Abstract
DsbA, a disulfide bond catalyst, is necessary for realization of the pathogenic potential of Shigella flexneri. Sh42, a mutant strain differing from wild-type M90TS solely because it expresses nonfunctional DsbA33G (substitution for 33C at the active site), secreted less IpaB and IpaC than M90TS in response to various stimuli in vitro. A kinetic study demonstrated that Sh42 responded more slowly to Congo red than M90TS. By modulating relative concentrations of functional and nonfunctional DsbA within bacteria, functional enzyme has been shown to be necessary for intercellular spread. By confocal microscopy, M90TS dividing in protrusions was shown to secrete Ipa proteins from the septation furrow, anticipating lysis of protrusions, while Sh42 showed minimal Ipa secretion in this location. In the light of a previous demonstration that DsbA is not necessary for entry of epithelial cells, we conclude that a role in virulence of this disulfide bond catalyst lies in facilitating secretion of Ipa proteins specifically within epithelial protrusions, in turn allowing cell-to-cell spread of S. flexneri.
Shigella flexneri is an intracellular pathogen that causes the acute infectious inflammatory enteritis in humans and primates known as shigellosis. In vitro, S. flexneri efficiently invades cultured epithelial cells, a process involving several steps: entry, escape from the phagocytic vacuole, intracellular spread, and passage into neighboring cells via protrusions (28). Genes essential for invasion are encoded by three contiguous operons—ipa, mxi, and spa—in a 31-kb pathogenicity island on a 220-kb virulence plasmid (13, 17). When the bacterium contacts the epithelial cell surface or responds to other environmental stimuli, it secretes IpaB, -C, and -D through the type III secretion apparatus constituted by the Mxi and Spa proteins (28). The secreted Ipa proteins form a complex (21) that interacts with the host cell, involving binding to the α5β1 integrin and CD44 on the cell membrane (33, 35). This binding triggers at least two major signaling pathways. The proto-oncoprotein pp60c-src-mediated pathway induces tyrosine phosphorylation of cortactin, a cytoskeleton-associated tyrosine kinase substrate (9), and a small GTP-binding protein (Rho)-mediated pathway leads to tyrosine phosphorylation of a 125-kDa focal adhesion kinase, pp125FAK, and of paxillin (1, 36). As a consequence, actin polymerization occurs at bacterial attachment sites, which leads to cell cytoskeletal rearrangement, which in turn leads to bacterial entry (35) by parasite-directed phagocytosis.
Once taken up, the bacteria rapidly destroy the phagocytic vacuole and escape into the host cell cytosol, where they proliferate (14). IpaB was first identified as the Shigella hemolysin responsible for lysis of the phagocytic membrane. A nonpolar ipaB mutant was unable to lyse erythrocytes or to escape from the phagocytic vacuole in macrophage-like J774 cells (14). A recent study has shown that IpaC also plays an active role in both entry and lysis of the phagocytic vacuole (5, 8). Furthermore, purified IpaC or IpaB alone is able to cause lysis of calcein-loaded lipid vesicles, suggesting that both proteins interact with the host cell membrane in vivo to fulfil the dual role of inducing ruffling upon entry and lysis of the phagocytic membrane thereafter (28).
Inside the epithelial cytosol, S. flexneri can move rapidly in a random fashion by polymerizing actin, which forms a “comet tail” behind the moving organism. This actin-based movement is mediated by the 120-kDa outer membrane protein IcsA, encoded by the gene borne by the virulence plasmid (25). IcsA is expressed only at one pole of the bacterium, and its processing requires a specific protease, SopA (11). IcsA was discovered as a factor promoting tissue dissemination, and an icsA deletion mutant was strongly attenuated (20, 30). Presumably, actin-based movement provides a driving force for protrusion formation when a moving organism collides with the inner face of the cytoplasmic membrane (28). There is evidence that protrusions form at the intermediate junction of epithelial cells, where cadherin is a key molecule interacting with the bacterium and its actin tail to lead to the formation of a rigid protrusion structure (31). After a protrusion enters a neighboring cell, the bacterium rapidly destroys the now double-membrane-bounded compartment to lie free once more in the host cell cytosol. Lysis of protrusions was thought to depend on IcsB, also encoded by a gene borne by the virulence plasmid (2). A recent study has shown, however, that a nonpolar icsB mutant has no defect in spread and that the original insertional mutation in the icsB mutant appears to have a polar effect on Ipa protein expression (26). Furthermore, other recent studies have shown that IpaB, IpaC, their specific molecular chaperone IpgC, and the Mxi-Spa secretion system are all required for intercellular spread (24, 32). These studies suggest that secreted Ipa proteins are most likely responsible for the lysis of protrusion membranes.
