Abstract
Transthyretin cardiac amyloidosis (ATTR-CM) is a rare and under-recognized disorder characterized by the aggregation of transthyretin-derived insoluble amyloid fibrils in the myocardium. Heterogeneity of symptoms at presentation, makes its diagnosis often delayed. An expert panel gathered on a virtual platform across India to conduct a meeting for developing a guiding tool for ATTR-CM diagnosis. The panel recommended younger age (≥40 years) for suspecting ATTR-CM and thick-walled non-dilated hypokinetic ventricle was considered as one of the important red flags. Electrocardiogram (ECG) and echocardiography (ECHO) findings were recommended as primary tests to raise the suspicion while nuclear scintigraphy and hematological tests were recommended to confirm the diagnosis and rule out amyloid light-chain (AL) amyloidosis. Cardiac magnetic resonance (CMR) and biopsy were recommended in case of ambiguity in the presence of red flags. Considering the lack of expert guidelines in the Indian scenario, a standardized diagnostic algorithm was also proposed.
Keywords: Transthyretin cardiac amyloidosis (ATTR-CM), Rare disorder, Amyloidosis, Cardiac dysfunction
1. Introduction
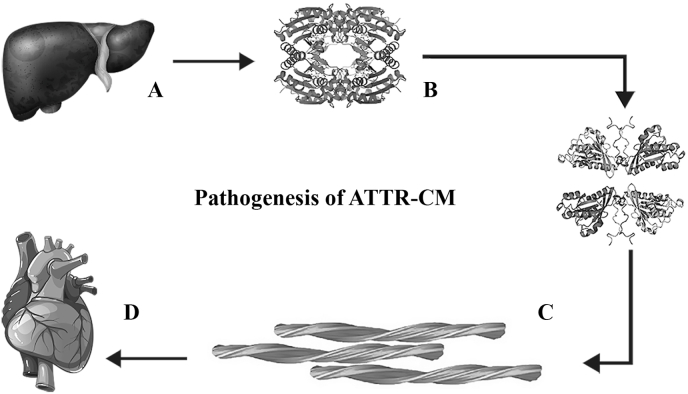
Transthyretin amyloid cardiomyopathy (ATTR-CM) is a life-threatening, progressive and infiltrative cardiomyopathy caused by the myocardial deposition of transthyretin amyloid fibrils and is considered as under-recognized cause of heart failure (HF) in adults.1,2 Transthyretin (TTR or TBPA) is a transport protein secreted by liver and found in the serum and cerebrospinal fluid that carries thyroid hormone, thyroxine (T4) and retinol-binding protein bound to retinol. When transthyretin misfolds, it gets deposited in various organs causing amyloid diseases.3 There are >120 known heritable (autosomal dominant) mutations in the TTR gene.4 Fig. 1 shows the pathogenesis of ATTR-CM.2
Fig. 1.
Pathogenesis of transthyretin cardiac amyloidosis a) Transthyretin is produced in the liver as a tetrameric protein b) The tetramer dissociates into monomer subunits c) The monomers misfolds and reaggregates into toxic dimers and oligomers and insoluble amyloid fibrils d) The amyloid fibrils get deposited extracellularly in the interstitial space of the myocardium.
ATTR-CM has two subtypes, hereditary ATTR-CM (ATTRv) and wild-type ATTR-CM (ATTRwt).5 ATTRwt, also called as senile amyloidosis is typically seen in older patients, while ATTRv can affect younger patients with men and women affected equally and is more aggressive of the two.4,6, 7, 8, 9 Epidemiology of ATTR-CM is not well characterized in India.10 However, the available case reports on cardiac amyloidosis reported a different profile of the disease in India than the West, with a higher prevalence in younger patients.11,12 With advances in diagnostic techniques, recent studies have reported an exponential increase in the incidence of ATTR-CM, in particular, ATTRwt-CM is now being diagnosed more than twice as frequently as ATTRv-CM.2,13 Despite increased recognition, ATTR patients experience poor quality of life, which can be due to the substantial delay in diagnosis.13,14 In addition, there is low life expectancy of ∼2–5 years once diagnosed which points towards the necessity of early suspicion and accurate diagnosis to forestall disease progression and prevent significant morbidity and mortality. Other major challenge apart from the poor disease awareness and lack of knowledge are indistinct identification of the hallmark features that can raise the suspicion, often leading to delayed and misdiagnosis.14,15 Multiple signs and predominant symptoms of cardiomyopathy or progressive polyneuropathy, compounded by the fact that the signs and symptoms are non-specific and seen in many other cardiac conditions that delay the actual treatment.16
2. Need for guiding tool for ATTR-CM in India
ATTR-CM is not only a complicated and rare disorder but is often diagnosed very late. In India, there are multiple challenges to clearly understand the burden of this disease. There is limited region-specific literature available on ATTR-CM looking at the burden of the disease and there is lack of specific diagnostic guidelines to aid raising suspicion and diagnosis. A battery of specialized tests has to be employed which are available at select specialized centers and there are no uniform recommendations to guide the interpretation. Due to these challenges and looking at the prognosis and drugs being available in the near future, there is an urgent need to develop consensus on how to diagnose ATTR-CM and sensitize the doctors and regulatory bodies to increase the awareness of this rare cardiovascular disorder in India.
