Abstract
For electromechanical coupling of cardiomyocytes, intercalated discs (ICDs) are pivotal as highly specialized intercellular contact areas. ICD consists of adhesive contacts, such as desmosomes and adherens junctions (AJs) that are partially intermingled and thereby form an area composita to provide mechanical strength, as well as gap junctions (GJ) and sodium channels for excitation propagation. In contrast, in epithelia, mixed junctions with features of desmosomes and AJs are regarded as transitory primarily during the formation of desmosomes. The anatomy of desmosomes is defined by a typical ultrastructure with dense intracellular plaques anchoring the cadherin‐type adhesion molecules to the intermediate filament cytoskeleton. Desmosomal diseases characterized by impaired adhesive and signalling functions of desmosomal contacts lead to arrhythmogenic cardiomyopathy when affecting cardiomyocytes and cause pemphigus when manifesting in keratinocytes or present as cardiocutaneous syndromes when both cell types are targeted by the disease, which underscores the high biomedical relevance of these cell contacts. Therefore, comparative analyses regarding the structure and regulation of desmosomal contacts in cardiomyocytes and epithelial cells are helpful to better understand disease pathogenesis. In this brief review, we describe the structural properties of ICD compared to epithelial desmosomes and suggest that mechanisms regulating adhesion may at least in part be comparable. Also, we discuss whether phenomena such as hyperadhesion or the bidirectional regulation of desmosomes to serve as signalling hubs in epithelial cells may also be relevant for ICD.
Keywords: area composita, cell adhesion, desmoglein, desmoplakin, desmosomes, intercalated disc, plakoglobin and plakophilin
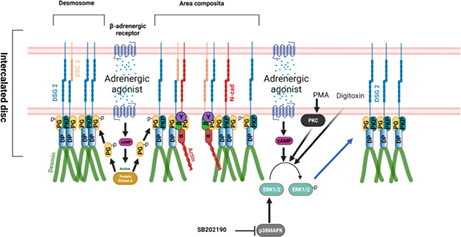
Adrenergic agonists act via PKA‐dependent PG phosphorylation at S665, leading to enhanced translocation of DSG2 to the desmosomes or area composita of the ICD and thereby increasing cardiomyocyte adhesion. On the other hand adrenergic agonists, PKC activation by PMA ,p38MAPK inhibition by SB202190 and digitoxin enhanced cardiomyocyte adhesion, in an ERK1/2‐dependent manner.
1. INTRODUCTION
Desmosomes are adhesive intercellular contacts required for intercellular adhesion, which is reflected by the abundance of desmosomes in tissues subjected to extensive mechanical strain such as the epidermis and the myocardium (Getsios et al., 2004; Holthofer et al., 2007; North et al., 1999; Waschke, 2008). Desmosomes are composed of members of at least three different protein families, that is, cadherin‐type adhesion proteins which belong to the desmoglein (Dsg) and desmocollin (Dsc) subfamily, armadillo proteins such as plakoglobin (Pg) and plakophilin (Pkp2) isoforms and the plakin family member desmoplakin (Dp) (Green et al., 2019). The turnover of the desmosomes is tightly regulated by lipid raft membrane domains and assembly and disassembly are controlled by post‐translational modifications of plaque proteins including Dp phosphorylation which fine‐tunes the cytoskeletal anchorage of desmosomes (Bartle et al., 2020; Broussard et al., 2020; Zimmer and Kowalczyk, 2020).
The high biomedical relevance of desmosomes, which are present in all vertebrates but not insects, can be appreciated from desmosomal diseases, which are caused by failure of adhesive or signalling functions of desmosomes (Waschke, 2019). These desmosomal diseases comprise genetic disorders such as arrhythmogenic cardiomyopathy (AC) (Basso et al., 2011; Delmar and McKenna, 2010), which can occur combined with hair and skin abnormalities including blistering pathologies (Lee and McGrath, 2021; Nitoiu et al., 2014). These disorders are caused by mutations in desmosomal components referred to above (Mohammed and Chidgey, 2021) and present in about 50%–60% of patients leading to sudden cardiac death or heart failure (Austin et al., 2019). Besides genetic disorders, the autoimmune blistering skin disease pemphigus is a desmosomal disorder caused by autoantibodies against desmosomal adhesion proteins such as Dsg1, Dsg3 and Dsc3 (Kasperkiewicz et al., 2017; Schmidt et al., 2019; Spindler et al., 2018). Interestingly, recently, it was reported that in AC patients autoantibodies against ICD proteins including Dsg2 can be detected (Caforio et al., 2020; Chatterjee et al., 2018). Finally, impaired function of desmosomes caused by cytokines or lack of neurotrophic factors also can contribute to the pathogenesis of inflammatory bowel diseases, although the latter is not regarded as desmosomal diseases (Schlegel et al., 2021).
Taken together, accumulative evidence indicates that mutations and autoantibodies can cause comparable and overlapping clinical phenotypes predominantly involving the heart and epidermis, which is explained by parallels in the composition and regulation of desmosomes in ICD and keratinocytes. Since desmosomes in epithelial cells have been characterized more profoundly in terms of their regulation but ICDs are much more complex in structure, we will describe desmosomes in ICD in comparison to typical desmosomes in epithelia.
2. THE STRUCTURE OF EPITHELIAL DESMOSOMES
Desmosomes as was described using transmission electron microscopy (TEM) from a keratinocyte in intact human epidermis typically are 0.2–0.5 μm in diameter and consist of two electron‐dense plaques separated by an intercellular space of 25–30 nm (Odland, 1958). The less opaque inner dense plaque anchors the desmosomes to keratin filaments in epithelial cells (Kelly, 1966), which is mediated by Dp, whereas in the outer dense plaque, the cytoplasmic tails of Dsg and Dsc molecules interact with Pg, DP and Pkp isoforms (Figure 1) (Green et al., 2019). Besides these canonical structural components of desmosomes, functional regulators such as Ndel1 and its binding protein Lis1, which are required for robust keratin linkage of desmosomes, were found recently (Kim et al., 2021). In contrast, it was shown that desmosomes can also act as organizing centres for the keratin filament cytoskeleton (Moch et al., 2020).
FIGURE 1.
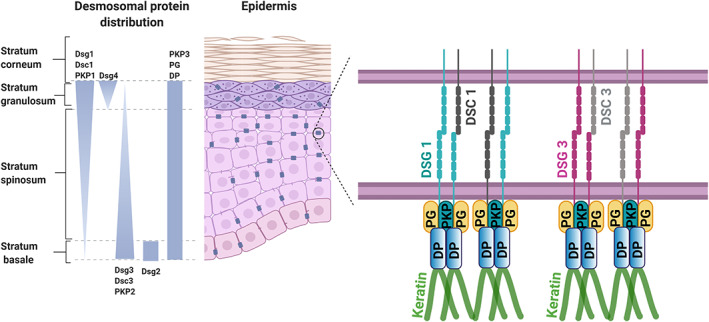
Schematic showing the localization of components of epithelial desmosomes. The epidermis of the skin consists of multiple layers such as basal proliferating layer (stratum basale), differentiating spinous layer (stratum spinosum), granular layer (stratum ganulosum) and fully differentiated cornified layer (stratum corneum). Stratified epithelia express four isoforms of desmogleins (DSG1–4) and three types of desmocollin (DSC1–3), the latter of which also are present in a shorter and longer variant. But the expression pattern of these isoforms varies across the epidermis as shown above. The armadillo proteins plakoglobin (PG) and plakophilin (PKP)1–3 and desmoplakin (DP) are constantly expressed along with the epidermis. Among the desmogleins expressed in the epidermis DSG1 and DSG3 have higher expression in an inverted fashion across the epidermis. Homophilic and heterophilic interactions of DSG1 and DSC1, and DSG3 and DSC3 are the most predominant interactions present in the epidermis of the skin. Schematic was created with BioRender.Com
Interestingly, in simple epithelial cells such as enterocytes only the pair of Dsg2 and Dsc2, both of which contribute to cell adhesion and barrier function (Gross et al., 2018; Raya‐Sandino et al., 2021; Schlegel et al., 2011; Ungewiss et al., 2017), are expressed in desmosomes typically arranged underneath tight junctions (TJ) and AJ and form junctional complexes (Farquhar and Palade, 1963). In contrast, stratified epithelia express four isoforms of Dsg (Dsg1–4) and three types of Dsc (Dsc1–3), the latter of which also are present in a shorter and longer variant (Green et al., 2019). The fact that both Dsg and Dsc molecules are expressed in the same cell raised the question of whether homophilic or heterophilic interaction may be predominant. Since in artificial systems such as bead assays or cell‐free atomic force microscopy for some Dsg isoforms either more homophilic or more heterophilic interactions have been observed, whereas experiments in cultured keratinocytes demonstrated homophilic interactions only, this topic is still a matter of debate (Vielmuth et al., 2018). Most likely, both types of interactions occur to some extent.