We have been studying the role of the bacterial periplasmic protein disulfide bond catalyst DsbA in Shigella pathogenesis (38). This enzyme is required for oxidative folding of Spa32, an outer membrane protein constituent of the Shigella type III secretion apparatus which subserves Ipa protein secretion (34). We have focused on the role of DsbA in intracellular processes which follow initial cell entry, once organisms lie within the glutathione-rich reducing environment of the cytoplasm (12). Our previous work with the dsbA knockout mutant Sh4 revealed a failure of bacterial spread from cell to cell and an associated loss of virulence. The mutant was recovered in greatly reduced viable counts from an infected HeLa cell monolayer during a 4-h experiment, and transmission electron microscopy revealed the cause to be disintegration of organisms proliferating within intercellular epithelial protrusions that fail to lyse and so to give rise to productive cell-to-cell spread (38). The insertion of the kan cassette in dsbA to construct Sh4 potentially altered expression of the adjacent gene yihE, with which dsbA is partly cotranscribed (6). Growth of Sh4 in vitro was significantly slower (doubling time, 56 min) than that of the wild type (35 min) (our unpublished data), and a concern remained that the phenotype reflected more than simply loss of oxidoreductase activity. In order to resolve this, we have now constructed a nonpolar DsbA active-site mutant, Sh42, and used this to evaluate the effect of dsbA mutation on Ipa secretion and cell-to-cell spread.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. S. flexneri strains were routinely grown at 37°C overnight on tryptic soy agar (TSA) plates containing 0.01% Congo red. Red colonies were inoculated into tryptic soy broth and grown to an appropriate turbidity at 37°C with shaking (180 rpm) for subsequent experiments. Escherichia coli strains were routinely grown at 37°C in Luria-Bertani medium (L broth or 1.5% L agar). Dithiothreitol (10 mM) was added as a supplement to TSA to confirm dsbA mutants. Antibiotic supplementation, when necessary, was to the following final concentrations: 100 μg/ml for streptomycin, 200 μg/ml for ampicillin, and 50 μg/ml for kanamycin.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| XL-1 Blue | recA1 lac endA1 gyrA96 thi hsdR17 supE44 rclA1 (F′ proAB lacIqlacZΔM15 Tn10) Δ(ara-leu) araD ΔlacX74 galE galK phoA20 | Pharmacia |
| CC118 λpir | thi-1 rpsE rpoB argE(Am) recA1 λpir phage lysogen | 11 |
| JCB571 | phoR mutant, zih-12::Tn10 dsbA::mini-kan Tetr | 4 |
| S. flexneri | ||
| M90TS | Wild type, serotype 5, Strr | 2 |
| Sh4 | dsbA::kan mutant, Strr Kanr | 38 |
| Sh42 | dsbA33G Strr | This study |
| Plasmids | ||
| pCVD442 | Suicide cloning vector | 11 |
| pJYU15 | Derivative of pCVD442 for delivery of the dsbA33G mutation | This study |
| pMMB66 | Broad-host-range plasmid with ptac promoter at the multiple-cloning site | 27 |
| pJYU5 | Derivative of pMMB66 expressing functional recombinant DsbAHis | This study |
| pBEJ18 | Derivative of pMMB66 expressing nonfunctional recombinant DsbA33GHis | This study |
Plasmid construction.
Red-Hot DNA polymerase (Advanced Biotechnologies) was used for PCR amplification. The oligonucleotide primers used for amplification of the yihE-dsbA operon were OA1 (5′-CGTGTCTGTCTCAAGAGTAA-3′) and OB1 (5′-CCTTCAATATCCAGGTTAG-3′), derived from the published E. coli sequence (GenBank accession no. X80762). The PCR product was cloned and modified for construction of a dsbA33G mutant of S. flexneri as described in Results. Primers used for amplification of the dsbA coding sequence were dsbA1 (5′-GGAATTCGGAGAGAGTAGATCATGAA-3′) and dsbA3b (5′-CCCGGATCCTTAGTGATGGTGATGGTGATGCGATCCTCTTTTTTTCTCGGACAGATAT-3′), derived from the S. flexneri dsbA sequence (GenBank accession no. D38253). The underlined bases encode a His6 tail at the carboxyl terminus of DsbA. The PCR product was cloned into pGEM-T (Promega) and then subcloned into a broad-range expression vector, pMMB66 (25). The resultant plasmid, pJYU5, expresses high levels of recombinant DsbAHis when the promoter ptac is induced by the addition of IPTG (isopropyl-β-d-thiogalactopyranoside). pJYU5 was subsequently subjected to site-directed mutagenesis using a QuikChange kit (Stratagene) with the primers AG33EF (5′-CTTCTGCCCGCATGGATATCAGTTTGAAG-3′) and AG33ER (5′-CTTCAAACTGATATCCATGCGGGCAGAAG-3′). The underlined bases in the primers were incorporated in order to replace codon Cys33 with Gly33 at the active site and to introduce an EcoRV site for screening of mutant clones. Thus, a clone, pBEJ18, that was able to produce the nonfunctional protein DsbA33GHis was constructed.
Purification of recombinant DsbAHis and preparation of polyclonal antibody against DsbA.
A dsbA-null E. coli strain, JCB571 (4), was transformed with pJYU5. The transformant was grown to exponential phase in L broth, and expression of DsbAHis was induced by addition of IPTG to a final concentration of 2 mM. After a further 2 h of incubation at 37°C, bacteria were collected by centrifugation and the DsbAHis was purified from the periplasm extract using a QIAexpress (nickel-nitrilotriacetic acid) system (Qiagen) under native conditions. Purified DsbAHis protein was used to immunize a rabbit for preparation of polyclonal antibodies (Immune System Ltd., Paignton, United Kingdom). Polyclonal antiserum was obtained and absorbed with the null dsbA mutant Sh4 to abolish cross-reaction with other Shigella proteins, leaving a specific anti-DsbA reagent with a titer of 1:3,000 for immunoblot assays.
Determination of the redox status of DsbA in vivo.
Determination of the redox status of DsbA in vivo was carried out as described by Kobayashi et al. (19). Briefly, whole-cell proteins were precipitated by direct treatment of a culture with trichloroacetic acid at a final concentration of 5% to avoid any subsequent reduction of DsbA after cell disruption. Protein precipitates were washed once with acetone and dissolved in freshly prepared solution containing 1% sodium dodecyl sulfate, 50 mM Tris-HCl (pH 7.5), and 15 mM 4-acetamido-4′-maleimidyl-stilbene-2,2′-disulfonate. Proteins were then separated by sodium dodecyl sulfate–15% polyacrylamide gel electrophoresis without using any reducing reagent and transferred to nitrocellulose membranes. DsbA and its recombinant derivatives (DsbA33G, DsbAHis, and DsbA33GHis) were detected either with anti-DsbA antibodies followed by horseradish peroxidase (HRP)-conjugated goat anti-rabbit immunoglobulin G (IgG) (DAKO) or with monoclonal anti-His6 antibody (Sigma) followed by HRP-conjugated rabbit anti-mouse IgG (DAKO) and visualized by ECL (Amersham).
