Abstract
Gasotransmitters such as NO, H2S, and CO have emerged as key players in the regulation of various pathophysiological functions, prompting the development of gas therapy for various pathogeneses. Deficient production of gasotransmitters has been linked to various diseases such as hypertension, endothelial dysfunction, myocardial infarction, ischemia, and impaired wound healing, as they are involved in the regulatory action of angiogenesis. A better understanding of the regulatory mechanisms has given new hope to address the vascular impairment caused by the breakthroughs in gasotransmitters as therapeutics. However, the unstable nature and poor target specificity of gas donors limit the full efficacy of drugs. In this regard, biomaterials that possess excellent biocompatibility and porosity are ideal drug carriers to deliver the gas transmitters in a tunable manner for therapeutic angiogenesis. This review article provides a comprehensive discussion of biomaterial-based gasotransmitter delivery approaches for therapeutic angiogenesis. The critical role of gasotransmitters in modulating angiogenesis during tissue repair as well as their challenges and future directions are demonstrated.
1. Introduction
The phenomenon of angiogenesis is a complex process involving different steps mediated by different signaling pathways and multiple pro-angiogenic and antiangiogenic factors.1 Angiogenesis is regulated by the balance between pro-angiogenic and antiangiogenic factors rather than a single angiogenic factor. An imbalance in angiogenic factors can lead to either excessive angiogenesis, leading to tumors and inflammatory diseases, or defective angiogenesis, causing impaired healing of injuries and ischemia.2 Any impairment of the vascular system causes various diseases such as cardiovascular diseases, inflammatory diseases, ischemia, skin diseases, etc.3 To address the vascular complications, biomedical innovation has explored various therapeutics, and one of them is the gasotransmitters endogenously present in the system, which offer more therapeutic value.
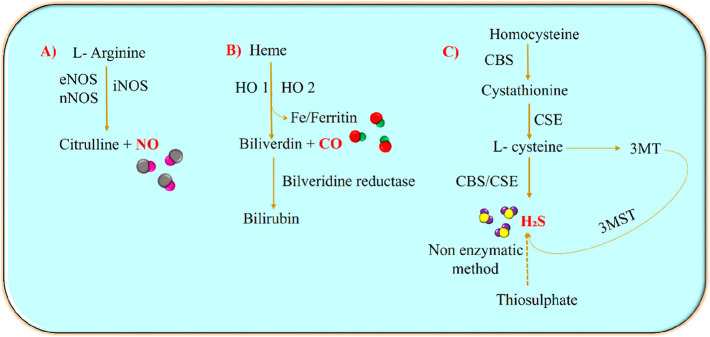
For a long time, the gasotransmitters were considered toxic due to their adverse effects on the biological system, until the functional side of the therapeutic molecules was explored. In addition to angiogenic factors, increasing evidence has shown the involvement of gasotransmitters in the formation and development of the vasculature.4,5 The body’s own gas transmitters nitric oxide (NO), carbon monoxide (CO), and hydrogen sulfide (H2S) can regulate their physiological functions via various signaling pathways.6,7 The catalytic mechanisms for the biosynthesis of NO, H2S, and CO are depicted in Figure 1. These gaseous molecules regulate multiple biological processes such as inflammation, vasodilation, vascularization, and oxidative stress, offering greater therapeutic potential.8 However, few studies have reported both the antiproliferative and pro-tumorigenic effects of the gasotransmitters in tumors.9 For example, when the effect of gasotransmitters such as NO and H2S was studied on colon rectal cancer cells, the NO donors showed antiapoptotic effects, whereas the H2S donors exhibited a decrease in proliferation rate.10
Figure 1.
Catalytic biosynthesis of NO, H2S, and CO: NO, CO, and H2S are synthesized enzymatically from various enzymes such as nitric oxide synthase (NOS), heme oxygenase (HO), and cystathionine synthase (CBS)/cystathionine lyase (CSE), respectively. (A) The three isoforms of NOS catalyze the endogenous production of NO in the physiological system by mediating the oxidation of its substrate l-arginine to citrulline. (B) Heme oxidation via HO 1/HO 2 forms iron, biliverdin, and CO. The biliverdin is then converted to bilirubin with the help of biliverdin reductase. (C) H2S is synthesized using enzymes such as CSE and CBS that catalyze the oxidation of homocysteine, cystathione, and l-cysteine. H2S can also be generated from 3-mercaptopyruvate using 3-mercaptopyruvate sulfur transferase (3-MST) and from thiosulfates via nonenzymatic reactions.
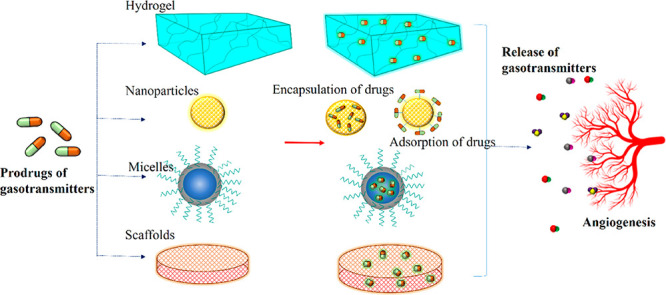
Although the gasotransmitters have proven to be of great therapeutic value, delivering the molecule directly to the target presents several difficulties. There are many small-molecule precursors of gasotransmitters which can release the gas under physiological conditions, but the drug donors are easily disintegrated in vivo before reaching the target sites, reducing the bioavailability of the gasotransmitters.11 Short lifespan, overdose due to the sudden release of the molecule, and the unstable nature of the gas necessitate the use of effective therapies for controlled release of the gas molecules.12 Moreover, an ideal drug carrier should possess greater biocompatibility and biodegradability for it to deliver the drugs without inducing any toxicity in vivo.13 On that aspect, the biomaterials act as an excellent carrier platform for sustained drug delivery which can further degrade into nontoxic metabolites, thus ensuring the biosafety within the body.11 The biomaterials are particularly attractive as they afford platforms that are highly modular, tunable, dynamic, and responsive to small changes in their environments.14 The tunable nature of biomaterial can benefit the therapeutic efficacy of drugs by tailoring physicochemical properties of the material.15 The endeavors to develop materials for gasotransmitter delivery like hydrogels, inorganic/organic hybrids, nanoparticles, microparticles, micelles, and polymeric composites continue to gain strength.16,17 Although drug delivery via a biomaterial system has not been exploited in clinical trials, a large body of in vitro and in vivo research has been conducted toward its sustained therapeutic potential, which is discussed in this review.
Even though the free gas donors without a carrier have a lot of biological potential, their precise delivery to the specific site is not very significant. This limitation can be overcome by designing biomaterial-based drug carriers for efficient delivery of gasotransmitters to the target site. Material scientists design biomaterials or any drug carriers by specifically focusing on the physicochemical nature and the biological potential of the polymers. However, the interaction of the drugs with the microenvironment and their downstream action is neglected, which is essential to understand for designing biomaterials. Here, we aim to delineate this gap of knowledge by discussing the biomaterial-based delivery of gasotransmitters and their role in modulating angiogenesis and various pathways involved in regulating the microenvironment.
2. Endogenous NO in a Physiological System
NO has been considered as a relaxing factor originating from endothelial cells and contributes to various physiological functions. It is a biologically active free radical gasotransmitter molecule that has a shorter lifespan. They are known to induce vasodilation, reduce inflammation, inhibit platelet aggregation, and regulate apoptosis. It is a potent proangiogenic inducer that can mediate endothelial cell proliferation and migration, thus stimulating the formation of new blood vessels from the existing ones.18 At lower nM concentrations, the NO molecules regulate physiological properties, while at higher μM concentrations they exhibit toxic effects.19 Any interference with the NO synthesis pathway through metabolic or genetic effects would affect angiogenesis. Several NO inhibitors are also known to disrupt the angiogenic pathway, indicating the association of NO with angiogenesis.20 The endogenous NO molecule is synthesized from arginine enzymatically and nonenzymatically via the nitrate–nitrite pathway.18
The biosynthesis of NO is regulated by the constitutive expression of endothelial NO synthase (eNOS), neuronal NO synthase (nNOS), and inducible NO synthase (iNOS). The three isozymes use l-arginine as a substrate to synthesize NO and l-citrulline molecules.21 All NOS enzymes contain an N-terminal oxygenase domain and a reductase domain to which the substrate molecules and other regulatory molecules bind. The active form of the NOS enzyme exists as a homodimer with a zinc ion attached to four cysteine residues on the surface of the dimer. In the inactive state it is found bound to the caveolin protein in the caveolar region of the membrane. The increased intracellular influx of Ca2+ and calmodulin activates the NOS by detaching the caveolin from the enzyme, leading to a conformational change and activation of the substrate-binding domains.5l-Arginine is the precursor molecule of NO, which normally acts as a substrate for NOS under physiological conditions.22 In particular, the terminal guanidine residue of arginine accepts five electrons via an oxidative catalytic mechanism and uses molecular oxygen and NADPH as cosubstrates to generate NO and citrulline. The nitric oxide synthase enzyme (NOS) first hydroxylates the terminal guanidine residue of l-arginine to generate nitric oxide-hydroxyl-l-arginine (NOHA) as an intermediate which is further oxidized to form the final products.23 This catalytic reaction is very specific as even the d-form of arginine cannot act as a substrate for the enzyme NOS.24 In addition, the bioavailability of the substrates also plays a major role in determining the functional activity of the enzyme.5
2.1. Intracellular Signaling Pathways of NO and Angiogenesis
In the physiological system, angiogenesis is a well-organized and tightly regulated process since any impairment would lead to cancer, inflammatory diseases, and metabolic disorders. Of all the angiogenic factors, VEGF is the most critical factor that regulates blood vessel formation.25 At the same time, NO has inducible effects on angiogenic factors such as VEGF and regulates proliferation, differentiation, and apoptosis in tissue regeneration. NO can stimulate VEGF-induced angiogenesis to form new blood vessels from existing blood vessels by activating multiple signaling pathways. In this sense, NO-based therapy has received a lot of attention in the field of regenerative medicine to repair the damaged tissue.26 The signaling pathways involving NO and angiogenic factors for the regulation of vascularization are discussed below.
2.1.1. VEGF Pathway
The VEGF pathway is one of the main pathways for NO to carry out its proangiogenic functions.27 VEGF, which is a central mediator of angiogenesis, is thought to stimulate the eNOS pathway for NO synthesis by activating VEGFR-1 and VEGFR-2 to regulate angiogenesis. Endothelial cell proliferation, migration, and differentiation are further regulated by downstream signaling of protein kinase C (PKC) and phosphatidylinositol-3-kinase (PI3K) and Akt pathways.28 Studies have demonstrated the role of NO in VEGF-induced angiogenesis. For example, eNOS (−/−) mice showed a weak angiogenic response to treatment with VEGF, specifying the role of eNOS in VEGF-induced vascular development.29
2.1.2. NO/SGC/cGMP Pathway
Soluble guanylyl cyclase (sGC) serves as an intracellular receptor for NO, converting guanosine triphospahte (GTP) to cyclic guanosine monophosphate (cGMP). sGC consists of heterodimers α and β and subunits, with the NO-binding heme domain present in the β subunit.30 Both subunits must be connected to each other for the catalytic domain to be active. The increased level of cGMP in turn opens the Ca2+ channels, which further activates the Protein Kinase G ( PKG) to initiate neovascularization.31 The association of cGMP signaling in NO-induced angiogenesis was illustrated using a rat hindlimb ischemia model. Rats treated with a NO donor, MPC-1011, showed improved angiogenesis with increased levels of proangiogenic factors such as VEGF and cGMP. However, the NO inhibitor responded with reduced angiogenic effects, explaining the involvement of the cGMP signaling pathway in NO-induced angiogenesis.32
2.1.3. PI3K/Akt Pathway
NO-induced angiogenesis via PI3K/Akt was demonstrated in HUVEC cells by simulating microgravity, electric field, and ultrasound, and the influence of NO was evaluated by the quantification of eNOS expression.33−35 The electric field, which is a positive regulator of angiogenesis, stimulated eNOS expression and induced activation of the Akt pathway. In contrast, when the PI3K pathway was inhibited, NO production and the subsequent angiogenesis process were impeded, demonstrating that the PI3K/Akt pathway is one of the major signaling pathways involved in NO-mediated angiogenesis.35
2.2. NO in Placental Angiogenesis
NO is one of the most important biological molecules in the physiological system during pregnancy as it acts as the main vasodilator in the placenta.36 Impaired NO regulation during pregnancy would result in vascular disease, hypertensive pregnancy disorders, intrauterine growth restriction, gestational diabetes, and preeclampsia. NO stimulates the proliferation of endothelial cells in the fetus and regulates the growth of blood vessels. It also regulates VEGF and angiopoietin-signaling molecules to induce vasculogenesis and angiogenesis in the placenta.28 It is also involved in the regulation of vascular tone and placental development and apparently plays an important role in normal placental development. For example, when pregnant IUGR patients were treated with NO donors, increased expression of an epidermal growth factor like domain 7 (EGFL7) and enhanced fetal development were observed, suggesting the association of NO and angiogenic factors in placental development.37 Thus, angiogenesis mediated by NO via the eNOS pathway plays a crucial role in placental development.
