 26).
Human challenge protocol.
Healthy adult male and female volunteers over 18 years of age were recruited for the study. Informed consent was obtained from the subjects for participation in the study and for performance of HIV serological analyses, in accordance with the human experimentation guidelines of the U.S. Department of Health and Human Services and the Institutional Review Board of Indiana University-Purdue University Indianapolis. Enrollment procedures, exclusion criteria, preparation of the bacteria, method of inoculation, determination of the estimated delivered dose (EDD), clinical observations, and surface cultures were done as described in detail elsewhere (2, 5, 48, 49, 52). The area of papular erythema was calculated by multiplying the vertical and horizontal diameters, which were recorded by a physician who was blinded to the identity of the inoculum used at each site. Papule areas were compared by random-effects ANOVA models to account for any within-subject correlation. Pairwise comparisons were adjusted by using the Tukey-Kramer procedure.
A modification of an escalating dose-response study was used to compare the virulence of strains 35000HP and 35000HP-SMS4 exactly as described previously (2). The rationale for the design is described in detail elsewhere (3, 38). Subjects were observed until they reached a clinical endpoint, defined as either 14 days after inoculation, development of a painful pustule, or resolution of infection at all sites. When a clinical endpoint was achieved, the code was broken and up to two sites with active disease (one inoculated with the parent and one inoculated with the mutant), if present, were biopsied with punch forceps. The subjects were then treated with oral ciprofloxacin as described previously.
Biopsies.
Each biopsy specimen was cut into portions. One portion was cultured semiquantitatively as described previously (48, 49). One portion was fixed in formalin, and immunohistological studies were done as previously described (37, 48, 49). The slides were coded and read by a dermatopathologist who was unaware of the code. One portion was fixed in 4% paraformaldehyde and cryosectioned for confocal microscopy as described previously (1, 6).
Phenotypes of recovered bacteria.
Individual colonies from the inocula, surface cultures, and biopsy specimen cultures were picked, suspended in freezing medium, and frozen in 96-well plates. The colonies were scored for susceptibility to chloramphenicol on chloramphenicol-containing chocolate agar plates. If available, sufficient colonies (n ≥ 30) from an individual specimen were scored so that there was a 95% probability that ≤11% of the colonies would have the incorrect phenotype (2).
Confocal microscopy.
Biopsy specimens were obtained from a parent-inoculated and a mutant-inoculated site on volunteer no. 141. Both biopsy specimens were culture positive for H. ducreyi and no other bacterial species. Samples were processed and stained as described previously (1, 6), except that during primary staining, sections were stained with MAb 3B9 (13) followed by a mixture of 3B9 and rabbit polyclonal anti-H. ducreyi serum (24). Fluorescein isothiocyanate (FITC)-labeled goat anti-mouse immunoglobulin G (IgG) and indodicarbocyanine (Cy5)-labeled goat anti-rabbit IgG (Jackson ImmunoResearch Labs, West Grove, Pa.) were the secondary antibodies used. Samples were examined on a Bio-Rad MRC 1024 confocal laser-scanning microscope. Images for FITC and Cy5 signals were collected separately, and the images were colorized and combined by using Metamorph software (Universal Imaging Corp., West Chester, Pa.) to demonstrate areas of colocalizing signals. Negative controls included omitting the primary antibodies and staining sections of uninfected upper-arm skin.
RESULTS
Genomic analysis of the PAL ORF.
The pal gene is located at the 3′ end of the tol gene cluster, as is P6, its homologue in the H. influenzae Rd genome. The gene order is tolB-pal. The TolB protein sequence is 63% identical to the sequence of the Rd protein (HI0382), while the Pal protein is 62% identical to the P6 (PAL) protein of H. influenzae (HI0381). A putative Rho-independent transcriptional terminator is located 3′ of the pal gene (47). In H. ducreyi, the gene encoding a DnaJ-like protein, designated djlA, is found downstream of the tol-pal gene cluster. Two hundred seventeen nucleotides separate the pal gene and the djlA gene. The H. influenzae homologue (HI0271) is 67% identical to the djlA gene product, but the gene encoding HI0271 is not located immediately 3′ of the H. influenzae pal gene. Taken together, the data suggest that the H. ducreyi djlA gene is probably transcribed independently of the pal gene. Further, the gene order in the pal region is not conserved between H. influenzae and H. ducreyi, although the gene products are highly conserved.
Construction of H. ducreyi 35000HP-SMS4.
Prior attempts to construct an isogenic PAL-deficient mutant by allele exchange utilizing pHD18pal:mTn3(Cm) yielded only single-crossover events (47). The mTn3(Cm) cassette was located 138 bp downstream of the transcriptional start of pal, and cat was in the same orientation as pal (reference 47 and data not shown). Thus, allele exchange with this construct was not very likely to have polar effects on genes downstream of pal. A 4.3-kb fragment from pHD18pal:mTn3(Cm) was moved into pRSM1791, which contains lacZ as a counterselectable marker and facilitates resolution of cointegrates (12). H. ducreyi 35000HP was electroporated with pRSM1791pal:mTn3(Cm), and 153 Cmr colonies were obtained (12, 47). The colonies were grown on plates containing chloramphenicol and X-Gal, and 26 white Cmr colonies were screened by Western blotting with MAb 3B9. Five 3B9-negative colonies were obtained, and one was designated strain 35000HP-SMS4.
Comparison of strains 35000HP-SMS4 and 35000HP.
For Southern blotting, genomic DNAs from strains 35000HP and 35000HP-SMS4 were digested with XbaI and probed with the pal ORF and cat. The pal probe bound to a 4.2-kb DNA fragment of the parent genome and a 5.6-kb DNA fragment of the mutant genome. The cat probe did not bind to 35000HP DNA but it did bind to a 5.6-kb band of the 35000HP-SMS4 DNA (data not shown).