To address this, we came up with the recommendations to be used as a guiding tool for the clinicians in diagnosing the patients of ATTR-CM. Additionally, this consensus document focuses on patient journey, the common red flags to raise the suspicion, and the most recommended tools for diagnosis in the Indian context.
3. Methodology
A panel of 12 subject matter experts (SME) participated from India to discuss the global and regional recommendations of ATTR-CM and to develop India specific diagnostic approach protocol to raise the awareness for suspecting and diagnosing ATTR-CM. The multidisciplinary expert panel included SMEs from the domain of cardiology (n = 7), nuclear medicine (n = 4) and hematology (n = 1). Consensus recommendations on ATTR-CM from United States and Europe were reviewed and used as reference documents. Panel discussions were held on the following topics in four sessions: i) patient journey and red flags; ii) tools for suspicion and diagnosis; iii) review of global and regional recommendations for diagnosis and management of ATTR-CM; iv) expert recommendations for development of India specific diagnostic approach protocol. Following every session, a set of questions on each of these topics were addressed by panellists to be incorporated into the India specific diagnostic recommendations. To the best of our knowledge, this is the first time that an expert panel meeting from India explored the adaptation of global guidelines for ATTR-CM, which in turn lead to the development of India specific diagnostic protocol approach.
4. Discussion and recommendations
4.1. Patient journey and when to suspect ATTR-CM
As there is limited information available on ATTR-CM patient journey, there is a strong need towards understanding the difficulties of patients in the journey to diagnosis to promote earlier intervention to not only improve the quality of life but also for better prognosis.17
“Red flags” or warning signals refer to signs and/or symptoms that support a high degree of suspicion of ATTR-CM, many of which can be identified from an initial physical examination, assessment of patient history and routine investigations.18 These red flags or warning signals can be grouped into cardiac and extracardiac (Table 1).
Key recommendations by panel on patient journey and red flags.
Patient journey is lengthy that currently takes many years from the onset of symptoms to diagnosis and is often missed due to overlap of signs and symptoms with multiple other cardiac conditions
-
•
ATTR-CM should be suspected in younger age group (≥40 years) with red flags considering the propensity of Indian people to develop heart conditions earlier as compared to western population.
-
•
HFpEF, left ventricular (LV) thickness (>11mm), global longitudinal strain (GLS), aortic stenosis, arrythmias, cardiac conduction abnormalities should be considered as red flags. “Thick walled non dilated hypokinetic ventricle” should be considered an important red flag.
-
•
Suspect ATTR-CM with pseudo infarct pattern with low/decreased QRS voltage, increased left ventricular (LV) thickness, atrial fibrillation together with conduction system disorders examined in ECG/ECHO.
-
•
Conditions like carpel tunnel syndrome (CTS), lumbar spinal stenosis (LSS), ophthalmological and neurological manifestations, liver and kidney disorders, edema and swelling should be considered as extracardiac signs to raise suspicion of ATTR amyloidosis.
-
•
ATTR-CM should be actively looked for in HF patient (≥65 years), aortic stenosis, hypotension or normotensive if previously hypertensive, sensory involvement and autonomic dysfunction.
-
•
CMR although should be reserved in case of ambiguity but it can provide important clues to suspect ATTR-CM.
Table 1.
Red flags of ATTR-CM.
| Cardiac | Extracardiac |
|---|---|
|
|
ECG, electrocardiogram; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LGE, late gadolinium enhancement; LV, left ventricular; NT-proBNP, N-terminal pro b-type natriuretic peptide.
4.2. Stepwise diagnostic approach
4.2.1. ATTR presentation
ATTR can present in many ways depending on the organ system involved. When heart is the major organ involved then ATTR may present mimicking conditions such as heart failure with HFpEF (13%) or heart failure with reduced ejection fraction (HFrEF) (11%), left ventricular hypertrophy (LVH)/hypertrophic cardiomyopathy (HCM) 5% of all, aortic stenosis (5% of surgical aortic valve replacement [AVR] and 10–16% of transcatheter AVR) and conduction disturbance (2%).19, 20, 21, 22, 23
4.2.2. Criteria for diagnosis
Once the suspicion of ATTR-CM is raised by history, physical examination and findings on investigations like chest X-ray, electrocardiogram (ECG) and echocardiography (ECHO) or cardiac magnetic resonance (CMR), diagnosis can be confirmed using non-invasive or invasive methods which includes cardiac or extracardiac biopsy or fat aspiration biopsy. Although CMR is generally reserved if ECHO findings are ambiguous or inconclusive, but it can also sometimes help raise suspicion of ATTR-CM before confirmation of diagnosis. It has a disadvantage that it cannot distinguish ATTR-CM from amyloid light-chain (AL) amyloidosis.