Interaction of desmosomal cadherins occurs most likely in antiparallel alignment via their most distal extracellular domains EC1 and EC2, with both molecules from the same cell (cis) and the opposite cell (trans) engaging in forming interaction nodes (Sikora et al., 2020). During transinteraction, EC1 domains exchange the tryptophan residue 2 by insertion into a hydrophobic binding pocket of the partner molecule (Al‐Amoudi et al., 2007; Blaschuk et al., 1990).
For single Dsg and Dsc molecules, it is established that they bind in a Ca2+‐dependent manner (Chitaev and Troyanovsky, 1997), but Ca2+‐independent interaction has been observed as well for Dsg2 with Dsc2 (Shafraz et al., 2018). More complex is the situation for entire desmosomes. It has been proposed that most desmosomes in intact epidermis differentiate to become hyperadhesive and, in this state, to become Ca2+‐independent (Garrod and Kimura, 2008; Garrod et al., 2005). In this state, desmosomal anchorage to keratin filaments via Dp is enhanced, resulting in desmosomal components becoming locked in hyperadhesive desmosomes so that exchange of proteins and thus shifting the turnover towards desmosome disassembly is reduced (Bartle et al., 2020). In this scenario, not all desmosomal cadherins adapt their molecular binding properties indicating that hyperadhesion may be achieved by some Dsg isoforms such as Dsg3 but not others as shown for Dsg1 (Fuchs et al., 2020). It has been suggested that the dense midline typical for the extracellular space of epithelial desmosomes is the ultrastructural correlate for hyperadhesive desmosomes (Garrod and Kimura, 2008; Garrod et al., 2005). However, since in cultured keratinocytes, hyperdahesive desmosomes were lacking electron‐dense midlines (Bartle et al., 2020), it is possible that the midline results from the high number of EC1 and EC2 domains interacting in this plane in highly differentiated desmosomes of intact tissue and thus does not correlate with the adhesive state (Sikora et al., 2020).
3. INTERCALATED DISCS SHARE MANY STRUCTURAL FEATURES FOUND IN TYPICAL DESMOSOMES BUT ARE MORE COMPLEX
Many aspects described for epithelial desmosomes are also found in desmosomes from ICD (Green et al., 2019). In epithelial desmosomes, hybrid junctions containing Dsg2 or Dsg3 molecules interacting with E‐cadherin and in association with actin filaments are regarded as transitory during the formation of new desmosomes (Rotzer et al., 2015; Shafraz et al., 2018). In contrast, in ICD, it is a typical hallmark that desmosomal components including Dsg2, Dsc2, Pkp2, Dp and Pg as well as AJ proteins such as N‐cadherin, α‐catenin and β‐catenin are found intermingled in junctional areas of the ICD ultrastructurally resembling both desmosomes or fasciae adherents (Borrmann et al., 2006; Franke et al., 2006). Therefore, the term area composita was established for this hybrid junction. The two junction types were proposed to coalesce in the first year after birth in mice (Pieperhoff and Franke, 2007). This complex composition, which exists only in mammalian species, appears to be of high functional relevance for ICD integrity (Li and Radice, 2010). It was shown that the α‐catenin family member αT‐catenin is required for Pkp2 recruitment and GJ integrity and αT‐catenin‐deficient mice suffered from cardiomyopathy and arrhythmias (Li et al., 2012).
The complex structure of ICD was first described when TEM was applied in cell biology (Fawcett and McNutt, 1969; Van Breemen, 1953). The interdigitated structure of the ICD became readily apparent with GJ plaques, also called Nexus located primarily in the longitudinal/interplicate regions and adhesive contacts now referred to as area composita primarily covering the transverse/plicate regions between adjacent cardiomyocytes (Figure 2). Meanwhile, super‐resolution imaging helped to refine the molecular composition of these different segments of the ICD. In the plicate regions, some Nav1.5 channels are detectable, whereas most of Nav1.5 is located in the area surrounding GJ plaques, where the β1 subunit of the channel was demonstrated to stabilize intercellular adhesion by homophilic transinteraction (Veeraraghavan et al., 2018). Moreover, nanoscale signalling revealed that Nav1.5 with N‐cadherin forms clusters that may regulate intercellular adhesion (Leo‐Macias et al., 2016). To these sites, Nav1.5 may be restricted by ankyrin G, a cytoskeletal adaptor molecule also serving to couple Cx43 of GJ to Pkp2 of the area composite (Sato et al., 2011). This coupling of channel complexes and GJ components to desmosomal contacts of the ICD not only allows to explain how mutations in desmosomal components such as Pkp2 cause arrhythmia in AC but also suggest that excitation propagation in addition to electrotonic transmission via GJ may also include ephaptic contributions via Nav1.5 channels in the perinexus Gourdie, Anatom Rec. 2018.
FIGURE 2.
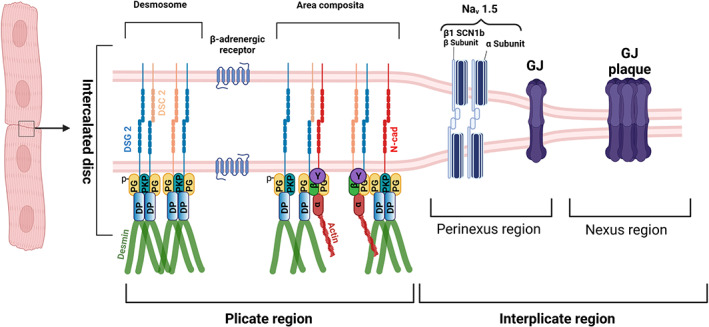
Schematic of cardiomyocyte intercalated disc (ICD). The intercalated disc of the cardiomyocyte consists of plicate and interplicate regions, which in general lie perpendicular to each other. For convenience, we depicted here the two regions as parallel structures. The plicate region consists of the desmosome, as an individual mechanical component, and area composita or fascia adherens where both the desmosomes and adherens junction proteins are intermingled. Desmosomes are formed by the homo or heterophilic interactions of desmoglein 2 (DSG2) and desmocollin 2 (DSC2), where AJs are formed by the N‐cadherin (N‐cad). Desmosomes are anchored to the intermediate filament protein, desmin via the armadillo proteins plakoglobin (PG) also known as γ‐catenin and plakophillin (PKP) 2 which further interacts with the plaque protein desmoplakin, which then interacts with desmin. AJs are very well known to be anchored to the actin cytoskeleton via the alpha‐catenin (α), beta‐catenin (β) and the gamma‐catenin (known also as PG). But in the area composita, it could also be likely that desmosome and AJs are intermingled in such a way that desmosomes are anchored to the actin cytoskeleton and AJs to the desmin. In the interplicate region, there exists two regions, perinexus and nexus, respectively. In the perinexus region sodium channel complexes (Nav 1.5) concentrated at the edge of GJs, which are found in the nexus region, are involved in ephaptic conduction in the heart. The sodium channel β1 subunit with its extracellular adhesion domain plays a critical for this mechanism to function. Another important structure in ICD, that plays a major role in cardiomyocyte adhesion, is the β‐adrenergic receptor. Schematic was created with BioRender.Com
Besides typical structural components of desmosomes, also other regulatory proteins have been localized to ICD, including iASPP, SORBS2 and COP9, the loss of which causes AC in mice (Ding et al., 2020; Liang et al., 2021; Notari et al., 2015). Interestingly, COP9 organizes a signalosome regulating the targeted desmosome proteome degradation, which is altered by mutations in desmosomal components found in patients suffering from AC. Since in cardiomyocytes, the contractile machinery of the myofibrils takes up large sections of the cytoplasm, it is conceivable that the ICD becomes the major structural platform to coordinate intercellular adhesion with other functions. This may explain why a loss of different regulatory proteins located at the ICD causes a phenotype similar to mutations in desmosomal components which cause AC.