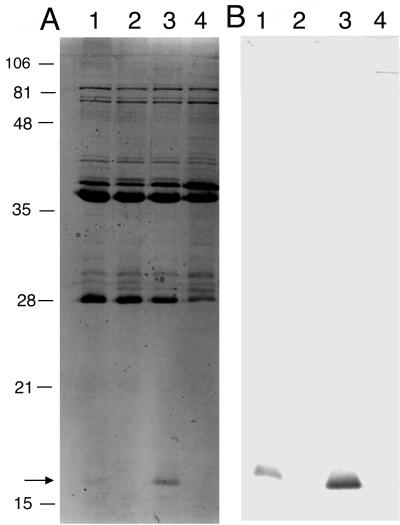
The colonial morphology of 35000HP-SMS4 differed from that of 35000HP in that the mutant colonies were smaller and transparent. However, colonies of 35000HP-SMS4 could be pushed intact across chocolate agar plates, which is a characteristic of H. ducreyi. 35000HP and 35000HP-SMS4 had similar growth rates in broth (data not shown). OMPs and LOS prepared from 35000HP-SMS4 and 35000HP were analyzed by SDS-PAGE. Both isolates had similar LOS (data not shown) and OMP profiles, except that 35000HP-SMS4 lacked a 18-kDa band corresponding to PAL (Fig. 1). In Western blot analysis, MAb 3B9 and the anti-PAL polyclonal serum bound to 35000HP but not to 35000HP-SMS4 (Fig. 1 and data not shown). As reported previously, 35000HP was not killed by NHS and grew during the 3-h incubation period (Table 1). There was no difference in the survival rates of 35000HP-SMS4 in untreated NHS or in heat-inactivated NHS, but the mutant did not survive as well as the parent in untreated NHS or in heat-inactivated NHS (Table 1).
FIG. 1.
(A) SDS–15% PAGE and Coomassie blue staining of OMPs prepared from strains 35000HP (lane 1), 35000HP-SMS4 (lane 2), 35000HP-SMS4(pLSKSpal) (lane 3), and 35000HP-SMS4(pLSKS) (lane 4). Note the location of the 18-kDa PAL (arrow). (B) Western blot of an identical gel whose proteins were transferred to a nylon membrane and probed with MAb 3B9. Positions of molecular mass standards are shown on the left (in kilodaltons).
TABLE 1.
Effect of active and inactive NHS
| % NHSa | % Survival of inoculum of strainb:
|
|
|---|---|---|
| 35000HP | 35000HP-SMS4 | |
| 50 (a) | 167 ± 83 | 66 ± 31 |
| 50 (i) | 113 ± 51 | 48 ± 34 |
| 25 (a) | 121 ± 89 | 83 ± 53 |
| 25 (i) | 136 ± 82 | 55 ± 20 |
| 12.5 (a) | 135 ± 83 | 61 ± 14 |
| 12.5 (i) | 115 ± 53 | 83 ± 49 |
Bacteria were incubated in active NHS (a) or in NHS that was heat inactivated (i).
Percent survival was calculated as the geometric-mean CFU in a test well divided by the geometric-mean CFU in the control well multiplied by 100. Values are means ± SD of data from three experiments.
Complementation of strain 35000HP-SMS4.
Sequences containing the pal promoter, ORF, and transcription terminator were amplified by PCR and ligated into the shuttle vector pLSKS to form the plasmid pLSKSpal. 35000HP-SMS4 was electroporated with pLSKS or pLSKSpal. When grown on chocolate agar plates containing streptomycin, 35000HP-SMS4(pLSKS) formed small, translucent colonies while 35000HP-SMS4(pLSKSpal) formed large, opaque colonies that resembled those of strain 35000HP (data not shown). In Western blot analysis, 35000HP-SMS4(pLSKSpal) expressed an 18-kDa band that bound MAb 3B9 and the anti-PAL polyclonal serum (Fig. 1 and data not shown). OMP profiles showed that 35000HP-SMS4(pLSKS) retained the mutant phenotype while 35000HP-SMS4(pLSKSpal) resembled 35000HP. Thus, complementation of the pal mutant in trans restored the major phenotypic differences between the mutant and the parent.
Antibiotic susceptibilities.
tol-pal mutants are usually hypersusceptible to antibiotics (29). To examine the effect of the pal mutation on antibiotic susceptibility, zones of inhibition around antibiotic-impregnated disks were measured. For erythromycin, ciprofloxacin, and cefotaxime, zones of inhibition were significantly smaller with 35000HP than with 35000HP-SMS4 (Table 2). On streptomycin-containing plates, 35000HP-SMS4(pLSKSpal) had significantly smaller zones of inhibition than 35000HP-SMS4(pLSKS) (Table 2). Pairwise comparisons adjusted for multiple tests showed that 35000HP and 35000HP-SMS4(pLSKSpal) were different from 35000HP-SMS4 and 35000HP-SMS4(pLSKS) (P = 0.0001). Thus, the pal mutation was responsible for the hypersusceptibility phenotype.
TABLE 2.
Antibiotic susceptibilitiesa
| Antibiotic | Zone of inhibition (cm) for strain:
|
|||
|---|---|---|---|---|
| 35000HP | 35000HP-SMS4 | 35000HP-SMS4 (pLSKSpal) | 35000HP-SMS4 (pLSKS) | |
| Erythromycin | 5.0 ± 0.1 | 5.7 ± 1.0 | 5.1 ± 0.2 | 5.5 ± 0.3 |
| Cefotaxime | 5.5 ± 0.0 | 7.2 ± 1.0 | 5.8 ± 0.4 | 7.4 ± 0.4 |
| Ciprofloxacin | 5.5 ± 0.1 | 6.6 ± 0.3 | 5.6 ± 0.2 | 6.3 ± 0.3 |
Diameters of zones of inhibition were measured. Values are means ± SD of data from three experiments.