2.3. Role of NO in Wound Healing
NO can promote the migration of epithelial cells and induce the formation of new blood vessels and can therefore be used for tissue regeneration and wound healing.38 The NO produced by the cells can also induce antioxidant activity against the oxy radicals in the wound niche, thereby enhancing the healing process.39 An appropriate level of NO in inflammatory and proliferative stages of the wound could benefit the healing process of cutaneous wounds. However, uncontrolled exposure of NO to the wound environment could negatively regulate the angiogenesis and lead to chronic wounds.40 Nevertheless, the inflammation-regulating potential and potent antimicrobial property make NO a therapeutic of high value in wound healing.21
2.3.1. NO and Embryonic Wounds
Generally, acute inhibition of NOS significantly reduces the NO production, further negatively regulating the wound healing process.20 For that reason, the rate of wound closure was slower in the NO-inhibited embryos compared to the control embryos. The wounds of the normal embryo closed after 90 min, whereas healing and wound closure in the NOS-inhibited embryos stopped after 30 min, indicating the chronic establishment of a NO-free wound. In addition, the immunohistochemical studies revealed the accumulation of cells at the wound edges, preventing cell migration in NOS-inhibited embryos. It also increased stress levels at the wound margin due to the increased expression of stress response genes. Thus, NO turns out to be crucial for the regulation of the healing process in embryonic wounds, especially in the early phase.41
2.3.2. NO and Diabetic Wounds
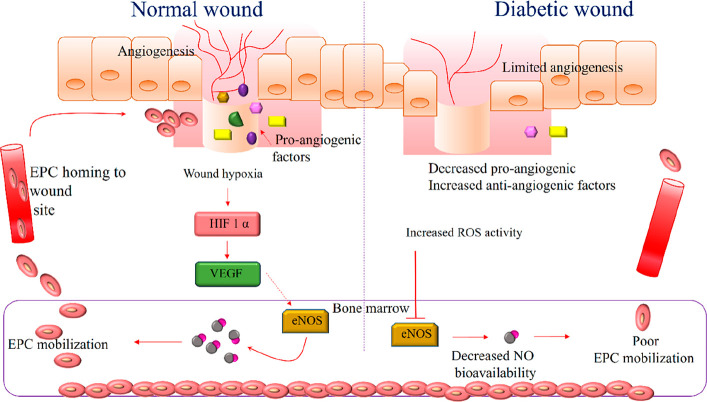
Diabetic wounds are chronic, nonhealing wounds with an increased risk of infection that can eventually lead to amputation. A better understanding of the pathophysiology and molecular functions of diabetic ulcers is important to develop improved therapies and treatments for this complication.42 Diabetes is physiologically characterized by the decreased bioavailability of NO in the wound environment. In normal wounds, acute tissue hypoxia at the wound site activates the hypoxia-inducible factor-1α (HIF-1α) signaling pathway, which promotes VEGF release in the epithelial cells. The VEGF phosphorylates and activates eNOS in the bone marrow, resulting in increased NO levels. The NO further stimulates the mobilization of endothelial progenitor cells, which are the key regulators of vascular repair at the wound site for neovasculogenesis. In diabetic wounds, phosphorylation and activation of eNOS are inhibited, further impeding the mobilization and function of endothelial progenitor cells, resulting in impaired wound healing.43 The regulatory pathway of angiogenesis mediated by NO in normal and diabetic wounds is depicted in Figure 2.
Figure 2.
Angiogenesis in normal and diabetic wounds: In normal wounds, the hypoxic niche stimulates the activation of HIF factors, which mediate the synthesis of VEGF via signaling pathways. The VEGF activates the eNOS pathway for the synthesis of NO in bone marrow cells, which mobilizes the EPC to the wound site for angiogenesis. In the case of a diabetic wound on the right, an increased level of ROS inhibits eNOS activity, which consequently limits EPC mobilization, resulting in impaired healing.
In addition, sustained hyperglycemia in the diabetic state increases superoxide levels in the vasculature, which subsequently inhibits NO production. This was reported in a study in which the increase in glucose proportionally increased superoxide levels in streptozotocin-induced type 1 diabetic mice. When the diabetic mice were treated with manganese superoxide dismutase (MnSOD) gene therapy, superoxide was significantly reduced as NO levels increased, further improving the wound healing process.44 In another study, when polyvinyl alcohol (PVA) sponges containing the NO donor molsidomine were implanted into diabetic wounds in rats, an increased level of hydroxylproline corresponding to the formation of new collagen was observed. Higher concentrations of metalloproteinase 2 (MMP2), which contribute to matrix remodeling, were also observed in the treated diabetic rats, reflecting the influence of NO on MMP2.45 Similarly, polyvinyl methyl ether-co-maleic anhydride (PVMMA) and polyvinylpyrrolidone (PVP) have been reported as drug delivery complexes for the controlled release of NO at the diabetic wound site. The NO precursor S-nitrosothiols (RSNO) were immobilized on the interpolymer complex, resulting in improved stability. Topical application of the polymer complex to the wounds of the diabetic rat model showed improved wound healing with sustained release of NO from the drug carrier.46
2.3.3. Influence of NO on Collagen Deposition
Collagen accumulation during wound healing is significantly associated with nitrate and nitrite molecules, implying that NO plays a crucial role in collagen deposition in dermal fibroblasts and confers mechanical strength to the skin.47 According to the reports, when NOS inhibitors were administered to the wound area, delayed wound healing with lower collagen accumulation rate and skin firmness was observed. On the other hand, delivery of NO and transfection of iNOS enhanced the matrix synthesis and collagen deposition.44
2.4. NO-Based Biomaterials for Therapeutic Angiogenesis
Therapies involving the synergistic use of polymer-based biomaterials with NO donors will have significant implications for tissue engineering. The biological and pathophysiological role of NO has triggered its exogenous delivery to targets for various biomedical applications. In this regard, the precursors of NO can be incorporated into the biomaterials to efficiently deliver the therapeutics to the target site. The low molecular weight free prodrugs have disadvantages such as burst release and nonspecific delivery, while the drugs can be delivered to specific targets within the biomaterial in a sustained manner.48 The biomaterials used for carrying the NO prodrugs are listed in Table 1. Gaseous molecules incorporated into biomaterials such as hydrogels, nanoparticles, surface coatings, and microspheres as angiogenic therapeutics are discussed below.
Table 1. Biomaterials as Carriers for NO Prodrugs in Various Applications.
| composition | biomaterial | stimuli | NO prodrugs | application | ref |
|---|---|---|---|---|---|
| CS-PVA | Hydrogel | SNAP | Wound healing | (56) | |
| CS | Injectable hydrogel | Glycosidase | Diazeniumdiolates | Inhibited platelet adhesion, promotes angiogenesis | (62) |
| PLGA | Nanoparticle | GSNO | Antibacterial effects | (129) | |
| Cerium oxide | Nanoparticle | SNAP | Antimicrobial effects | (130) | |
| Alginate, pectin, PEG | Hydrogel | GSNO | Antibacterial effects, promotes angiogenesis | (131) | |
| PEG–PNORM–PEG | Micelle | Light | DNP | (132) | |
| Keratin/doxorubicin | Nanoparticles | pH/GSH/trypsin | GSNO | Inhibits cancer cell proliferation | (133) |
| Graphene oxide zeolitic imidazolate | Nanoparticle composite | H2O2 | l-Arginine | Tumor cell apoptosis | (134) |
| PVA | Injectable microvesicles | H2O2, magnetic responsive | l-Arginine | Blood glucose homeostasis | (135) |
| Alginate–PVA | Scaffold | Temperature responsive | GSNO | Fibroblast migration | (136) |
2.4.1. Hydrogels
Hydrogels are porous, hydrophilic biomaterials with tunable properties that mimic the ECM and can serve as a suitable biomaterial for tissue regeneration. In addition, they also expand their potential applications in the field of drug delivery and serve as excellent drug carrier systems.49 A well-designed drug delivery system can overcome the problems of traditional delivery approaches and maximize the therapeutic efficacy of the drug. Hydrogels can also ensure sustained release of drugs, and the porosity helps in the formation of new blood vessels. In addition, certain polymeric hydrogels such as hyaluronic acid and collagen hydrogel contribute to angiogenic and healing properties.50
a. NO-Releasing Hydrogels with Stem Cells
NO-releasing hydrogels seeded with stem cells are capable of enhanced angiogenesis and increased regenerative potential for tissue repair. A CS-based NO-releasing hydrogel was synthesized to transplant mesenchymal stem cells (MSCs) for treatment of hindlimb ischemia in mice. The NO donor diazeniumdiolate (NONOate) was grafted onto chitosan together with β galactose; under the action of β galactosidase, β galactose is detached from the donor; and the free NONOate releases the NO in the physiological state.51 Delivery of NO via the NO donor NONOate enhanced the angiogenic effects of the stem cells with increased capillary density and improved ischemic limbs in the mouse model. Here, the prepared hydrogels with NO donors offered drug delivery in a sustained manner without burst release and overdose.52
In another approach, programmed release of NO from hydrogel synthesized by transglutaminase-catalyzed enzymatic cross-linking of gelatin has been reported. Here, the transglutaminase enzyme mediates accumulation of NH3 in gelatin, which is partially oxidized by the biological cycles such as NO and urea cycles to synthesize endogenous NO.53 For further studies, the adipose-derived mesenchymal cells and bone-marrow-derived mesenchymal cells were cultured in the NO gels, which exhibit excellent tubular formation along with the upregulation of proangiogenic genes such as the platelet EC adhesion molecule 1 (PECAM1) and the fetal liver kinase 1 (Flk1).18 In a similar study, FFGGG peptide-grafted naphthalene hydrogel was encapsulated with a NO donor surrounded by β galactose. In the presence of β galactosidase, the hydrogel can release NO in a sustained manner because the galactosidase enzyme can cleave the galactose molecule, leaving the NO donor free. Furthermore, the adipose-derived mesenchymal stem cells (AD-MSCS) were seeded onto the hydrogel to determine the effect of NO on the therapeutic and angiogenic efficacy of adipose-derived mesenchymal stem cells on myocardial infarction in mice. The NO-based hydrogel seeded with MSC showed improved HUVEC migration in the scratch assay, whereas the stem cells seeded on hydrogel with a NO inhibitor showed poor cell migration. The study also suggested that the NO-based hydrogel showed increased VEGFR2 production via signaling pathways and significant improvement in blood vessel density in myocardial infraction hearts.54 Studies have shown that the NO can stimulate the expression of the cytoskeletal proteins in the endothelium which could be the consequence of an improved migration rate.55
b. Cell-Free NO-Releasing Hydrogels
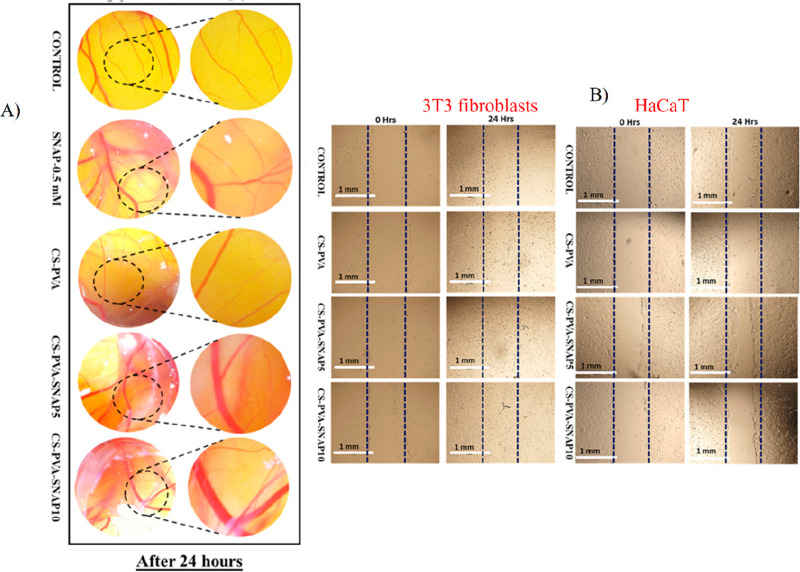
Hydrogels synthesized from chitosan (CS) and PVA polymers encapsulated with NO donor S-nitroso-N-acetylpenicillamine (SNAP) showed enhanced angiogenic and healing properties. The polymer blend hydrogel loaded with 10% SNAP showed an increased level of new blood vessel formation when injected into a chick embryo model (Figure 3).56 In another example, a NO-releasing injectable redox hydrogel (NO-RIG) composed of an A–B–A-type triblock copolymer (PArg–PEG–PArg) and PMNT–PEG–PMNT coupled with poly(acrylic acid) (PAAc) was developed to treat cardiovascular disorders by controlling the NO level and redox equilibrium57 simultaneously at the local injection sites. After 7 days of intradermal injection, the mice treated with RIG without NO showed no to very little new blood vessel formation, whereas the mice treated with RIG with NO showed higher induction of angiogenesis.22 However, here the redox imbalance was stabilized by the antioxidant potential of the polymers used, and the NO had no effect on the scavenging activity.58
Figure 3.
In vitro and in vivo angiogenic effects of SNAP-loaded CS PVA hydrogel: (A) The in vitro cell migration assay on 3T3 and HaCaT cells. Complete closure of wounds in CS–PVA–SNAP after 24 h was observed in fibroblast cells, while only partial closure was observed in HaCat cells. (B) In vivo angiogenesis in the chick embryo model showing the newly formed blood vessels. The CS–PVA–SNAP-treated chick embryo showed improved pro-angiogenic activity with increased blood vessel size and length at 24 h. (Adapted with permission from Zahid et al. Copyright 2019 Elsevier.)