4. DESMOSOMES AS SIGNALLING HUBS
It has been shown early that phosphorylation of Dp or Pg regulates adhesion via the cytoskeletal anchorage of desmosomes (Bornslaeger et al., 1996; Hobbs and Green, 2012; Lorch et al., 2004; Stappenbeck et al., 1994; Troyanovsky et al., 1993; Troyanovsky et al., 1994) and Dp phosphorylation is now known to regulate the switch to a hyperadhesive state of desmosomes (Bartle et al., 2020). Moreover, Dsg1 was shown to inhibit EGFR signalling via Erbin‐mediated regulation of Shoc2 to allow epidermal differentiation (Getsios et al., 2009). Together with the finding that Dsg3 directly interacts with p38MAPK, which is activated after application of Dsg3‐specific autoantibodies or peptides (Spindler et al., 2013), desmosomal contacts emerged as signalling hubs in healthy tissues and to contribute to the pathogenesis of skin diseases such as pemphigus (Johnson et al., 2014; Spindler and Waschke, 2014; Waschke and Spindler, 2014). Meanwhile, it was found that Dsg molecules organize isoform‐specific signalling complexes, where Dsg1 and Dsg3 in keratinocytes interact with p38MAPK, PLC, PKC, PI4K and EGFR (Rotzer et al., 2015; Schmitt et al., 2021) to regulate cell adhesion, whereas Dsg2 in enterocytes binds to EGFR and PI3K to control cell adhesion and barrier function (Burkard et al., 2021; Ungewiss et al., 2018).
In epithelial cells from complex epithelia such as keratinocytes, the role of different Dsg and presumably also Dsc isoforms in the regulation of signalling is not equal. For example, both Dsg1 and Dsg3 can activate p38MAPK, whereas Dsg3 also activates EGFR in Src‐dependent manner and Dsg1 is involved in PLC‐mediated Ca2+ influx and ERK activation, as has been found in studies using pemphigus autoantibodies (Berkowitz et al., 2005; Berkowitz et al., 2006; Berkowitz et al., 2008; Schmitt et al., 2021; Walter et al., 2017; Walter et al., 2019). On the other hand, Dsg2 was found not to regulate p38MAPK but rather to act as a compensation molecule for adhesion and thus is expressed in pemphigus lesions when Dsg3 adhesion is impaired (Hartlieb et al., 2013; Hartlieb et al., 2014; Sigmund et al., 2020). Dsc3 may also be involved in the activation of EGFR and p38MAPK (Hudemann et al., 2021; Spindler et al., 2009).
In enterocytes, which are similar to cardiomyocytes in expressing Dsg2 and Dsc2 only, Dsg2 regulates EGFR and p38MAPK (Ungewiss et al., 2017; Ungewiss et al., 2018), suggesting that this could also be the case at ICD. However, since activation of signalling pathways including p38MAPK, Src and RhoA was found to be mediated by extradesmosomal Dsg3, that is free Dsg3 molecules outside of desmosomes and thus not coupled to Dp and keratin filaments but rather interacting with actin filaments (Hartlieb et al., 2014; Rotzer et al., 2015; Tsang et al., 2012), it was proposed that extradesmosomal Dsg contacts may serve as adhesion‐dependent receptors (Waschke and Spindler, 2014). This was supported by the fact that depletion of Pg but not of Dp, both of which causes loss of cell adhesion, was followed by activation of p38MAPK (Spindler et al., 2014). Since Dsg2 in keratinocytes is mainly localized in desmosomes, whereas Dsg2 in enterocytes is detectable without Dp (Hartlieb et al., 2013; Ungewiss et al., 2017), these results would correlate with the different functions of Dsg2 in the regulation of signalling in these two cell types. At present, not much is known for Dsg2 signalling in cardiomyocytes.
The different signalling pathways in keratinocytes contribute to loss of cell adhesion in pemphigus presumably because they are involved in the regulation of the desmosome turnover (Schmitt and Waschke, 2021). Using an ex vivo human skin model, first attempts were made to attribute the different signalling pathways involved in the loss of cell adhesion to different steps of desmosome turnover. Data indicate that some signalling mechanisms are central in this respect such as p38MAPK, which drives desmosome disassembly and negatively affects keratin anchorage (Egu et al., 2017). Others such as ERK regulate desmosome size, whereas PLC controls keratin insertion only (Egu et al., 2019). Although not much is known about the regulation of desmosomes in mucous membranes, the role of p38MAPKs appears less significant compared to the epidermis (Egu et al., 2020).
Besides the regulation of signalling mechanisms to control cell adhesion, desmosomes were recently found to control differentiation and stratification of the epidermis and to maintain epidermal barrier functions via Dsg1‐mediated TJ formation (Broussard et al., 2021; Kugelmann et al., 2019). Taken together, the signalling function of desmosomes in epithelial cells is partially understood. However, most data come from studies on pemphigus pathogenesis which explains why pemphigus is a model disease to study the regulation of desmosomes.
5. ADHESION OF INTERCALATED DISCS IS TIGHTLY REGULATED BY SIGNALLING PATHWAYS
Wnt signalling was found to be activated in cardiomyocytes which are involved in the fibrofatty replacement of cardiomyocytes in AC (Garcia‐Gras et al., 2006). Similarly, the Hippo pathway was found to be controlled by molecules located at the ICD such as YAP (Chen et al., 2014; Guo et al., 2020; Vite et al., 2018). However, it is unclear whether these mechanisms regulate cardiomyocyte adhesion. Similarly, GSK3β was found to localize to ICD in hearts from AC patients suggesting a role in disease pathogenesis which may or may not involve regulation of adhesion (Chelko et al., 2016). Thus, until a few years, it was not known whether cardiomyocytes adhesion is controlled or is just kept strong constantly which would make sense during contraction of the myocardium. The situation was similar until the middle of the 90s the first signalling pathways were discovered to be altered by autoantibodies in pemphigus (Esaki et al., JID 1995; Seishima et al., JID 1995).
