Abstract
A series of nitrogen-doped porous carbon nanosheets (NPCNs) doped with transition-metal-supported Pt catalysts were prepared by colloidal deposition and evaluated for the selective oxidation of glycerol to glyceric acid (GLYA) under nonalkaline conditions. The transition metal contained in the catalyst was found to affect its performance and selectivity for GLYA, with the Pt/Zr@NPCN catalyst showing the highest catalytic activity and selectivity. These materials were characterized using Brunauer–Emmett–Teller surface area analysis, transmission electron microscopy, X-ray diffraction, X-ray photoelectron spectroscopy, and CO2 temperature-programmed desorption. The results showed that the small size of the Pt nanoparticles, the interaction between the Pt nanoparticles and the support, and the unique textural properties of the catalyst all promoted glycerol conversion and GLYA selectivity. A Zr concentration of 1.5 wt % and a support preparation temperature of 800 °C were found to provide a catalyst with the optimal performance that exhibited a glycerol conversion and selectivity for GLYA of 68.62 and 77.29%, respectively, at an initial O2 pressure of 10 bar and 60 °C after 6 h. Even after being recycled five times, this material provided a GLYA selectivity of approximately 75%, although the glycerol conversion decreased from 68 to 50%. The insights may provide new suggestions on the design of efficient support for the selective oxidation of polyols.
1. Introduction
In recent years, biodiesel has attracted increasing attention as a renewable energy source.1−3 Consequently, biodiesel production has continually increased and so has the quantity of glycerol generated as a byproduct.4 Therefore, it is important to develop means of effectively transforming glycerol into high-value-added products, such as selective oxidation,1,5 steam reforming reaction,6−8 hydrogenolysis,9,10 transesterification11 and pyrolysis.12 which could reduce the production cost of biodiesel while improving the associated economics. In particular, selective catalytic oxidation is a promising approach to the molecular transformation of biomass-based substances as well as a potential path to the conversion and utilization of glycerol.13 In fact, various high-value-added fine chemical products can be obtained through the catalytic oxidation of glycerol, including glyceric acid (GLYA),14−17 dihydroxyacetone (DHA),18−21 tartaric acid (TA),22 lactic acid (LA),23−25 and glycolic acid (GLYC).26 Among them, DHA, LA, and GLYA are currently in especially high demand. Therefore, the identification of catalysts exhibiting high selectivity for specific products and the clarification of the reaction paths associated with glycerol oxidation are of interest. The goal is to develop the targeted oxidation of glycerol to improve the selectivity of active components, although this remains highly challenging.
Previous studies have found that supported Au catalysts will only promote glycerol oxidation under alkaline conditions. Even so, the addition of alkaline substances to the reaction solution can lead to environmental pollution and also require the use of expensive equipment and product separation procedures, such that the use of supported Au catalysts is limited to a certain extent.27−29 Compared with supported Au catalysts, supported Pt catalysts tend to exhibit better catalytic activity under neutral and acidic conditions.30−36 However, the catalytic activity and product selectivity strongly depend on the structure and the status of Pt, support, and reaction conditions. Recently, it was recommended that adding alloying Pt with a second element is an effective approach to change the status of Pt and further improve its catalytic activity, for example, Sb@PtSb2/NC,16 PtRu/MCM-41,17 PtCo/MCM-41,37 Pt9Sn1/C,15 Pt-CeO2/carbon nanotube (CNT),32 Pt/ZCA-10,38 and Au–Pt.39 By introducing a second metal with Pt, the catalytic activity and selectivity of oxidation products can be effectively improved. At the same time, the structure of Pt nanoparticles is also a key factor affecting the catalytic activity and product selectivity. Zhou et al.40 used a sol-fixation method to load Pt nanoparticles having various morphologies onto carbon nanotubes (CNTs) and obtained nanoparticles having cubic, spherical, or tetrahedral shapes. To understand the relationship between the intrinsic facet structure and the catalytic property, the supported Pt nanocubes enclosed by Pt(1 0 0) facets are found to exhibit much higher catalytic activity and stability. Furthermore, the support also affects catalytic activity and product selectivity, and carbon-based materials are widely used as supports because of their superior stability.41−44 As an example, Zhan et al.45 prepared N-doped mesoporous carbon via a one-pot method and used this material to catalyze the oxidation of glycerol after loading with Pt. Under the optimized conditions, 87.2% glycerol conversion and 60.2% selectivity for TA were obtained over the Pt/N-doped mesoporous carbon catalyst. Tang et al.31 introduced Pt nanoparticles onto ZIF-67 support with subsequent calcination at high temperatures. When applied to the oxidation of glycerol without an alkaline medium, this material gave up to 61% glycerol conversion.