Human inoculation experiments.
Twelve healthy adults (10 females and 2 males; all Caucasian; age range, 19 to 50 years; mean age ± SD, 30.2 ± 11.1 years) volunteered for the study. An escalating dose-response study was performed to compare the virulence of the mutant with that of the parent. In the first iteration, we inoculated three subjects at six sites on both arms. Our goal was to inoculate one arm at three sites with the mutant at EDDs of 25, 50, and 100 CFU. The other arm was to be inoculated at two sites with the parent at an EDD of 50 CFU and at a third site with the heat-killed mutant at the highest dose. The EDDs in the first iteration were 41 CFU for 35000HP and 28, 55, and 110 CFU for 35000HP-SMS4. One papule developed at each of three sites inoculated with 110 CFU of the heat-killed mutant, and they had resolved by day 5. Papules developed at six of six sites inoculated with the parent and at eight of nine sites inoculated with the mutant. The papules at six of the nine sites inoculated with the mutant resolved (Table 3). Pustules developed at five of six sites inoculated with the parent and at one of nine sites inoculated with the mutant. The single pustule that formed at a mutant-inoculated site was atypical in that it lacked surrounding erythema. This pustule reverted to a papule at day 12 and remained a papule at the clinical endpoint.
TABLE 3.
Responses to inoculation of live H. ducreyi strainsa
| Subject no. | Observation period (days) | Isolate | No. of initial papules | Final outcome of initial papule
|
||
|---|---|---|---|---|---|---|
| No. of papules | No. of pustules | No. resolved | ||||
| 134 | 14 | |||||
| 35000HP | 2 | 1 | 1 | |||
| 35000HP-SMS4 | 3 | 1 | 2 | |||
| 135 | 14 | |||||
| 35000HP | 2 | 1 | 1b | |||
| 35000HP-SMS4 | 2 | 1c | 1 | |||
| 136 | 6 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-SMS4 | 3 | 3 | ||||
| 139 | 9 | |||||
| 35000HP | 2 | 1 | 1 | |||
| 35000HP-SMS4 | 2 | 2 | ||||
| 140 | 12 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-SMS4 | 3 | 3 | ||||
| 141 | 7 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-SMS4 | 3 | 2 | 1 | |||
| 144 | 8 | |||||
| 35000HP | 2 | 2 | ||||
| 35000HP-SMS4 | 3 | 3 | ||||
| 145 | 14 | |||||
| 35000HP | 2 | 2b | ||||
| 35000HP-SMS4 | 3 | 3 | ||||
| 147 | 7 | |||||
| 35000HP | 2 | 1 | 1 | |||
| 35000HP-SMS4 | 3 | 3 | ||||
Each volunteer was inoculated at two sites with 35000HP and at three sites with 35000HP-SMS4. Volunteers 134 to 136 were inoculated in the first iteration, volunteers 139 to 141 were inoculated in the second iteration, and volunteers 144, 145, and 147 were inoculated in the third iteration.
Pustules formed at three parent-inoculated sites but resolved by day 12 or 14.
A pustule formed at one mutant-inoculated site but reverted to a papule by day 12.
Since the ability of the mutant to cause pustules seemed to be impaired, we infected three more subjects and attempted to increase the dose of the mutant. In the second iteration, three subjects were inoculated with 35000HP at an EDD of 68 CFU and with 35000HP-SMS4 at EDDs of 39, 78, and 155 CFU. Two papules developed at sites inoculated with 155 CFU of the heat-killed mutant, and they resolved in 5 to 7 days. Papules developed at six of six parent-inoculated sites and at eight of nine mutant-inoculated sites (Table 3). At the clinical endpoint, one parent-inoculated site and six mutant-inoculated sites resolved. Pustules developed at five of six parent-inoculated sites and at two of nine mutant-inoculated sites. The two mutant inoculation site pustules formed in one subject. Thus, the results of the first and second iterations were similar in that the ability of the mutant to form pustules seemed to be impaired.
We challenged three additional volunteers in the third iteration. Each subject was inoculated with 35000HP at an EDD of 89 CFU, with 35000HP-SMS4 at EDDs of 200, 400, and 800 CFU, and with the heat-killed mutant at an EDD of 800 CFU. Three papules developed at sites inoculated with the heat-killed control, and they resolved in 5 to 7 days. Papules developed at all parent- or mutant-inoculated sites. At the clinical endpoint, five parent-inoculated sites and all mutant-inoculated sites had resolved. Pustules developed at three of six parent-inoculated sites and at none of nine mutant-inoculated sites (Table 1). In volunteer no. 145, two of the parent pustules subsequently resolved by day 14.
The cumulative results for the three iterations showed that papules developed at 100% (95% confidence interval [CI], 84.7 to 100%) of sites inoculated with 35000HP and at 93% (95% CI, 75.7 to 99.1%) of sites inoculated with 35000HP-SMS4 (one-tailed Fisher's exact test, P = 0.36). However, we noted that the papules on one arm seemed smaller than those on the other arm. Although there was no significant difference in papule formation rates, the surface areas of the mutant-induced papules were significantly smaller than the parent-induced papules at 24 and 48 h (Table 4). The surface areas of the mutant-induced papules were similar to those for the heat-killed controls (Table 4). Pustules formed at 13 of 18 (72%; 95% CI, 46.5 to 90.3%) sites inoculated with 35000HP and at 3 of 27 (11%; 95% CI, 2.4 to 29.2%) sites inoculated with 35000HP-SMS4 (one-tailed Fisher's exact test, P < 0.0001). These data suggest that relative to the parent, the mutant was impaired during the initial stages of infection and in its ability to progress to pustules.
TABLE 4.