Activation and detection of secreted Ipa proteins.
Activation and detection of secreted Ipa proteins were carried out as described by Bahrani et al. (3). Briefly, 109 CFU were collected from mid-log-phase cultures and resuspended in 1 ml of phosphate-buffered saline (PBS) containing either Congo red (0.01%), fibronectin (50 μg/ml), or laminin (50 μg/ml). The bacterial suspensions were incubated at 37°C for 30 min or for a series of times to activate Ipa protein secretion. Bacteria were spun down, washed once with PBS, resuspended in 100 μl of sample buffer, and boiled for 5 min, and 10 μl was loaded on the gel. The supernatants were passed through a 0.45-μm-pore-size filter, and proteins were precipitated with 10% trichloroacetic acid, washed with pure ethanol, dissolved in 25 μl of sample buffer, boiled for 5 min, and loaded on the gel. IpaB and IpaC were captured by the monoclonal antibodies H16 (anti-IpaB) and J22 (anti-IpaC), respectively (22), followed by incubation with goat anti-mouse IgG conjugated with HRP (DAKO), and were visualized by ECL (Amersham). Immunoblots was scanned using an imaging densitometer (model GS-690; Bio-Rad) for semiquantitation.
Infection of cultured cells.
Plaque assays were carried out using CaCo-2 cells according to the method of Oaks et al. (23). Infection of HeLa cells was performed as described by Sansonetti et al. (30).
Fluorescent labeling of infected HeLa cells and confocal laser scanning microscopy.
Two hours postinfection, HeLa cells were washed with PBS, fixed in 3.7% (wt/vol) paraformaldehyde for 20 min at room temperature, permeabilized with 0.1% Triton X-100 for 10 min at room temperature, and blocked in 8% bovine serum albumin overnight at 4°C. Rabbit antiserum against Shigella lipopolysaccharide (LPS) was used to capture bacteria, followed by secondary labeling with Texas red-conjugated goat anti-rabbit polyclonal IgG (Jackson). IpaB and IpaC were localized with mouse monoclonal antibody H16 or J22, followed by secondary labeling with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (20). A confocal laser scanning microscope (LSM-510; Zeiss, Göttingen, Germany) was used to scan a series of optical sections at <1-μm intervals. Pictures were processed using Adobe Photoshop software.
RESULTS
Construction of Sh42 expressing nonfunctional DsbA33G.
To avoid the problems of polarity theoretically contributing to the attenuated phenotype of the dsbA-knockout strain Sh4, site-directed mutagenesis was used to replace one of the cysteines crucial for catalytic activity (37). The dsbA locus (2,742 bp), including a complete upstream gene, yihE, and part of the downstream gene orfB, was amplified from M90TS using the primers OA1 and OB1 and cloned into pGEM-T (Promega). The resultant clone was subjected to site-directed mutagenesis as described in Materials and Methods, creating a Cys33-to-Gly33 substitution at the active site. A mutant clone was identified by endonuclease digestion at the novel EcoRV site and confirmed by DNA sequencing. The mutant clone with dsbA33G was subcloned into pCVD442 to generate a suicide plasmid, pJYU15, which was used in an allelic exchange to replace wild-type dsbA in M90TS by the method of Donnenberg and Kaper (10). An S. flexneri dsbA33G mutant, Sh42, was first identified by its growth defect on TSA-dithiothreitol plates, and its identity was confirmed by direct sequencing. In vitro, Sh42 grew more rapidly than Sh4 (doubling time, 45 min), although still more slowly than the wild type.
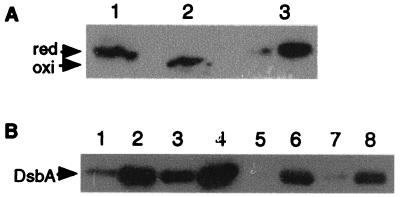
The expression of mutant protein DsbA33G from Sh42 was confirmed by immunoblotting using anti-DsbA antibodies (Fig. 1A). In the absence of added reducing agents, DsbA from wild-type M90TS is found in the fast-migrating oxidized state. In contrast, DsbA33G expressed from Sh42 migrated more slowly, comparable to the speed of reduced DsbA, reflecting the loss of one cysteine needed to form the internal disulfide bond (4).
FIG. 1.
Expression of DsbA from S. flexneri. (A) Analysis of the redox status of wild-type and mutant DsbA33G during laboratory growth. Lanes 1 and 2, DsbA from M90TS, treated with reducing (lane 1) and nonreducing (lane 2) buffer; lane 3, DsbA33G from Sh42 treated with nonreducing buffer. Anti-DsbA polyclonal antiserum was used for the immunoblot. (B) Expression of recombinant DsbA from plasmids during bacterial growth. Lanes 1 and 5, M90TS/pBEJ18 (noninduced); lanes 2 and 6, M90TS/pBEJ18 (induced); lanes 3 and 7, Sh42/pJYU5 (noninduced); lanes 4 and 8, Sh42/pJYU5 (induced). DsbA in lanes 1 to 4 was detected by anti-DsbA antiserum and in lanes 5 to 8 was detected by anti-His6 antibody (Sigma). red, reduced form; oxi, oxidized form.
Functional DsbA is required for intercellular dissemination.