Therefore, we were very much intrigued by the finding that the adrenergic β1 receptor is localized at ICD (Schlipp et al., 2014) and we started to investigate whether the sympathetic nervous system, besides its other function in heart muscle, also regulates cardiomyocyte adhesion. This led to the finding that cAMP downstream of adrenergic agonists induces translocation of Dsg2 molecules towards cell junctions and enhances Dsg2 binding strength on a single molecule level (Schinner et al., 2017). This stabilizes cardiomyocyte adhesion and also increases the length and diameter of area composita plaques and therefore, we refer to this new function of adrenergic signalling as positive adhesiotropy (Yeruva et al., 2020). The molecular mechanism strictly depends on Pg expression and involves PKA‐mediated phosphorylation of Pg on S665 (Schinner et al., 2017; Yeruva et al., 2020). Moreover, Dsg2 also appears to be required for cAMP formation by the adrenergic β1 receptor and Dsg2 and Pg to be required for regular excitation propagation across cardiomyocyte monolayers (Schinner et al., 2019). This indicates that Dsg2 is important for coordinating cell adhesion and excitation propagation in cardiomyocytes which are supported by the finding that peptide crosslinking Dsg2 molecules can reduce arrhythmia in the murine AC model (Schinner, Erber, et al., 2020).
These results brought up the question of whether other external stimuli or drugs also can regulate cardiomyocyte adhesion and signalling pathways known to regulate cell adhesion in keratinocytes and enterocytes may be relevant in this scenario. Surprisingly, digitoxin, which is used to treat heart failure, also stabilizes cardiomyocyte adhesion in an ERK‐dependent manner (Schinner, Olivares‐Florez, et al., 2020). In line with this, PKA and PKC found to stabilize cardiomyocyte adhesion and p38MAPK to reduce adhesion were found to be dependent on ERK1/2 in cultured cells as well as cardiac slice cultures (Shoykhet et al., 2020). Interestingly, adrenergic stabilization but not effects of PKC and p38MAPK required Pg indicating that the downstream mechanisms are in part different (Figure 3).
FIGURE 3.
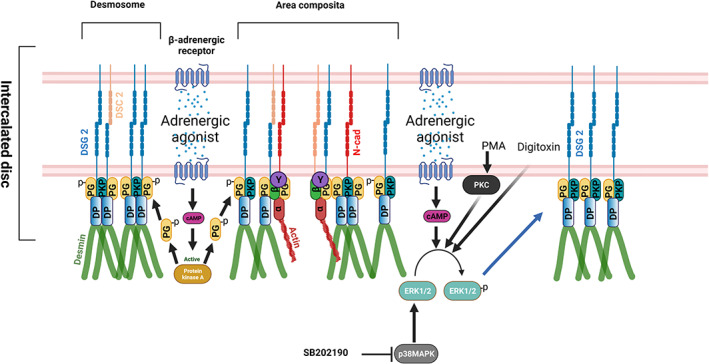
Schematic overview of signalling mechanisms regulating cardiomyocyte adhesion. Adrenergic agonists like the combination of forskolin and rolipram, and isoprenaline acts via PKA‐dependent PG phosphorylation at S665 leading to enhanced translocation of DSG2 to the desmosomes or area composita of the ICD and thereby increases cardiomyocyte adhesion. On the other hand, adrenergic agonists, PKC activation by PMA, p38MAPK inhibition by SB202190 and digitoxin enhanced cardiomyocyte adhesion, in an ERK1/2‐dependent manner through alterations in the interactions of the desmosomal proteins PG, PKP2 and DP that eventually lead to increased DSG2 translocation to the cell borders. Schematic was created with BioRender.Com
An interesting question in cardiomyocyte adhesion is whether desmosomes in ICD can acquire a hyperadhesive state similar to keratinocytes. If the dense midline in the extracellular space of desmosomal contacts is the ultrastructural hallmark of hyperadhesive desmosomes as has been proposed (Garrod and Kimura, 2008; Garrod et al., 2005), this likely is the case since at least in cat hearts midlines have been observed at least in some segments of areae composita (Fawcett and McNutt, 1969). This is in line with the finding that adrenergic signalling as well as PKC activation and p38MAPK inhibition render cardiomyocyte adhesion resistant against Ca2+ chelation in cultured cells and slice cultures, at least to some extent (Schinner et al., 2017; Shoykhet et al., 2020). Surprisingly, the proportion of desmosomal components anchored to the cytoskeleton was not enhanced during hyperadhesion. This could be explained if the situation is similar to keratinocytes where during hyperadhesion the exchange of desmosomal components in and out of desmosomes is reduced (Bartle et al., 2020), which would not necessarily alter the number of proteins coupled to the cytoskeleton.
This brings up a limitation of studies on the regulation of cardiomyocyte adhesion conducted so far because it is unknown how Pg665 phosphorylation downstream of PKA or by which targets the different signalling pathways regulate adhesion and whether this involves modulation of desmin anchorage or actin dynamics. In addition, it has been shown that Pg is necessary for the maintenance of the cortical actin skeleton (Kofron et al., 2002), which acts as a scaffold for actin assembly and organization (Daly, 2004; Weed and Parsons, 2001). This shows the need for a new field of research and is promising because signalling pathways regulating desmosomal adhesion in keratinocytes such as p38MAPK, RhoA or PLC/Ca2+ induce uncoupling of desmosomes from keratin filaments and cause profound alterations of actin filaments (Berkowitz et al., 2005; Gliem et al., 2010; Schmitt et al., 2021; Spindler et al., 2007; Waschke et al., 2005; Waschke et al., 2006). It is intriguing that rho‐kinase inhibition during early cardiac development leads to AC in mice (Ellawindy et al., 2015), further supporting the necessity to delineate the molecular signalling that regulated cardiomyocyte adhesion.
Recently, ICDs have been described as mechanosensing signalling nodes (Pruna and Ehler, 2020; Zhao et al., 2019). Among all pathways investigated so far, best evidence has been collected that Wnt and the Hippo pathways are locally controlled at the ICD since YAP has been shown to interact with Pg and β‐catenin and to be regulated by α‐catenin as well (Chen et al., 2014; Vite et al., 2018). Moreover, Xinβ, a regulator of the Hippo pathway, was reported to localize to and thereby to recruit NF2 (Merlin) to ICD (Guo et al., 2020). However, this implies only that signalling molecules located at ICD control cardiomyocyte differentiation and cell fate which is disturbed during cardiac diseases. Whether adhesion proteins of the area composita such as Dsg2, Dsc2 or N‐cadherin directly are involved in mechanosignalling in cardiomyocytes is unknown at present.
6. CONCLUSION: INTERCALATED DISCS AS SIGNALLING PLATFORMS
Until some years ago, not much was known about the bidirectional regulation of cardiomyocyte adhesion at ICD. Now, we can conclude that the function of ICD is beyond electromechanical coupling. Rather, ICD serves as a signalling hub comparably to desmosomes in epithelia. Similar to desmosomes in keratinocytes, which besides cell adhesion also regulate cell migration and wound healing (Rotzer et al., 2016; Waschke, 2019) or desmosomes in enterocytes, which control epithelial barrier properties, cell proliferation and cell death (Kamekura et al., 2014; Nava et al., 2007; Raya‐Sandino et al., 2021; Ungewiss et al., 2018; Yulis et al., 2018), ICD may also regulate different functions in cardiomyocytes. This possibility is intriguing because, for example adrenergic signalling has various functions in the heart and it is tempting to speculate that functions such as positive chronotropy and inotropy may dependent on enhanced cardiomyocyte intercellular binding, which we refer to as positive adhesiotropy (Schinner et al., 2017), between cells in the cardiac conduction system or the ventricular myocardium respectively. Since AC is a disease where mutations in desmosomal adhesion and plaque proteins cause arrhythmia, it is important to find out which signalling mechanisms may be involved in this context.
CONFLICT OF INTEREST
None declared.