Similarly, using another polymer, an alginate hydrogel with diethylenetriamine/diazeniumdiolate (DETA/NONOate) as the NO donor was developed to accelerate healing of infected wounds. This hydrogel together with its bactericidal effect increased the rate of cell proliferation and induced tissue remodeling in the mouse model. After 6 days of hydrogel application, the mouse wound surfaces showed reduced infection and also improved wound closure. At the same time, alginate/DETA without NO showed no improvement in healing and no reduction in bacterial load, while by day 12, 96% of wound closure without a scar was observed with the NO-loaded gel, indicating the importance of NO on the wound healing process.59 In a related study, an injectable hydrogel of chitosan and hyaluronic acid incorporated with NO-donor RSNO was developed to accelerate wound healing in infected wounds. The hydrogel showed potent antibacterial activity of 99% and 98% against E. coli and S. epidermidis, respectively, after 1.5 h. Furthermore, the in vitro scratch wound test showed the improved migration of NIH 3T3 with 100% closure within 48 h.60
A reduction in NO bioavailability leads to endothelial dysfunction, which is one of the pathophysiological features of critical limb ischemia. Therefore, therapeutic angiogenesis to increase blood flow in tissues and alleviate the ischemic condition has become an important strategy for treating critical limb ischemia.61 The unstable nature and uncontrolled release of NO complicate its application for therapeutic approaches. To solve the problem, researchers developed terminally galactose-protected NO donor compounds that can only be cleaved using glycosidase to release NO. Additionally, an azide group was introduced into the NO donor for the covalent attachment of NO to the chitosan polymer. In vivo studies in diabetic mice with hindlimb ischemia point out that injection of the hydrogel improved capillary densities with microvessel formation in the CS–NO-treated mice compared to the CS-treated mice, indicating the angiogenic potential of NO.62 The NO from eNOS potentially plays a vital role in the microvascular stability and the microvascular tone regulation, which are consistent with the findings.63
c. Nanoparticle-Loaded NO Hydrogel
Graphene oxide functionalized with BNN6 (NO donor) and β-cyclodextrin nanocarriers was incorporated into the polymer blend of methacrylated gelatin and HA doped with dopamine to develop a photothermal hydrogel for efficient wound healing. The released NO showed potential antibacterial and wound healing activities in both in vivo and in vitro studies. Furthermore, more well-structured collagen deposition, re-epithelialization, and raised fibroblasts with more capillaries were observed with the synthesized hydrogel than in the control groups.64
2.4.2. Microspheres
Microspheres are small spherical particles ranging in diameter from 1 to 1000 μm that can provide high bioavailability with sustained release of drugs.65l-Arginine incorporated into sodium alginate microspheres was formulated to delay the release of NO at the target wound site for accelerated healing of diabetic wounds. The microspheres were integrated into chitosan hydrogel, and the CS–hydrogel composite was further layered on their outer shell with sodium alginate/AgNPs. The former sustainably deliver the NO to the target site, and the latter provide antibacterial activity at the wound site. The in vivo studies also demonstrated the enhanced re-epithelialization, granulation, and collagen deposition of the NO-based microspheres on diabetic wounds, highlighting the influence of NO on angiogenesis.66
2.4.3. Nanomaterials
Nanoparticles used for drug delivery systems are generally <100 nm in at least one dimension, with excellent properties such as specificity and increased cell uptake due to the surface charge and size.67 A nanosystem of gelatin and siloxane nanoparticles was designed to deliver NO at the target site for improved vascular homeostasis. RSNO, the NO donor, was functionalized to the nanoparticle composite for the controlled release of NO at the target site. The inhibition of aortic smooth muscle cell (AoSMC) proliferation and stimulation of HUVEC proliferation took place in a concentration-dependent manner which is significant for preventing restenosis (narrowing of lumen).68 The size of the nanoparticles confers a greater advantage as they can penetrate through the cell membrane and can deliver the drugs to the target sites, avoiding the loss of drugs outside the niche.69
2.4.4. Surface Coatings
NO donors coated on the 3D-printed vascular grafts were designed for the sustained release of NO under physiological conditions to enhance angiogenic potential and endothelial cell regeneration. Conventional vascular grafts typically have a poor success rate due to infection and obstruction during implantation. However, the NO-donor SNAP-coated vascular graft of PEG and polylactic acid (PLA) showed antibacterial effects against both Gram-positive and Gram-negative bacteria. The NO-based grafts were also able to mimic the native endothelial cells and induced increased neovascularization compared to the non-NO-coated control grafts.70 The increased migration rate of endothelial cells could be due to the upregulation of cytoskeletal proteins upon treatment with NO, which can subsequently accelerate the tissue regeneration process.71
2.4.5. Microneedle
Microneedles synthesized from hydrogels offer a minimally invasive method for transdermal drug delivery. These delivery systems have higher drug loading capacity and controlled drug release. The biocompatibility of the polymeric microneedle can overcome the toxicity induced by traditional metallic microneedles.72 Hydrogel-forming microneedles were developed by integrating the NO donor S-nitrosoglutathione (GSNO) with PVA at freezing temperatures. The NO is said to be released gradually with increasing temperature and in response to mild infrared radiation. The hydrogel’s microneedle structure disrupts the biofilm formed on the wound surface and delivers NO directly to the site, thereby fixing regeneration in infected wounds.73 Here, the infrared radiation as a stimuli can precisely deliver the NO to the target area, thus favoring the healing process. This suggests that, in addition to the angiogenic potential of NO, the delivery method also imparts its role in promoting angiogenesis and vasodilation.74
3. Physiological Role of H2S in Angiogenesis
H2S is the recently explored gasotransmitter with potential biological values that play significant roles in homeostasis and pathophysiology. A growing body of evidence has shown that delivery of H2S to endothelial cells can significantly enhance cell proliferation, capillary tube formation, and cell migration. H2S is diffusible and spreads its function to neighboring cells via a paracrine action, just like other gas transmitters mediating cellular activities.4 The physiological H2S level in the tissue is estimated to be between 15 nM and 300 μM.60In vivo studies have also shown the positive regulation of H2S in neovascularization and blood vessel growth.
The endogenous H2S can be synthesized by both enzymatic and nonenzymatic mechanisms and is widely distributed in all mammalian cells and tissues. The enzymes involved in the synthesis of H2S are CBS, CSE, and 3-MST. The first two enzymes catalyze the conversion of methionine to cysteine in the presence of pyridoxal-5-phosphate (PLP) as a cofactor through transsulfuration. The latter enzyme converts mercaptopyruvate into pyruvate and H2S without using PLP as a cofactor.75 The nonenzymatic mode uses organic compounds such as glucose, glutathione, and elemental sulfur to synthesize H2S, and these pathways are not well elucidated.
3.1. H2S Donors
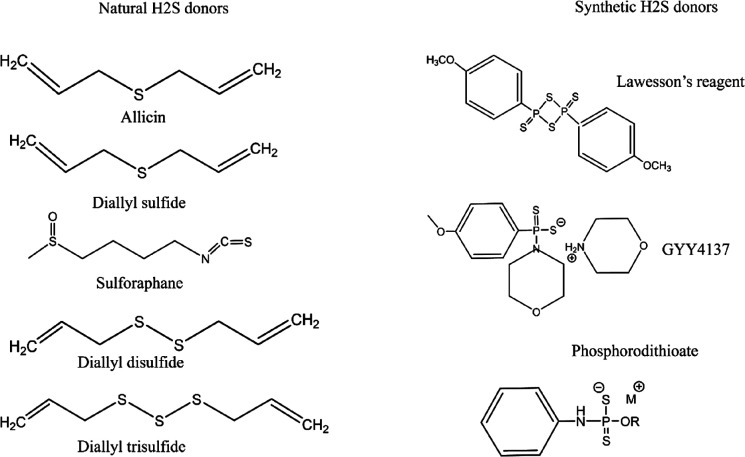
H2S donors are compounds that can release the H2S molecule in response to a trigger or stimuli under physiological conditions. The H2S donors such as sodium hydrosulfide (NaHS) and sodium sulfide (Na2S) are in the form of sulfide salts. In addition, naturally occurring compounds such as garlic can also release H2S from the existing thiosulfonates. An example of thiosulfonate in garlic is allicin, which can be broken down into diallyl disulfide (DADS), diallyl sulfide (DAS), and diallyl trisulfide (DATS) to release H2S in the presence of free thiols in the physiological system.76 Cruciferous vegetables contain natural isothiocyanates such as sulforaphane, allyl isothiocyanate, benzyl isothiocyanate, 4-hydroxybenzyl isothiocyanate, and erucine, which possess H2S-releasing activity.77 Another H2S-releasing compound is Lawesson’s reagent, which releases H2S in a controlled manner but lacks water solubility.78
Although the naturally occurring donors release H2S in a sustained manner in the physiological system, they show poor pharmacokinetic profiles and generate undesirable byproducts. This shifted the researchers’ attraction to the synthetic donors, which are categorized as hydrolysis-triggered donors, pH-controllable H2S donors, thiol-triggered donors, and enzyme-triggered donors.79 The different donors of H2S are represented in Figure 4.
Figure 4.
Natural and synthetic donors of H2S.
3.2. Regulatory Pathways Involved in the Pro-Angiogenic Effects of H2S
Like NO, H2S also mediates various biological functions via different signaling pathways. It is said to mediate both vasoconstriction and vasodilation, with a lower concentration of H2S (<100 μM) stimulating vasoconstriction and a higher concentration promoting vasodilation. The higher H2S level changes the membrane potential of KATP channels to become more negative (hyperpolarized) in vascular smooth muscle cells. This further closes the membrane Ca2+ channel and decreases intracellular Ca2+, resulting in smooth muscle vaso-relaxation.80 Studies on the effect of H2S on KATP channels in piglet cerebral arteriole smooth muscle cells showed that the gas molecule can mediate the opening of KATP channels. Treatment of the cerebral arterioles with Na2S dilates the narrowed arteries by activating the KATP channel and reducing intracellular Ca2+, implying the H2S-induced vasodilation.81 Another study stated the role of K+ in Na2S-induced relaxation of CRC mesenteric arteries. It demonstrated that H2S activates the KATP channels of the mesenteric arteries which stimulates the membrane hyperpolarization by inhibiting the Ca2+ influx, resulting in vasodilation.82
3.2.1. PI3K/Akt Signaling Pathway
PI3K/Akt is an important signaling pathway that regulates various angiogenic activities such as cell migration, proliferation, new blood vessel formation, and tube formation. Dose-dependent treatment of endothelial cells with NaHS had a significant impact on Akt signaling. The exogenous H2S increased Akt phosphorylation, while PI3K inhibitors attenuated H2S-induced phosphorylation, specifying the role of H2S in Akt signaling. The in vivo studies suggested that, when injected intraperitoneally with NaHS, H2S showed increased cellular infiltration and new blood vessel formation.83 The Akt pathway generally induces cell survival by inhibiting the pro-apoptotic factors such as BAD and caspase 9. However, the detailed mechanism of how Akt regulates cell migration and proliferation for angiogenesis is not yet understood.
3.2.2. VEGF Pathway
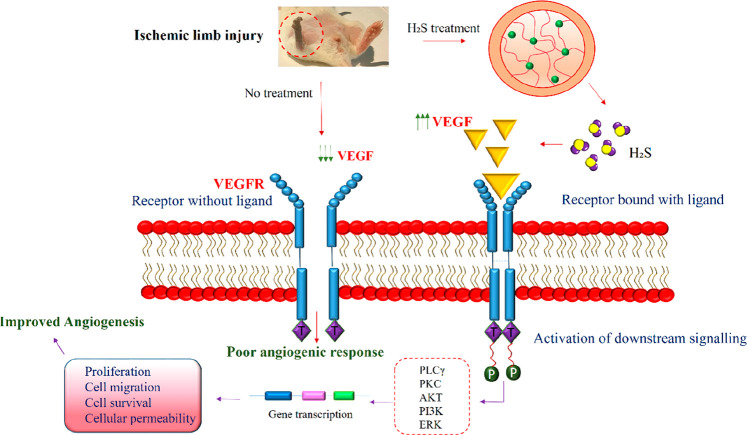
In VEGF signaling, the binding of VEGF ligands to the VEGFR receptors dimerizes and autophosphorylates the tyrosine kinase receptors and subsequently regulates the transduction of downstream signaling. The downstream molecules such as PLC-γ, PKC, Akt, PI3K, and ERK are activated for angiogenic processes such as cell survival, migration, and endothelial cell proliferation.80 The ischemic tissues of diabetic mice showed reduced phosphorylation of VEGFR, Akt, and ERK proteins and a negative effect on the angiogenic process. When the ischemic limb of diabetic mice was treated with H2S, serum levels of VEGF and PDGF were significantly increased with improved wound healing, indicating the involvement of VEGFR signaling in angiogenesis.83 The H2S-mediated VEGF-induced angiogenesis pathway in ischemic limb injury is illustrated in Figure 5
Figure 5.
H2S-mediated VEGF signaling for angiogenesis in ischemic limb injury: H2S treatment of ischemic limb injury stimulated increased VEGF production which subsequently binds to the VEGFR receptor that further activates the downstream signaling molecules PLCγ, PKC, Akt, PI3K, and ERK. These factors regulate the expression of various genes responsible for cell proliferation, cell migration, permeability, and cell survival, thereby mediating angiogenesis. The untreated mice did not evoke the production of VEGF, thus resulting in poor angiogenic response.
3.3. Role of H2S and Angiogenesis in Diabetes
Several studies have supported the role of H2S in modulating the angiogenic factors for the systematic formation of blood vessels in diabetic complications such as chronic wounds and ischemic injuries.84,85 The H2S deficit in diabetic models affects angiogenesis by creating an imbalance between the antiangiogenic and the proangiogenic factors. In the normal physiological state, microRNA (miR-126–3p) acts as a pro-angiogenic factor and promotes angiogenesis, while in diabetics, plasma levels of miR-126–3p drop drastically and affect angiogenesis. The influence of H2S on (miR)-126–3p and angiogenesis was determined by treating the high-glucose HUVEC cells with H2S. The exogenously introduced H2S enhanced migration of HUVEC cells and upregulated miR-126–3p, which further mediated angiogenic activities.86
Similarly, NaHS delivery mediated collagen deposition and inhibited MMP 9 in the granulated tissues of wounded obese mice.87 Diabetes-induced ischemic injured mice were treated with H2S donor sodium bisulfide, and angiogenic transcription factors were quantitated using real-time PCR and Western blotting. Increased expression of VEGF, EGF, PDGF, HIF 1α, and angiogenic factor receptors was observed in the treated animals, indicating the role of H2S in angiogenesis-related complications of diabetes.83 Although studies have shown the positive regulation of H2S on diabetes-impaired angiogenesis, the precise regulatory mechanisms are not well understood.