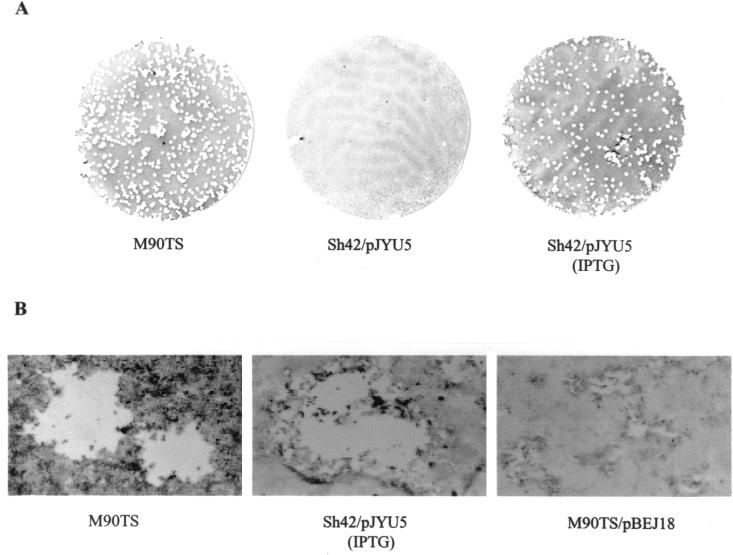
Like Sh4, Sh42 failed to elicit keratoconjunctivitis in guinea pigs (data not shown), a virulence phenotype dependent on intraepithelial growth and cell-to-cell spread. Sh42 multiplied within the bacterial cytoplasm (albeit more slowly than the wild type) but was retained in epithelial protrusions and (as previously reported for the mutant Sh4 [38]) disintegrated when protrusions lysed, identifying a defect in cell-to-cell spread as the basis for attenuation of virulence. To address the specific role of DsbA in bacterial dissemination, we carried out a plaque assay using cultured CaCo-2 cell monolayers. Sh42 was unable to form plaques when it was not transformed with cloned dsbA (data not shown) or when it harbored pJYU5 in the uninduced state (Fig. 2A). However, when the promoter ptac was induced by IPTG supplementation of the overlay medium, Sh42/pJYU5 formed plaques that were similar in size and in numbers to those of M90TS (Fig. 2). As IPTG induction resulted in an increase of DsbAHis expression from pJYU5 (Fig. 1B), it could be concluded that the elevated expression of DsbAHis was responsible for restoration of bacterial dissemination. Furthermore, the IPTG-containing overlay also contained gentamicin (50 μg/ml) and was applied after extensive washing of the infected monolayers, which ensured that bacteria could not enter the cells after application of the overlay. The elevated expression of DsbAHis from pJYU5, therefore, played no role in bacterial entry, rather only permitting already internalized bacteria to disseminate in the CaCo-2 cell monolayers. These data indicate that DsbA is necessary for intercellular dissemination but is not crucial at entry, confirming our previous finding that Sh4 penetrated HeLa cells with an efficiency comparable to that of the wild type (38).
FIG. 2.
Plaque assays. CFU (106) from each strain were used to infect CaCo-2 cell monolayers in a 35-mm-diameter culture dish. (A) Whole plates; (B) individual plaques.
The effect of a mixture of functional (DsbAHis) and nonfunctional (DsbA33GHis) DsbA on the dissemination phenotype was explored in this merodiploid system. S. flexneri M90TS was transformed with pBEJ18 expressing DsbA33GHis. Under noninducing conditions, M90TS/pBEJ18 formed tiny plaques, visible only under the microscope (Fig. 2B). When induced by the addition of IPTG in the overlay medium, M90TS/pBEJ18 was no longer able to produce even microscopic plaques (Fig. 1B).
Wild-type S. flexneri M90TS secretes Ipa proteins in protrusions in a DsbA-dependent process.
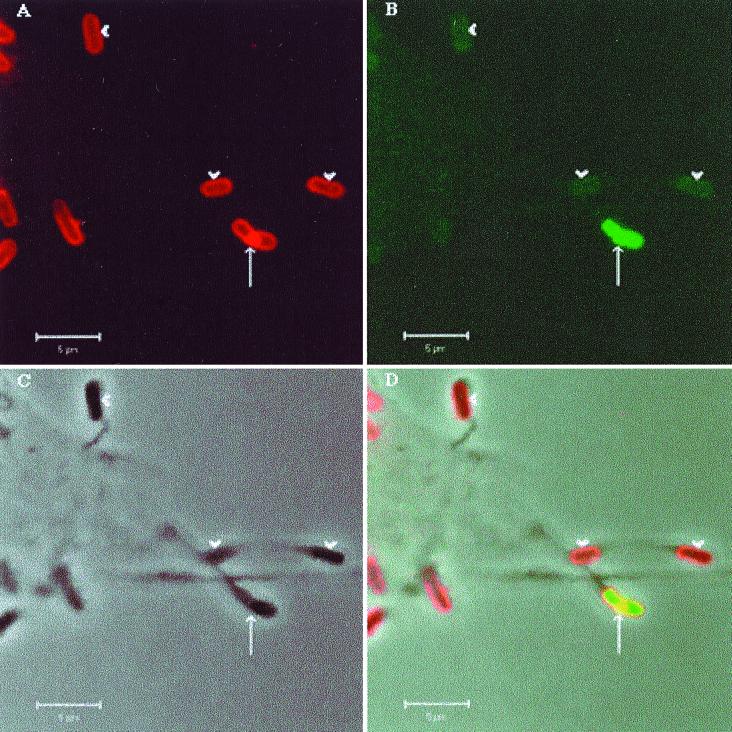
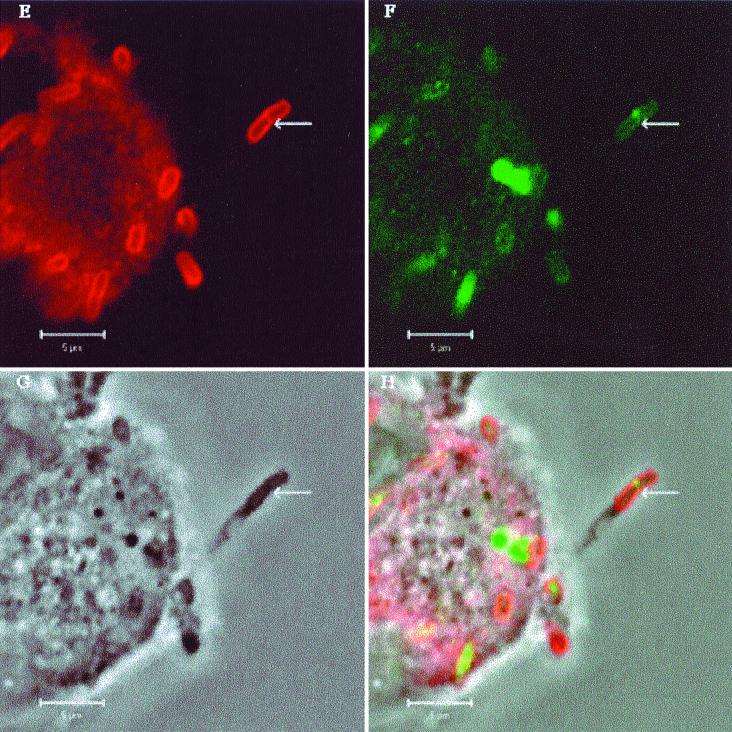
Ipa proteins secreted via the type III secretion system (24, 32) are required for Shigella dissemination. As DsbA catalyzes the oxidative folding of Spa32, a constituent of the type III machinery which is critical for maintaining the function of the secretion system (34), we have investigated whether there was a difference between M90TS and Sh42 in the secretion of Ipa proteins into protrusions. Confocal microscopy was used to examine HeLa cells infected with M90TS or Sh42 after double fluorescence labeling to localize LPS and IpaB or IpaC. No fluorescence was detected when labeled secondary antibodies were used alone or mismatched to the primary antibodies (data not shown). Bacteria could be identified both associated with the cell body (within the cytosol or on the