Papule surface area
| Day | Papule surface area (mm2) when inoculated witha:
|
||
|---|---|---|---|
| H | M | P | |
| 1 | 27.8 ± 40.4 | 36.6 ± 33.1b | 71.2 ± 60.7b |
| 2 | 26.0 ± 37.1 | 47.1 ± 64.3c | 116.5 ± 114.4c |
H, heat-killed control; M, 35000HP-SMS4; P, 35000HP. Values are means ± SD for data from 9 heat-killed, 27 mutant, and 18 parent sites.
P = 0.009 versus control.
P = 0.004 versus control.
Cellular infiltrate of mutant- and parent-induced lesions.
We examined the cellular infiltrates in six parent-inoculated and three mutant-inoculated sites that were present at the clinical endpoint. In biopsy specimens obtained from the parent-inoculated sites, micropustules with polymorphonuclear leukocytes (PMNs) were present in the epidermis. The dermis contained a perivascular infiltrate of mononuclear cells and some PMNs, and the venules were lined with reactive endothelial cells. Only one lesion produced by the mutant isolate was a pustule at the time of biopsy, and its histopathology was similar to that of the parent-induced lesions (data not shown). In both the parent and the mutant specimens, the majority of the mononuclear cells stained with a CD3 marker (data not shown).
In vivo detection of bacteria in lesions.
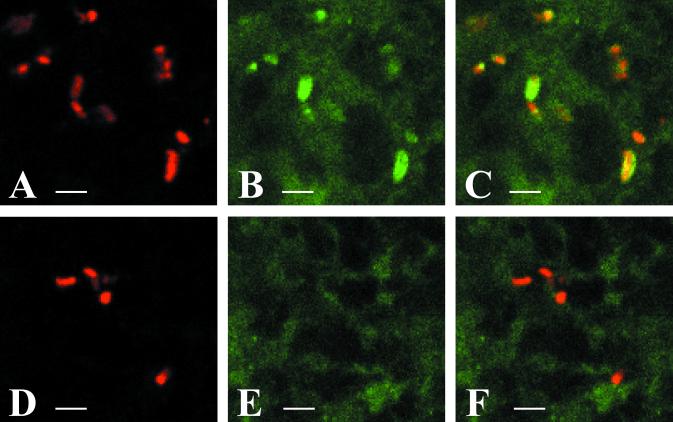
We have shown previously that H. ducreyi 35000HP expresses PAL in vivo (6). To confirm the phenotype of 35000HP-SMS4 in vivo, we stained sections of parent- or mutant-inoculated sites from one volunteer with the PAL-specific MAb 3B9. Numerous bacteria were detected in both samples by confocal microscopy when stained with a polyclonal H. ducreyi antiserum. However, only the bacteria in the parent-inoculated site reacted with MAb 3B9, confirming that the mutant bacteria did not express PAL (Fig. 2). Thus, the lesions at the mutant-inoculated sites were not caused by cross-contamination with the parent or by reversion of the mutation to wild type.
FIG. 2.
PAL expression in vivo. Tissues from parent-inoculated (A to C) or mutant-inoculated (D to F) sites from volunteer no. 141 were stained with rabbit polyclonal anti-H. ducreyi serum and MAb 3B9. (A and D) Bacteria stained with polyclonal antiserum, detected with Cy5-labeled goat anti-rabbit IgG (red); (B and E) bacteria stained with 3B9, detected with FITC-labeled goat anti-mouse IgG (green); (C and F) combined images of panels A and B and of panels D and E, respectively. Yellow-orange signals demonstrate colocalization of the two primary antibodies. Bars, 2.5 μm.
Recovery of bacteria from lesions.
No bacteria were recovered from sites inoculated with the heat-killed bacteria. H. ducreyi was recovered intermittently from the surface of the parent sites. The recovery rate of H. ducreyi from cultures obtained from parent-inoculated sites with active disease (n = 130) was 8% (95% CI, 4.3 to 14.6%), and that for mutant-inoculated sites (n = 120) was 0% (95% CI, 0.0 to 2.5%) (P < .001). All biopsy specimens were cultured semiquantitatively. Bacteria were recovered from six of seven parent inoculation site biopsy specimens and from one of three mutant inoculation site biopsy specimens. The yield ranged from 3.5 × 104 to 2.2 × 105 CFU per g of tissue for the parent inoculation site biopsy specimens, and it was 3.0 × 104 CFU per g of tissue for the mutant inoculation site biopsy specimen.
Confirmation of the phenotype of the recovered bacteria.
For the three parent and three mutant broth cultures used to prepare the inocula, all 144 parent colonies and 131 mutant colonies tested were phenotypically correct (mutant, Cmr; parent, Cms). All colonies obtained from surface cultures (n = 177) and biopsy specimens (n = 282) from parent sites were phenotypically correct (Cms). Colonies (n = 37) obtained from one mutant inoculation site biopsy specimen were phenotypically correct (Cmr, 3B9 negative). Thus, all tested colonies from the inocula, surface cultures, and biopsy specimens had the expected phenotype.
Complications.
Three volunteers were excluded from the analysis because they were inadvertently inoculated with a culture that was contaminated with Candida parapsilosis. The EDD of the contaminant was <1 CFU, and the EDD of H. ducreyi was 57 CFU. Within 24 h of inoculation, the volunteers developed a disease typical of experimental chancroid. When the low level of contamination was noted on subculture, the volunteers were treated with two doses of oral ciprofloxacin. Their lesions resolved, and the subjects were released from the study.