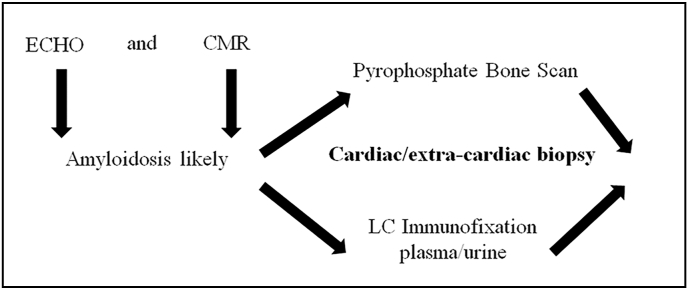
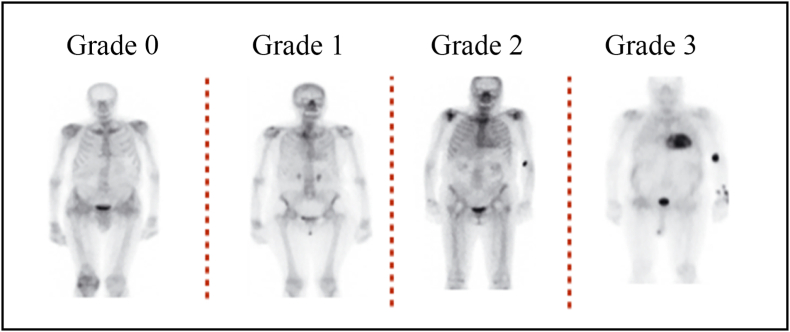
Nuclear scintigraphy using Tc-99m-DPD (3,3-diphosphono-1,2-propanodicarboxylicacid), Tc-99m-HMDP (hydroxy methylene diphosphonate) and Tc-99m-PYP (pyrophosphate) scans have high sensitivity and specificity for the diagnosis of ATTR-CM and is usually relied upon to confirm the diagnosis of ATTR-CM.24 The radiotracer eventually collects in the area of the body being examined, where it gives off energy in the form of gamma rays. Cardiac uptake is visually scored using Perugini grading system, and is categorized as follows: Grade 0 – no cardiac uptake and normal bone uptake; Grade 1 – cardiac uptake which is less intense than the bone signal; Grade 2 – cardiac uptake with intensity similar or greater than bone signal; and Grade 3 – cardiac uptake with much attenuated or absent bone signal.25,26 An uptake of Grade 2 and above is considered significant; Grade 2 and Grade 3 scans are reported to have 100% positive predictive value for detecting ATTR with 87% specificity and 97% sensitivity. For Grade 1, the non-invasive diagnosis is not possible and histological confirmation (cardiac or extracardiac) is required. There is a false positive rate of 13% in patients with AL amyloidosis.27 It is also necessary to simultaneously rule out AL amyloidosis which is done by hematological tests such as serum free light chain (FLC) assay, serum (SPIE) and urine (UPIE) protein electrophoresis with immunofixation in combination with nuclear scintigraphy. The combination of serum and urine immunofixation and quantification of serum free light chains has 99% sensitivity for identifying AL amyloidosis.28 (Fig. 2).
Fig. 2.
Simultaneous screening by bone scans, biopsy and immunofixation.
Invasive diagnosis of ATTR-CM employs cardiac or extracardiac biopsy. Extra-cardiac biopsies, such as abdominal fat pad, may be preferred as it gives a good diagnostic yield, but the diagnostic accuracy can be particularly low in ATTR amyloidosis. Similarly, for rectal and tongue biopsies, ATTR has lower pick up rate. Although cardiac biopsy provides confirmatory diagnosis, it should be reserved for only those cases where diagnosis could not be made using non-invasive methods or when clinical suspicion is high despite negative non-invasive diagnostic criteria. Histologic confirmation is still needed in cases where both bone scintigraphy and tests for monoclonal protein (suggestive of possible AL amyloidosis) are abnormal/inconclusive, to confirm and type amyloid deposits by immunohistochemistry or mass spectrometry.
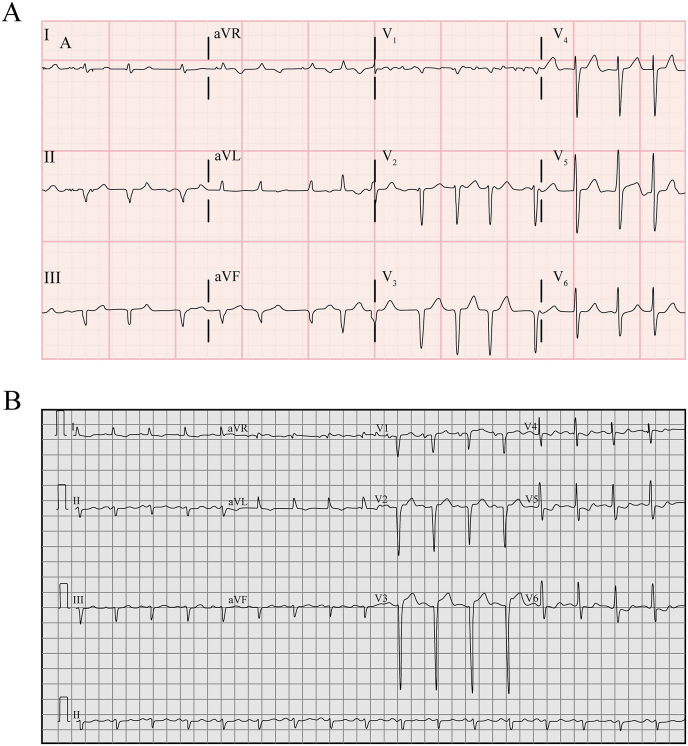
Fig. 3.
(a): ECG pattern showing old infarct 3(b): Goldberger triad and RV dysfunction.