surface) and within protrusions (Fig. 3). In nearly 100 protrusions formed by M90TS, we observed in each case substantial secretion of Ipa proteins at the septation furrow of the dividing bacterium, closely resembling in appearance the secretion of Ipa proteins in another context, that upon contact of bacteria with the surfaces of epithelial cells (22). Figure 3A to D show a typical protrusion. The dividing bacterium within the protrusion has apparently accumulated a large amount of LPS at the obvious septation furrow, where Ipa proteins were secreted, consistent with the hypothesis that assembly of the secretion machinery is coupled with LPS biosynthesis at this location (22). Such protrusions stained for IpaC showed the same characteristics (data not shown). Figure 3A to D also show protrusions containing nondividing bacteria, now only weakly stained for LPS and IpaB, further indicating that secretion of Ipa proteins into protrusions is associated with bacterial division. In the case of Sh42, although protrusions often contained dividing bacteria, in all of 50 protrusions examined, both LPS and Ipa proteins were stained much more weakly at the septation furrows. Figure 3E to H show a representative example. While the dividing bacterium in one protrusion (3F) has secreted very little IpaB, in striking contrast, organisms apparently attached to the host cell surface colocalize with a substantial quantity of the protein, strongly suggesting that the secretion of Ipa proteins from Sh42 was specifically impaired in the protrusions. Locally secreted Ipa proteins are thus strongly implicated as the effectors of protrusion lysis, in a process depending on functional DsbA.
FIG. 3.
Localization of IpaB in S. flexneri-infected HeLa cells by double fluorescence labeling and confocal microscopy. (A to D) HeLa cells infected with M90TS. Arrows indicate protrusions containing a dividing bacterium; arrowheads indicate nondividing bacteria in protrusions. (E to H) HeLa cells infected with Sh42. Arrows indicate protrusions containing Sh42. Other bacteria lie within the cell or on the surface. (A and E) Bacteria binding rabbit anti-Shigella LPS antiserum or goat anti-rabbit IgG conjugated with Texas red. (B and F) Localization of IpaB with mouse monoclonal antibody H16 or FITC-conjugated goat anti-mouse IgG. Panels C and G are phase-contrast images of the fields shown in panels A and B and E and F, respectively. (D and F) Overlays of A, B, and C and E, F, and G, respectively.
Ipa protein secretion from Sh42 is impaired in vitro.
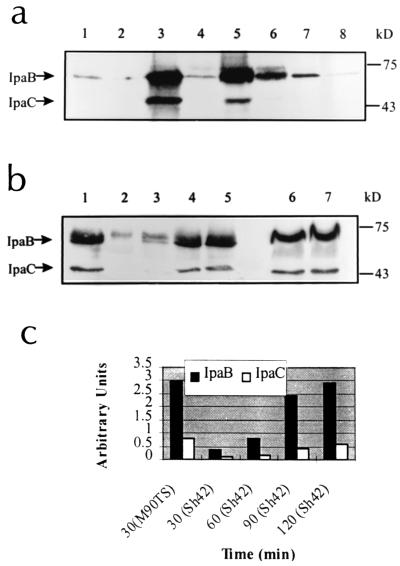
One explanation for the failure of Sh42 to secrete Ipa proteins into protrusions might be that stimuli there, while sufficient to trigger release of Ipa proteins from M90TS, are insufficient to do so for Sh42. To study this possibility, we examined secretion of IpaB and IpaC in vitro in response to Congo red, fibronectin, and laminin by the method of Bahrani et al. (3). Although laminin appeared to be a weak stimulus, Congo red and fibronectin stimulated massive secretion of IpaB and IpaC from M90TS in 30 min. In comparison, Sh42 secreted less Ipa protein than M90TS in response to each of the stimuli, although fibronectin appeared to be more potent than others (Fig. 4a). As the total intracellular Ipa protein contents of Sh42 and M90TS were comparable (Fig. 4b), the reduction of secreted Ipa proteins from Sh42 indicated an impaired secretion process.
