Abstract
This study examines the cytokine/chemokine profile of a 62-year-old African American male with progressive multiple sclerosis (MS). MRI images of the MS patient demonstrated generalized white matter involvement with multiple lesions in the periventricular area. A 42-plex Discovery Assay® (Eve Technologies) of the patient’s plasma and peripheral blood mononuclear cells (PBMCs) supernatant or PBMC-derived T cell supernatant samples from two separate clinic visits revealed vastly differing cytokine/chemokine levels. In addition, certain cytokine/chemokine profiles had notable differences when compared to the larger patient group or patients’ PBMCs treated with a calpain inhibitor in vitro. Interestingly, large numbers of cytokines/chemokines and growth factors in MS PBMCs are modulated by calpain inhibition, suggesting the clinical significance of these findings in designing better therapeutics against progressive MS.
Keywords: Progressive multiple sclerosis, Cytokine/chemokine, PBMCs, T cells, Calpain
Introduction
Multiple sclerosis (MS) is an inflammatory autoimmune demyelinating and neurodegenerative disease of the central nervous system (CNS) with unknown triggering cause and for which there is no cure. About 1 million people are affected by MS in the United States. There are approximately 2.5 million cases worldwide. Twice as many women as men develop MS, and most people afflicted with the disease are diagnosed between the ages of 20 and 40. Additionally, the incidence is highest among those that grew up in cold climates and did not leave before 12–15 years of age, and Caucasians of Northern European descent regardless of geographic location (Tullman, 2013).
According to the 1996 nomenclature (Lublin and Reingold, 1996), the clinical course of MS was classified as relapsing-remitting (RR), primary progressive (PP), secondary progressive (SP) or progressive relapsing (PR). In 2013, revisions were made, and MS was characterized as two core phenotypes: (a) relapsing, which includes RR, SP, and PR, and (b) progressive, which includes PP, SP, and PR (Lublin et al., 2014). Recommendations were also made that clinical descriptions be updated to specify an assessment of disease activity and progression over time, to eliminate the PRMS designation, instead classifying those patients as PPMS with active disease, and to incorporate clinically isolated syndrome (CIS) as an MS phenotype. Nevertheless, the course and rate of disease progression in MS is highly unpredictable, and few treatments to target both the immune and neurodegenerative components of the disease are available. MS pathology is characterized by the infiltration of myelin-specific T cells into the CNS (Trager et al., 2014). The predominance of Th1/Th17 cells, pro-inflammatory chemokines (MIP-1α, RANTES etc.), cytokines (IFNγ, IL-17, IL-6, IL-12, IL-21, IL-23 etc.) over Th2/Treg cells is a hallmark of the active disease phase, but Th2/Treg cells and associated anti-inflammatory cytokines (e.g. IL-4, IL-5, IL-10, and IL-13) predominate in remission (Trager et al., 2013). Evaluation of the disease in active phase and the corresponding murine EAE experimental autoimmune encephalomyelitis (EAE) model have shown that the resultant pro-inflammatory attack on the CNS can lead to neurodegeneration and axonal damage.
Previous studies reported a relationship between MS and other autoimmune disease within the families, but there was no significant association with non-autoimmune diseases (Cardenas-Roldan et al., 2013, Kalman and Leist, 2004). Recent evidence suggests that the interaction of multiple genetic and environmental factors may contribute to the development of MS. Thus, analyzing samples from MS, as well as other autoimmune diseases from families and siblings should provide valuable data for determining genetic factors and/or other biomarkers of the disease. Interestingly, several strategies have been explored for treating EAE/MS. Most therapies are directed towards immunomodulation, which has not significantly improved efficacy or halted disease progression. MS is not only an inflammatory autoimmune disease, it also has an additional neurodegenerative component that adds to the complexity of MS pathology. Therefore, therapy should be directed to both immunomodulation and neurodegeneration. One strategy has been to target proteases that degrade myelin and axonal proteins, which can destabilize myelin and degenerate axons, thus contributing to disability (Aktas et al. 2010). While the exact etiology of MS/EAE is unknown, both acidic and neutral proteases are thought to play a role in development of the neurodegenerative component of the disease. One such protease with increased activity in MS is the Ca2+-activated neutral protease calpain (Banik et al., 1987). The degeneration of axons and neuronal death mediated by calpain has also been suggested in EAE (Shields et al., 1999, Schaecher et al., 2001). Previous studies demonstrated increased calpain activity and cell-specific overexpression in neural cells (astrocytes, microglia) and other cell types (macrophages, T cells) in EAE/MS, implicating a pivotal role for calpain in immunological aspects (T cell activation) and myelin breakdown in these diseases (Shields et al., 1998, Shields and Banik, 1998, Shields et al., 1999). In contrast, treatment with calpain inhibitors decrease EAE disease signs in rats and mice by reducing inflammatory cytokines/chemokines and neurodegenerative events (Guyton et al., 2005, Guyton et al., 2010, Guyton et al., 2009). Recently, treatment of EAE animals with calpain inhibitors has been shown to protect neuronal cells and preserve axons with reduction of disease severity (Guyton et al., 2006, Smith et al., 2011a). In addition, calpain inhibition with calpeptin has been shown to attenuate inflammatory T cell proliferation (Th1/Th17) and increase anti-inflammatory Th2/Treg cells in human MS patient peripheral blood mononuclear cells (PBMCs) (Payne et al., 2012, Chandran et al., 2018). These findings indicate increased calpain is not only involved in inflammatory response and T cell proliferation, but may also promote neurodegeneration in EAE. Subsequently, treatment of EAE animals with calpain inhibitor has been found to attenuate development and progression of disease, possibly by preventing both inflammatory and neurodegenerative processes (Trager et al., 2014). This suggests that effective therapy for MS should have an agent(s) that could take care of both immunomodulation and neurodegeneration. In our evaluation of the effect of calpain inhibition on cytokine and chemokine profiles in MS PBMCs, one particular patient was found to have several outlying factors within the cytokine and chemokine profile that will be further examined in this report.