Metal–organic frameworks (MOFs) have certain advantages in various territories as a consequence of their precise crystal morphologies, adjustable topological structures, and ultrahigh surface areas.46−48 In addition, MOFs derived from porous carbon materials can be obtained via a pyrolysis process under an inert gas atmosphere, which inherited the structure features of MOFs precursor to a large extent. At the same time, it may also be homogeneously doped with impurities to obtain improved catalytic effects in the catalysis system. As a result, many porous materials show even better performance than the parent MOFs.49−51 Kim et al.52 successfully prepared hierarchically porous N-doped carbon (HPDC) materials, and the HPDCs prepared in this study feature physical and electrochemical advantages of hierarchically porous carbons such as high electrochemically accessible surface area, short diffusion distance, and high mass-transfer rate, thus demonstrating significantly improved ion diffusion and surface-enhanced high specific capacitance at high charge–discharge rates. They53 presented different treatments of sugarcane biomass to alter the lignocellulose compositions to obtain low-cost porous carbon as NIB’s anode; the sugarcane biomass-derived carbon displays an ultrastable capacity with almost no attenuation even after 2000 cycles. The porous structures of these substances allow doping with various N-based ligands to provide numerous active sites and greater durability. Various N donor ligands can be used for this purpose; hexamethylenetetramine (HMT), in which four N atoms lie at the corners of the tetrahedron, adopts different coordination models from the terminal to the bridge connect of the double bond and has been shown to provide a metal–HMT coordination framework.
In the present work, MOFs were synthesized from HMT (which acted as a source of N and C) and nitrate, after which the heating of these materials at high temperatures under argon generated M@nitrogen-doped porous carbon nanosheets (NPCNs) (M = Mn, Ni, Fe, Cu, Zn, Zr, Ce). Finally, Pt/M@NPCN catalysts were prepared using a colloidal deposition process. Among these materials, the Pt/Zr@NPCNs exhibited up to 77.3% selectivity for GLYA. The effects of reaction conditions on catalytic activity and selectivity for GLYA were also examined in this work and are discussed herein.
2. Results and Discussion
2.1. TG Analyses
TG analyses of the transition-metal-modified MOF precursors were carried out in a nitrogen atmosphere, and the results are shown in Figure 1. It can be seen that each sample exhibited three mass loss stages. The first stage began below 180 °C. Except for the Ce-MOF precursor, which was stable at low temperatures, each specimen exhibited varying degrees of mass loss (approximately 10%) within the range of 30–180 °C, primarily as a result of the loss of surface adsorbed water and evaporation of water associated with the decomposition of some oxygen-containing functional groups.54 The second mass loss occurred between 180 and 350 °C, during which each precursor showed a rapid mass loss in the range of 30–70%. These data suggest that the original framework of each material collapsed in association with carbonization as a consequence of the decomposition of the organic ligands. The Zn-, Fe-, and Cu-based precursors (Figure 1a–c) exhibited losses of approximately 40, 60, and 50%, respectively, while the Ni, Zr, and Mn samples showed mass losses of approximately 50, 40, and 55% upon heating to 220 °C, respectively (Figure 1d–f) and the Ce-MOFs (Figure 1g) underwent a mass loss on the order of 70% upon heating to 230 °C. During the third stage, from 350 to 600 °C, only the Fe-MOFs, Ni-MOFs, and Zr-MOFs showed a mass loss, possibly because the Fe, Ni, and Zr ions were embedded in the MOFs and formed a relatively stable structure and thus failed to decompose completely at low temperatures.
Figure 1.

Thermogravimetric analysis of MOF precursors modified with (a) Zn, (b) Fe, (c) Cu, (d) Ni, (e) Zr, (f) Mn, and (g) Ce.