FIG. 4.
Secreted Ipa proteins from S. flexneri in vitro. (a) Secreted Ipa proteins in response to different stimuli. Lanes 1, 3, 5, and 7, M90TS; lanes 2, 4, 6, and 8, Sh42; lanes 1 and 2, unactivated controls; lanes 3 and 4, Congo red-activated samples; lanes 5 and 6, fibronectin-activated samples; lanes 7 and 8, laminin-activated samples. IpaB and IpaC are indicated by arrows, and molecular mass markers are indicated. (b) Kinetics of the secretion of Ipa proteins in response to Congo red. Lane 1, secreted Ipa proteins from M90TS at 30 min; lanes 2, 3, 4, and 5, secreted Ipa proteins from Sh42 at 30, 60, 90, and 120 min, respectively; lanes 6 and 7, cell-associated Ipa proteins from M90TS and Sh42, respectively. (c) Semiquantitation of the secreted Ipa proteins shown in panel b (lanes 1 to 5) by image densitometry.
In a previous study, it was shown that Sh4 (dsbA::kan) can efficiently secrete Ipa proteins in vitro (40). This now requires qualification. In that study, Congo red was added to the culture and bacteria were exposed to the compound for at least 1 h. Under these conditions, Sh4 secreted a quantity of Ipa protein comparable to that secreted by M90TS. Indeed, under the same conditions, Sh42 can also secrete IpaB and IpaC to levels comparable to those seen with M90TS (data not shown). However, in a kinetic study using Congo red as the secretion stimulus, it took Sh42 120 min to secrete amounts of IpaB and IpaC comparable to those that M90TS secreted in 30 min (Fig. 4b and c). Under these conditions, Sh42 responds to Congo red significantly more slowly than M90TS. A defect operating comparably in vivo may form the basis for the trapping of dsbA mutants in protrusions, resulting in defective intercellular dissemination.
DISCUSSION
Cell-to-cell spread via interepithelial protrusions is a primary virulence property of S. flexneri. This process depends on bacteria entering the epithelial cytosol and proliferating within cells, protrusion formation, and the lysis of the double-membrane-bound protrusion vacuole to release bacteria into the adjacent cell. The successful orchestration of this sequence of events depends on a series of bacterial components. These fall into two functional groups: products affecting the biogenesis of IcsA, a bacterial actin polymerase that generates movement (25), and those involved in synthesis or secretion of Ipa proteins which are involved in epithelial membrane lysis (24, 32). The first group includes IcsA itself (26); SopA, which is a protease specific for the processing of IcsA (11); proteins involved in synthesis of LPS (15); and outer membrane proteins (7) which affect the processing or distribution of IcsA on the bacterial envelope. The second group includes the Ipa proteins themselves, their chaperone (24), components of the type III secretion system (24, 32), and gene products which lead to hypersecretion of Ipa proteins (16).
A wild-type DsbA background may be important for the normal functioning of several of these gene products, but existing evidence has suggested that, among them, Spa32 is preeminent. This component of the type III secretion system, involved in Ipa secretion and release, requires DsbA for folding to a functional form. Using a model experimental system with a dsbA-knockout strain, Watarai et al. (34) suggested that the disulfide bond catalyst was necessary for Ipa-mediated Shigella virulence functions. However, the point in the pathogenetic sequence of epithelial invasion and spread at which DsbA plays its crucial role has not previously been established directly. In their recent report (32), Schuch et al. have elegantly demonstrated that Ipa proteins are necessary for epithelial cell entry and that the Mxi-Spa type III secretion system is required to support an intercellular spread function. The inference that Ipa protein production in the protrusion is necessary for spread is strong, but until now this has not been explicitly demonstrated. Here we have shown that Ipa proteins are produced in protrusions and that cell-to-cell invasion occurs when the folding defect in Spa32 that depends on the absence of DsbA is corrected. While at the epithelial surface, bacterial-host cell contact triggers Ipa secretion, leading to entry (22), our previous work has suggested that DsbA is not necessary at this stage (38). In vitro also, the experimental Ipa secretion stimuli Congo red and fibronectin lead to (slow) Ipa secretion by Sh42 despite the absence of DsbA (Fig. 4). We infer that under these conditions, and at the epithelial surface, Ipa secretion takes place independently of Spa32. Shigella has a conditional need for functional Spa32, requiring DsbA, which is manifest within protrusions but not at the epithelial surface.