Experimental procedure
Patient history
The patient discussed herein is a 62-year-old African American male with a history of secondary progressive multiple sclerosis. He was diagnosed with multiple sclerosis (MS) in 2004 and was on Rebif® from 2004 to 2013, until it was discontinued due to site reactions, ineffectiveness, and lack of modifying effects on MS. He was also on Dalfampridine (AMPYRA®) from July 2012 to January 2013, which is used to treat MS-related weakness. This drug was stopped as the patient was obtaining no clear benefit. The patient started on another disease modifying MS drug OCREVUS® at the end of 2017. Other medical conditions in addition to the MS include hyperthyroidism, now post ablation and hypothyroid; hypertension, hyper-cholesterolemia, and anemia. The two blood samples from this patient were taken on May 19, 2015 and on August 10, 2015. Relevant medical history around this time period includes an upper endoscopy in February 2015 and a cystoscopy in June 2015 (in which he received a one-day prescription for ciprofloxacin). The patient had a prolonged admission from September 2013 to September 2014 due to a stage IV decubitus ulcer with underlying osteomyelitis. His hospital course was complicated by pulmonary embolus. He also underwent a diverting colostomy and bilateral flap surgeries during the hospitalization.
Isolation and stimulation of PBMCs
After obtaining patient consent according to IRB protocol, approximately 8–10 mL of blood was collected from the patient in a heparinized tube. 1–2 mL of the blood sample were then set aside and separated by centrifugation at 7000 RPM for 2 min at 4 °C. The plasma layer was collected and stored at −80 °C. The remaining 4–9 mL of blood sample was diluted 1:1 with Hank’s Balanced Salt Solution (HBSS) (ThermoFisher, Catalog number: 14170161). Ficoll (GE Healthcare, Piscataway, NJ) separation was performed with centrifugation at 2000 RPM for 20–25 min at room temperature, and the PBMC layer was collected. This layer was then washed twice with HBSS and washed with Tcell media (complete RPMI) (ThermoFisher, Catalog number: 11875093) at a sample to media ratio of 1:5.
Following PBMC isolation, cells were counted and resuspended at 3 × 106 cells/mL in complete RPMI medium. Cells (2 × 106 /well) were treated in duplicate with 10 μg/mL of α-CD3 and 5 μg/mL of α-CD28 in the presence or absence of 10 μM calpeptin dissolved in dimethyl sulfoxide (DMSO) for 24 h. The final concentration of DMSO in PBMC culture was <0.001%. Treated cells were then spun at 1200 RPM for 10 min at 4 °C, and supernatant (incubation medium) was collected and stored at −80 °C until further experimentation.
Chemokine/cytokine profiling
Plasma and PBMC or activated T cell supernatants from MS patient blood samples were run with the Human Cytokine Array / Chemokine Array 42-Plex with IL-18 (HD42) kit (Eve Technologies, Calgary, AB Canada T2N 0 M4) according to the manufacturer’s protocol.
Statistical analysis
Statistical analyses were performed using Microsoft Excel and GraphPad Prism (version 4.0; GraphPad Software). PBMC supernatants from the first and second visits were compared via Two Way Analysis of Variance (ANOVA) followed by Tukey’s HSD with statistical significance determined at p < 0.05.
Results
The MRI of the MS patient demonstrated generalized white matter involvement (Fig. 1). The periventricular area is involved on both sides. The shrinkage of brain tissue due to a decrement in the size of the cell is common in aging and is called brain atrophy (De Stefano et al., 2010). In MS, this process of brain atrophy is accelerated and can cause cognitive decline, a major disabling result of MS. Comparing the two axial images (control and adjacent image), the MRI image of MS brain demonstrates periventricular T2 hyperintensities, while the control image does not (Fig. 1). In general, MS patients experience about three to four times more annual brain volume loss than a healthy person. Thus, measuring brain atrophy in MS patients should help identify patients’ risk for physical and cognitive decline as suggested previously (Alroughani et al., 2016). MRI images also showed some of the classic findings with Dawson’s fingers (arrows) in the MS patient.
Fig. 1.