3.4. H2S-Based Biomaterials for Therapeutic Angiogenesis
3.4.1. Hydrogel
A pH-controllable H2S donor JK1 was incorporated into a hyaluronic acid (HA) hydrogel to evaluate the angiogenic potential of the H2S–HA hydrogel system. The hydrogel encapsulated with JK1 showed a better pH-dependent release profile of H2S than JK1 alone. The H2S-based gel induced polarization of macrophages toward M2, which reduced inflammation and promoted improved healing.88 In this approach, the M2 macrophages could have offered their role in angiogenic processes through cell proliferation and secretion of VEGF factors as reported in other studies.89 In another report, alginate hydrogel was incorporated with free H2S molecules to determine the wound healing potential of the dressing. In vivo studies on mice wounds showed improved granulation tissue formation and re-epithelialization along with faster wound closure than control, demonstrating the role of H2S in wound healing.90 In another example for cardiac tissue repair, a novel composite H2S-releasing hydrogel made up of polyethylene glycol–fibrinogen hydrogel (PFHy) was modified by embedding perfluorohexane-filled bovine serum albumin microbubbles. They were coated with rhodanese (TST), which catalyzes the production of H2S in the physiological system. The in vitro study showed increased proliferation of hCPC cells, suggesting the possibility that the gasotransmitters induce proliferation.91
Scaffolds are crucial biomaterials used in regenerative medicine due to their surface properties and porous nature. The topographical properties of scaffolds favor tissue regeneration and vascularization, fuelling researchers’ interest in integrating therapeutics with scaffolds for drug delivery and regenerative medicine. In this context, a silk fibroin polymer scaffold loaded with GYY4137 (morpholin-4-ium-4-methoxyphenylphosphinodithioate), a slow-releasing H2S donor, was prepared to determine the osteogenic potential of bone cells. The scaffold released the H2S for 12 h at a concentration of 3.5 μM to 10 μM in which osteogenic proliferation and differentiation were also observed. The expression of the mRNA of the angiogenic nuclear factor VEGF was higher in the H2S-loaded hydrogel than in the silk fibroin hydrogel. This demonstrates the role of H2S in VEGF regulation to stimulate angiogenesis.92 A functional sodium alginate sponge encapsulated with JK-1 was developed that was able to release H2S in a controlled manner in the acidic pH of the wound environment. The sodium alginate/JK-1 enhanced the proliferation, migration, and revascularization of fibroblasts, implying the angiogenic potential of the nanomaterial incorporated with H2S.93 The sponges exhibit excellent ability to absorb the excess exudates from the wound while maintaining the moist environment which could also have benefited the healing process.94
Furthermore, the role of the immune system in H2S-induced angiogenesis was demonstrated in immunocompetent and immunocompromised mice (lacking T cells). For this, mice were implanted with a poly(d,l-lactide-co-caprolactone) scaffold and injected intraperitoneally with NaHS, while control mice received saline. The immunocompromised mice had compromised vasculature and angiogenesis, whereas vascularization in the immunocompetent mice showed a significant increase, indicating the role of the immune system in H2S-mediated angiogenesis.95 As per the report, T cells are involved in the H2S-mediated angiogenesis with the fact that H2S activates DNA methylation of Foxp3 T regulatory cells to promote immune tolerance,which can induce anti-inflammatory responses.96
3.4.2. Fibrous Membranes
Due to their large surface-to-volume ratio, fiber membranes are suitable carrier systems for gas transmitters that can stimulate the controlled release of gas molecules such as H2S. Here, the garlic H2S donor was integrated into the PLA fibrous membrane to assess the impact of H2S on human cardiac MSC. The cells on the fibrous membrane proliferated after 24 h of growth and also showed antimicrobial activity.97 An electrospun fiber membrane was fabricated using polycaprolactone (PCL) with integrated H2S donor JK1 for skin regeneration. In the acidic environment of the wound, the pH-dependent H2S donor released the gas molecule faster than that in the basic environment. In vivo skin wound models showed faster wound closure in PCL containing JK1 compared to PCL nanofibers. Furthermore, the tissues treated with the former group showed complete re-epithelialization and granulation, while the latter group without H2S showed delayed healing. The H2S also induced the formation of many new blood vessels and stimulated collagen deposition in the wound model tissues, demonstrating the potential angiogenic effects of H2S.98
3.4.3. Composite Polymers and Particles
A H2S-based polymer composite was developed by conjugating Na2S with a silicone–polycarbonate–urethane polymer. The composite polymer showed cytocompatibility against HUVEC and HLE cell lines. The fibroblasts were able to proliferate within 24 h and fully migrate within 48 h in in vitro studies, indicating the potential of the composite for use in regenerative medicine.99
Sodium thiosulfate, which releases H2S upon certain stimuli, was encapsulated in polylactic-co-glycolic acid and showed sustained release of H2S over 24 h. The formation and branching of the tubular network apparently increased in HUVEC cells when treated with the H2S-donor-encapsulated nanomaterial.100 In a related study, mesoporous silica nanoparticles were synthesized and loaded with an H2S-releasing compound, DATS. The silica nanoparticles have greater affinity for cell wall phospholipids, and the particle size of the nanomaterial provides easier uptake and facilitates drug delivery to the specified target site. The H2S released from nanoparticles enhanced endothelial proliferation and tubular formation. In addition, the treated cells activated the ERK and p38 pathway, which plays a crucial role in angiogenesis via endothelial migration and proliferation.101
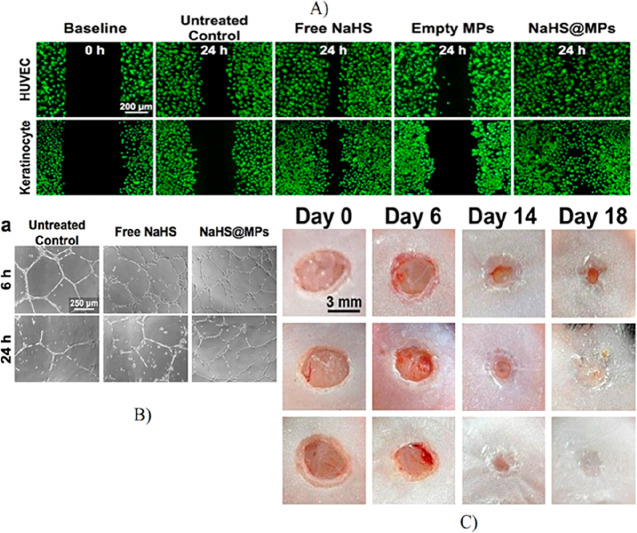
A H2S-based polylactic-co-glycolic acid microparticle system conjugated with donor DATS was developed to enhance angiogenesis in ischemic diseases. The H2S was released sustainably over a long period of time, leading to improved vascular branching in the HUVEC cells.102 Another microparticle system consisting of hydrophobic phase change materials tetradecanol and paraffin wax encapsulated with NaHS was incorporated into the wound dressing. The phase change materials were used to synthesize solidified emulsions that would likely minimize drug loss. Because NaHS is a water-labile compound, NaHS reacts with water in the wound environment to release H2S in a controlled manner. In the treated group of animals, which healed the wounds faster than the untreated groups, increased wound vasculature and complete re-epithelialization were observed (Figure 6).103
Figure 6.
Angiogenic potential of NaHS-conjugated microparticles: microparticle-loaded NaHS demonstrated the sustained release of H2S that supports the increased migration of HUVEC and keratinocytes. In contrast, the spontaneously released free NaHS showed a lower rate of gap closure than the H2S-loaded microparticles. (B) The tubular network formed from HUVEC treated with microparticle-loaded NaHS showed higher branch points than the control. (C) The in vivo studies showed complete closure of wounds treated with microparticle-loaded NaHS by day 14, whereas the mice treated with free NaHS showed 100% closure by day 18. (Adapted with permission from Lin et al. Copyright 2017 Elsevier.)
4. Physiological Role of CO in Angiogenesis
Carbon monoxide, once feared as a toxic molecule, has come a long way to become one of the therapeutic gas transmitters that modulate various biological functions. It is a colorless, odorless, and tasteless gas that interacts with the hemoglobin in blood cells to form carboxyhemoglobin while replacing the oxygen bound to the hemoglobin. Although the toxic nature and physiological effects of the gas have been widely debated, later studies have demonstrated the biological properties of CO with therapeutic and beneficial effects. CO is generated endogenously within the physiological system by catalytic degradation of heme by a HO enzyme.104 HO degrades its substrate heme to form iron and byproducts such as CO and biliverdin. Two types of HO exist in the physiological system, namely, inducible HO-1 and constitutively expressed HO-2. The inducible HO-1 catalyzes its action in response to specific stimuli such as hypoxia, hyperoxia, growth factors, NO, lipids, etc., while the HO-2 is constitutively expressed in the brain, endothelium, and testes. Several studies have shown that endogenous CO production induces synthesis of angiogenic factors such as VEGF, SDF, IL-8, PDGF, and TGF β and inhibition of antiangiogenic factors such as VEGFR-1 and soluble endoglin. This regulation of angiogenic factors promotes endothelial cell proliferation and cell migration and inhibits apoptosis.105,106
4.1. Regulatory Pathways Involving CO and Angiogenesis
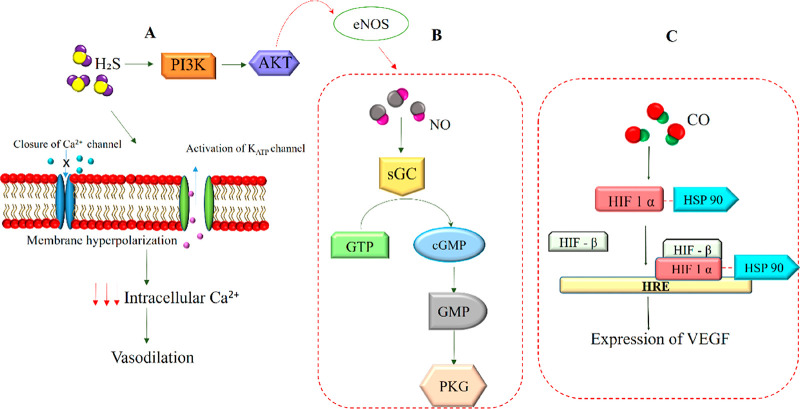
4.1.1. sGC Pathway
CO can also act as a signaling molecule for biological functions such as growth and regeneration of tissues and also maintains tissue homeostasis. It is involved in the signaling pathway of sGC, which forms a complex with CO, increases cGMP formation, and subsequently stimulates vasodilation and blood pressure regulation.107 Guanylyl cyclase can also be induced by NO, which further triggers VEGF production for angiogenesis. Therefore, it is speculated that both NO and CO via VEGF have a synergistic effect on endothelial cell neovascularization. In addition, during the hypoxic state, when NOS and NO are suppressed, HO-1 takes over the role of VEGF production. This was proved by a report stating that HO-1 inhibitors downregulated hypoxia-induced angiogenesis, while the NOS inhibitors did not affect hypoxia-induced angiogenesis.108
4.1.2. HIF 1α–VEGF Pathway
In addition to sGC signaling, CO also stimulates the HIF 1α protein to promote angiogenesis via increased VEGF secretion and other cellular activities.105 The active form of HIF 1α binds to the hypoxia-response elements of the genes responsible for VEGF, erythropoietin, and certain metabolic enzymes in the nucleus.109,110 These then regulate angiogenesis and other cell survival mechanisms. The CO can induce angiogenesis by activating the HIF 1α via two possible pathways. The former mechanism involves activation of the translation proteins p70 S6 kinase and eIF-4E via the PI3K/Akt and MEK/ERK signaling pathways, and the latter mechanism protects HIF 1α from degradation by stabilizing the HIF protein with HSP90α. The resulting stimulation of HIF 1α leads to increased production of VEGF in the endothelial cells, which further modulates angiogenesis. Glutamate-stimulated CO production in astrocytes also functions as a signaling molecule that regulates vascular homeostasis through vasodilation.106 The role of CO on VEGF production has also been reported in several studies, showing that a lower dose of 1% CO increased VEGF levels, while 5% CO levels negatively affected VEGF production.108
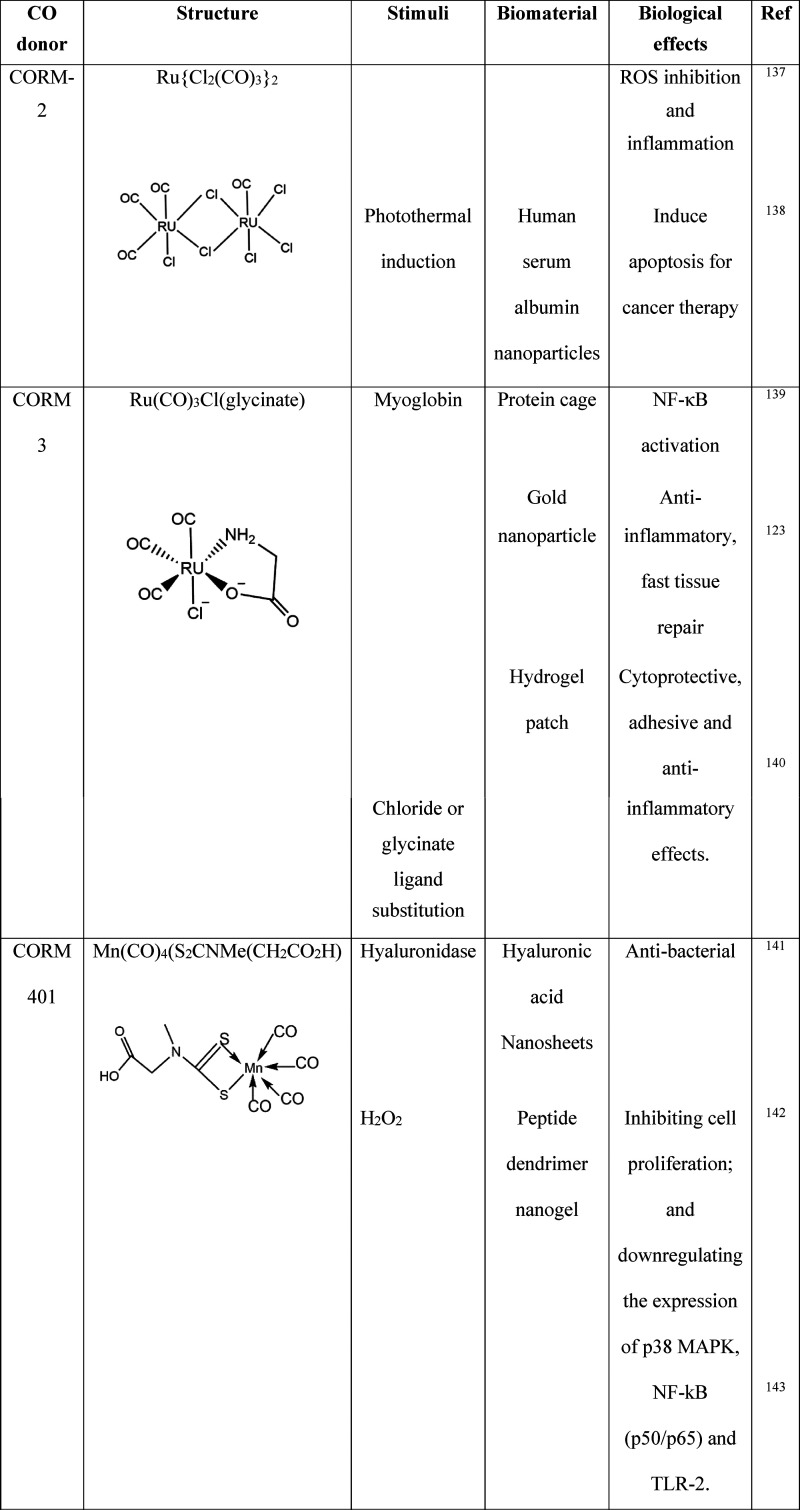
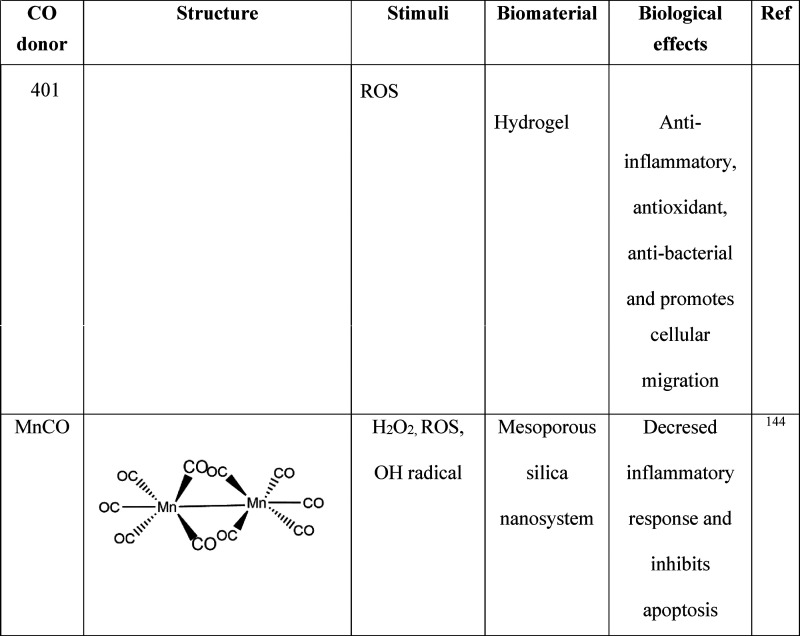
The CO molecule has thus developed a new paradigm that it can contribute to various physiological functions as a therapeutic agent. However, the exogenous delivery of CO gas directly to the biological system is not well regulated and presents difficulties in reaching the therapeutic potential of the gas molecule. Furthermore, like other gases, CO has the disadvantage of an uncontrolled release, and an overdose can lead to CO poisoning.111 Therefore, several CO-releasing metal carbonyls (CORM) such as CORM 1, CORM 2, and CORM 3 have been identified and reported that regulate a safe and controlled delivery of CO to the target site. CORMs are small metal center molecules with branched carbonyl groups for CO release. The CORM can release the CO gas molecule at the specific site within the biological system for its action to take place.112 The different CO donors and their biological effects are listed in Table 2.