2.2. X-ray Diffraction (XRD) Analyses
The XRD patterns of MOF precursors and M@NPCN samples are shown in Figure S1. Figure 2 shows the XRD patterns of the Pt/M@NPCN catalysts. After heating at a high temperature, all samples showed characteristic diffraction peaks related to modified metal elements or oxides, indicating that all seven transition metals had been successfully embedded in the porous carbon. However, the position of the diffraction peak of the support changed, and the intensity of the diffraction peak increased, indicating that the morphology of the support was changed by high-temperature calcination. After supported Pt nanoparticles, the Pt/Zn@NPCNs generated characteristic ZnO diffraction peaks at 31.7, 34.4, 36.3, and 56.5° along with the characteristic Pt peaks (PDF# 87-0640) at 39.9, 46.4, and 67.7°. These peaks corresponded to the (111), (200), and (220) crystal planes and were accompanied by a broad peak at 25° attributed to the (002) plane of carbon with a low degree of graphitization.55,56 Interestingly, the other catalysts only produced the characteristic diffraction peaks related to the corresponding metal or metal oxide, with no Pt peaks, indicating that the Pt particles on these specimens were small and highly dispersed on the surface of the support.
Figure 2.
XRD patterns of Pt/M@NPCN catalysts.
2.3. Brunauer–Emmett–Teller (BET) Surface Area and Pore Size Analyses
The surface area and pore size of a solid catalyst are important parameters affecting its performance, and the data acquired for the present materials based on adsorption analyses are shown in Figure S2. According to the International Union of Pure and Applied Chemistry classification system, each material generated a typical type IV isotherm with an obvious hysteresis loop. The Pt/Zr@NPCN and Pt/Zn@NPCN catalysts produced H4 hysteresis loops, possibly as a result of the porous structures formed by particle accumulation. These hysteresis loops confirmed the mesoporous nature of each catalyst. Table 1 shows the specific surface area, pore size, and pore volume of each catalyst. Compared with the other samples, the Pt/Zr@NPCNs and Pt/Zn@NPCNs had larger specific surface areas and pore volumes, indicating that the incorporation of different transition metals affected the specific surface area by modifying the structure of the support. According to the pore size distribution diagram (Figure S3), the pore size distributions of Pt/Zr@NPCNs, Pt/Zn@NPCNs, and Pt/Zr@NPCNs were relatively uniform, mainly concentrated at 15 nm, and the other distributions are not concentrated enough, which is consistent with the results of scanning electron microscopy (SEM). This is mainly attributed to the characteristics of the morphology of carbon nanosheets modified with different metals.
Table 1. Textural Properties, Pt Contents, and Pt 4f X-ray Photoelectron Spectroscopy (XPS) Analysis Results for Each Catalyst.
| Pt 4f |
|||||||
|---|---|---|---|---|---|---|---|
| catalysts | SBET (m2·g–1) | Dp (nm) | Vp (m3·g–1) | Pt content (wt %) | species | binding energy (eV) | relative intensity (%) |
| Pt/Zn@NPCNs | 70 | 2.64 | 0.30 | 2.54 | Pt(0) | 71.17 | 81.1 |
| Pt(II) | 71.98 | 18.9 | |||||
| Pt/Fe@NPCNs | 8 | 12.46 | 0.03 | 2.46 | Pt(0) | 70.60 | 69.0 |
| Pt(II) | 71.23 | 31.0 | |||||
| Pt/Cu@NPCNs | 4 | 6.67 | 0.01 | 2.63 | Pt(0) | 71.11 | 72.3 |
| Pt(II) | 72.77 | 27.7 | |||||
| Pt/Ni@NPCNs | 2 | 6.63 | 0.01 | 2.78 | Pt(0) | 71.08 | 80.0 |
| Pt(II) | 71.86 | 20.0 | |||||
| Pt/Zr@NPCNs | 90 | 6.77 | 0.15 | 2.75 | Pt(0) | 71.01 | 71.9 |
| Pt(II) | 71.83 | 28.1 | |||||
| Pt/Mn@NPCNs | 16 | 12.82 | 0.05 | 2.70 | Pt(0) | 71.28 | 74.5 |
| Pt(II) | 71.88 | 25.5 | |||||
| Pt/Ce@NPCNs | 4 | 12.34 | 0.01 | 2.85 | Pt(0) | 70.61 | 59.9 |
| Pt(II) | 71.31 | 40.5 | |||||
2.4. CO2 Temperature-Programmed Desorption (TPD) Analyses
Figure 3 shows the CO2-TPD curves obtained from the Pt/M@NPCN catalysts. Pt/Zn@NPCNs generated four peaks, indicating that this material had both weak and strong alkaline sites. According to the intensities of these peaks, there were significantly more moderate alkaline sites than weak or strong alkaline sites. The Pt/Cu@NPCN, Pt/Zr@NPCN, and Pt/Ce@NPCN catalysts all generated intense peaks at 370, 380, and 376 °C, respectively, showing that these samples were moderate alkaline and had more alkaline sites than the other catalysts. This difference can be attributed to the interaction between the two metals because the N atoms would be expected to provide electrons to Pt nanoparticles. This effect would promote the nucleation and growth of Pt nanoparticles on the N-doped carbon support, making the interaction between the metal and the support stronger. Pt/Fe@NPCNs and Pt/Ni@NPCNs showed weak peaks at 330 °C, indicating that these two catalysts had fewer moderate alkaline sites. Finally, Pt/Mn@NPCNs produced a wide peak at high temperatures, demonstrating that complex high alkaline sites were present on this material.