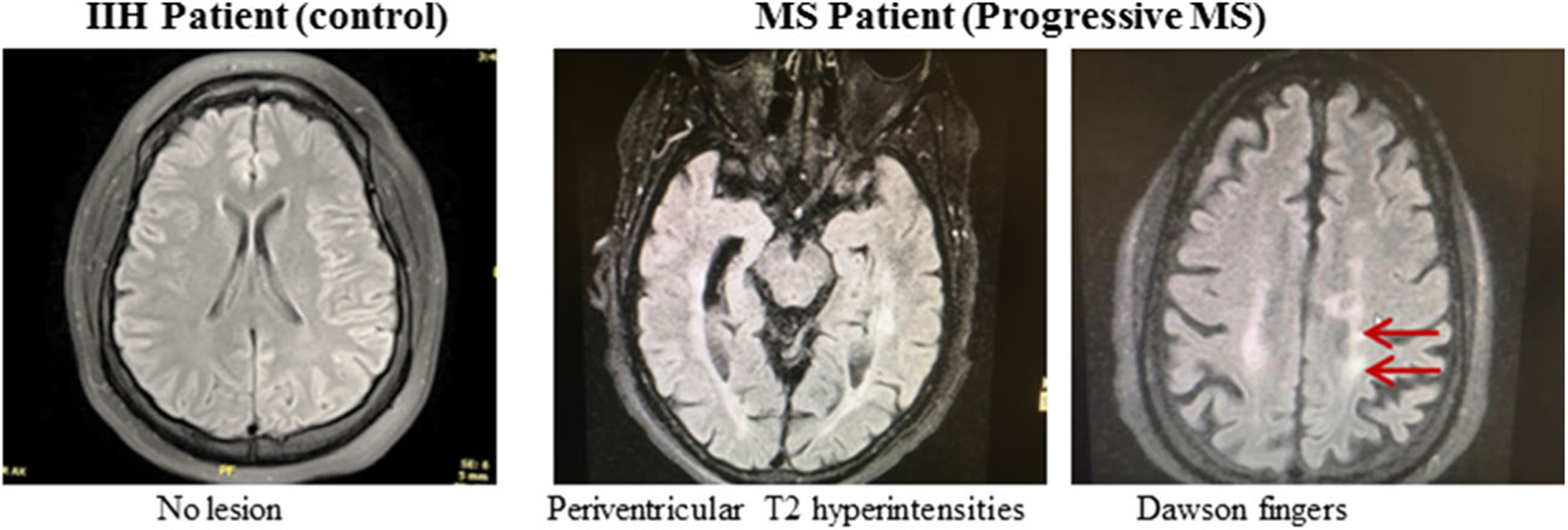
Representative MRI Images of a control IIH patient and a progressive MS patient. Arrows indicate abnormalities possibly due to multiple lesions in the white matter
To characterize the inflammatory cascade of cytokines and chemokines in MS patients, a 42-plex Discovery Assay® (Eve Technologies) was performed on plasma and supernatant samples to analyze the expression of 42 different cytokines and chemokines in MS PBMCs.
Cytokines/chemokines in plasma
The patient’s plasma and PBMC-culture supernatant growth factors and chemokine/cytokine profiles showed marked overexpression of GRO-α (1843.44 pg/mL), BDNF (3991.44 pg/mL), PDGF-AA (674.11 pg/mL), PDGF-AB (8301.37 pg/mL), PDGF-BB (14,597.15 pg/mL), PAI-1 (14,008.76 pg/mL), and RANTES (27,167.78 pg/mL) in the plasma sample from the first visit. After the second visit, the levels of each of these cytokines/chemokines were reduced to levels that mirrored those observed in some remission patient samples (Fig. 2). IL-12p40 (39.31 pg/mL) was markedly increased in the first sample as compared to other patients and was too high or out of range to detect in the second visit sample. In the second visit PBMC plasma sample, fractalkine (3.2 pg/mL in the first sample) and IL-17A (1.49 pg/mL in the first sample) were increased to a point out of the range of detection. Levels of IFNα2 were too high out of range to quantify in both visits. These are unusual events in MS relapse or remission patients, suggesting that dysregulation of cytokines/chemokines could play important roles in progressive MS.
Fig. 2.