Genetic testing should be considered in younger people with high suspicion of HF and in conditions of peripheral neuropathy. The test though should not be a rate limiting step to put the patient on treatment. However, it must be offered for the first-degree family members of the patients with an inheritable form of ATTR-CM, at an early age or as soon as the symptoms compatible with amyloidosis develop.18,29,30 It is important to stress that biopsy need not to be included in the diagnostic protocol, however; it should be reserved only in case of ambiguity.1,30
Fig. 4.
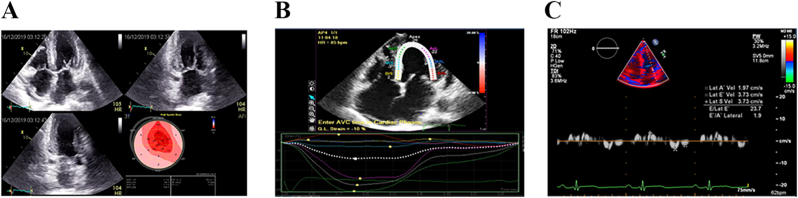
(a): Thick-walled LV: LVEF/longitudinal strain >4 Apical sparing 4(b): ABS index >3:1 4(c): Tissue doppler 5-5-5 sign.
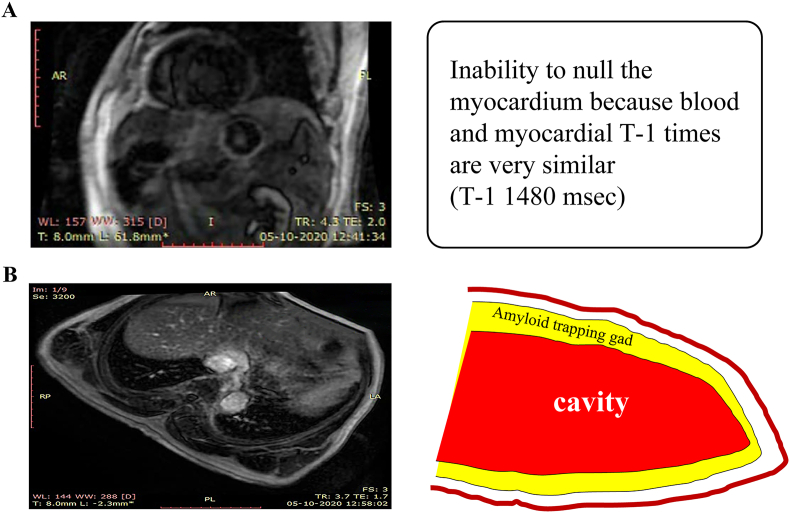
Fig. 5.
(a): Amyloidotic HF (male, 65 years) 5(b): Phase-sensitive myocardial delayed enhancement at 20 min after gad injection in 4CV.
Typical diagnostic features of various invasive and non-invasive tests which may raise the suspicion of ATTR-CM in patients with HF, are summarized in Table 2.
Key recommendations by the panel on tools for diagnosis.
-
•
Red flags, ECG and ECHO should be used to raise the suspicion of ATTR-CM and nuclear scintigraphy should be considered to confirm the diagnosis.
-
•
Hematological tests should be done simultaneously with ATTR-CM to rule out AL amyloidosis.
-
•
Nuclear scintigraphy must be performed on suspicion by patient history and ECHO/ECG. It should be preferred over CMR, considering the sensitivity, availability and cost.
-
•
No need of performing biopsy in all patients and should not be part of diagnostic algorithm.
-
•
CMR and biopsy should be utilized to confirm the diagnosis in case of ambiguity.
Fig. 6.
Bone scintigraphy grades.
Table 2.
Tools and its characteristics to raise the suspicion of ATTR-CM.
| Tests and hallmarks | Presentation |
|---|---|
|
ECG Pseudo infarct pattern: ECG showing old infarct pattern with low voltage. Commonly seen in ATTRwt (63–65%) and ATTRv (18–69%) Diagnostic yield of 60–65% LVF without any infarction HF with conduction disorders; left BBB, right BBB and first degree AV blocks and other AV blocks Goldberger triad (Low QRS voltage in limb leads, normal voltage in precordial leads, poor R wave progression (V1–V3) RV dysfunction (R wave in aVR, positive T wave in aVR) Isolated AF |
[ReferFig. 3A and B] |
|
ECHO Thick-walled LV, RV, RA RCM or hypokinetic nondilated CM Markedly reduced GLS LVEF/Longitudinal strain >4 ‘Bulls eye pattern’ due to apical sparing Apical strain/mid basal strain >3:1 Tissue doppler 5-5-5 sign |
[ReferFig. 4A, B, 4C] |
| CMR | |
| T-1> 1400msec ECV >42% Positive global subendocardial LGE Thick-walled ventricle and atrium Pleural effusion DIR is a type of “black blood"
|
[ReferFig. 5A and B] |
| Biochemical marker: persistent increase in the levels of Troponin T > 0.05 ng/mL, NT-proBNP >3000 pg/mL | |
| Bone scintigraphy | |
| Semi-quantitative visual Grade of 2 or 3, target to background (LV myocardium to blood pool) ratio >1.5 and retention index >0.030/min. If cardiac uptake is Grade 1, histological confirmation of amyloid deposits (could be extracardiac) is required as non-invasive diagnosis is not possible. |
[ReferFig. 6] |
| Hematology | |
| Serum free kappa: lambda light chain ratio >3 and free light chain>18 mg/dL is suggestive to go for hematological testing; immunofixation electrophoresis of urine and serum | |
AF, atrial fibrillation; ATTRv, hereditary ATTR-CM; ATTRwt, wild-type ATTR-CM; AV, atrioventricular; aVR, augmented vector right; BBB, bundle branch block; CM, cardiomyopathy; CMR, cardiac magnetic resonance; DIR, double inversion recovery; ECG, electrocardiogram; ECHO, echocardiography; ECV, extracellular volume; GLS, global longitudinal strain; HF, heart failure; LGE, late gadolinium enhancement; LV, left ventricle; LVEF, left ventricular ejection fraction; LVF, left ventricular failure; NT-proBNP, N-terminal pro B-type natriuretic peptide; RA, right atrium; RV, right ventricle; RCM, restrictive cardiomyopathy.