DISCUSSION
In this study, we constructed an isogenic PAL-deficient mutant. The colonial morphology and OMP profile of the PAL mutant were different from those of the parent, and, unlike the parent, the mutant was hypersusceptible to antibiotics. In human volunteers, the mutant formed papules at a rate similar to that of the parent, but the mutant papules were significantly smaller and did not progress to the pustular stage of disease as frequently. In a previous study, an isogenic HgbA receptor mutant formed papules similar to those of its parent but was unable to form pustules (reference 2 and unpublished observations). With the caveat that we are not permitted to test a complemented mutant in human subjects, this is the first demonstration that a putative virulence factor of H. ducreyi facilitates both papule and pustule formation in humans.
For sites inoculated with 1 to 120 CFU of the parent in this and previous trials, 73 of 166 papules spontaneously resolved while 93 progressed to pustules (reference 5 and unpublished observations). In this trial, three parent-induced pustules resolved and one mutant-induced pustule reverted to a papule before the subjects became symptomatic. We had never previously seen pustules resolve during the 14-day observation period (5). Since pustules usually contain microulcerations in the epidermis, we had hypothesized that pustule formation represented a commitment to progression to the ulcerative stage of disease (5). Although spontaneous resolution of pustules is a rare event, these data indicate that humans are capable of resolving both the papular and pustular stages of infection in the model. Similarly, in the preantibiotic era, untreated chancroidal ulcers typically resolved spontaneously after several months (50).
Although both 35000HP and 35000HP-SMS4 were able to initiate papule formation, the mutant-induced papules were significantly smaller than those caused by the parent strain. The pustule formation rates were also significantly different (72% at 18 parent-inoculated sites and 11% at 27 mutant-inoculated sites [P < 0.0001]). In the model, H. ducreyi is intermittently shed from papules and pustules (5). In this trial, H. ducreyi was recovered intermittently from surface cultures of parent-inoculated sites but not those of mutant-inoculated sites. H. ducreyi was recovered from six of seven biopsy specimens from parent-inoculated sites but from only one of three biopsy specimens from mutant-inoculated sites. tol-pal mutants have unstable outer membranes (29), and an S. typhimurium tolB mutant is unable to survive in macrophages and cause fatal infection in mice (11). Our data suggest that the PAL mutant was not fit enough to survive host defenses and therefore could not progress to the pustular stage of disease as well as the parent. Thus, an intact tol-pal system seems to be an important virulence determinant for several gram-negative organisms.
Although the mutant formed smaller colonies on plates than the parent, the growth rates of the mutant and parent in broth were similar. H. ducreyi clumps less when grown in broth than on plates, and we have only infected human volunteers with mid-log-phase bacteria. Whether either in vitro growth condition is relevant to growth in vivo is unknown. We cannot exclude the possibility that the mutant was impaired simply because it grew less well than the parent in vivo.
Experimental infection with H. ducreyi does not protect against subsequent challenge, and the bulk of the clinical data for natural infection suggests that chancroid is a nonimmunizing disease (4). The cutaneous immune response to experimental infection consists of two major components: an epidermal infiltrate of PMNs and a dermal infiltrate that consists primarily of CD45RO+ CD4+ αβ T cells, macrophages, and dendritic cells (37, 48). Whether some components of this immune response help contain the infection or contribute to pathology is unclear. Although we have not yet analyzed T cells for the presence of the cutaneous lymphocyte antigen (CLA), it is likely that the CD45RO+ CD4+ αβ T cells present in experimental lesions represent the homing of CLA+ memory/effector cells into the skin (9, 39, 40). Lipoproteins bind to toll-like receptors on macrophages and can stimulate innate and adaptive immune responses (14, 30, 32). When injected into the skin of human volunteers, lipoproteins and synthetic lipoprotein analogs derived from T. pallidum elicit an infiltrate that predominantly consists of neutrophils, CLA+ CD45RO+ lymphocytes, macrophages, and dendritic cells (Justin D. Radolf and Timothy J. Sellati, personal communication). It is possible that the PAL-deficient mutant was less efficient than the parent at recruiting inflammatory cells to the skin and caused milder pathology.
We did not test the complemented mutant in human subjects. Complementation of H. ducreyi mutants in trans has been achieved using derivatives of pLS88 such as pLSKS, which has a very broad host range and carries cassettes encoding resistance to several antibiotics (20, 57, 58). To avoid acquisition of such plasmids by normal flora, the use of a construct containing a virulence determinant in the pLS88 background is not permitted by the local or national biosafety committees that review our protocols. Thus, we cannot exclude the possibility that the decreased virulence of 35000HP-SMS4 was due to a secondary mutation or a polar effect of the cat cassette. However, complementation of the PAL-deficient mutant in trans did restore several mutant phenotypes, such as altered colonial morphology and OMP profile as well as antibiotic hypersusceptibility, to the parental phenotype. The chromosomal location of pal and its relationship to neighboring genes also makes it unlikely that the insertion of the cat cassette resulted in other phenotypic differences that were not apparent.
H. ducreyi is not known to have genes that are subject to phase or antigenic variation. Although we tested a mutant that occurred after limited in vitro passaging of its parent, we also cannot exclude the possibility that phase or antigenic variation of another bacterial surface component was responsible for the decreased virulence of the pal mutant.
In summary, these data show that expression of PAL is required for full expression of virulence. PAL is homologous to P6 of H. influenzae (47). P6 is a vaccine candidate because it is genetically and phenotypically conserved, antigenically stable, and surface exposed and it binds antibodies found in NHS that are bactericidal (27, 35, 36). The level of anti-P6 IgG in middle-ear fluid inversely correlates with the number of CFU of H. influenzae present in the fluid of infected children (60), and immunization of chinchillas with P6 reduced either the incidence (19) or the severity (23) of experimental H. influenzae otitis media. H. ducreyi PAL has many of the above-mentioned features of P6. Future studies will be directed at examining whether PAL is a suitable vaccine candidate for H. ducreyi infection.
ACKNOWLEDGMENTS
The first two authors contributed equally to this work.