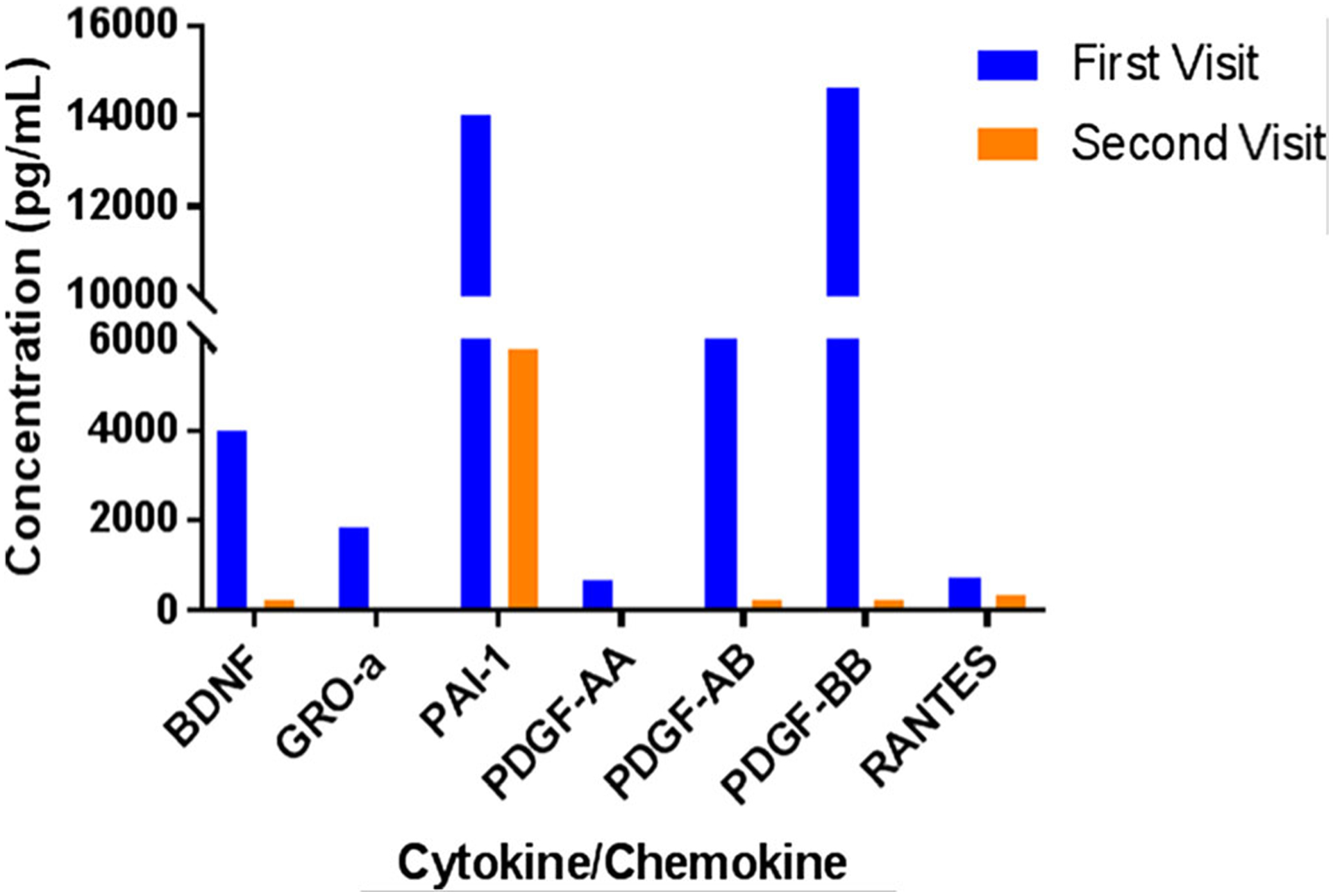
Cytokine/Chemokine Profile from Human Cytokine Array/Chemokine Array 42-Plex with IL-18 (HD42) kit (Eve Technologies) of MS patient plasma sample from two clinic visits
Cytokines/chemokines in activated PBMC supernatant
The cytokine/chemokine profile for calpain inhibition of this progressive MS patient’s activated PBMC supernatant revealed several interesting effects and drastic differences in profiles between the first and second visit (Fig. 3). Calpain inhibition by calpeptin increased levels of EGF (first visit: 209.27 pg/mL to 233.69 pg/mL; second visit: 812.46 pg/mL to 894.57 pg/mL) and IL-4 (first visit: 217.23 pg/mL to 380.88 pg/mL; second visit: 75.86 pg/mL to 81.78 pg/mL) in supernatant samples from both patient visits when compared to α-CD3/CD28 stimulated cells. IP-10, MIP-1β, IL1RA, and FGF expression were increased with calpeptin treatment of activated PBMC supernatant from the first visit. Calpain inhibition decreased levels of GM-CSF, IL-10, MDC, sCD40L, IL-17A, IL-1α, IL-3, TNF-α, and VEGF-A. Calpeptin treatment also reduced GRO-α expression from 7437.63 pg/mL to 6593.94 pg/mL in the first visit, but expression levels from the second visit sample were out of range for detection. The second visit sample showed decreased expression of IFNα2, IL-1β, IL-8, and MIP-1α with calpain inhibition.
Fig. 3.