The merodiploid experimental systems used in this study support the suggestion that the more functional DsbA there is available, the more likely it is that sufficient Spa32 will fold to its functional form, in turn facilitating Ipa protein secretion. In E. coli, mutant DsbA with the same Cys33 substitution forms permanent disulfide bonds via its reactive Cys30 with DsbB, an inner membrane protein responsible for recycling DsbA to its active (oxidized) form (18). The excess of DsbA33G in IPTG-uninduced Sh42/pJYU5 or IPTG-induced M90TS/pBEJ18 may in each case cause DsbB to react, preventing wild-type DsbA from functioning normally. Glutathione in the reducing host cell cytosol (12), which can penetrate the bacterial outer membrane, creates a reducing redox potential that further impairs oxidative protein folding in the bacterial periplasm. This, however, does not apparently critically affect bacterial proliferation in the host cell cytosol, but the survival of organisms in protrusions is affected. On electron microscopic examination, while the dsbA mutant Sh4 was seen to divide in protrusions, organisms apparently disintegrated as the enveloping protrusion membranes lysed, which resulted in the frequent observation of empty vacuoles in infected HeLa cells (38). Furthermore, in contrast to an mxiM mutant whose intracellular growth could be rescued by addition of bafilomycin (which inhibits the host V-ATPase, preventing acidification of endocytic vesicles) (32), dsbA mutants do not respond to bafilomycin at all (J. Yu, unpublished data). These results suggest that, apart from their defect in Ipa protein secretion, dsbA mutants are vulnerable to unidentified bacterial or host cellular processes that lead to their disintegration together with that of protrusion vacuoles.
ACKNOWLEDGMENTS
We thank Philippe Sansonetti for kindly providing monoclonal antibodies H16 and J22 and Gill Collar, John de Felice, and Lynn Wicks for electron microscopy and confocal microscopy services.
O.N. is a recipient of a Travelling Research Fellowship from the Wellcome Trust. This work was financed by a Career Development Award (047657/Z/96/Z) to Jun Yu from the Wellcome Trust.
REFERENCES
- 1.Adam T, Giry M, Boquet P, Sansonetti P J. Rho-dependent membrane folding causes Shigella entry into epithelial cells. EMBO J. 1996;15:3315–3321. [PMC free article] [PubMed] [Google Scholar]
- 2.Allaoui A, Mounier J, Prevost M C, Sansonetti P J, Parsot C. icsB: a Shigella flexneri virulence gene necessary for the lysis of protrusions during intercellular spread. Mol Microbiol. 1992;6:1605–1616. doi: 10.1111/j.1365-2958.1992.tb00885.x. [DOI] [PubMed] [Google Scholar]
- 3.Bahrani F K, Sansonetti P J, Parsot C. Secretion of Ipa proteins by Shigella flexneri: inducer molecules and kinetics of activation. Infect Immun. 1997;65:4005–4010. doi: 10.1128/iai.65.10.4005-4010.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulphide bond formation in vivo. Cell. 1991;67:581–589. doi: 10.1016/0092-8674(91)90532-4. [DOI] [PubMed] [Google Scholar]
- 5.Barzu S, Benjelloun-Touimi Z, Phalipon A, Sansonetti P J, Parsot C. Functional analysis of the Shigella flexneri IpaC invasin by insertional mutagenesis. Infect Immun. 1997;65:1599–1605. doi: 10.1128/iai.65.5.1599-1605.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belin P, Boquet P L. The Escherichia coli dsbA gene is partly transcribed from the promoter of a weakly expressed upstream gene. Microbiology. 1994;140:3337–3348. doi: 10.1099/13500872-140-12-3337. [DOI] [PubMed] [Google Scholar]
- 7.Bernardini M, Sanna D G, Fontaine A, Sansonetti P J. OmpC is involved in invasion of epithelial cells by Shigella flexneri. Infect Immun. 1993;61:3625–3635. doi: 10.1128/iai.61.9.3625-3635.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Geyter C, Vogt B, Benjelloun-Touimi Z, Sansonetti P J, Ruysschaert J-M, Parsot C, Cabiaux V. Purification of IpaC, a protein involved in entry of Shigella flexneri into epithelial cells and characterization of its interaction with lipid membranes. FEBS Lett. 1997;400:149–154. doi: 10.1016/s0014-5793(96)01379-8. [DOI] [PubMed] [Google Scholar]
- 9.Dehio C, Prevost M C, Sansonetti P J. Invasion of epithelial cells by Shigella flexneri induces tyrosine phosphorylation of cortactin by a pp. 60c-src-mediated signalling pathway. EMBO J. 1995;14:2471–2482. doi: 10.1002/j.1460-2075.1995.tb07244.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Egile C, d'Hauteville H, Parsot C, Sansonetti P J. SopA, an outer membrane protease achieving secretion and polar localisation of IcsA in S. flexneri. Mol Microbiol. 1997;23:1063–1073. doi: 10.1046/j.1365-2958.1997.2871652.x. [DOI] [PubMed] [Google Scholar]
- 12.Freedman R B. Protein disulfide isomerase: multiple roles in the modification of nascent secretory proteins. Cell. 1989;57:1069–1072. doi: 10.1016/0092-8674(89)90043-3. [DOI] [PubMed] [Google Scholar]
- 13.Galan J E. Molecular genetic bases of Salmonella entry into host cells. Mol Microbiol. 1996;20:263–271. doi: 10.1111/j.1365-2958.1996.tb02615.x. [DOI] [PubMed] [Google Scholar]
- 14.High N, Mounier J, Prevost M C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong M, Payne S M. Effect of mutations in Shigella flexneri chromosomal and plasmid-encoded lipopolysaccharide genes on invasion and serum resistance. Mol Microbiol. 1997;24:779–791. doi: 10.1046/j.1365-2958.1997.3731744.x. [DOI] [PubMed] [Google Scholar]