4.3. Global and regional recommendations for diagnosis and management of ATTR-CM
Expert recommendations are available for diagnosis of ATTR-CM in the United States and Europe.18,31 These recommendations were discussed in detail in the meeting and analysed for adaptation to Indian population for raising early suspicion and diagnosing these patients. The salient features of both recommendations discussed in the meeting are highlighted in Table 3.
Key recommendations by the panel on comparison of global ATTR-CM guidelines.
-
•
Minor differences exist between AHA and ESC guidelines and the Indian panel recommended a personalized diagnostic approach.
-
•
Lower age limit ≥40 years with red flags should be considered as the cut off limit to suspect ATTR-CM.
-
•
Lab tests like troponins and ECG, ECHO in addition to clinical findings should be used for raising suspicion as screening tests.
-
•
Nuclear scintigraphy may be used after suspicion has been raised based on clinical symptoms and investigations.
-
•
CMR should be used in case of ambiguity or when suspicion is high despite negative tests.
-
•
Genetic testing should be offered for the relatives first degree family members of the patients with an inheritable form of CA.
Table 3.
| Key Points | AHA | ESC | Indian panel recommendations |
|---|---|---|---|
| Aim of position papers | To help practicing cardiologists focus on diagnosis and management of ATTR-CM | To help cardiologists and other physicians in suspecting, diagnosing, and treating patients with CA
|
To develop India specific diagnostic approach protocol to help cardiologists in India to raise the suspicion and diagnosis of ATTR-CM |
| When to suspect | Presence of moderate to severe left ventricular (LV) thickening (wall thickness ≥14 mm) triggers consideration of ATTR-CM especially if there is discordance between wall thickness on ECG and QRS voltage on ECG | Presence of LV wall thickness >12 mm along with either heart failure, aortic stenosis, or red flag signs/symptoms, particularly if older than 65 years | Considering propensity of Indians to develop heart conditions at an earlier age, age limit should be kept lower (≥40 years) for suspecting ATTR-CM Thick-walled non-dilated hypokinetic ventricle should be considered as an important red flag HFpEF, LV thickness ≥11 mm), GLS, aortic stenosis, arrythmias, cardiac conduction abnormalities are some of the other common red flags |
| Non-invasive diagnostic tests | |||
| ECG | Recommends ECG in the diagnostic protocol Also important in patients with advanced diseases as <40% of such patients show low voltage on ECG Absence of low voltage does not rule out ATTR-CM |
Recommends ECG at the time of first suspicion and every 6 months | Recommends ECG, chest x-ray and ECHO as the primary and mandatory screening test The tests should also be used for follow up periodically |
| ECHO | Does not recommend it for diagnosis of ATTR-CM since, it cannot distinguish between ATTRv and ATTRwt. However, can identify nonamyloid causes of LV Thickening (HCM, aortic stenosis, and Fabry disease) |
Recommends ECHO under following conditions: Unexplained LV thickness (≥12 mm) plus 1 or 2:
|
Recommends ECHO for raising suspicion of ATTR-CM It is recommended as the cornerstone for early diagnosis of all types of cardiac amyloidosis; to identify increased LV thickness, granular sparkling of myocardium, and pericardial effusion. Left ventricular wall thickness (>11 mm), right ventricular wall thickness, free valves of the right atrium, LV longitudinal strain are some of important features seen on ECHO |
| Nuclear scintigraphy | Scans may be positive even in AL amyloidosis, and a bone scintigraphy scan alone, without concomitant testing for light chains, is neither appropriate nor valid for distinguishing ATTR-CM from AL-CM Assessment of ATTR-CM with bone scintigraphy is accomplished by quantitative approaches comparing heart to rib uptake Grade 0 is no cardiac and normal rib uptake Grade 1 is cardiac less than rib uptake Grade 2 is cardiac equal to rib uptake Grade 3 is cardiac greater than rib uptake with mild/absent rib uptake In the absence of a light chain abnormality, the 99mTc-PYP scintigraphy is diagnostic of ATTR-CM if there is Grade 2 to 3 cardiac uptake or a heart/contralateral chest ratio >1.5 |
While recommending scintigraphy, SPIE, UPIE and serum FLC, four scenarios should be considered a) Scintigraphy does not show cardiac uptake and assessments for monoclonal proteins are negative – Amyloidosis unlikely
|
It is considered as a gold standard for confirming the diagnosis of ATTR-CM as it is accurate, cheap, interpretation is simple and has high sensitivity and specificity. Pyrophosphate scans are recommended |