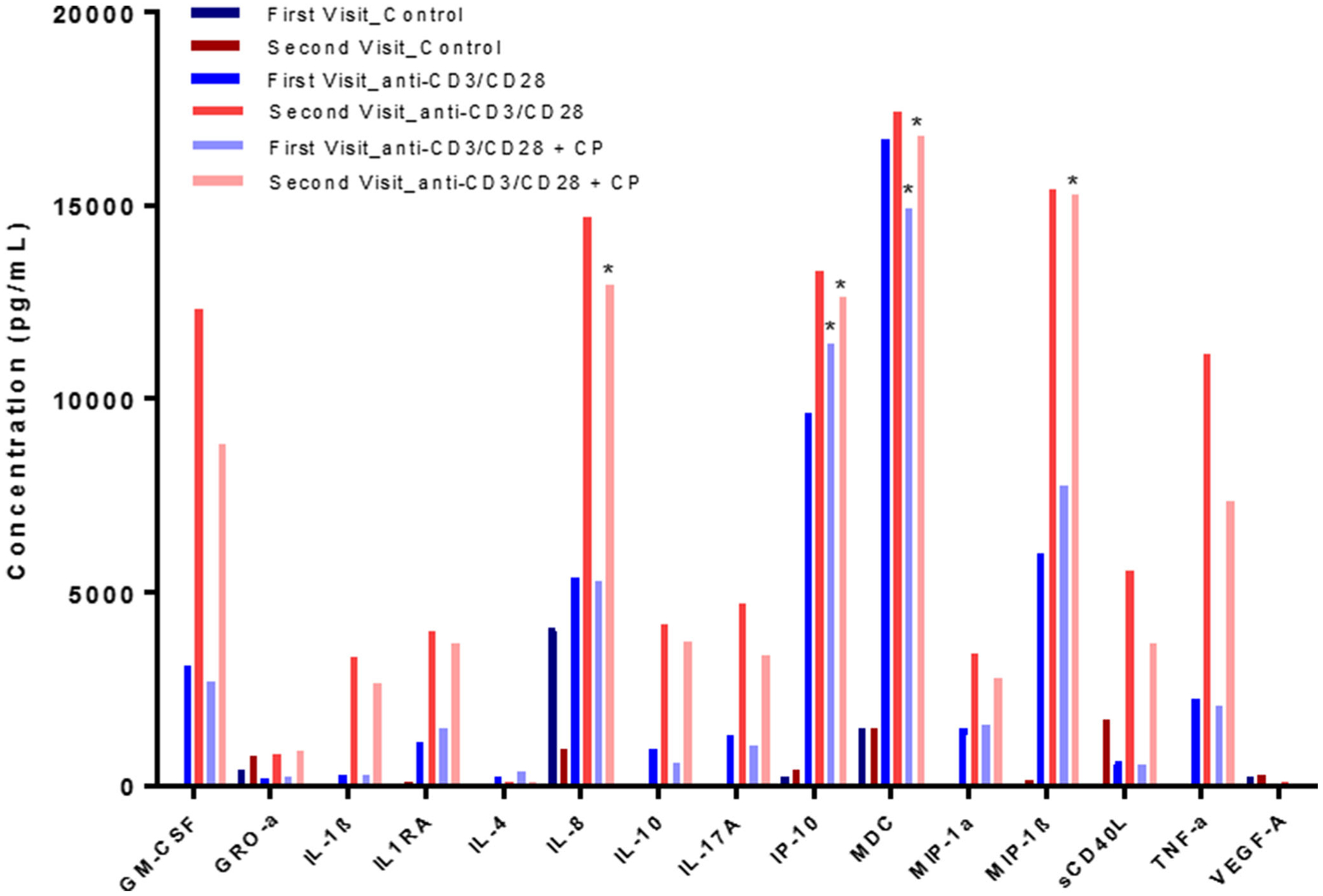
MS patient PBMC supernatant cytokine/chemokine profile. PBMCs were stimulated with α-CD3/CD28 antibody in the presence or absence of calpain inhibitor, calpeptin. The experiment was performed in duplicate. Statistical significance was determined by Two Way ANOVA (*p < 0.05)
When compared to other patient profiles (data not shown) and the first patient visit, IL-3, IL-6, IL-8, MIP-1α, TNF-α, GM-CSF, PDGF-AA, sCD40L, IL-17A, and IL-1α expression were markedly upregulated in α-CD3/CD28 and α-CD3/CD28 + CP conditions. Levels of IL-4 were notably upregulated in treatment groups from the patient’s first visit when compared to other patient profiles, and the addition of calpeptin to the α-CD3/CD28-stimulated PBMCs led to a greater increase in IL-4 expression (217.23 pg/mL to 380.88 pg/mL). These data suggest that calpain inhibition may help attenuate inflammation in unusual MS cases regardless of dysregulation of cytokine/chemokine levels in the host.
Two Way ANOVA followed by Tukey’s HSD indicated that IP-10 and MDC levels from the first visit sample were significantly increased with α-CD3/CD28 and α-CD3/CD28 + CP stimulation as compared to unstimulated controls. MIP-1β levels showed a trend towards significance (p = 0.0911) in α-CD3/CD28 + CP stimulated PBMCs as compared to controls but not for α-CD3/CD28-stimulated PBMCs compared to unstimulated controls or α-CD3/CD28 + CP compared to α-CD3/CD28-stimulated PBMCs. In the second visit PBMC sample, IL-8, IP-10, MDC, and MIP-1β were significantly upregulated with α-CD3/CD28 and α-CD3/CD28 + CP stimulation as compared to unstimulated controls. GM-CSF levels were significantly upregulated in α-CD3/CD28-stimulated PBMCs from the second visit, but no significant upregulation was noted for the α-CD3/CD28 + CP condition when compared to controls or the α-CD3/CD28 levels compared to α-CD3/CD28 + CP. TNF-α showed a trend towards significance (p = 0.0622) in upregulation associated with α-CD3/CD28 stimulation as compared to unstimulated controls, but no significance was observed in α-CD3/CD28 vs α-CD3/CD28 + CP conditions or α-CD3/CD28 + CP stimulation as compared to unstimulated controls. Further information is needed to fully understand the effects of calpeptin treatment on cytoine/chemokine expression following PBMC stimulation.