- 16.Hong M, Gleason Y, Wyckoff E E, Payne S M. Identification of two Shigella flexneri chromosomal loci involved in intercellular spreading. Infect Immun. 1998;66:4700–4710. doi: 10.1128/iai.66.10.4700-4710.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kishigami S, Kanaya E, Kikuchi M, Ito K. DsbA-DsbB interaction through their active site cysteines. J Biol Chem. 1995;270:17072–17074. doi: 10.1074/jbc.270.29.17072. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Kishigami S, Sone M, Inokuchi H, Mogi T, Ito K. Respiratory chain is required to maintain oxidized states of the DsbA-DsbB disulfide bond formation system in aerobically growing Escherichia coli cells. Proc Natl Acad Sci USA. 1997;94:11857–11862. doi: 10.1073/pnas.94.22.11857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makino S, Sasakawa C, Kamata K, Kurata T, Yoshikawa M. A genetic determinant required for continuous reinfection of adjacent cells on large plasmid in Shigella-flexneri 2a. Cell. 1986;46:551–555. doi: 10.1016/0092-8674(86)90880-9. [DOI] [PubMed] [Google Scholar]
- 21.Menard R, Sansonetti P J, Pasort C, Vasselo T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of Shigella flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 22.Mounier J, Bahrani F K, Sansonetti P J. Secretion of Shigella flexneri Ipa invasins on contact with epithelial cells and subsequent entry of the bacterium into cells are growth stage dependent. Infect Immun. 1997;65:774–782. doi: 10.1128/iai.65.2.774-782.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oaks E V, Hale T L, Formal S B. Plaque formation by virulent Shigella flexneri. Infect Immun. 1985;48:124–129. doi: 10.1128/iai.48.1.124-129.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page A-L, Ohayon H, Sansonetti P J, Parsot C. The secreted IpaB and IpaC invasins and their cytoplasmic chaperone IpgC are required for intercellular dissemination of Shigella flexneri. Cell Microbiol. 1999;1:183–193. doi: 10.1046/j.1462-5822.1999.00019.x. [DOI] [PubMed] [Google Scholar]
- 25.Prevost M C, Lesourd M, Arpin M, Vernel F, Mounier J, Hellio R, Sansonetti P J. Unipolar reorganization of F-actin layer at bacterial division and bundling of actin filaments by plastin correlate with movement of Shigella flexneri within HeLa cells. Infect Immun. 1992;60:4088–4099. doi: 10.1128/iai.60.10.4088-4099.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathman M, Jouirhi N, Allaoui A, Sansonetti P, Parsot C, Nhieu G T V. The development of a FACS-based strategy for the isolation of Shigella flexneri mutants that are deficient in intercellular spread. Mol Microbiol. 2000;35:974–990. doi: 10.1046/j.1365-2958.2000.01770.x. [DOI] [PubMed] [Google Scholar]
- 27.Sandkvist M, Hirst T R, Bagdasarian M. Alterations at the carboxyl terminus change assembly and secretion properties of the B subunit of Escherichia coli heat-labile enterotoxin. J Bacteriol. 1987;169:4570–4576. doi: 10.1128/jb.169.10.4570-4576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sansonetti P J, Egile C. Molecular bases of epithelial cell invasion by Shigella flexneri. Antonie Leeuwenhoek Int J Gen Mol Microbiol. 1998;74:191–197. doi: 10.1023/a:1001519806727. [DOI] [PubMed] [Google Scholar]
- 29.Sansonetti P J, Ryter A, Clerc P, Maurelli A T, Mounier J. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect Immun. 1986;51:461–469. doi: 10.1128/iai.51.2.461-469.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sansonetti P J, Arondel J, Fontaine C, d'Hauteville H, Bernardini M L. OmpB (osmo-regulation) and icsA (cell-to-cell spread) mutants of Shigella flexneri. Evaluation as vaccine candidates. Probes to study the pathogenesis of shigellosis. Vaccine. 1991;9:416–422. doi: 10.1016/0264-410x(91)90128-s. [DOI] [PubMed] [Google Scholar]
- 31.Sansonetti P J, Mounier J, Prevost M C, Mege R M. Cadherin expression is required for the spread of Shigella flexneri between epithelial cells. Cell. 1994;76:829–839. doi: 10.1016/0092-8674(94)90358-1. [DOI] [PubMed] [Google Scholar]
- 32.Schuch R, Sandlin R C, Maurelli A T. A system for identifying post-invasion functions of invasion genes: requirements for the Mxi-Spa type III secretion pathway in intercellular dissemination of Shigella flexneri. Mol Microbiol. 1999;34:675–689. doi: 10.1046/j.1365-2958.1999.01627.x. [DOI] [PubMed] [Google Scholar]
- 33.Skoudy A, Mounier J, Aruffo A, Ohayon H, Gounon P, Sansonetti P, Nhieu G T V. CD44 binds to the Shigella IpaB protein and participates in bacterial invasion of epithelial cells. Cell Microbiol. 2000;2:19–33. doi: 10.1046/j.1462-5822.2000.00028.x. [DOI] [PubMed] [Google Scholar]
- 34.Watarai M, Tobe T, Yoshikawa M, Sasakawa C. Disulphide oxidoreductase activity of Shigella flexneri is required for release of Ipa proteins and invasion of epithelial cells. Proc Natl Acad Sci USA. 1995;92:4927–4931. doi: 10.1073/pnas.92.11.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watarai M, Funato S, Sasakawa C. Interaction of Ipa proteins of Shigella flexneri with α5β1 integrin promotes entry of the bacteria into mammalian cells. J Exp Med. 1996;183:991–999. doi: 10.1084/jem.183.3.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watarai M, Kamata Y, Kozaki S, Sasakawa C. rho, a small GTP-binding protein, is essential for Shigella invasion of epithelial cells. J Exp Med. 1997;185:281–292. doi: 10.1084/jem.185.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu J. Cloning and active site mutagenesis of Vibrio cholerae DsbA, a periplasmic enzyme that catalyzes disulfide bond formation. J Biol Chem. 1993;268:4326–4330. [PubMed] [Google Scholar]
- 38.Yu J. Inactivation of DsbA, but not DsbC and DsbD, affects Shigella flexneri intracellular survival and virulence. Infect Immun. 1998;66:3909–3917. doi: 10.1128/iai.66.8.3909-3917.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]