| CMR Imaging | Consider CMR as the appropriate test when an infiltrative cardiomyopathy is suspected but ATTR-CM is less likely, as in younger patients or those with findings suggestive of other infiltrative/inflammatory or restrictive cardiomyopathies | Characteristic CMR findings (a and b have to be present):
|
CMR is useful if ECHO findings are inconclusive or ambiguous, recommended as optional (depending on the cost availability and need) |
| Hematologic consideration | Based on history, ECHO and ECG findings suggestive of amyloidosis Scintigraphy along with serum FLC and serum and urine IFE (Measurement of serum IFE, urine IFE, and serum FLC is >99% sensitive for AL amyloidosis) |
Based on clinical, laboratory and ECG suspicion Scintigraphy coupled to assessment for monoclonal proteins by SPIE, UPIE, and quantification of serum FLC is recommended |
Based on clinical findings: Combination of SPIE, UPIE and serum FLC to rule out AL amyloidosis, has 99% sensitivity for abnormal pro-amyloidotic precursor in AL amyloidosis |
| Genetic testing | Recommends genetic testing to distinguish ATTRv and ATTRwt after confirmation of ATTR-CM from bone scintigraphy or EMB | Strongly recommends genetic testing once ATTR-CM is confirmed in order to differentiate between ATTRwt and ATTRv Genetic testing should be performed even in elderly patients, as a significant number of patients can have TTR mutations |
Once diagnosis of ATTR-CM is confirmed the first-degree relatives should be offered genetic testing It should not be a rate limiting step in the initiation of treatment Not recommended as a mandatory test |
| Invasive diagnostic tests | |||
| EMB |
It is mandatory in other 3 scenarios:
|
It is recommended to confirm ATTR-CM in case of any discrepancy Demonstrates amyloid deposits after Congo red staining irrespective of the degree of LV wall thickness Diagnosis of CA in case of MGUS (or any hematological disorder that produces FLC), AL amyloidosis, or coexistence of both AL and ATTR amyloidosis require histology with amyloid typing, usually via EMB |
Biopsy may not be needed to confirm diagnosis of amyloidosis Fat aspiration biopsy may be positive in 80% of cases of AL and 40% cases of ATTR |
AHA, American Heart Association; AL-CM, amyloid light-chain amyloidosis; ATTR-CM, transthyretin amyloid cardiomyopathy; CMR, cardiac magnetic resonance; ECV, extracellular volume fraction; ECG, echocardiogram; ECHO, echocardiography; EMB, endomyocardial biopsy; ESC, European Society of Cardiology; HCM, hypertrophic cardiomyopathy; FLC, free light chain; GLS, global longitudinal strain; HFpEF, heart failure with preserved ejection fraction; IVS, interventricular septum; IFE, immunofixation electrophoresis; LV, left ventricle; LVEDD, left ventricular end diastolic diameter; LGE, late gadolinium enhancement; PWT, posterior wall thickness; 99mTc-PYP, technetium pyrophosphate; TAPSE, tricuspid annular plane systolic excursion; MGUS, monoclonal gammopathy of undetermined significance; SPIE, serum protein electrophoresis with immunofixation; UPIE, urine protein electrophoresis with immunofixation.
5. Proposed diagnostic algorithm
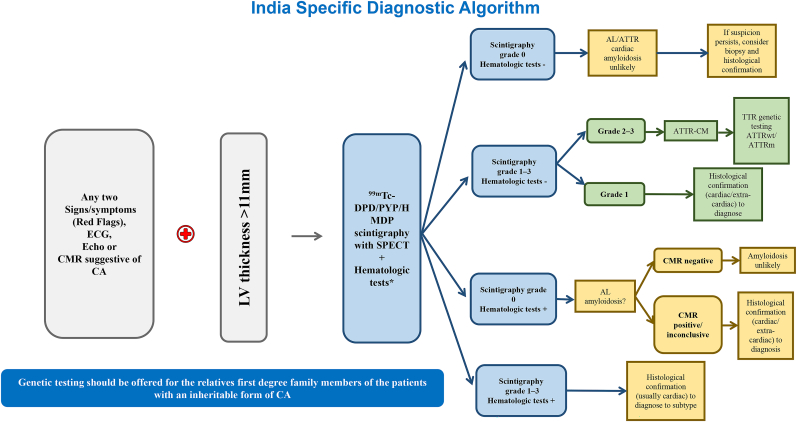
A timely, definitive diagnosis should be obtained as patient outcomes depend largely on early initiation of therapy. On the basis of above discussions and key recommendations, a stepwise standardized diagnostic algorithm was proposed which would be used as a guiding tool for diagnosing patients with ATTR-CM across India. Fig. 7 shows the diagnostic algorithm including red flags and recommended tests. It should be noted that this is the first attempt to standardise the suspicion and diagnosis of ATTR-CM in India, however, there are currently no experimental data to support the algorithm.
Fig. 7.
India specific diagnostic algorithm for ATTR-CM NOTE: EMB is not recommended for ATTR-CM, though it can be helpful to confirm AL amyloidosis. ∗Serum free-light chain quantification and serum, and urine immunofixation. AL, light-chain amyloidosis; ATTR-CM, transthyretin amyloid cardiomyopathy; ATTRv, hereditary transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; CA, cardiac amyloidosis; CM, cardiomyopathy; CMR, cardiac magnetic resonance; ECG, electrocardiogram; ECHO, echocardiography; EMB, endomyocardial biopsy; LV, left ventricle;; 99mTc-PYP, technetium pyrophosphate; SPECT, single photon emission computed tomography; TTR, transthyretin; 99mTc-DPD/PYP/HMDP, Technetium 3,3-diphosphono-1,2-propanodicarboxylicacid/pyrophosphate/hydroxy methylene diphosphonate.