Table 2. Biomaterial Carriers of CO Donors and Their Biological Effects137 −145.
4.2. Hemeoxygenase and Angiogenesis
The HO-1 enzyme has demonstrated its vascular protective function in various experimental model diseases, elucidating its role in pathophysiology. During an inflammatory state, HO-1 has a dual role of initiating the anti-inflammatory effect by inhibiting leukocyte infiltration and then triggering the synthesis of angiogenic factors such as VEGF to promote angiogenesis.113 Based on the reported studies, the HO-1 might increase the proliferation rate of endothelial cells but negatively regulate the vascular smooth muscle cells. Likewise, overexpression of HO-1 in coronary microvascular endothelial cells upregulated the rate of proliferation, indicating the role of HO-1 in endothelial proliferation. Expression of cell cycle inhibitors p21 and p27 increases, with a decrease in HO-1 and upregulated HO-1 enzymes increasing VEGF production and angiogenic activity in endothelial cells.108 The vascular protective effects of HO-1 were demonstrated in a rat hindlimb ischemia model, which showed improved blood vessel density and neovascularization when the HO-1 gene was vectored and transplanted into endothelial progenitor cells.114 Another report of impaired HO in murine bone marrow resulted in reduced CO, which also affected the VEGF, SDF-1, and placental growth factor, further reducing revascularization in the bone cells.115 Activation of VEGF by HO-1 for the formation of new blood vessels in the endothelial cells increased VEGF at the cellular level, further activating the HO-1, creating a positive feedback loop.116
4.3. Biomaterials
The release of free CO affects the physiological system as it binds to hemoglobin, and the diffusible gas cannot achieve target-specific release; therefore, CO is normally released via CORM. The integration of the CORM into carrier molecules such as macromolecules or nanomaterials offers a greater advantage for stabilized CORM complexes with specific cellular uptake and drug localization.117 The delivery of CORM via various biomaterials and their biological responses are represented in Table 2. In addition, the macromolecular carriers offer a larger surface area118 that can hold a higher amount of CO precursors, while the nanocarriers improve permeability and reduce systemic toxicity.119
4.3.1. Nanomaterials
A light-responsive manganese-based carbonyl complex was conjugated with mesoporous silica nanoparticles. The photosensitive carbonyl complex releases the CO when exposed to a lower range of visible light. The released gas molecule induced vasodilation of the rat model aortic muscle rings by activating the cyclic GMP pathway. The CO donor thus delivered the CO in a controlled manner to the specified target site to carry out its biological functions.120
A stimuli-responsive nanocarrier was designed from the glutathione-conjugated adhesion peptides REDV-GG-TAT-GC-POSS to encapsulate the CORM-401 for the treatment of ischemic injuries. In addition, pZNF580 was conjugated to the peptide CO system to enhance angiogenic function. The nanoparticle composite showed increased expression of pro-angiogenic factors compared to the single drug system, indicating the synergistic potential of the nanomaterial and the drug molecule.121 Similarly, CORM 2 has been incorporated into solid nanoparticles to minimize the after-effects of spinal cord injury by protecting endothelial cells and the blood–spinal cord barrier. The CORM 2 nanosystem preserved the integrity of the tight junctions, protecting the barrier after injury. Angiopoeitin-1 protein, which was reduced due to leakage of the vasculature after injury, increased significantly after treatment with the CORM nanosystem, thus playing an important role in neural regeneration.122 Gold nanoparticles incorporated with CORM 3 showed potential anti-inflammatory effects and wound healing activities. Cell migration was faster for the cells treated with nanoconjugated CORM than for the free CORM. In addition, the nanomaterial-based CORM can reduce the inflammatory response compared to the free CORM. According to the reported study, this may be due to the stabilized uptake of the nanocarrier and the sustained release of CO at the target site.123 The stress-induced cardiomyocytes showed increased viability after treatment with the CO-based gel compared to the control gel without CO. This study thus proves the cardioprotective effect of CO on oxidatively stressed rat cardiomyocytes.8
4.3.2. Micelles
A polymeric micelle composed of a hydrophilic poly(ethylene glycol) block, a poly(ornithineacrylamide) block containing Ru(CO)3Cl(ornithine) units, and a hydrophobic poly(n-butylacrylamide) block was synthesized to encapsulate the CO-releasing complex. The CO was released from the polymeric micellar compound in response to the cysteine molecule present in the physiological system. The micelles with CO-releasing molecules inhibited LPS-induced NF kβ activation, which regulates multiple inflammatory responses.124 Although the free CO donors are cytotoxic to the cells as stated by different reports,125,126 transporting the donors through the micelle delivery system showed significant reduction in the cytotoxicity which implicates the therapeutic usefulness of biomaterial-based delivery systems.
4.3.3. Microparticles
CO donors, Re CORM 2 and B12Re CORM 2, were incorporated into the silica microparticles synthesized from diatoms to assess the potential of diatoms as drug delivery vehicles. The drugs were chemisorbed onto the silica microparticles, and blood vessel formation was determined in vivo in the zebra fish model. The nontoxicity and drug delivering ability of diatoms made them a potential candidate for drug delivery applications. Neovascularization and increased vessel density were observed at lower doses (5–10 μM) of the CO-releasing molecule, and angiogenic inhibition was observed at higher doses (above 25 μM). Thus, the CORM induced dose-dependent angiogenesis, but the carrier molecule showed no toxicity even at higher doses.127
4.4. Hybrid Gasotransmitters Releasing Biomaterials
The three gas molecules mediate various physiological functions by sharing the signaling pathway with each other through either direct or indirect interactions to regulate functions such as vascular homeostasis, cell proliferation, differentiation, and inflammatory responses. Therefore, the synergistic application of the gaseous molecules would increase the efficiency of the treatment to a greater extent. The signaling pathways regulating the three gasotransmitters are depicted in Figure 7. The combinatorial NO/H2S hybrid system was studied by designing polymeric nanoparticles conjugated with the gasotransmitters. The nanocarrier composed of mPEG–PLGH was integrated with thiobenzamide and DETA NONate to release H2S and NO molecules. The hybrid nanoparticle system with the therapeutic gaseous molecules showed higher tube formation than the nanoparticle coated with single gaseous molecules. It was also found that sprouting of new vessels is higher in the hybrid nanoparticle delivery system, demonstrating the angiogenic potential of the gas-based nanomaterials.12 In a related study, the synthetic hybrid NO/H2S molecule ZYZ-803 demonstrated higher angiogenic activities by regulating the VEGF/cGMP pathway. The increased rate of cell proliferation, migration, and tubularization along with angiogenic induction in rat aortic rings was observed in the hybrid molecule compared to that of the solitary NO or H2S molecule.128
Figure 7.
Signaling pathways involved in gasotransmitter-mediated angiogenesis. (A) The H2S stimulates activation of the PI3K/Akt pathway that mediates angiogenesis by activating the eNOS pathway. The H2S also activates the KATP channel, causing hyperpolarization of the membrane, leading to a decrease in intracellular Ca2+. This further promotes the relaxation of blood vessels in smooth muscle cells. (B) NO generated by eNOS induces activation of the sGC pathway, which catalyzes the conversion of GTP to cGMP and further promotes downstream signaling to mediate angiogenesis. (C) CO stabilizes HIF1α through the HSP 90 protein, which protects the HIF factor from degradation. This promotes the binding of HIF1α and HIFβ to HRE, which subsequently mediates the expression of VEGF.
5. Conclusion and Future Perspectives
The discovery of the physiological properties of gasotransmitters a few decades ago stimulated researchers to explore an entirely new state of matter as a therapeutic agent for various pathologies. Although the signaling pathways such as sGC, PI3K/Akt, and VEGF pathways associated with the gasotransmitters and angiogenesis are relatively explored, the precise regulatory mechanism and the involvement of other signaling pathways have yet to be understood. The therapeutic effectiveness of gasotransmitters has led to the development of dispensers that can release the gas molecules in a sustained manner. Extensive in vitro and in vivo research has been conducted to determine the angiogenic potential of these gas transmitters; however, clinical research on these gas molecules is not well established.
Because the concentration above physiological levels produces toxic effects, it is important to understand the biological effects and the concentration required to maintain homeostasis. The release rates of gas from the donor molecule can vary under in vitro and in vivo conditions, and it is difficult to quantify release rates in in vivo models. Therefore, controlled drug release within the in vivo model is still a challenge, since the delivery of gas molecules with sudden release or higher concentration entails various risks that cause physiological effects. Future research is expected to focus on developing biocompatible donor molecules with fewer byproducts or nontoxic byproducts. Furthermore, the researches on using smart materials with stimuli-responsive potential are the future prospects that need to be addressed for more efficient delivery of the gasotransmitters in the coming years. Extensive research and a better understanding of signaling pathways will enable future researchers to conclude that gas-transmitter-based therapeutics have a greater positive impact on human health.
Acknowledgments
The author Dr. Sadhasivam Subramaniam acknowledges the Department of Biotechnology (DBT), India, for the financial support provided by the Ramalingaswami Re-entry fellowship (Order No. BT/RLF/Re-entry/55/2013).
The authors declare no competing financial interest.