This work was supported by Public Health Service grants AI27863, AI31494, and MO1RR00750. The H. ducreyi genome project was supported by NIH grant R01AI45091 (to R.S.M.). The clinical trial was supported by the Sexually Transmitted Diseases Clinical Trials Unit through NIAID contract N01-AI75329.
We thank Byron Batteiger for advice and assistance with the manuscript.
REFERENCES
- 1.Al-Tawfiq J A, Bauer M E, Fortney K R, Katz B P, Hood A F, Ketterer M, Apicella M A, Spinola S M. A pilus-deficient mutant of Haemophilus ducreyi is virulent in the human model of experimental infection. J Infect Dis. 2000;181:1176–1179. doi: 10.1086/315310. [DOI] [PubMed] [Google Scholar]
- 2.Al-Tawfiq J A, Fortney K R, Katz B P, Elkins C, Spinola S M. An isogenic hemoglobin receptor-deficient mutant of Haemophilus ducreyi is attenuated in the human model of experimental infection. J Infect Dis. 2000;181:1049–1054. doi: 10.1086/315309. [DOI] [PubMed] [Google Scholar]
- 3.Al-Tawfiq J A, Harezlak J, Katz B P, Spinola S M. Cumulative experience with Haemophilus ducreyi in the human model of experimental infection. Sex Transm Dis. 2000;27:111–114. doi: 10.1097/00007435-200002000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Al-Tawfiq J A, Palmer K L, Chen C-Y, Haley J C, Katz B P, Hood A F, Spinola S M. Experimental infection of human volunteers with Haemophilus ducreyi does not confer protection against subsequent challenge. J Infect Dis. 1999;179:1283–1287. doi: 10.1086/314732. [DOI] [PubMed] [Google Scholar]
- 5.Al-Tawfiq J A, Thornton A C, Katz B P, Fortney K R, Todd K D, Hood A F, Spinola S M. Standardization of the experimental model of Haemophilus ducreyi infection in human subjects. J Infect Dis. 1998;178:1684–1687. doi: 10.1086/314483. [DOI] [PubMed] [Google Scholar]
- 6.Bauer M E, Spinola S M. Localization of Haemophilus ducreyi at the pustular stage of disease in the human model of infection. Infect Immun. 2000;68:2309–2314. doi: 10.1128/iai.68.4.2309-2314.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Behets F M-T, Andriamiadana J, Randrianasolo D, Randriamanga R, Rasamilalao D, Chen C-Y, Weiss J B, Morse S A, Dallabetta G, Cohen M S. Chancroid, primary syphilis, genital herpes, and lymphogranuloma venereum in Antananarivo, Madagascar. J Infect Dis. 1999;180:1382–1385. doi: 10.1086/315005. [DOI] [PubMed] [Google Scholar]
- 8.Behets F M-T, Brathwaite A R, Hylton-Kong T, Chen C-Y, Hoffman I, Weiss J B, Morse S A, Dallabetta G, Cohen M S, Figueroa J P. Genital ulcers: etiology, clinical diagnosis, and associated human immunodeficiency virus infection in Kingston, Jamaica. Clin Infect Dis. 1999;28:1086–1090. doi: 10.1086/514751. [DOI] [PubMed] [Google Scholar]
- 9.Berg E L, Yoshino T, Rott L S, Robinson M K, Warnock R A, Kishimoto T K, Picker L J, Butcher E C. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernadac A, Gavioli M, Lazzaroni J-C, Raina S, Lloubès R. Escherichia coli tol-pal mutants form outer membrane vesicles. J Bacteriol. 1998;180:4872–4878. doi: 10.1128/jb.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowe F, Lipps C J, Tsolis R M, Groisman E, Heffron F, Kusters J G. At least four percent of the Salmonella typhimurium genome is required for fatal infection of mice. Infect Immun. 1998;66:3372–3377. doi: 10.1128/iai.66.7.3372-3377.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozue J A, Tarantino L, Munson R S., Jr Facile construction of mutations in Haemophilus ducreyi using lacZ as a counter-selectable marker. FEMS Microbiol Lett. 1998;164:269–273. doi: 10.1111/j.1574-6968.1998.tb13097.x. [DOI] [PubMed] [Google Scholar]
- 13.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brightbill H D, Libraty D H, Krutzik S R, Yang R-B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 15.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Summary of notifiable diseases, United States, 1998. Morbid Mortal Weekly Rep. 1999;47:78–79. [PubMed] [Google Scholar]
- 17.Chen C Y, Ballard R C, Beck-Sague C M, Dangor Y, Radebe F, Schmid S, Weiss J B, Tshabalala V, Fehler G, Htun Y, Morse S A. Human immunodeficiency virus infection and genital ulcer disease in South Africa. The herpetic connection. Sex Transm Dis. 2000;27:21–29. doi: 10.1097/00007435-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Clavel T, Germon P, Vianney A, Portalier R, Lazzaroni J C. TolB protein of Escherichia coli K-12 interacts with the outer membrane peptidoglycan-associated proteins Pal, Lpp and OmpA. Mol Microbiol. 1998;29:359–367. doi: 10.1046/j.1365-2958.1998.00945.x. [DOI] [PubMed] [Google Scholar]
- 19.DeMaria T F, Murwin D M, Leake E R. Immunization with outer membrane protein P6 from nontypeable Haemophilus influenzae induces bactericidal antibody and affords protection in the chinchilla model of otitis media. Infect Immun. 1996;64:5187–5192. doi: 10.1128/iai.64.12.5187-5192.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dixon L G, Albritton W L, Willson P J. An analysis of the complete nucleotide sequence of the Haemophilus ducreyi broad-host-range plasmid pLS88. Plasmid. 1994;32:228–232. doi: 10.1006/plas.1994.1060. [DOI] [PubMed] [Google Scholar]