6. Conclusion
ATTR-CM is a complicated and rare disorder that is often missed or misdiagnosed due to its heterogeneous nature of symptoms mimicking other cardiac conditions such as HF. Though its prevalence is reported all over the world, there is a lack of data from the Asian region, particularly in India. Additionally, there is a need to raise the awareness of this rare disorder among all patients and health care professionals. Guidelines for the diagnosis and management of ATTR-CM are available in the United States and Europe while no such recommendations are available from the Asian region. It is expected that this expert opinion effort would bring standardization in the diagnosis of ATTR-CM which in turn would reduce morbidity and mortality with timely treatment.
Declaration of competing interest
None of the authors have any conflicts of interest to declare. All authors have received honorarium from Pfizer for their services as a member of the ATTR-CM Expert Panel Meeting.
Acknowledgements
The authors acknowledge Pfizer India for bringing the issue into notice among all and show keen interest to support them for the further steps to be taken in the respective direction. Pfizer was not involved in designing, writing and conceptualizing, editing, or final approval for publication of this manuscript. Medical writing services were provided by Indegene Pvt. Ltd, Bangalore, India and funded by Pfizer India.
Contributor Information
Jagdish Chander Mohan, Email: a51hauzkhas@gmail.com.
Jamshed Dalal, Email: jjdalal@hotmail.com.
Vijay Kumar Chopra, Email: chopravk@gmail.com.
Calambur Narasimhan, Email: calambur1@gmail.com.
Prafulla Kerkar, Email: prafullakerkar@hotmail.com.
Abraham Oomman, Email: drabrahamoomman@gmail.com.
Saumitra Ray Fcsi, Email: saumitraray64@gmail.com.
Anshu Rajnish Sharma, Email: anshurajneesh@rediffmail.com.
Pankaj Dougall, Email: pdougall@maxhealthcare.com.
Shelley Simon, Email: shelleysimon@rediffmail.com.
Atul Verma Drm, Email: atul.verma@fortishealthcare.com.
Vivek Radhakrishnan, Email: drvivekradhakrishnan@yahoo.com.
References
- 1.Witteles R.M., Bokhari S., Damy T., et al. Screening for transthyretin amyloid cardiomyopathy in everyday practice. JACC Hear Fail. 2019;7(8):709–716. doi: 10.1016/j.jchf.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 2.Ruberg F.L., Grogan M., Hanna M., Kelly J.W., Maurer M.S. Transthyretin amyloid cardiomyopathy: JACC state-of-the-art review. J Am Coll Cardiol. 2020;73(22):2872–2891. doi: 10.1016/j.jacc.2019.04.003. [Transthyretin] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeldenrust S.R., Benson M.D. Familial and senile amyloidosis caused by transthyretin. Protein Misfolding Dis Curr Emerg Princ Ther. 2010:795–815. doi: 10.1002/9780470572702.ch36. Published online. [DOI] [Google Scholar]
- 4.González-López E., López-Sainz Á, Garcia-Pavia P. Diagnosis and treatment of transthyretin cardiac amyloidosis. Progress and hope. Rev Española Cardiol. 2017;70(11):991–1004. doi: 10.1016/j.rec.2017.05.036. English Ed. [DOI] [PubMed] [Google Scholar]
- 5.Ruberg F.L., Berk J.L. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012;126(10):1286–1300. doi: 10.1161/CIRCULATIONAHA.111.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma G. Hereditary ATTR amyloidosis: burden of illness and diagnostic challenges. Am J Manag Care. 2017;(23):S107–S112. [PubMed] [Google Scholar]
- 7.Rapezzi C., Quarta C.C., Obici L., et al. Disease profile and differential diagnosis of hereditary transthyretin-related amyloidosis with exclusively cardiac phenotype: an Italian perspective. Eur Heart J. 2013;34(7):520–528. doi: 10.1093/eurheartj/ehs123. [DOI] [PubMed] [Google Scholar]
- 8.Arbustini E., Merlini G. Early identification of transthyretin-related hereditary cardiac amyloidosis. JACC Cardiovasc Imaging. 2014;7(5):511–514. doi: 10.1016/j.jcmg.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 9.Donnelly J.P., Hanna M. Cardiac amyloidosis: an update on diagnosis and treatment. Cleve Clin J Med. 2017;84:12–26. doi: 10.3949/CCJM.84.S3.02. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins P.N., Ando Y., Dispenzeri A., Gonzalez-Duarte A., Adams D., Suhr O.B. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–638. doi: 10.3109/07853890.2015.1068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agarwal H., Ghosh T., Arava S., Ray R., Seth S. Cardiac amyloidosis in India: a clinicopathological study. J Pract Cardiovasc Sci. 2021;7(2):121. doi: 10.4103/jpcs.jpcs_35_21. [DOI] [Google Scholar]
- 12.Ghosh S., Khanra D., Krishna V., Thakur A.K. Wild type transthyretin cardiac amyloidosis in a young individual: a case report. Medicine (Baltim) 2021;100(17) doi: 10.1097/MD.0000000000025462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lane T., Fontana M., Martinez-Naharro A., et al. Natural history, quality of life, and outcome in cardiac transthyretin amyloidosis. Circulation. 2019;140(1):16–26. doi: 10.1161/CIRCULATIONAHA.118.038169. [DOI] [PubMed] [Google Scholar]