References
- Fallah A.; Sadeghinia A.; Kahroba H.; Samadi A.; Heidari H. R.; Bradaran B.; Zeinali S.; Molavi O. Therapeutic targeting of angiogenesis molecular pathways in angiogenesis-dependent diseases. Biomed. Pharmacother. 2019, 110, 775–785. 10.1016/j.biopha.2018.12.022. [DOI] [PubMed] [Google Scholar]
- Cheng Z.; Kishore R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox Biol. 2020, 37, 101704 10.1016/j.redox.2020.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steven S.; Frenis K.; Oelze M.; Kalinovic S.; Kuntic M.; Bayo Jimenez M. T.; Vujacic-Mirski K.; Helmstadter J.; Kroller-Schon S.; Munzel T.; Daiber A. Vascular inflammation and oxidative stress: major triggers for cardiovascular disease. Oxid. Med. Cell. Longevity. 2019, 2019, 1. 10.1155/2019/7092151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabó C.; Papapetropoulos A. Hydrogen sulphide and angiogenesis: mechanisms and applications. Br. J. Pharmacol. 2011, 164 (3), 853–865. 10.1111/j.1476-5381.2010.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry R. K.; Brewer A. C. Redox regulation of gasotransmission in the vascular system: A focus on angiogenesis. Free Radical Biol. Med. 2017, 108, 500–516. 10.1016/j.freeradbiomed.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluru G. K.; Shen X.; Kevil C. G. A tale of two gases: NO and H2S, foes or friends for life?. Redox Biol. 2013, 1 (1), 313–318. 10.1016/j.redox.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olas B. Carbon monoxide is not always a poison gas for human organism: Physiological and pharmacological features of CO.. Chem.-Biol. Interact. 2014, 222, 37–43. 10.1016/j.cbi.2014.08.005. [DOI] [PubMed] [Google Scholar]
- Wang L.; Xie X.; Ke B.; Huang W.; Jiang X.; He G. Recent advances on endogenous gasotransmitters in inflammatory dermatological disorders. J. Adv. Res. 2022, 38, 261–274. 10.1016/j.jare.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihi A.; Al-Naqshabandi M. A.; Khudhur Z. O.; Housein Z.; Hama H. A.; Abdullah R. M.; Hussen B. M.; Alkasalias T. Gasotransmitters in the tumor microenvironment: Impacts on cancer chemotherapy. Mol. Med. Rep. 2022, 26 (1), 1–10. 10.3892/mmr.2022.12749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housein Z.; Kareem T. S.; Salihi A. In vitro anticancer activity of hydrogen sulfide and nitric oxide alongside nickel nanoparticle and novel mutations in their genes in CRC patients. Sci. Rep. 2021, 11 (1), 1–11. 10.1038/s41598-021-82244-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y.; Matson J. B. Gasotransmitter delivery via self-assembling peptides: treating diseases with natural signaling gases. Adv. Drug Delivery Rev. 2017, 110, 137–156. 10.1016/j.addr.2016.06.017. [DOI] [PubMed] [Google Scholar]
- Lee J.; Yang C.; Ahn S.; Choi Y.; Lee K. Enhanced NO-induced angiogenesis via NO/H 2 S co-delivery from self-assembled nanoparticles. Biomater. Sci. 2021, 9 (15), 5150–5159. 10.1039/D1BM00448D. [DOI] [PubMed] [Google Scholar]
- Cai A. Y.; Zhu Y. J.; Qi C. Biodegradable inorganic nanostructured biomaterials for drug delivery. Adv. Mater. Interfaces. 2020, 7 (20), 2000819 10.1002/admi.202000819. [DOI] [Google Scholar]
- Sahoo J. K.; Braegelman A. S.; Webber M. J. Immunoengineering with supramolecular peptide biomaterials. J. Indian Inst. Sci. 2018, 98 (1), 69–79. 10.1007/s41745-018-0060-x. [DOI] [Google Scholar]
- Fenton O. S.; Olafson K. N.; Pillai P. S.; Mitchell M. J.; Langer R. Advances in biomaterials for drug delivery. Adv. Mater. 2018, 30 (29), 1705328 10.1002/adma.201705328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccio D. A.; Schoenfisch M. H. Nitric oxide release: Part I. Macromolecular scaffolds. Chem. Soc. Rev. 2012, 41 (10), 3731–3741. 10.1039/c2cs15272j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T.-K.; Selvanayagam R.; Ho K. K. K.; Chen R.; Kutty S. K.; Rice S. A.; Kumar N.; Barraud N.; Duong H. T. T.; Boyer C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016, 7 (2), 1016–1027. 10.1039/C5SC02769A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M.-L.; Kim H.-S.; You J.; Choi Y. S.; Kwon B.-J.; Park C. H.; Baek W.; Kim M. S.; Lee Y. J.; Im G.-I.; Yoon J.-K.; Lee J. B.; Sung H.-J. Hydrogel cross-linking–programmed release of nitric oxide regulates source-dependent angiogenic behaviors of human mesenchymal stem cell. Sci. Adv. 2020, 6 (9), eaay5413 10.1126/sciadv.aay5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummanapalli S. S.; Kuppusamy R.; Yeo J. H.; Kumar N.; New E. J.; Willcox M. D. The role of nitric oxide in ocular surface physiology and pathophysiology. Ocul. Surf. 2021, 21, 37–51. 10.1016/j.jtos.2021.04.007. [DOI] [PubMed] [Google Scholar]
- Gao Y.; Zhou S.; Xu Y.; Sheng S.; Qian S. Y.; Huo X. Nitric oxide synthase inhibitors 1400W and L-NIO inhibit angiogenesis pathway of colorectal cancer. Nitric Oxide. 2019, 83, 33–39. 10.1016/j.niox.2018.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malone-Povolny M. J.; Maloney S. E.; Schoenfisch M. H. Nitric oxide therapy for diabetic wound healing. Adv. healthcare Mater. 2019, 8 (12), 1801210 10.1002/adhm.201801210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vong L. B.; Bui T. Q.; Tomita T.; Sakamoto H.; Hiramatsu Y.; Nagasaki Y. Novel angiogenesis therapeutics by redox injectable hydrogel-Regulation of local nitric oxide generation for effective cardiovascular therapy. Biomaterials. 2018, 167, 143–152. 10.1016/j.biomaterials.2018.03.023. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J. Enzymes of the L-arginine to nitric oxide pathway. J. Nutr. 2004, 134 (10), 2748S–2751S. 10.1093/jn/134.10.2748S. [DOI] [PubMed] [Google Scholar]
- Epstein F. H.; Moncada S.; Higgs A. The L-arginine-nitric oxide pathway. N. Engl. J. Med. 1993, 329 (27), 2002–2012. 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Kimura H.; Esumi H. Reciprocal regulation between nitric oxide and vascular endothelial growth factor in angiogenesis. Acta Biochim. Pol. 2003, 50 (1), 49–59. 10.18388/abp.2003_3713. [DOI] [PubMed] [Google Scholar]
- Jozkowicz A.; Cooke J. P.; Guevara I.; Huk I.; Funovics P.; Pachinger O.; Dulak J. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc. Res. 2001, 51 (4), 773–783. 10.1016/S0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- Zheng Y.; Wang J.; Zhao T.; Wang L.; Wang J. Modulation of the VEGF/AKT/eNOS signaling pathway to regulate liver angiogenesis to explore the anti-hepatic fibrosis mechanism of curcumol. J. Ethnopharmacol. 2021, 280, 114480 10.1016/j.jep.2021.114480. [DOI] [PubMed] [Google Scholar]
- Yang C.; Hwang H. H.; Jeong S.; Seo D.; Jeong Y.; Lee D. Y.; Lee K. Inducing angiogenesis with the controlled release of nitric oxide from biodegradable and biocompatible copolymeric nanoparticles. Int. J. Nanomed. 2018, 13, 6517. 10.2147/IJN.S174989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumura D.; Gohongi T.; Kadambi A.; Izumi Y.; Ang J.; Yun C.-O.; Buerk D. G.; Huang P. L.; Jain R. K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. U. S. A. 2001, 98 (5), 2604–2609. 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan S. M.; Kraehling J. R.; Eitner F.; Bénardeau A.; Sandner P. The impact of the nitric oxide (NO)/soluble guanylyl cyclase (sGC) signaling cascade on kidney health and disease: a preclinical perspective. Int. J. Mol. Sci. 2018, 19 (6), 1712. 10.3390/ijms19061712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aicher A.; Heeschen C.; Feil S.; Hofmann F.; Mendelsohn M. E.; Feil R.; Dimmeler S. cGMP-dependent protein kinase I is crucial for angiogenesis and postnatal vasculogenesis. PLoS One. 2009, 4 (3), e4879 10.1371/journal.pone.0004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes de Almeida Schirmer B.; Crucet M.; Stivala S.; Vucicevic G.; da Silva Barcelos L.; Vanhoutte P. M.; Pellegrini G.; Camici G. G.; Seebeck P.; Pfundstein S.; Stein S.; Paneni F.; Luscher T. F.; Simic B. The NO-donor MPC-1011 stimulates angiogenesis and arteriogenesis and improves hindlimb ischemia via a cGMP-dependent pathway involving VEGF and SDF-1α. Atherosclerosis. 2020, 304, 30–38. 10.1016/j.atherosclerosis.2020.05.012. [DOI] [PubMed] [Google Scholar]
- Shi F.; Wang Y.-C.; Zhao T.-Z.; Zhang S.; Du T.-Y.; Yang C.-B.; Li Y.-H.; Sun X.-Q. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3K-Akt-eNOS signal pathway. PloS one. 2012, 7 (7), e40365 10.1371/journal.pone.0040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. J.; Shi Y. Q.; Li R. L.; Hu A.; Lu Z. Y.; Weng L.; Duan J. L. Angiogenesis effect of therapeutic ultrasound on HUVECs through activation of the PI3K-Akt-eNOS signal pathway. Am. J. Transl. Res. 2015, 7 (6), 1106. [PMC free article] [PubMed] [Google Scholar]; https://pubmed.ncbi.nlm.nih.gov/26279754.
- Wei X.; Guan L.; Fan P.; Liu X.; Liu R.; Liu Y.; Bai H. Direct current electric field stimulates nitric oxide production and promotes NO-dependent angiogenesis: Involvement of the PI3K/Aktsignaling pathway. J. Vasc. Res. 2020, 57 (4), 195–205. 10.1159/000506517. [DOI] [PubMed] [Google Scholar]
- Zullino S.; Buzzella F.; Simoncini T. Nitric oxide and the biology of pregnancy. Vasc. Pharmacol. 2018, 110, 71–74. 10.1016/j.vph.2018.07.004. [DOI] [PubMed] [Google Scholar]
- Massimiani M.; Tiralongo G. M.; Salvi S.; Fruci S.; Lacconi V.; La Civita F.; Mancini M.; Stuhlmann H.; Valensise H.; Campagnolo L. Treatment of pregnancies complicated by intrauterine growth restriction with nitric oxide donors increases placental expression of Epidermal Growth Factor-Like Domain 7 and improves fetal growth: A pilot study. Transl. Res. 2021, 228, 28–41. 10.1016/j.trsl.2020.08.002. [DOI] [PubMed] [Google Scholar]
- Witte M. B.; Barbul A. Role of nitric oxide in wound repair. Am. J. Surg. 2002, 183 (4), 406–412. 10.1016/S0002-9610(02)00815-2. [DOI] [PubMed] [Google Scholar]
- Shekhter A. B.; Serezhenkov V. A.; Rudenko T. G.; Pekshev A. V.; Vanin A. F. Beneficial effect of gaseous nitric oxide on the healing of skin wounds. Nitric oxide. 2005, 12 (4), 210–219. 10.1016/j.niox.2005.03.004. [DOI] [PubMed] [Google Scholar]
- He M.; Sun L.; Fu X.; McDonough S. P.; Chu C. C. Biodegradable amino acid-based poly (ester amine) with tunable immunomodulating properties and their in vitro and in vivo wound healing studies in diabetic rats’ wounds. Acta Biomater. 2019, 84, 114–132. 10.1016/j.actbio.2018.11.053. [DOI] [PubMed] [Google Scholar]
- Abaffy P.; Tomankova S.; Naraine R.; Kubista M.; Sindelka R. The role of nitric oxide during embryonic wound healing. BMC genomics. 2019, 20 (1), 1–21. 10.1186/s12864-019-6147-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura L. I.; Dias A. M.; Carvalho E.; de Sousa H. C. Recent advances on the development of wound dressings for diabetic foot ulcer treatment—A review. Acta Biomater. 2013, 9 (7), 7093–7114. 10.1016/j.actbio.2013.03.033. [DOI] [PubMed] [Google Scholar]
- Gallagher K. A.; Liu Z.-J.; Xiao M.; Chen H.; Goldstein L. J.; Buerk D. G.; Nedeau A.; Thom S. R.; Velazquez O. C. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1α. J. Clin. Investig. 2007, 117 (5), 1249–1259. 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J. D.; Chen A. F. Nitric oxide: a newly discovered function on wound healing. Acta Pharmacol. Sinica. 2005, 26 (3), 259–264. 10.1111/j.1745-7254.2005.00058.x. [DOI] [PubMed] [Google Scholar]
- Witte M. B.; Kiyama T.; Barbul A. Nitric oxide enhances experimental wound healing in diabetes. Br. J. Surg. 2002, 89 (12), 1594–1601. 10.1046/j.1365-2168.2002.02263.x. [DOI] [PubMed] [Google Scholar]
- Li Y.; Lee P. I. Controlled nitric oxide delivery platform based on S-nitrosothiol conjugated interpolymer complexes for diabetic wound healing. Mol. Pharmaceutics. 2010, 7 (1), 254–266. 10.1021/mp900237f. [DOI] [PubMed] [Google Scholar]
- Han G.; Nguyen L. N.; Macherla C.; Chi Y.; Friedman J. M.; Nosanchuk J. D.; Martinez L. R. Nitric oxide–releasing nanoparticles accelerate wound healing by promoting fibroblast migration and collagen deposition. Am. J. Pathol. 2012, 180 (4), 1465–1473. 10.1016/j.ajpath.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Zhou X.; Zhang J.; Feng G.; Shen J.; Kong D.; Zhao Q. Nitric oxide-releasing biomaterials for biomedical applications. Curr. Med. Chem. 2016, 23 (24), 2579–2601. 10.2174/0929867323666160729104647. [DOI] [PubMed] [Google Scholar]
- Robert B.; Chenthamara D.; Subramaniam S. Fabrication and biomedical applications of Arabinoxylan, Pectin, Chitosan, soy protein, and silk fibroin hydrogels via laccase-Ferulic acid redox chemistry. Int. J. Biol. Macromol. 2022, 201, 539–556. 10.1016/j.ijbiomac.2021.12.103. [DOI] [PubMed] [Google Scholar]
- Giraudo M. V.; Di Francesco D.; Catoira M. C.; Cotella D.; Fusaro L.; Boccafoschi F. Angiogenic Potential in Biological Hydrogels. Biomedicines. 2020, 8 (10), 436. 10.3390/biomedicines8100436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J.; Zheng W.; Zhang J.; Guan D.; Yang Z.; Kong D.; Zhao Q. Enzyme-controllable delivery of nitric oxide from a molecular hydrogel. Chem. Commun. 2013, 49 (80), 9173–9175. 10.1039/c3cc45666h. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Chen X.; Li H.; Feng G.; Nie Y.; Wei Y.; Li N.; Han Z.; Han Z.-c.; Kong D.; Guo Z.; Zhao Q.; Li Z. A nitric oxide-releasing hydrogel for enhancing the therapeutic effects of mesenchymal stem cell therapy for hindlimb ischemia. Acta Biomater. 2020, 113, 289–304. 10.1016/j.actbio.2020.07.011. [DOI] [PubMed] [Google Scholar]