- 21.Fleming D T, Wasserheit J N. From epidemiological synergy to public health policy and practice: the contribution of other sexually transmitted diseases to sexual transmission of HIV infection. Sex Transm Infect. 1999;75:3–17. doi: 10.1136/sti.75.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldman R C, White D, Leive L. Identification of outer membrane proteins, including known lymphocyte mitogens, as the endotoxin protein of Escherichia coli O111. J Immunol. 1981;127:1290–1294. [PubMed] [Google Scholar]
- 23.Green B A, Vazquez M E, Zlotnick G W, Quigley-Reape G, Swarts J D, Green I, Cowell J L, Bluestone C D, Doyle W J. Evaluation of mixtures of purified Haemophilus influenzae outer membrane proteins in protection against challenge with nontypeable H. influenzae in the chinchilla otitis media model. Infect Immun. 1993;61:1950–1957. doi: 10.1128/iai.61.5.1950-1957.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiltke T J, Bauer M E, Klesney-Tait J, Hansen E J, Munson R S, Jr, Spinola S M. Effect of normal and immune sera on Haemophilus ducreyi 35000HP and its isogenic MOMP and LOS mutants. Microb Pathog. 1999;26:93–102. doi: 10.1006/mpat.1998.0250. [DOI] [PubMed] [Google Scholar]
- 25.Hiltke T J, Campagnari A A, Spinola S M. Characterization of a novel lipoprotein expressed by Haemophilus ducreyi. Infect Immun. 1996;64:5047–5052. doi: 10.1128/iai.64.12.5047-5052.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hitchcock P J, Brown T M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983;154:269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karalus R J, Murphy T F. Purification and characterization of outer membrane protein P6, a vaccine antigen of non-typeable Haemophilus influenzae. FEMS Microbiol Lett. 1999;26:159–166. doi: 10.1111/j.1574-695X.1999.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 28.Lazzaroni J-C, Portalier R. The excC gene of Escherichia coli K-12 required for cell envelope integrity encodes the peptidoglycan-associated lipoprotein (PAL) Mol Microbiol. 1992;6:735–742. doi: 10.1111/j.1365-2958.1992.tb01523.x. [DOI] [PubMed] [Google Scholar]
- 29.Lazzaroni J C, Germon P, Ray M-C, Vianney A. The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability. FEMS Microbiol Lett. 1999;177:191–197. doi: 10.1111/j.1574-6968.1999.tb13731.x. [DOI] [PubMed] [Google Scholar]
- 30.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 31.Mertz K J, Trees D, Levine W C, Lewis J S, Litchfield B, Pettus K S, Morse S A, St. Louis M E, Weiss J B, Schwebke J, Dickes J, Kee R, Reynolds J, Hutcheson D, Green D, Dyer I, Richwald G A, Novotny J, Weisfuse I, Goldberg M, O'Donnell J A, Knaup R. Etiology of genital ulcers and prevalence of human immunodeficiency virus coinfection in 10 US cities. J Infect Dis. 1998;178:1795–1798. doi: 10.1086/314502. [DOI] [PubMed] [Google Scholar]
- 32.Modlin R L, Brightbill H D, Godowski P J. The toll of innate immunity on microbial pathogens. N Engl J Med. 1999;340:1834–1835. doi: 10.1056/NEJM199906103402312. [DOI] [PubMed] [Google Scholar]
- 33.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morse S A, Trees D L, Htun Y, Radebe F, Orle K A, Dangor Y, Beck-Sague C M, Schmid S, Fehler G, Weiss J B, Ballard R C. Comparison of clinical diagnosis and standard laboratory and molecular methods for the diagnosis of genital ulcer disease in Lesotho: association with human immunodeficiency virus infection. J Infect Dis. 1997;175:583–589. doi: 10.1093/infdis/175.3.583. [DOI] [PubMed] [Google Scholar]
- 35.Nelson M B, Munson R S, Jr, Apicella M A, Sikkema D J, Molleston J P, Murphy T F. Molecular conservation of the P6 outer membrane protein among strains of Haemophilus influenzae: analysis of antigenic determinants, gene sequences, and restriction fragment length polymorphisms. Infect Immun. 1991;59:2658–2663. doi: 10.1128/iai.59.8.2658-2663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nelson M B, Murphy T F, van Keulen H, Rekosh D, Apicella M A. Studies on P6, an important outer-membrane protein antigen of Haemophilus influenzae. Rev Infect Dis. 1988;10:S331–S336. doi: 10.1093/cid/10.supplement_2.s331. [DOI] [PubMed] [Google Scholar]
- 37.Palmer K L, Schnizlein-Bick C T, Orazi A, John K, Chen C-Y, Hood A F, Spinola S M. The immune response to Haemophilus ducreyi resembles a delayed-type hypersensitivity reaction throughout experimental infection of human subjects. J Infect Dis. 1998;178:1688–1697. doi: 10.1086/314489. [DOI] [PubMed] [Google Scholar]
- 38.Palmer K L, Thornton A C, Fortney K R, Hood A F, Munson R S, Jr, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 39.Picker L J, Treer J R, Ferguson-Darnell B, Collins P A, Bergstresser P R, Terstappen L W M M. Control of lymphocyte recirculation in man. J Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- 40.Pitzalis C, Kingsley G H, Covelli M, Meliconi R, Markey A, Panayi G S. Selective migration of the human helper-inducer memory T cell subset: confirmation by in vivo cellular kinetic studies. Eur J Immunol. 1991;21:369–376. doi: 10.1002/eji.1830210218. [DOI] [PubMed] [Google Scholar]
- 41.Ray M-C, Germon P, Vianney A, Portalier R, Lazzaroni J C. Identification by genetic suppression of Escherichia coli TolB residues important for TolB-Pal interaction. J Bacteriol. 2000;182:821–824. doi: 10.1128/jb.182.3.821-824.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riley B S, Oppenheimer-Marks N, Hansen E J, Radolf J D, Norgard M V. Virulent Treponema pallidum activates human vascular endothelial cells. J Infect Dis. 1992;165:484–493. doi: 10.1093/infdis/165.3.484. [DOI] [PubMed] [Google Scholar]