Yeruva, S , Waschke, J (2023) Structure and regulation of desmosomes in intercalated discs: Lessons from epithelia. Journal of Anatomy. 242:81–90. 10.1111/joa.13634
Funding informationThis work was supported by the Deutsche Forschungsgemeinschaft DFG (grant number WA2474/11‐1 to J.W.).
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- Al‐Amoudi, A. , Diez, D.C. , Betts, M.J. & Frangakis, A.S. (2007) The molecular architecture of cadherins in native epidermal desmosomes. Nature, 450, 832–837. [DOI] [PubMed] [Google Scholar]
- Austin, K.M. , Trembley, M.A. , Chandler, S.F. , Sanders, S.P. , Saffitz, J.E. , Abrams, D.J. et al. (2019) Molecular mechanisms of arrhythmogenic cardiomyopathy. Nature Reviews Cardiology, 16, 519–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartle, E.I. , Rao, T.C. , Beggs, R.R. , Dean, W.F. , Urner, T.M. , Kowalczyk, A.P. et al. (2020) Protein exchange is reduced in calcium‐independent epithelial junctions. The Journal of Cell Biology, 219(6), e201906153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso, C. , Bauce, B. , Corrado, D. & Thiene, G. (2011) Pathophysiology of arrhythmogenic cardiomyopathy. Nature Reviews Cardiology, 9(4), 223–233. [DOI] [PubMed] [Google Scholar]
- Berkowitz, P. , Chua, M. , Liu, Z. , Diaz, L.A. & Rubenstein, D.S. (2008) Autoantibodies in the autoimmune disease pemphigus foliaceus induce blistering via p38 mitogen‐activated protein kinase‐dependent signaling in the skin. The American Journal of Pathology, 173, 1628–1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowitz, P. , Hu, P. , Liu, Z. , Diaz, L.A. , Enghild, J.J. , Chua, M.P. et al. (2005) Desmosome signaling. Inhibition of p38MAPK prevents pemphigus vulgaris IgG‐induced cytoskeleton reorganization. The Journal of Biological Chemistry, 280(25), 23778–23784. [DOI] [PubMed] [Google Scholar]
- Berkowitz, P. , Hu, P. , Warren, S. , Liu, Z. , Diaz, L.A. & Rubenstein, D.S. (2006) p38MAPK inhibition prevents disease in pemphigus vulgaris mice. Proceedings of the National Academy of Sciences of the United States of America, 103, 12855–12860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaschuk, O.W. , Sullivan, R. , David, S. & Pouliot, Y. (1990) Identification of a cadherin cell adhesion recognition sequence. Developmental Biology, 139, 227–229. [DOI] [PubMed] [Google Scholar]
- Bornslaeger, E.A. , Corcoran, C.M. , Stappenbeck, T.S. & Green, K.J. (1996) Breaking the connection: displacement of the desmosomal plaque protein desmoplakin from cell‐cell interfaces disrupts anchorage of intermediate filament bundles and alters intercellular junction assembly. The Journal of Cell Biology, 134, 985–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrmann, C.M. , Grund, C. , Kuhn, C. , Hofmann, I. , Pieperhoff, S. & Franke, W.W. (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. II. Colocalizations of desmosomal and fascia adhaerens molecules in the intercalated disk. European Journal of Cell Biology, 85, 469–485. [DOI] [PubMed] [Google Scholar]
- Broussard, J.A. , Jaiganesh, A. , Zarkoob, H. , Conway, D.E. , Dunn, A.R. , Espinosa, H.D. et al. (2020) Scaling up single‐cell mechanics to multicellular tissues—the role of the intermediate filament‐desmosome network. Journal of Cell Science, 133(6), jcs228031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussard JA, Koetsier JL, Hegazy M, Green KJ (2021) Desmosomes polarize and integrate chemical and mechanical signaling to govern epidermal tissue form and function. Current Biology, 31, 3275–3291 e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkard, N. , Meir, M. , Kannapin, F. , Otto, C. , Petzke, M. , Germer, C. T. et al. (2021) Desmoglein2 regulates claudin2 expression by sequestering PI‐3‐kinase in intestinal epithelial cells. Frontiers in Immunology, 12, 756321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caforio, A.L.P. , Re, F. , Avella, A. , Marcolongo, R. , Baratta, P. , Seguso, M. et al. (2020) Evidence from family studies for autoimmunity in arrhythmogenic right ventricular cardiomyopathy: associations of circulating anti‐heart and anti‐intercalated disk autoantibodies with disease severity and family history. Circulation, 141(15), 1238–1248. [DOI] [PubMed] [Google Scholar]
- Chatterjee, D. , Fatah, M. , Akdis, D. , Spears, D.A. , Koopmann, T.T. , Mittal, K. et al. (2018) An autoantibody identifies arrhythmogenic right ventricular cardiomyopathy and participates in its pathogenesis. European Heart Journal, 39(44), 3932–3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelko, S.P. , Asimaki, A. , Andersen, P. , Bedja, D. , Amat‐Alarcon, N. , DeMazumder, D. et al. (2016) Central role for GSK3beta in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight, 1(5), e85923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S.N. , Gurha, P. , Lombardi, R. , Ruggiero, A. , Willerson, J.T. & Marian, A.J. (2014) The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy. Circulation Research, 114, 454–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitaev, N.A. & Troyanovsky, S.M. (1997) Direct Ca2+−dependent heterophilic interaction between desmosomal cadherins, desmoglein and desmocollin, contributes to cell‐cell adhesion. The Journal of Cell Biology, 138, 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly, R.J. (2004) Cortactin signalling and dynamic actin networks. Biochemical Journal, 382, 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmar, M. & McKenna, W.J. (2010) The cardiac desmosome and arrhythmogenic cardiomyopathies: from gene to disease. Circulation Research, 107, 700–714. [DOI] [PubMed] [Google Scholar]
- Ding, Y. , Yang, J. , Chen, P. , Lu, T. , Jiao, K. , Tester, D.J. et al. (2020) Knockout of SORBS2 protein disrupts the structural integrity of intercalated disc and manifests features of arrhythmogenic cardiomyopathy. Journal of the American Heart Association, 9, e017055.(17), e017055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egu, D.T. , Kugelmann, D. & Waschke, J. (2019) Role of PKC and ERK signaling in epidermal blistering and desmosome regulation in pemphigus. Frontiers in Immunology, 10, 2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egu, D.T. , Sigmund, A.M. , Schmidt, E. , Spindler, V. , Walter, E. & Waschke, J. (2020) A new ex vivo human oral mucosa model reveals that p38MAPK inhibition is not effective in preventing autoantibody‐induced mucosal blistering in pemphigus. The British Journal of Dermatology, 182, 987–994. [DOI] [PubMed] [Google Scholar]
- Egu, D.T. , Walter, E. , Spindler, V. & Waschke, J. (2017) Inhibition of p38MAPK signalling prevents epidermal blistering and alterations of desmosome structure induced by pemphigus autoantibodies in human epidermis. The British Journal of Dermatology, 177, 1612–1618. [DOI] [PubMed] [Google Scholar]
- Ellawindy, A. , Satoh, K. , Sunamura, S. , Kikuchi, N. , Suzuki, K. , Minami, T. et al. (2015) Rho‐kinase inhibition during early cardiac development causes arrhythmogenic right ventricular cardiomyopathy in mice. Arteriosclerosis, Thrombosis, and Vascular Biology, 35(10), 2172–2184. [DOI] [PubMed] [Google Scholar]