- 14.Rozenbaum M.H., Large S., Bhambri R., et al. Impact of delayed diagnosis and misdiagnosis for patients with transthyretin amyloid cardiomyopathy (ATTR-CM): a targeted literature review. Cardiol Ther. 2021;10(1):141–159. doi: 10.1007/s40119-021-00219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ladefoged B., Dybro A., Povlsen J.A., Vase H., Clemmensen T.S., Poulsen S.H. Diagnostic delay in wild type transthyretin cardiac amyloidosis – a clinical challenge. Int J Cardiol. 2020;304:138–143. doi: 10.1016/j.ijcard.2019.12.063. [DOI] [PubMed] [Google Scholar]
- 16.Nativi-Nicolau J.N., Karam C., Khella S., Maurer M.S. Screening for ATTR amyloidosis in the clinic: overlapping disorders, misdiagnosis, and multiorgan awareness. Heart Fail Rev. 2021 doi: 10.1007/s10741-021-10080-2. Published online. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vera-Llonch M., Reddy S.R., Chang E., Tarbox M.H., Pollock M. The patient journey toward a diagnosis of hereditary transthyretin (ATTRv) amyloidosis. Orphanet J Rare Dis. 2021;16(1):1–11. doi: 10.1186/s13023-020-01623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia-Pavia P., Rapezzi C., Adler Y., et al. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J. 2021;42(16):1554–1568. doi: 10.1093/eurheartj/ehab072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.González-López E., Gallego-Delgado M., Guzzo-Merello G., et al. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36(38):2585–2594. doi: 10.1093/eurheartj/ehv338. [DOI] [PubMed] [Google Scholar]
- 20.López-Sainz Á, de Haro-del Moral F.J., Dominguez F., et al. Prevalence of cardiac amyloidosis among elderly patients with systolic heart failure or conduction disorders. Amyloid. 2019;26(3):156–163. doi: 10.1080/13506129.2019.1625322. [DOI] [PubMed] [Google Scholar]
- 21.Damy T., Costes B., Hagège A.A., et al. Prevalence and clinical phenotype of hereditary transthyretin amyloid cardiomyopathy in patients with increased left ventricular wall thickness. Eur Heart J. 2016;37(23):1826–1834. doi: 10.1093/eurheartj/ehv583. [DOI] [PubMed] [Google Scholar]
- 22.Treibel T.A., Fontana M., Gilbertson J.A., et al. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis. Circ Cardiovasc Imaging. 2016;9(8):1–10. doi: 10.1161/CIRCIMAGING.116.005066. [DOI] [PubMed] [Google Scholar]
- 23.Castano A., Narotsky D.L., Hamid N., et al. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J. 2017;38(38):2879–2887. doi: 10.1093/eurheartj/ehx350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bokhari S., Castaño A., Pozniakoff T., Deslisle S., Latif F.M.M. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imaging. 2013 Mar 1;6(2):195–201. doi: 10.1007/978-1-4419-6848-7_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perugini E., Guidalotti P.L., Salvi F., et al. Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scintigraphy. J Am Coll Cardiol. 2005;46(6):1076–1084. doi: 10.1016/j.jacc.2005.05.073. [DOI] [PubMed] [Google Scholar]
- 26.Hutt D.F., Fontana M., Burniston M., et al. Prognostic utility of the Perugini grading of 99m Tc-DPD scintigraphy in transthyretin (ATTR) amyloidosis and its relationship with skeletal muscle and soft tissue amyloid. Eur Heart J Cardiovasc Imaging. 2017;18(12):1344–1350. doi: 10.1093/ehjci/jew325. [DOI] [PubMed] [Google Scholar]
- 27.Gillmore J.D., Maurer M.S., Falk R.H., et al. Nonbiopsy diagnosis of cardiac transthyretin amyloidosis. Circulation. 2016;133(24):2404–2412. doi: 10.1161/CIRCULATIONAHA.116.021612. [DOI] [PubMed] [Google Scholar]
- 28.Maurer M.S., Elliott P., Comenzo R., Semigran M.R.C. Addressing common questions encountered in the diagnosis and management of cardiac amyloidosis. Circulation. 2017;135(14):1357–1377. doi: 10.1161/CIRCULATIONAHA.116.024438. [Addressing] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Conceição I., Coelho T., Rapezzi C., et al. Assessment of patients with hereditary transthyretin amyloidosis–understanding the impact of management and disease progression. Amyloid. 2019;26(3):103–111. doi: 10.1080/13506129.2019.1627312. [DOI] [PubMed] [Google Scholar]
- 30.Maurer M.S., Bokhari S., Damy T., et al. Expert consensus recommendations for the suspicion and diagnosis of transthyretin cardiac amyloidosis. Circ Hear Fail. 2019;12(9) doi: 10.1161/CIRCHEARTFAILURE.119.006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kittleson MM, Maurer MS, Ambardekar A V., et al. Cardiac amyloidosis: evolving diagnosis and management: a scientific statement from the American heart association. Circulation. Published online 2020:E7-E22. doi:10.1161/CIR.0000000000000792. [DOI] [PubMed]