Discussion
Inflammation is one of the most common factors involved in a majority of neurodegenerative diseases. MS, an autoimmune inflammatory disorder, is no exception. This study has focused on a progressive MS case study determining the changes in inflammatory cytokines/chemokines in patient plasma samples and PBMC culture supernatants following calpain inhibition by a pharmacological inhibitor in vitro.
Active MS conditions are characterized by a greater presence of Th1/Th17 cells that promote pro-inflammatory cascades of chemokines/cytokines, e.g. IFNγ, TGFβ, IL-1, IL-6, IL-12, IL-21, and IL-23 (Trager et al., 2013). In our evaluation of MS patients with relapsing-remitting MS (RRMS) and progressive MS, we found several outliers among this patient’s cytokine/chemokine profile when compared to the rest of the group. In the patient’s first visit (but not second visit) plasma sample, expression of GRO-α, BDNF, PDGF-AA, PDGF-AB, PDGF-BB, PAI-1, and RANTES were significantly overexpressed as compared to the other patient samples tested (Chandran et al., 2018).
Growth-related oncogene-alpha (GRO-α, CXCL1) can be expressed in activated microglia on the border of MS patient brain lesions–a finding consistent with the chemokine’s inflammatory role in the disease (Filipovic et al., 2003). However, GRO-α is not expressed in early oligodendrocyte progenitors in MS patients like it is in the developing brain, which may explain why remyelination is inefficient in MS (Filipovic et al., 2003). In this study, a notable increase was found in plasma levels of GRO-α at the patient’s first visit as compared to the second. However, with the exception of a slight increase in IL-5 concentration, no notable increases in levels of other pro-inflammatory cytokines/chemokines (e.g. IFNγ, IL-1α, IL-1β, MCP-1, and TNF-α) were detected in comparison to the second visit and other patient samples. IL-12p40, thought to suppress Th1-type responses in chronic inflammation (Kalinski et al., 2001), was markedly upregulated in this patient’s first visit plasma sample, which could perhaps account for the other cytokine/chemokine expression levels observed.
Platelet-derived growth factors (PDGF) promote cell proliferation and division via chemotactic activity (Kawada et al., 2009). PDGF signaling defects underlie clinical progression in MS, and it is thought that enhancing PDGF signaling could help maintain brain reserve and attenuate neuronal damage in progressive MS (Mori et al., 2013). The increased PDGF expression in the first visit plasma could indicate a period of reduced disease activity (remission phase) as compared to the second visit. It is interesting to note that PDGF-AA levels in supernatant revealed the reverse pattern of that in the plasma sample.
Calpeptin treatment reduced levels of sCD40L in α-CD3/CD28 activated supernatant from this MS patient’s PBMCs and increased IL-4 expression in the first visit sample. One of the therapeutic goals of MS is to reduce the numbers of inflammatory T cells and their production of cytokines/chemokines that damage the myelin sheath around axons. Recent studies suggest that treatment with IL-4 attenuates neuronal and axonal damage, improving function (Casella et al., 2016, Rossi et al., 2018). Thus, calpain inhibition may reduce inflammatory cytokines/chemokines in MS patients, resulting in an induction of neuronal and axonal protection. sCD40L is believed to disrupt the blood-brain barrier in MS conditions (Masuda et al., 2017) and was markedly increased in supernatant from the second visit but not in plasma. IL-4 is an anti-inflammatory cytokine that modulates microglial activation and can be detected in increased concentrations during EAE remission (Payne et al., 2012). It is possible that the vast differences in the patient chemokine/cytokine profile between the first and second visits could be attributed to a period of remission or reduced disease activity during the first visit and a period of worsening disease during the second visit. Interestingly, VEGF-A expression did not noticeably differ from the other patient samples. Although there were no noticeable changes in patient’s neurological function between the two visits, the observed changes in cytokine and chemokine expression between the two visits may have resulted from the disease progression or modification because of the treatment. Apparently, variations in cytokine/chemokine expression between the 2 visits did not happen because of the infection or a major alteration of the patient’s health.
Calpain activity and expression, concomitant with inflammatory cytokines/chemokines, have been previously found upregulated in PBMCs of relapsing/remitting MS patients (Smith et al., 2011b). This study investigated whether changes in cytokine, chemokines, and growth factors in a progressive MS patient sample are influenced by calpain inhibition. Calpain expression and activity are increased in MS patients as well as in the animal models of MS (Trager et al., 2014). In animal models, the clinical signs of the disease attenuated dramatically following calpain inhibition. It is not unusual to find increased levels of inflammatory cytokines/chemokines and other inflammatory factors during the progression of the disease in MS. The therapy should bring down the inflammatory status and reduce the degenerative process by blocking neural cell death as seen in the animal model, EAE. Although calpain is a proven target in different animal models of MS, calpain inhibitor is has not been taken to the clinic thus far. It remains to be seen if/when the inhibitor is approved by the FDA and its efficacy tested in humans.