- Farquhar, M.G. & Palade, G.E. (1963) Junctional complexes in various epithelia. The Journal of Cell Biology, 17, 375–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett, D.W. & McNutt, N.S. (1969) The ultrastructure of the cat myocardium. I. Ventricular papillary muscle. The Journal of Cell Biology, 42, 1–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke, W.W. , Borrmann, C.M. , Grund, C. & Pieperhoff, S. (2006) The area composita of adhering junctions connecting heart muscle cells of vertebrates. I. Molecular definition in intercalated disks of cardiomyocytes by immunoelectron microscopy of desmosomal proteins. European Journal of Cell Biology, 85, 69–82. [DOI] [PubMed] [Google Scholar]
- Fuchs, M. , Sigmund, A.M. , Waschke, J. & Vielmuth, F. (2020) Desmosomal hyperadhesion is accompanied with enhanced binding strength of desmoglein 3 molecules. Biophysical Journal, 119, 1489–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Gras, E. , Lombardi, R. , Giocondo, M.J. , Willerson, J.T. , Schneider, M.D. , Khoury, D.S. et al. (2006) Suppression of canonical Wnt/beta‐catenin signaling by nuclear plakoglobin recapitulates phenotype of arrhythmogenic right ventricular cardiomyopathy. The Journal of Clinical Investigation, 116(7), 2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod, D. & Kimura, T.E. (2008) Hyper‐adhesion: a new concept in cell‐cell adhesion. Biochemical Society Transactions, 36, 195–201. [DOI] [PubMed] [Google Scholar]
- Garrod, D.R. , Berika, M.Y. , Bardsley, W.F. , Holmes, D. & Tabernero, L. (2005) Hyper‐adhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. Journal of Cell Science, 118, 5743–5754. [DOI] [PubMed] [Google Scholar]
- Getsios, S. , Huen, A.C. & Green, K.J. (2004) Working out the strength and flexibility of desmosomes. Nature Reviews. Molecular Cell Biology, 5, 271–281. [DOI] [PubMed] [Google Scholar]
- Getsios, S. , Simpson, C.L. , Kojima, S. , Harmon, R. , Sheu, L.J. , Dusek, R.L. et al. (2009) Desmoglein 1‐dependent suppression of EGFR signaling promotes epidermal differentiation and morphogenesis. The Journal of Cell Biology, 185(7), 1243–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliem, M. , Heupel, W.M. , Spindler, V. , Harms, G.S. & Waschke, J. (2010) Actin reorganization contributes to loss of cell adhesion in pemphigus vulgaris. American Journal of Physiology. Cell Physiology, 299, C606–C613. [DOI] [PubMed] [Google Scholar]
- Green, K.J. , Jaiganesh, A. & Broussard, J.A. (2019) Desmosomes: essential contributors to an integrated intercellular junction network. F1000Res, 8, 2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, A. , Pack, L.A.P. , Schacht, G.M. , Kant, S. , Ungewiss, H. , Meir, M. et al. (2018) Desmoglein 2, but not desmocollin 2, protects intestinal epithelia from injury. Mucosal Immunology, 11(6), 1630–1639. [DOI] [PubMed] [Google Scholar]
- Guo, H. , Lu, Y.W. , Lin, Z. , Huang, Z. P. , Liu, J. , Wang, Y. et al. (2020) Intercalated disc protein Xinbeta is required for Hippo‐YAP signaling in the heart. Nature Communications, 11(1), 4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlieb, E. , Kempf, B. , Partilla, M. , Vigh, B. , Spindler, V. & Waschke, J. (2013) Desmoglein 2 is less important than desmoglein 3 for keratinocyte cohesion. PLoS One, 8, e53739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartlieb, E. , Rotzer, V. , Radeva, M. , Spindler, V. & Waschke, J. (2014) Desmoglein 2 compensates for desmoglein 3 but does not control cell adhesion via regulation of p38 mitogen‐activated protein kinase in keratinocytes. The Journal of Biological Chemistry, 289, 17043–17053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbs, R.P. & Green, K.J. (2012) Desmoplakin regulates desmosome hyperadhesion. The Journal of Investigative Dermatology, 132, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holthofer, B. , Windoffer, R. , Troyanovsky, S. & Leube, R.E. (2007) Structure and function of desmosomes. International Review of Cytology, 264, 65–163. [DOI] [PubMed] [Google Scholar]
- Hudemann, C. , Maglie, R. , Llamazares‐Prada, M. , Beckert, B. , Didona, D.T. , ikkanen, R. et al. (2021) Human desmocollin 3–specific IgG antibodies are pathogenic in a humanized HLA class ii transgenic mouse model of pemphigus. The Journal of Investigative Dermatology. [DOI] [PubMed] [Google Scholar]
- Johnson, J.L. , Najor, N.A. & Green, K.J. (2014) Desmosomes: regulators of cellular signaling and adhesion in epidermal health and disease. Cold Spring Harbor Perspectives in Medicine, 4, a015297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamekura R., Kolegraff K.N., Nava P., Hilgarth R.S., Feng M., Parkos C.A. & Nusrat, A. (2014) Loss of the desmosomal cadherin desmoglein‐2 suppresses colon cancer cell proliferation through EGFR signaling. Oncogene, 33(36), 4531–4536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkiewicz, M. , Ellebrecht, C.T. , Takahashi, H. , Yamagami, J. , Zillikens, D. , Payne, A. S. et al. (2017) Pemphigus. Nature Reviews. Disease Primers, 3, 17026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly, D.E. (1966) Fine structure of desmosomes, hemidesmosomes, and an adepidermal globular layer in developing newt epidermis. The Journal of Cell Biology, 28, 51–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, Y.B. , Hlavaty, D. , Maycock, J. & Lechler, T. (2021) Roles for Ndel1 in keratin organization and desmosome function. Molecular Biology of the Cell, 32, ar2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron, M. , Heasman, J. , Lang, S.A. & Wylie, C.C. (2002) Plakoglobin is required for maintenance of the cortical actin skeleton in early Xenopus embryos and for cdc42‐mediated wound healing. The Journal of Cell Biology, 158, 695–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kugelmann D, Radeva MY, Spindler V, Waschke J (2019) Desmoglein 1 deficiency causes lethal skin blistering. The Journal of Investigative Dermatology, 139, 1596–1599 e2. [DOI] [PubMed] [Google Scholar]
- Lee, J.Y.W. & McGrath, J.A. (2021) Mutations in genes encoding desmosomal proteins: spectrum of cutaneous and extracutaneous abnormalities. The British Journal of Dermatology, 184, 596–605. [DOI] [PubMed] [Google Scholar]
- Leo‐Macias, A. , Agullo‐Pascual, E. , Sanchez‐Alonso, J.L. , Keegan, S. , Lin, X. , Arcos, T. et al. (2016) Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nature Communications, 7, 10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Goossens, S. , van Hengel, J. , Gao, E. , Cheng, L. , Tyberghein, K. et al. (2012) Loss of alphaT‐catenin alters the hybrid adhering junctions in the heart and leads to dilated cardiomyopathy and ventricular arrhythmia following acute ischemia. Journal of Cell Science, 125(Pt 4), 1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. & Radice, G.L. (2010) A new perspective on intercalated disc organization: implications for heart disease. Dermatology Research and Practice, 2010, 207835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang, Y. , Lyon, R.C. , Pellman, J. , Bradford, W.H. , Lange, S. , Bogomolovas, J. et al. (2021) Desmosomal COP9 regulates proteome degradation in arrhythmogenic right ventricular dysplasia/cardiomyopathy. The Journal of Clinical Investigation, 131(11), e137689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch, J.H. , Klessner, J. , Park, J.K. , Getsios, S. , Wu, Y.L. , Stack, M.S. et al. (2004) Epidermal growth factor receptor inhibition promotes desmosome assembly and strengthens intercellular adhesion in squamous cell carcinoma cells. The Journal of Biological Chemistry, 279(35), 37191–37200. [DOI] [PubMed] [Google Scholar]