- Nagasaka H.; Yorifuji T.; Egawa H.; Inui A.; Fujisawa T.; Komatsu H.; Tsukahara H.; Uemoto S.; Inomata Y. Characteristics of NO cycle coupling with urea cycle in non-hyperammonemic carriers of ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2013, 109 (3), 251–254. 10.1016/j.ymgme.2013.04.013. [DOI] [PubMed] [Google Scholar]
- Yao X.; Liu Y.; Gao J.; Yang L.; Mao D.; Stefanitsch C.; Li Y.; Zhang J.; Ou L.; Kong D.; Zhao Q.; Li Z. Nitric oxide releasing hydrogel enhances the therapeutic efficacy of mesenchymal stem cells for myocardial infarction. Biomaterials. 2015, 60, 130–140. 10.1016/j.biomaterials.2015.04.046. [DOI] [PubMed] [Google Scholar]
- Segal M. S.; Shah R.; Afzal A.; Perrault C. M.; Chang K.; Schuler; Beem E.; Shaw L. C.; Li Calzi S.; Harrison J. K.; Tran-Son-Tay R. Nitric oxide cytoskeletal–induced alterations reverse the endothelial progenitor cell migratory defect associated with diabetes. Diabetes 2006, 55 (1), 102–109. 10.2337/diabetes.55.01.06.db05-0803. [DOI] [PubMed] [Google Scholar]
- Zahid A. A.; Ahmed R.; urRehman S. R.; Augustine R.; Tariq M.; Hasan A. Nitric oxide releasing chitosan-poly (vinyl alcohol) hydrogel promotes angiogenesis in chick embryo model. Int. J. Biol. Macromol. 2019, 136, 901–910. 10.1016/j.ijbiomac.2019.06.136. [DOI] [PubMed] [Google Scholar]
- Nakagawa H.; Matsumoto Y.; Matsumoto Y.; Miwa Y.; Nagasaki Y. Design of high-performance anti-adhesion agent using injectable gel with an anti-oxidative stress function. Biomaterials. 2015, 69, 165–173. 10.1016/j.biomaterials.2015.08.018. [DOI] [PubMed] [Google Scholar]
- Thangavel S.; Yoshitomi T.; Sakharkar M. K.; Nagasaki Y. Redox nanoparticles inhibit curcumin oxidative degradation and enhance its therapeutic effect on prostate cancer. J. Controlled Release 2015, 209, 110–119. 10.1016/j.jconrel.2015.04.025. [DOI] [PubMed] [Google Scholar]
- Hasan N.; Lee J.; Kwak D.; Kim H.; Saparbayeva A.; Ahn H. J.; Yoon I. S.; Kim M. S.; Jung Y.; Yoo J. W. Diethylenetriamine/NONOate-doped alginate hydrogel with sustained nitric oxide release and minimal toxicity to accelerate healing of MRSA-infected wounds. Carbohydr. Polym. 2021, 270, 118387 10.1016/j.carbpol.2021.118387. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Zhou Y.; Li Y.; Guo L.; Zhou J.; Chen J. Injectable and self-healing hydrogel containing nitric oxide donor for enhanced antibacterial activity. React. Funct. Polym. 2021, 166, 105003 10.1016/j.reactfunctpolym.2021.105003. [DOI] [Google Scholar]
- Samakova A.; Gazova A.; Sabova N.; Valaskova S.; Jurikova M.; Kyselovic J. The PI3k/Akt pathway is associated with angiogenesis, oxidative stress and survival of mesenchymal stem cells in pathophysiologic condition in ischemia. Physiol. Res. 2019, 68, S131–S138. 10.33549/physiolres.934345. [DOI] [PubMed] [Google Scholar]
- Zhao Q.; Zhang J.; Song L.; Ji Q.; Yao Y.; Cui Y.; Kong D. Polysaccharide-based biomaterials with on-demand nitric oxide releasing property regulated by enzyme catalysis. Biomaterials 2013, 34 (33), 8450–8458. 10.1016/j.biomaterials.2013.07.045. [DOI] [PubMed] [Google Scholar]
- Lenz I. J.; Plesnila N.; Terpolilli N. A. Role of endothelial nitric oxide synthase for early brain injury after subarachnoid hemorrhage in mice. J. Cereb. Blood Flow Metab. 2021, 41 (7), 1669–1681. 10.1177/0271678X20973787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S.; Liu H.; Liao K.; Hu Q.; Guo R.; Deng K. Functionalized GO nanovehicles with nitric oxide release and photothermal activity-based hydrogels for bacteria-infected wound healing. ACS Appl. Mater. Interfaces. 2020, 12 (26), 28952–28964. 10.1021/acsami.0c04080. [DOI] [PubMed] [Google Scholar]
- Prasad B. S.; Gupta V. R.; Devanna N.; Jayasurya K. Microspheres as drug delivery system-a review. J. Global Trends Pharm. Sci. 2014, 5 (3), 1961–1972. [Google Scholar]
- Liu G.; Zhou Y.; Xu Z.; Bao Z.; Zheng L.; Wu J. Janus hydrogel with dual antibacterial and angiogenesis functions for enhanced diabetic wound healing. Chin. Chem. Lett. 2022, 10.1016/j.cclet.2022.07.048. [DOI] [Google Scholar]
- Patra J. K.; Das G.; Fraceto L. F.; Campos E. V. R.; Rodriguez-Torres M. d. P.; Acosta-Torres L. S.; Diaz-Torres L. A.; Grillo R.; Swamy M. K.; Sharma S.; Habtemariam S.; Shin H.-S. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnol. 2018, 16 (1), 1–33. 10.1186/s12951-018-0392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q. Y.; Wang Z. Y.; Wen F.; Ren L.; Li J.; Teoh S. H.; Thian E. S. Gelatin–siloxane nanoparticles to deliver nitric oxide for vascular cell regulation: synthesis, cytocompatibility, and cellular responses.. J. Biomed. Mater. Res., Part A 2015, 103 (3), 929–938. 10.1002/jbm.a.35239. [DOI] [PubMed] [Google Scholar]
- Graczyk A.; Pawlowska R.; Jedrzejczyk D.; Chworos A. Gold nanoparticles in conjunction with nucleic acids as a modern molecular system for cellular delivery. Molecules. 2020, 25 (1), 204. 10.3390/molecules25010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabirian F.; Brouki Milan P.; Zamanian A.; Heying R.; Mozafari M. Nitric oxide-releasing vascular grafts: A therapeutic strategy to promote angiogenic activity and endothelium regeneration. Acta Biomater. 2019, 92, 82–91. 10.1016/j.actbio.2019.05.002. [DOI] [PubMed] [Google Scholar]
- Yang Z.; Yang Y.; Xiong K.; Li X.; Qi P.; Tu Q.; Jing F.; Weng Y.; Wang J.; Huang N. Nitric oxide producing coating mimicking endothelium function for multifunctional vascular stents. Biomaterials. 2015, 63, 80–92. 10.1016/j.biomaterials.2015.06.016. [DOI] [PubMed] [Google Scholar]
- Turner J. G.; White L. R.; Estrela P.; Leese H. S. Hydrogel-Forming Microneedles: Current Advancements and Future Trends. Macromol. Biosci. 2021, 21 (2), 2000307 10.1002/mabi.202000307. [DOI] [PubMed] [Google Scholar]
- Ma C. J.; He Y.; Jin X.; Zhang Y.; Zhang X.; Li Y.; Xu M.; Liu K.; Yao Y.; Lu F. Light-regulated nitric oxide release from hydrogel-forming microneedles integrated with graphene oxide for biofilm-infected-wound healing. Biomater. Adv. 2022, 134, 112555 10.1016/j.msec.2021.112555. [DOI] [PubMed] [Google Scholar]
- Yao S.; Wang Y.; Chi J.; Yu Y.; Zhao Y.; Luo Y.; Wang Y. Porous MOF microneedle array patch with photothermal responsive nitric oxide delivery for wound healing. Adv. Sci. 2022, 9 (3), 2103449 10.1002/advs.202103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsouda A.; Bibli S. I.; Pyriochou A.; Szabo C.; Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol. Res. 2016, 113, 175–185. 10.1016/j.phrs.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell C. R.; Dillon K. M.; Matson J. B. A review of hydrogen sulfide (H2S) donors: Chemistry and potential therapeutic applications. Biochem. Pharmacol. 2018, 149, 110–123. 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi V.; Martelli A.; Testai L.; Marino A.; Breschi M. C.; Calderone V. Hydrogen sulfide releasing capacity of natural isothiocyanates: is it a reliable explanation for the multiple biological effects of Brassicaceae?. Planta Med 2014, 80, 610. 10.1055/s-0034-1368591. [DOI] [PubMed] [Google Scholar]
- Citi V.; Martelli A.; Testai L.; Marino A.; Breschi M.; Calderone V. Hydrogen Sulfide Releasing Capacity of Natural Isothiocyanates: Is It a Reliable Explanation for the Multiple Biological Effiects of Brassicaceae?. Planta Med. 2014, 80, 610–613. 10.1055/s-0034-1368591. [DOI] [PubMed] [Google Scholar]
- Rose P.; Dymock B. W.; Moore P. K. GYY4137, a novel water-soluble, H2S-releasing molecule. Methods Enzymol. 2015, 554, 143–167. 10.1016/bs.mie.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Corvino A.; Frecentese F.; Magli E.; Perissutti E.; Santagada V.; Scognamiglio A.; Caliendo G.; Fiorino F.; Severino B. Trends in H2S-Donors Chemistry and Their Effects in Cardiovascular Diseases. Antioxidants 2021, 10 (3), 429. 10.3390/antiox10030429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson M.; Heymach J. V. Vascular endothelial growth factor (VEGF) pathway. J. Thorac. Oncol. 2006, 1 (8), 768–770. 10.1016/S1556-0864(15)30404-4. [DOI] [PubMed] [Google Scholar]
- Liang G. H.; Adebiyi A.; Leo M. D.; McNally E. M.; Leffler C. W.; Jaggar J. H. Hydrogen sulfide dilates cerebral arterioles by activating smooth muscle cell plasma membrane KATP channels. Am. J. Physiol. Heart Circ. Physiol. 2011, 300 (6), H2088–H2095. 10.1152/ajpheart.01290.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan A. Y.; Maulood I. M.; Salihi A. The vasodilatory mechanism of nitric oxide and hydrogen sulfide in the human mesenteric artery in patients with colorectal cancer. Exp. Ther. Med. 2021, 21 (3), 1–1. 10.3892/etm.2021.9646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G. G.; Li W. Hydrogen sulfide improves vessel formation of the ischemic adductor muscle and wound healing in diabetic db/db mice. Iranian J. Basic Med. Sci. 2019, 22 (10), 1192. 10.22038/ijbms.2019.36551.8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D.; Liao Y.; Cornel E. J.; Lv M.; Wu T.; Zhang X.; Du J.; et al. Polymersome wound dressing spray capable of bacterial inhibition and H2S generation for complete diabetic wound healing. Chem. Mater. 2021, 33 (20), 7972–7985. 10.1021/acs.chemmater.1c01872. [DOI] [Google Scholar]
- Liu F.; Chen D. D.; Sun X.; Xie H. H.; Yuan H.; Jia W.; Chen A. F. Hydrogen sulfide improves wound healing via restoration of endothelial progenitor cell functions and activation of angiopoietin-1 in type 2 diabetes. Diabetes. 2014, 63 (5), 1763–1778. 10.2337/db13-0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue W. L.; Chen R. Q.; Zhang Q. Q.; Li X. H.; Cao L.; Li M. Y.; Zhu Y. C.; et al. Hydrogen sulfide rescues high glucose-induced migration dysfunction in HUVECs by upregulating miR-126–3p. Am. J. Physiol. Cell Physiol. 2020, 318 (5), C857–C869. 10.1152/ajpcell.00406.2019. [DOI] [PubMed] [Google Scholar]
- Zhao H.; Lu S.; Chai J.; Zhang Y.; Ma X.; Chen J.; Guan Q.; Wan M.; Liu Y. Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J. Diabetes Its Complications. 2017, 31 (9), 1363–1369. 10.1016/j.jdiacomp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- Wu J.; Chen A.; Zhou Y.; Zheng S.; Yang Y.; An Y.; Xu K.; He H.; Kang J.; Luckanagul J. A.; Xian M.; Xiao J.; Wang Q. Novel H2S-Releasing hydrogel for wound repair via in situ polarization of M2 macrophages. Biomaterials. 2019, 222, 119398 10.1016/j.biomaterials.2019.119398. [DOI] [PubMed] [Google Scholar]
- Ferrante C. J.; Leibovich S. J. Regulation of macrophage polarization and wound healing. Adv. Wound Care. 2012, 1 (1), 10–16. 10.1089/wound.2011.0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarnezhada S.; Abbaszadeh-Goudarzi G.; Samadian H.; Khaksari M.; Ghatar J. M.; Khastar H.; Rezaei N.; Mousavi S. R.; Shirian S.; Salehi M. Alginate hydrogel containing hydrogen sulfide as the functional wound dressing material: In vitro and in vivo study. Int. J. Biol. Macromol. 2020, 164, 3323–3331. 10.1016/j.ijbiomac.2020.08.233. [DOI] [PubMed] [Google Scholar]
- Mauretti A.; Neri A.; Kossover O.; Seliktar D.; Nardo P. D.; Melino S. Design of a novel composite H2S-releasing hydrogel for cardiac tissue repair. Macromol. Biosci. 2016, 16 (6), 847–858. 10.1002/mabi.201500430. [DOI] [PubMed] [Google Scholar]
- Gambari L.; Amore E.; Raggio R.; Bonani W.; Barone M.; Lisignoli G.; Grigolo B.; Motta A.; Grassi F. Hydrogen sulfide-releasing silk fibroin scaffold for bone tissue engineering. Mater. Sci. Eng. C 2019, 102, 471–482. 10.1016/j.msec.2019.04.039. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Liu L.; An T.; Xian M.; Luckanagul J. A.; Su Z.; Lin Y.; Wang Q. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020, 104, 85–94. 10.1016/j.actbio.2019.12.032. [DOI] [PubMed] [Google Scholar]
- Lee K. Y.; Mooney D. J. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012, 37 (1), 106–126. 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smink A. M.; Najdahmadi A.; Alexander M.; Li S.; Rodriquez S.; van Goor H.; de Vos P.; et al. The Effect of a Fast-Releasing Hydrogen Sulfide Donor on Vascularization of Subcutaneous Scaffolds in Immunocompetent and Immunocompromised Mice. Biomolecules. 2020, 10 (5), 722. 10.3390/biom10050722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R.; Qu C.; Zhou Y.; Konkel J. E.; Shi S.; Liu Y.; Shi S.; et al. Hydrogen sulfide promotes Tet1-and Tet2-mediated Foxp3 demethylation to drive regulatory T cell differentiation and maintain immune homeostasis. Immunity. 2015, 43 (2), 251–263. 10.1016/j.immuni.2015.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciotti I.; Ciocci M.; Di Giovanni E.; Nanni F.; Melino S. Hydrogen sulfide-releasing fibrous membranes: Potential patches for stimulating human stem cells proliferation and viability under oxidative stress. Int. J. Mol. Sci. 2018, 19 (8), 2368. 10.3390/ijms19082368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J.; Li Y.; He C.; Kang J.; Ye J.; Xiao Z.; Wang Q.; et al. Novel H2S releasing nanofibrous coating for in vivo dermal wound regeneration. ACS Appl. Mater. Interfaces. 2016, 8 (41), 27474–27481. 10.1021/acsami.6b06466. [DOI] [PubMed] [Google Scholar]