- 43.Risbud A, Chan-Tack K, Gadkari D, Gangakhedkar R R, Shepherd M E, Bollinger R, Mehendale S, Gaydos C, Divekar A, Rompalo A, Quinn T C. The etiology of genital ulcer disease by multiplex polymerase chain reaction and relationship to HIV infection among patients attending sexually transmitted disease clinics in Pune, India. Sex Transm Dis. 1999;26:55–62. doi: 10.1097/00007435-199901000-00009. [DOI] [PubMed] [Google Scholar]
- 44.Seifert H S, So M, Heffron F. Shuttle mutagenesis: a method of introducing transposons into transformable organisms. In: Setlow J K, Hollander A, editors. Genetic engineering: principles and methods. New York, N.Y: Plenum Press; 1986. pp. 123–134. [Google Scholar]
- 45.Sellati T J, Wilkinson D A, Sheffield J S, Koup R A, Radolf J D, Norgard M V. Virulent Treponema pallidum, lipoprotein, and synthetic lipopeptides induce CCR5 on human monocytes and enhance their susceptibility to infection by human immunodeficiency virus type 1. J Infect Dis. 2000;181:283–293. doi: 10.1086/315209. [DOI] [PubMed] [Google Scholar]
- 46.Spinola S M, Griffiths G E, Bogdan J, Menegus M A. Characterization of an 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi that contains a conserved surface-exposed epitope. Infect Immun. 1992;60:385–391. doi: 10.1128/iai.60.2.385-391.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spinola S M, Hiltke T J, Fortney K, Shanks K. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL. Infect Immun. 1996;64:1950–1955. doi: 10.1128/iai.64.6.1950-1955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo P, Chen C-Y, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 49.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan M. Chancroid. Am J Syphilis Gonorrhea Vener Dis. 1940;24:482–521. [Google Scholar]
- 51.Theus S A, Harrich D A, Gaynor R, Radolf J D, Norgard M V. Treponema pallidum, lipoproteins, and synthetic lipoprotein analogues induce human immunodeficiency virus type 1 gene expression in monocytes via NF-κB activation. J Infect Dis. 1998;177:941–950. doi: 10.1086/515240. [DOI] [PubMed] [Google Scholar]
- 52.Throm R E, Al-Tawfiq J A, Fortney K R, Katz B P, Hood A F, Slaughter C A, Hansen E J, Spinola S M. Evaluation of an isogenic major outer membrane protein-deficient mutant in the human model of Haemophilus ducreyi infection. Infect Immun. 2000;68:2602–2607. doi: 10.1128/iai.68.5.2602-2607.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Totten P A, Kuypers J M, Chen C-Y, Alfa M J, Parsons L M, Dutro S M, Morse S A, Kiviat N B. Etiology of genital ulcer disease in Dakar, Senegal, and comparison of PCR and serologic assays for detection of Haemophilus ducreyi. J Clin Microbiol. 2000;38:268–273. doi: 10.1128/jcm.38.1.268-273.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Totten P A, Stamm W E. Clear broth and plate media for culture of Haemophilus ducreyi. J Clin Microbiol. 1994;32:2019–2023. doi: 10.1128/jcm.32.8.2019-2023.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wasserheit J N. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 57.Willson P J, Albritton W L, Slaney L, Setlow J K. Characterization of a multiple antibiotic resistance plasmid from Haemophilus ducreyi. Antimicrob Agents Chemother. 1989;33:1627–1630. doi: 10.1128/aac.33.9.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wood G E, Dutro S M, Totten P A. Target cell range of Haemophilus ducreyi hemolysin and its involvement in invasion of human epithelial cells. Infect Immun. 1999;67:3740–3749. doi: 10.1128/iai.67.8.3740-3749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu H C. Biosynthesis of lipoproteins. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1005–1014. [Google Scholar]
- 60.Yamanaka N, Faden H. Local antibody response to P6 of nontypeable Haemophilus influenzae in otitis-prone and normal children. Acta Oto-Laryngol. 1993;113:524–529. doi: 10.3109/00016489309135857. [DOI] [PubMed] [Google Scholar]
- 61.Young R S, Fortney K, Haley J C, Hood A F, Campagnari A A, Wang J, Bozue J A, Munson R S, Jr, Spinola S M. Expression of sialylated or paragloboside-like lipooligosaccharides are not required for pustule formation by Haemophilus ducreyi in human volunteers. Infect Immun. 1999;67:6335–6340. doi: 10.1128/iai.67.12.6335-6340.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang H, Kaur I, Niesel D W, Seetharamaiah G S, Peterson J W, Justement L B, Prabhakar B S, Klimpel G R. Yersinia enterocolitica envelope proteins that are crossreactive with thyrotropin receptor (TSHR) also have B-cell mitogenic activity. J Autoimmun. 1996;9:509–516. doi: 10.1006/jaut.1996.0068. [DOI] [PubMed] [Google Scholar]
- 63.Zhang H, Kaur I, Niesel D W, Seetharamaiah G S, Peterson J W, Prabhakar B S, Klimpel G R. Lipoprotein from Yersinia enterocolitica contains epitopes that cross-react with the human thyrotropin receptor. J Immunol. 1997;158:1976–1983. [PubMed] [Google Scholar]
- 64.Zhang H, Peterson J W, Niesel D W, Klimpel G R. Bacterial lipoprotein and lipopolysaccharide act synergistically to induce lethal shock and proinflammatory cytokine production. J Immunol. 1997;159:4868–4878. [PubMed] [Google Scholar]