- Moch, M. , Schwarz, N. , Windoffer, R. & Leube, R.E. (2020) The keratin‐desmosome scaffold: pivotal role of desmosomes for keratin network morphogenesis. Cellular and Molecular Life Sciences, 77, 543–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammed, F. & Chidgey, M. (2021) Desmosomal protein structure and function and the impact of disease‐causing mutations. Journal of Structural Biology, 213, 107749. [DOI] [PubMed] [Google Scholar]
- Nava, P. , Laukoetter, M.G. , Hopkins, A.M. , Laur, O. , Gerner‐Smidt, K. , Green, K.J. et al. (2007) Desmoglein‐2: a novel regulator of apoptosis in the intestinal epithelium. Molecular Biology of the Cell, 18(11), 4565–4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitoiu, D. , Etheridge, S.L. & Kelsell, D.P. (2014) Insights into desmosome biology from inherited human skin disease and cardiocutaneous syndromes. Cell Communication & Adhesion, 21, 129–140. [DOI] [PubMed] [Google Scholar]
- North, A.J. , Bardsley, W.G. , Hyam, J. , Bornslaeger, E.A. , Cordingley, H.C. , Trinnaman, B. et al. (1999) Molecular map of the desmosomal plaque. Journal of Cell Science, 112(Pt 23), 4325–4336. [DOI] [PubMed] [Google Scholar]
- Notari, M. , Hu, Y. , Sutendra, G. , Dedeic, Z. , Lu, M. , Dupays, L. et al. (2015) iASPP, a previously unidentified regulator of desmosomes, prevents arrhythmogenic right ventricular cardiomyopathy (ARVC)‐induced sudden death. Proceedings of the National Academy of Sciences of the United States of America, 112(9), E973–E981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odland, G.F. (1958) The fine structure of the interrelationship of cells in the human epidermis. The Journal of Biophysical and Biochemical Cytology, 4, 529–538. [PMC free article] [PubMed] [Google Scholar]
- Pieperhoff, S. & Franke, W.W. (2007) The area composita of adhering junctions connecting heart muscle cells of vertebrates ‐ IV: coalescence and amalgamation of desmosomal and adhaerens junction components ‐ late processes in mammalian heart development. European Journal of Cell Biology, 86, 377–391. [DOI] [PubMed] [Google Scholar]
- Pruna, M. & Ehler, E. (2020) The intercalated disc: a mechanosensing signalling node in cardiomyopathy. Biophysical Reviews, 12, 931–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya‐Sandino, A. , Luissint, A.C. , Kusters, D.H.M. , Narayanan, V. , Flemming, S. , Garcia‐Hernandez, V. et al. (2021) Regulation of intestinal epithelial intercellular adhesion and barrier function by desmosomal cadherin desmocollin‐2. Molecular Biology of the Cell, 32(8), 753–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzer, V. , Hartlieb, E. , Vielmuth, F. , Gliem, M. , Spindler, V. & Waschke, J. (2015) E‐cadherin and Src associate with extradesmosomal Dsg3 and modulate desmosome assembly and adhesion. Cellular and Molecular Life Sciences, 72, 4885–4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotzer, V. , Hartlieb, E. , Winkler, J. , Walter, E. , Schlipp, A. , Sardy, M. et al. (2016) Desmoglein 3‐dependent signaling regulates keratinocyte migration and wound healing. The Journal of Investigative Dermatology, 136(1), 301–310. [DOI] [PubMed] [Google Scholar]
- Sato, P.Y. , Coombs, W. , Lin, X. , Nekrasova, O. , Green, K.J. , Isom, L.L. et al. (2011) Interactions between ankyrin‐G, Plakophilin‐2, and Connexin43 at the cardiac intercalated disc. Circulation Research, 109(2), 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinner, C. , Erber, B.M. , Yeruva, S. & Waschke, J. (2019) Regulation of cardiac myocyte cohesion and gap junctions via desmosomal adhesion. Acta Physiologica (Oxford, England), 226, e13242. [DOI] [PubMed] [Google Scholar]
- Schinner, C. , Erber, B.M. , Yeruva, S. , Schlipp, A. , Rotzer, V. , Kempf, E. et al. (2020) Stabilization of desmoglein‐2 binding rescues arrhythmia in arrhythmogenic cardiomyopathy. JCI Insight, 5(9), e130141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinner, C. , Olivares‐Florez, S. , Schlipp, A. , Trenz, S. , Feinendegen, M. , Flaswinkel, H. et al. (2020) The inotropic agent digitoxin strengthens desmosomal adhesion in cardiac myocytes in an ERK1/2‐dependent manner. Basic Research in Cardiology, 115(4), 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinner, C. , Vielmuth, F. , Rotzer, V. , Hiermaier, M. , Radeva, M.Y. , Co, T.K. et al. (2017) Adrenergic signaling strengthens cardiac myocyte cohesion. Circulation Research, 120(8), 1305–1317. [DOI] [PubMed] [Google Scholar]
- Schlegel, N. , Boerner, K. & Waschke, J. (2021) Targeting desmosomal adhesion and signalling for intestinal barrier stabilization in inflammatory bowel diseases‐Lessons from experimental models and patients. Acta Physiologica (Oxford, England), 231, e13492. [DOI] [PubMed] [Google Scholar]
- Schlegel, N. , Meir, M. , Spindler, V. , Germer, C.T. & Waschke, J. (2011) Differential role of Rho GTPases in intestinal epithelial barrier regulation in vitro. Journal of Cellular Physiology, 226, 1196–1203. [DOI] [PubMed] [Google Scholar]
- Schlipp, A. , Schinner, C. , Spindler, V. , Vielmuth, F. , Gehmlich, K. , Syrris, P. et al. (2014) Desmoglein‐2 interaction is crucial for cardiomyocyte cohesion and function. Cardiovascular Research, 104(2), 245–257. [DOI] [PubMed] [Google Scholar]
- Schmidt, E. , Kasperkiewicz, M. & Joly, P. (2019) Pemphigus. Lancet, 394, 882–894. [DOI] [PubMed] [Google Scholar]
- Schmitt, T. , Egu, D.T. , Walter, E. , Sigmund, A.M. , Eichkorn, R. , Yazdi, A. et al. (2021) Ca(2+) signalling is critical for autoantibody‐induced blistering of human epidermis in pemphigus. The British Journal of Dermatology, 185(3), 595–604. [DOI] [PubMed] [Google Scholar]
- Schmitt, T. & Waschke, J. (2021) Autoantibody‐specific signalling in pemphigus. Frontiers in Medicine, 8, 701809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafraz, O. , Rubsam, M. , Stahley, S.N. , Caldara, A.L. , Kowalczyk, A.P. , Niessen, C.M. et al. (2018) E‐cadherin binds to desmoglein to facilitate desmosome assembly. eLife, 7, e37629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoykhet, M. , Trenz, S. , Kempf, E. , Williams, T. , Gerull, B. , Schinner, C. et al. (2020) Cardiomyocyte adhesion and hyperadhesion differentially require ERK1/2 and plakoglobin. JCI Insight, 5(18), e140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigmund, A.M. , Steinert, L.S. , Egu, D.T. , Bayerbach, F.C. , Waschke, J. & Vielmuth, F. (2020) Dsg2 upregulation as a rescue mechanism in pemphigus. Frontiers in Immunology, 11, 581370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikora, M. , Ermel, U.H. , Seybold, A. , Kunz, M. , Calloni, G. , Reitz, J. et al. (2020) Desmosome architecture derived from molecular dynamics simulations and cryo‐electron tomography. Proceedings of the National Academy of Sciences of the United States of America, 117(44), 27132–27140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler, V. , Dehner, C. , Hubner, S. & Waschke, J. (2014) Plakoglobin but not desmoplakin regulates keratinocyte cohesion via modulation of p38MAPK signaling. The Journal of Investigative Dermatology, 134, 1655–1664. [DOI] [PubMed] [Google Scholar]