- Pant J.; Mondal A.; Manuel J.; Singha P.; Mancha J.; Handa H. H2S-Releasing Composite: a Gasotransmitter Platform for Potential Biomedical Applications. ACS Biomater. Sci. Eng. 2020, 6 (4), 2062–2071. 10.1021/acsbiomaterials.0c00146. [DOI] [PubMed] [Google Scholar]
- Marwah M. K.; Shehzad S.; Shokr H.; Sacharczuk J.; Wang K.; Ahmad S.; Sanchez-Aranguren L. Novel controlled-release polylactic-co-glycolic acid (PLGA) nanoparticles for sodium thiosulphate, a hydrogen sulphide donor, retains pro-angiogenic potential of hydrogen sulphide. J. Exp. Nanosci. 2022, 17 (1), 197–213. 10.1080/17458080.2022.2060963. [DOI] [Google Scholar]
- Wang W.; Sun X.; Zhang H.; Yang C.; Liu Y.; Yang W.; Guo C.; Wang C. Controlled release hydrogen sulfide delivery system based on mesoporous silica nanoparticles protects graft endothelium from ischemia–reperfusion injury. Int. J. Nanomed. 2016, 11, 3255. 10.2147/IJN.S104604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh M.-H.; Tsai H.-W.; Lin K.-J.; Wu Z.-Y.; Hu H.-Y.; Chang Y.; Wei H.-J.; Sung H.-W. An in situ slow-releasing H2S donor depot with long-term therapeutic effects for treating ischemic diseases. Mater. Sci. Eng. C 2019, 104, 109954 10.1016/j.msec.2019.109954. [DOI] [PubMed] [Google Scholar]
- Lin W.-C.; Huang C.-C.; Lin S.-J.; Li M.-J.; Chang Y.; Lin Y.-J.; Wan W.-L.; Shih P.-C.; Sung H.-W. In situ depot comprising phase-change materials that can sustainably release a gasotransmitter H2S to treat diabetic wounds. Biomaterials. 2017, 145, 1–8. 10.1016/j.biomaterials.2017.08.023. [DOI] [PubMed] [Google Scholar]
- Ozaki K. S.; Kimura S.; Murase N. Use of carbon monoxide in minimizing ischemia/reperfusion injury in transplantation. Transplant. Rev. 2012, 26 (2), 125–139. 10.1016/j.trre.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak J.; Deshane J.; Jozkowicz A.; Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008, 117 (2), 231–241. 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. K.; Kim C.-K.; Lee H.; Jeoung D.; Ha K.-S.; Kwon Y.-G.; Kim K.-W.; Kim Y.-M. Carbon monoxide promotes VEGF expression by increasing HIF-1α protein level via two distinct mechanisms, translational activation and stabilization of HIF-1α protein. J. Biol. Chem. 2010, 285 (42), 32116–32125. 10.1074/jbc.M110.131284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M. J.; Cai W. J.; Zhu Y. C. Mechanisms of angiogenesis: role of hydrogen sulphide. Clin. Exp. Pharmacol. Physiol. 2010, 37 (7), 764–771. 10.1111/j.1440-1681.2010.05371.x. [DOI] [PubMed] [Google Scholar]
- Bussolati B.; Mason J. C. Dual role of VEGF-induced heme-oxygenase-1 in angiogenesis. Antioxid. Redox Signaling. 2006, 8 (7–8), 1153–1163. 10.1089/ars.2006.8.1153. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Sun M.; Wang L.; Jiao B. HIFs, angiogenesis, and cancer. J.Cell. Biochem 2013, 114 (5), 967–974. 10.1002/jcb.24438. [DOI] [PubMed] [Google Scholar]
- Tirpe A. A.; Gulei D.; Ciortea S. M.; Crivii C.; Berindan-Neagoe I. Hypoxia: overview on hypoxia-mediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 2019, 20 (24), 6140. 10.3390/ijms20246140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong A. M.; Luke P. P.; Bhattacharjee R. N. Carbon monoxide mechanism of protection against renal ischemia and reperfusion injury. Biochem. Pharmacol. 2022, 202, 115156 10.1016/j.bcp.2022.115156. [DOI] [PubMed] [Google Scholar]
- Kourti M.; Westwell A.; Jiang W.; Cai J. Repurposing old carbon monoxide-releasing molecules towards the anti-angiogenic therapy of triple-negative breast cancer. Oncotarget. 2019, 10 (10), 1132. 10.18632/oncotarget.26638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulak J.; Jozkowicz A.; Foresti R.; Kasza A.; Frick M.; Huk I.; Green C. J.; Pachinger O.; Weidinger F.; Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid. Redox Signaling. 2002, 4 (2), 229–240. 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- Long J.; Wang S.; Zhang Y.; Liu X.; Zhang H.; Wang S. The therapeutic effect of vascular endothelial growth factor gene-or heme oxygenase-1 gene-modified endothelial progenitor cells on neovascularization of rat hindlimb ischemia model. J. Vasc. Surg. 2013, 58 (3), 756–765. 10.1016/j.jvs.2012.11.096. [DOI] [PubMed] [Google Scholar]
- Grochot-Przeczek A.; Kotlinowski J.; Kozakowska M.; Starowicz K.; Jagodzinska J.; Stachurska A.; ozkowicz A.; et al. Heme oxygenase-1 is required for angiogenic function of bone marrow-derived progenitor cells: role intherapeutic revascularization. Antioxid. Redox Signaling. 2014, 20 (11), 1677–1692. 10.1089/ars.2013.5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. H.; Kim S. J.; Na H. K.; Han W.; Kim N. J.; Suh Y. G.; Surh Y. J. 15-Deoxy-Δ12, 14-prostaglandin J2 upregulates VEGF expression via NRF2 and heme oxygenase-1 in human breast cancer cells. Cells. 2021, 10 (3), 526. 10.3390/cells10030526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaba H.; Fujita K.; Ueno T. Design of biomaterials for intracellular delivery of carbon monoxide. Biomater. Sci. 2015, 3 (11), 1423–1438. 10.1039/C5BM00210A. [DOI] [PubMed] [Google Scholar]
- Aksakal R.; Mertens C.; Soete M.; Badi N.; Du Prez F. Applications of discrete synthetic macromolecules in life and materials science: recent and future trends. Adv. Sci. 2021, 8 (6), 2004038 10.1002/advs.202004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossen S.; Hossain M. K.; Basher M. K.; Mia M. N. H.; Rahman M. T.; Uddin M. J. Smart nanocarrier-based drug delivery systems for cancer therapy and toxicity studies: A review. J. Adv. Res. 2019, 15, 1–18. 10.1016/j.jare.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales M. A.; Han H.; Moyes A.; Radinos A.; Hobbs A. J.; Coombs N.; Oliver S. R. J.; Mascharak P. K. Light-triggered carbon monoxide delivery with Al-MCM-41-based nanoparticles bearing a designed manganese carbonyl complex. J. Mater. Chem. B 2014, 2 (15), 2107–2113. 10.1039/c3tb21309a. [DOI] [PubMed] [Google Scholar]
- Wang X.; Gao B.; Sebit Ahmed Suleiman G.; Ren X.-k.; Guo J.; Xia S.; Zhang W.; Feng Y. A “controlled CO release” and “pro-angiogenic gene” dually engineered stimulus-responsive nanoplatform for collaborative ischemia therapy. Chem. Eng. J. 2021, 424, 130430 10.1016/j.cej.2021.130430. [DOI] [Google Scholar]
- Joshi H. P.; Kumar H.; Choi U. Y.; Lim Y. C.; Choi H.; Kim J.; Han I. B.; et al. CORM-2-solid lipid nanoparticles maintain integrity of blood-spinal cord barrier after spinal cord injury in rats. Mol. Neurobiol. 2020, 57 (6), 2671–2689. 10.1007/s12035-020-01914-5. [DOI] [PubMed] [Google Scholar]
- Fernandes A. R.; Mendonça-Martins I.; Santos M. F.; Raposo L. R.; Mendes R.; Marques J.; Baptista P. V.; et al. Improving the anti-inflammatory response via gold nanoparticle vectorization of CO-releasing molecules. ACS Biomater. Sci. Eng. 2020, 6 (2), 1090–1101. 10.1021/acsbiomaterials.9b01936. [DOI] [PubMed] [Google Scholar]
- Hasegawa U.; Van Der Vlies A. J.; Simeoni E.; Wandrey C.; Hubbell J. A. Carbon monoxide-releasing micelles for immunotherapy. J. Am. Chem. Soc. 2010, 132 (51), 18273–18280. 10.1021/ja1075025. [DOI] [PubMed] [Google Scholar]
- Sarady J. K.; Otterbein S. L.; Liu F.; Otterbein L. E.; Choi A. M. Carbon monoxide modulates endotoxin-induced production of granulocyte macrophage colony-stimulating factor in macrophages. Am. J. Respir. Cell Mol. Biol. 2002, 27 (6), 739–745. 10.1165/rcmb.4816. [DOI] [PubMed] [Google Scholar]
- Chhikara M.; Wang S.; Kern S. J.; Ferreyra G. A.; Barb J. J.; Munson P. J.; Danner R. L. Carbon monoxide blocks lipopolysaccharide-induced gene expression by interfering with proximal TLR4 to NF-κB signal transduction in human monocytes. PloS one. 2009, 4 (12), e8139 10.1371/journal.pone.0008139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delasoie J.; Radakovic N.; Pavic A.; Zobi F. Neovascularization Effects of Carbon Monoxide Releasing Drugs Chemisorbed on Coscinodiscus Diatoms Carriers Characterized by Spectromicroscopy Imaging. Appl. Sci. 2020, 10 (20), 7380. 10.3390/app10207380. [DOI] [Google Scholar]
- Hu Q.; Wu D.; Ma F.; Yang S.; Tan B.; Xin H.; Zhu Y. Z.; et al. Novel angiogenic activity and molecular mechanisms of ZYZ-803, a slow-releasing hydrogen sulfide–nitric oxide hybrid molecule. Antioxid. Redox Signaling. 2016, 25 (8), 498–514. 10.1089/ars.2015.6607. [DOI] [PubMed] [Google Scholar]
- Lee J.; Kwak D.; Kim H.; Kim J.; Hlaing S. P.; Hasan N.; Cao J.; Yoo J.-W. Nitric oxide-releasing s-nitrosoglutathione-conjugated poly (Lactic-Co-Glycolic Acid) nanoparticles for the treatment of MRSA-infected cutaneous wounds. Pharmaceutics. 2020, 12 (7), 618. 10.3390/pharmaceutics12070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes L. M.; Singha P.; Singh S.; Sakthivel T. S.; Garren M.; Devine R.; Brisbois E. J.; Seal S.; Handa H. Characterization of a nitric oxide (NO) donor molecule and cerium oxide nanoparticle (CNP) interactions and their synergistic antimicrobial potential for biomedical applications. J. Colloid Interface Sci. 2021, 586, 163–177. 10.1016/j.jcis.2020.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.; Hlaing S. P.; Cao J.; Hasan N.; Ahn H. J.; Song K. W.; Yoo J. W. In situ hydrogel-forming/nitric oxide-releasing wound dressing for enhanced antibacterial activity and healing in mice with infected wounds. Pharmaceutics. 2019, 11 (10), 496. 10.3390/pharmaceutics11100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z.; He K.; Duan Y.; Shen Z.; Cheng J.; Zhang G.; Hu J. Photo-degradable micelles for co-delivery of nitric oxide and doxorubicin. J. Mater. Chem. B 2020, 8 (31), 7009–7017. 10.1039/D0TB00817F. [DOI] [PubMed] [Google Scholar]
- Li Y.; Lin J.; Zhi X.; Li P.; Jiang X.; Yuan J. Triple stimuli-responsive keratin nanoparticles as carriers for drug and potential nitric oxide release. Mater. Sci. Eng. C 2018, 91, 606–614. 10.1016/j.msec.2018.05.073. [DOI] [PubMed] [Google Scholar]
- Liu W.; Semcheddine F.; Guo Z.; Jiang H.; Wang X. Glucose-Responsive ZIF-8 Nanocomposites for Targeted Cancer Therapy through Combining Starvation with Stimulus-Responsive Nitric Oxide Synergistic Treatment. ACS Appl. Bio Mater. 2022, 5, 2902–2912. 10.1021/acsabm.2c00262. [DOI] [PubMed] [Google Scholar]
- Yang F.; Li M.; Liu Y.; Wang T.; Feng Z.; Cui H.; Gu N. Glucose and magnetic-responsive approach toward in situ nitric oxide bubbles controlled generation for hyperglycemia theranostics. J. Controlled Release 2016, 228, 87–95. 10.1016/j.jconrel.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Pant J.; Pedaparthi S.; Hopkins S. P.; Goudie M. J.; Douglass M. E.; Handa H. Antibacterial and cellular response toward a gasotransmitter-based hybrid wound dressing. ACS Biomater. Sci. Eng. 2019, 5 (8), 4002–4012. 10.1021/acsbiomaterials.9b00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C. W.; Wu C. H.; Chiang Y. C.; Chen Y. L.; Chang K. T.; Chuang C. C.; Lee I. T. Carbon monoxide releasing molecule-2 attenuates Pseudomonas aeruginosa-induced ROS-dependent ICAM-1 expression in human pulmonary alveolar epithelial cells. Redox Biol. 2018, 18, 93–103. 10.1016/j.redox.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H.; Yang Q.; Yong J.; Fang X.; Yang Z.; Liu Z.; Jiang X.; Miao W.; Li X. Mitochondria targeted nanoparticles to generate oxygen and responsive-release of carbon monoxide for enhanced photogas therapy of cancer. Biomater. Sci. 2021, 9 (7), 2709–2720. 10.1039/D0BM02028A. [DOI] [PubMed] [Google Scholar]
- Fujita K.; Tanaka Y.; Sho T.; Ozeki S.; Abe S.; Hikage T.; Kuchimaru T.; Kizaka-Kondoh S.; Ueno T. Intracellular CO release from composite of ferritin and ruthenium carbonyl complexes. J. Am. Chem. Soc. 2014, 136 (48), 16902–16908. 10.1021/ja508938f. [DOI] [PubMed] [Google Scholar]
- Kim I.; Han E. H.; Bang W.-Y.; Ryu J.; Min J.-Y.; Nam H. C.; Park W. H.; Chung Y.-H.; Lee E. Supramolecular carbon monoxide-releasing peptide hydrogel patch. Adv. Funct. Mater. 2018, 28 (47), 1803051 10.1002/adfm.201803051. [DOI] [Google Scholar]
- Liu J.; Li R. S.; He M.; Xu Z.; Xu L. Q.; Kang Y.; Xue P. Multifunctional SGQDs-CORM@ HA nanosheets for bacterial eradication through cascade-activated “nanoknife” effect and photodynamic/CO gas therapy. Biomaterials. 2021, 277, 121084 10.1016/j.biomaterials.2021.121084. [DOI] [PubMed] [Google Scholar]
- Yang G.; Fan M.; Zhu J.; Ling C.; Wu L.; Zhang X.; Cai X.; et al. A multifunctional anti-inflammatory drug that can specifically target activated macrophages, massively deplete intracellular H2O2, and produce large amounts CO for a highly efficient treatment of osteoarthritis. Biomaterials. 2020, 255, 120155 10.1016/j.biomaterials.2020.120155. [DOI] [PubMed] [Google Scholar]
- Chen J.; Chen D.; Chen J.; Shen T.; Jin T.; Zeng B.; Cai X.; et al. An all-in-one CO gas therapy-based hydrogel dressing with sustained insulin release, anti-oxidative stress, antibacterial, and anti-inflammatory capabilities for infected diabetic wounds. Acta Biomater. 2022, 146, 49–65. 10.1016/j.actbio.2022.04.043. [DOI] [PubMed] [Google Scholar]
- Zhang C.; Peng S.-Y.; Hong S.; Chen Q.-W.; Zeng X.; Rong L.; Zhong Z.-L.; Zhang X.-Z. Biomimetic carbon monoxide nanogenerator ameliorates streptozotocin induced type 1 diabetes in mice. Biomaterials. 2020, 245, 119986 10.1016/j.biomaterials.2020.119986. [DOI] [PubMed] [Google Scholar]