We have previously shown that calpain inhibition reduces inflammatory cytokines, including IL-12, IL-17, TNFα, and G-CSF in relapsed MS patient PBMCs (Imam et al., 2007). We have also shown that calpain inhibition differentially regulates IL-6, IL-8, IL-17, IL-23 and IL-12p40 cytokine gene expression in these MS-PBMCs. Further studies have also shown that calpain inhibition upregulates indoleamine 2,3-dioxygenase (IDO) mRNA levels in activated MS PBMCs. Reductions of inflammatory T cells by increased IDO production can help attenuate inflammatory cytokines/chemokines in MS (Smith et al., 2011b). Thus, the observed effects of calpain inhibition on a progressive MS patient PBMCs suggest that targeting calpain may help reduce inflammation and disease severity in MS. Because calpain also plays multiple roles, including neuron death and axonal degeneration in the pathophysiological events in MS, the approach of calpain inhibition may have the potential to be used in the clinic.
Analysis of this patient’s unique chemokine/cytokine profile has revealed several interesting factors to consider, and further research should be conducted to determine if similar patterns can be found in larger sample groups or in progressive MS patients. Furthermore, the deviation of this cytokine/chemokine profile from a healthy individual or a patient with lower risk of developing the disease suggests the importance of evaluating different patient populations for identifying and producing alternative or combination treatment strategies and regimens. Nonetheless, studies targeting cytokines/chemokines and their regulation by calpain inhibition may offer therapeutic approaches in MS.
Conclusions
The present study suggests that an involvement of a complex array of inflammatory and anti-inflammatory cytokines/chemokines may initiate and/or regulate the progression of the disease in MS. Investigating cytokines/chemokines and their modulation by calpain regulation in progressive MS may help identify mechanisms involved in the pathogenesis of the disease. Dissecting the variation of levels of cytokines/chemokines and other growth factors observed in two different visits may aid in monitoring the disease course and in evaluating responses to existing therapies, which could be helpful in designing novel therapeutics against progressive MS.
Acknowledgements
This study was made possible by grants from the Ralph H. Johnson Veterans Administration Medical Center, Charleston (1I01BX002349-01, 1 I01 BX004269-01A1) to NLB. This work was also supported by grants from the South Carolina Spinal Cord Injury Research Fund (SCIRF #2018 I-01) to AH.
Footnotes
Conflict of interest The authors have no financial conflicts of interest.
References
- Aktas O, Kieseier B, Hartung HP (2010) Neuroprotection, regeneration and immunomodulation: broadening the therapeutic repertoire in multiple sclerosis. Trends Neurosci 33:140–152 [DOI] [PubMed] [Google Scholar]
- Alroughani R, Deleu D, El Salem K, Al-Hashel J, Alexander KJ, Abdelrazek MA, Aljishi A, Alkhaboori J, Al Azri F, Al Zadjali N, Hbahbih M, Sokrab TE, Said M, Rovira A (2016) A regional consensus recommendation on brain atrophy as an outcome measure in multiple sclerosis. BMC Neurol 16:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banik NL, Chakrabarti AK, Hogan EL (1987) Distribution of calcium activated neutral proteinase (mM CANP) in myelin and cytosolic fractions in bovine brain white matter. Life Sci 41:1089–1095 [DOI] [PubMed] [Google Scholar]
- Cardenas-Roldan J, Rojas-Villarraga A, Anaya JM (2013) How do auto-immune diseases cluster in families? A systematic review and meta-analysis. BMC Med 11:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casella G, Garzetti L, Gatta AT, Finardi A, Maiorino C, Ruffini F, Martino G, Muzio L, Furlan R (2016) IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J Neuroinflammation 13:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran R, Capone M, Matzelle D, Polcyn R, Kau E, Haque A, Banik NL (2018) Distinct cytokine and chemokine expression in plasma and Calpeptin-treated PBMCs of a relapsing-remitting multiple sclerosis patient: A case report. Neurochem Res 43:2224–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefano N, Giorgio A, Battaglini M, Rovaris M, Sormani MP, Barkhof F, Korteweg T, Enzinger C, Fazekas F, Calabrese M, Dinacci D, Tedeschi G, Gass A, Montalban X, Rovira A, Thompson A, Comi G, Miller DH, Filippi M (2010) Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology 74:1868–1876 [DOI] [PubMed] [Google Scholar]
- Filipovic R, Jakovcevski I, Zecevic N (2003) GRO-alpha and CXCR2 in the human fetal brain and multiple sclerosis lesions. Dev Neurosci 25:279–290 [DOI] [PubMed] [Google Scholar]
- Guyton MK, Brahmachari S, Das A, Samantaray S, Inoue J, Azuma M, Ray SK, Banik NL (2009) Inhibition of calpain attenuates encephalitogenicity of MBP-specific T cells. J Neurochem 110: 1895–1907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton MK, Das A, Matzelle DD, Samantaray S, Azuma M, Inoue J, Ray S & Banik NL 2006. SJA6017 attenuates immune cell infiltration and Neurodegneration in EAE. 8th International Congress of Neuroimmunology, 107–112 [Google Scholar]