- Spindler, V. , Drenckhahn, D. , Zillikens, D. & Waschke, J. (2007) Pemphigus IgG causes skin splitting in the presence of both desmoglein 1 and desmoglein 3. The American Journal of Pathology, 171, 906–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler, V. , Eming, R. , Schmidt, E. , Amagai, M. , Grando, S. , Jonkman, M.F. et al. (2018) Mechanisms causing loss of keratinocyte cohesion in pemphigus. The Journal of Investigative Dermatology, 138(1), 32–37. [DOI] [PubMed] [Google Scholar]
- Spindler, V. , Heupel, W.M. , Efthymiadis, A. , Schmidt, E. , Eming, R. , Rankl, C. et al. (2009) Desmocollin 3‐mediated binding is crucial for keratinocyte cohesion and is impaired in pemphigus. The Journal of Biological Chemistry, 284(44), 30556–30564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler, V. , Rotzer, V. , Dehner, C. , Kempf, B. , Gliem, M. , Radeva, M. et al. (2013) Peptide‐mediated desmoglein 3 crosslinking prevents pemphigus vulgaris autoantibody‐induced skin blistering. The Journal of Clinical Investigation, 123(2), 800–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler, V. & Waschke, J. (2014) Desmosomal cadherins and signaling: lessons from autoimmune disease. Cell Communication & Adhesion, 21, 77–84. [DOI] [PubMed] [Google Scholar]
- Stappenbeck, T.S. , Lamb, J.A. , Corcoran, C.M. & Green, K.J. (1994) Phosphorylation of the desmoplakin COOH terminus negatively regulates its interaction with keratin intermediate filament networks. The Journal of Biological Chemistry, 269, 29351–29354. [PubMed] [Google Scholar]
- Troyanovsky, S.M. , Eshkind, L.G. , Troyanovsky, R.B. , Leube, R.E. & Franke, W.W. (1993) Contributions of cytoplasmic domains of desmosomal cadherins to desmosome assembly and intermediate filament anchorage. Cell, 72, 561–574. [DOI] [PubMed] [Google Scholar]
- Troyanovsky, S.M. , Troyanovsky, R.B. , Eshkind, L.G. , Krutovskikh, V.A. , Leube, R.E. & Franke, W.W. (1994) Identification of the plakoglobin‐binding domain in desmoglein and its role in plaque assembly and intermediate filament anchorage. The Journal of Cell Biology, 127, 151–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang, S.M. , Brown, L. , Lin, K. , Liu, L. , Piper, K. , O'Toole, E.A. et al. (2012) Non‐junctional human desmoglein 3 acts as an upstream regulator of Src in E‐cadherin adhesion, a pathway possibly involved in the pathogenesis of pemphigus vulgaris. The Journal of Pathology, 227(1), 81–93. [DOI] [PubMed] [Google Scholar]
- Ungewiss, H. , Rotzer, V. , Meir, M. , Fey, C. , Diefenbacher, M. , Schlegel, N. et al. (2018) Dsg2 via Src‐mediated transactivation shapes EGFR signaling towards cell adhesion. Cellular and Molecular Life Sciences, 75(22), 4251–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewiss, H. , Vielmuth, F. , Suzuki, S.T. , Maiser, A. , Harz, H. , Leonhardt, H. et al. (2017) Desmoglein 2 regulates the intestinal epithelial barrier via p38 mitogen‐activated protein kinase. Scientific Reports, 7(1), 6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen, V.L. (1953) Intercalated discs in heart muscle studied with the electron microscope. The Anatomical Record, 117, 49–63. [DOI] [PubMed] [Google Scholar]
- Veeraraghavan, R. , Hoeker, G.S. , Alvarez‐Laviada, A. , Hoagland, D. , Wan, X. & King, D.R. et al. (2018) The adhesion function of the sodium channel beta subunit (beta1) contributes to cardiac action potential propagation. eLife, 7, e37610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vielmuth, F. , Spindler, V. & Waschke, J. (2018) Atomic force microscopy provides new mechanistic insights into the pathogenesis of pemphigus. Frontiers in Immunology, 9, 485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vite, A. , Zhang, C. , Yi, R. , Emms, S. & Radice, G.L. (2018) alpha‐Catenin‐dependent cytoskeletal tension controls Yap activity in the heart. Development, 145(5), dev149823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, E. , Vielmuth, F. , Rotkopf, L. , Sardy, M. , Horvath, O.N. , Goebeler, M. et al. (2017) Different signaling patterns contribute to loss of keratinocyte cohesion dependent on autoantibody profile in pemphigus. Scientific Reports, 7(1), 3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter, E. , Vielmuth, F. , Wanuske, M.T. , Seifert, M. , Pollmann, R. , Eming, R. et al. (2019) Role of Dsg1‐ and Dsg3‐mediated signaling in pemphigus autoantibody‐induced loss of keratinocyte cohesion. Frontiers in Immunology, 10, 1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke, J. (2008) The desmosome and pemphigus. Histochemistry and Cell Biology, 130, 21–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke, J. (2019) Desmogleins as signaling hubs regulating cell cohesion and tissue/organ function in skin and heart ‐ EFEM lecture 2018. Annals of Anatomy, 226, 96–100. [DOI] [PubMed] [Google Scholar]
- Waschke, J. , Bruggeman, P. , Baumgartner, W. , Zillikens, D. & Drenckhahn, D. (2005) Pemphigus foliaceus IgG causes dissociation of desmoglein 1‐containing junctions without blocking desmoglein 1 transinteraction. The Journal of Clinical Investigation, 115, 3157–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waschke, J. & Spindler, V. (2014) Desmosomes and extradesmosomal adhesive signaling contacts in pemphigus. Medicinal Research Reviews, 34, 1127–1145. [DOI] [PubMed] [Google Scholar]
- Waschke, J. , Spindler, V. , Bruggeman, P. , Zillikens, D. , Schmidt, G. & Drenckhahn, D. (2006) Inhibition of Rho A activity causes pemphigus skin blistering. The Journal of Cell Biology, 175, 721–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed, S.A. & Parsons, J.T. (2001) Cortactin: coupling membrane dynamics to cortical actin assembly. Oncogene, 20, 6418–6434. [DOI] [PubMed] [Google Scholar]
- Yeruva, S. , Kempf, E. , Egu, D.T. , Flaswinkel, H. , Kugelmann, D. & Waschke, J. (2020) Adrenergic signaling‐induced ultrastructural strengthening of intercalated discs via plakoglobin is crucial for positive adhesiotropy in murine cardiomyocytes. Frontiers in Physiology, 11, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yulis, M. , Quiros, M. , Hilgarth, R. , Parkos, C.A. & Nusrat, A. (2018) Intracellular Desmoglein‐2 cleavage sensitizes epithelial cells to apoptosis in response to pro‐inflammatory cytokines. Cell Death & Disease, 9, 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, G. , Qiu, Y. , Zhang, H.M. & Yang, D. (2019) Intercalated discs: cellular adhesion and signaling in heart health and diseases. Heart Failure Reviews, 24, 115–132. [DOI] [PubMed] [Google Scholar]
- Zimmer, S.E. & Kowalczyk, A.P. (2020) The desmosome as a model for lipid raft driven membrane domain organization. Biochimica et Biophysica Acta ‐ Biomembranes, 1862, 183329. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