- Guyton MK, Das A, Samantaray S, Wallace GCT, Butler JT, Ray SK, Banik NL (2010) Calpeptin attenuated inflammation, cell death, and axonal damage in animal model of multiple sclerosis. J Neurosci Res 88:2398–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyton MK, Wingrave JM, Yallapragada AV, Wilford GG, Sribnick EA, Matzelle DD, TYOR WR, Ray SK, Banik NL (2005) Upregulation of calpain correlates with increased neurodegeneration in acute experimental auto-immune encephalomyelitis. J Neurosci Res 81:53–61 [DOI] [PubMed] [Google Scholar]
- Imam SA, Guyton MK, Haque A, Vandenbark A, Tyor WR, Ray SK, Banik NL (2007) Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol 190:139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalinski P, Vieira PL, Schuitemaker JH, De Jong EC, Kapsenberg ML (2001) Prostaglandin E(2) is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97:3466–3469 [DOI] [PubMed] [Google Scholar]
- Kalman B, Leist TP (2004) Familial multiple sclerosis and other inherited disorders of the white matter. Neurologist 10:201–215 [DOI] [PubMed] [Google Scholar]
- Kawada K, Upadhyay G, Ferandon S, Janarthanan S, Hall M, Vilardaga JP, Yajnik V (2009) Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol Cell Biol 29:4508–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin FD, Reingold SC (1996) Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 46:907–911 [DOI] [PubMed] [Google Scholar]
- Lublin f D, Reingold SC, Cohen JA, Cutter GR, Sorensen PS, Thompson AJ, Wolinsky JS, Balcer LJ, Banwell b, Barkhof F jr. Bebo B, Calabresi PA, Clanet M, Comi G, Fox RJ, Freedman MS, Goodman AD, Inglese M, Kappos L, Kieseier BC, Lincoln JA, Lubetzki C, Miller AE, Montalban X, O’connor PW, Petkau J, Pozzilli C, Rudick RA, Sormani MP, Stuve O, Waubant E, Polman CH (2014) Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology 83:278–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Mori M, Uchida T, Uzawa A, Ohtani R, Kuwabara S (2017) Soluble CD40 ligand contributes to blood-brain barrier breakdown and central nervous system inflammation in multiple sclerosis and neuromyelitis optica spectrum disorder. J Neuroimmunol 305:102–107 [DOI] [PubMed] [Google Scholar]
- Mori F, Rossi S, Piccinin S, Motta C, Mango D, Kusayanagi H, Bergami A, Studer V, Nicoletti CG, Buttari F, Barbieri F, Mercuri NB, Martino G, Furlan R, Nistico R, Centonze D (2013) Synaptic plasticity and PDGF signaling defects underlie clinical progression in multiple sclerosis. J Neurosci 33:19112–19119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne NL, Dantanarayana A, Sun G, Moussa L, Caine S, Mcdonald C, Herszfeld D, Bernard CC, Siatskas C (2012) Early intervention with gene-modified mesenchymal stem cells overexpressing interleukin-4 enhances anti-inflammatory responses and functional recovery in experimental autoimmune demyelination. Cell Adhes Migr 6:179–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi C, Cusimano M, Zambito M, Finardi A, Capotondo A, Garcia-Manteiga JM, Comi G, Furlan R, Martino G, Muzio L (2018) Interleukin 4 modulates microglia homeostasis and attenuates the early slowly progressive phase of amyotrophic lateral sclerosis. Cell Death Dis 9:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaecher KE, Shields DC, Banik NL (2001) Mechanism of myelin breakdown in experimental demyelination: a putative role for calpain. Neurochem Res 26:731–737 [DOI] [PubMed] [Google Scholar]
- Shields DC, Banik NL (1998) Putative role of calpain in the pathophysiology of experimental optic neuritis. Exp Eye Res 67:403–410 [DOI] [PubMed] [Google Scholar]
- Shields DC, Schaecher KE, Saido TC, Banik NL (1999) A putative mechanism of demyelination in multiple sclerosis by a proteolytic enzyme, calpain. Proc Natl Acad Sci U S A 96:11486–11491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields DC, Tyor WR, Deibler GE, Hogan EL, Banik NL (1998) Increased calpain expression in activated glial and inflammatory cells in experimental allergic encephalomyelitis. Proc Natl Acad Sci U S A 95:5768–5772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Das A, Guyton MK, Ray SK, Rohrer B, Banik NL (2011a) Calpain inhibition attenuates apoptosis of retinal ganglion cells in acute optic neuritis. Invest Ophthalmol Vis Sci 52:4935–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AW, Doonan BP, Tyor WR, Abou-Fayssal N, Haque A, Banik NL (2011b) Regulation of Th1/Th17 cytokines and IDO gene expression by inhibition of calpain in PBMCs from MS patients. J Neuroimmunol 232:179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager N, Butler JT, Haque A, Ray SK, Beeson C, Banik NL (2013) The involvement of Calpain in CD4(+) T helper cell Bias in Multple sclerosis. J Clin Cell Immunol 4:1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager N, Smith A, Wallace IV G, Azuma M, Inoue J, Beeson C, Haque A, Banik NL (2014) Effects of a novel orally administered calpain inhibitor SNJ-1945 on immunomodulation and neurodegeneration in a murine model of multiple sclerosis. J Neurochem 130:268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullman MJ (2013) Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care 19:S15–S20 [PubMed] [Google Scholar]