Figure 3.

CO2-TPD patterns of Pt/M@NPCN catalysts.
2.5. SEM Analyses
Figure 4 shows the morphologies of the seven different catalysts. Because of the distributions of nanoscale transition-metal particles on these materials, they have the appearance of solid sheets with smooth surfaces. It can be seen that Pt/Zn@NPCNs and Pt/Fe@NPCNs (Figure 4a,b) are mostly phospho-sheet carbon sheets; after magnification, it is found that the surface of both are relatively smooth and there are no metal particles on the surface. Pt/Ni@NPCNs and Pt/Ce@NPCNs (Figure 4c,d) are mostly 10 μm sheets with obvious stacking of carbon sheets, and the stacked carbon sheets are relatively compact. After surface magnification, carbon sheet stacking made more pores in the structure, and the stacked holes were larger. Pt/Zr@NPCNs and Pt/Cu@NPCNs (Figure 4e,f) are large lumpy flake particles on which a uniform pore size can be clearly seen. Pt/Mn@NPCNs (Figure 4f) are clearly granular, and these sheet solids were mainly composed of nanosized particles; the formation of obvious pores between the small particles was clearly visible after surface magnification. Compared with the other catalysts, the transition-metal particles on the surface of Pt/Zr@NPCNs were more densely distributed. The magnified views of the surfaces in the lower right corner of each SEM image confirm that pores were formed between the small metal particles, which are consistent with the observed physical adsorption characteristics of these specimens.
Figure 4.
SEM images of (a) Pt/Zn@NPCNs, (b) Pt/Fe@NPCNs, (c) Pt/Cu@NPCNs, (d) Pt/Ni@NPCNs, (e) Pt/Zr@NPCNs, (f) Pt/Mn@NPCNs, and (g) Pt/Ce@NPCNs.
2.6. Transmission Electron Microscopy (TEM) Analyses
Figure 5 presents the TEM images of the various catalysts along with histograms summarizing the Pt nanoparticle size distributions on the catalyst surfaces. Because the transition metals were embedded inside the supports, only the Pt nanoparticles can be observed in these images. These spherical nanoparticles were evenly dispersed on the support surfaces and tightly attached. The Nano Measurer software package was used to calculate the sizes of the Pt nanoparticles on each sample. The addition of transition metals made the support functional, and the size distributions of the Pt nanoparticles were uneven. As shown in these images, the Pt nanoparticles on the Pt/Ni@NPCNs and Pt/Zr@NPCNs were relatively small (less than 3 nm). These results are attributable to the addition of transition metals, which decreases the size of Pt nanoparticles. For this reason, the theoretical activities of these two catalysts were higher than those of the other specimens. In addition, the standard deviations of the particle size distributions in these two catalysts were smaller than those of the other materials, meaning that the Pt nanoparticles on Pt/Ni@NPCNs and Pt/Zr@NPCNs were more homogeneous. Meanwhile, TEM mapping was used to investigate the distribution of the element in the Pt/Zr@NPCN catalyst. As shown in Figure 6, the Pt, Zr, C, N, and O elements are well dispersed in the Pt/Zr@NPCN catalyst. The contents of C, Zr, and Pt were the highest, followed by N and O, which indicated that MOFs were mainly present in the form of carbon materials after calcination. Also, Zr and N were doped into carbon materials, and Pt nanoparticles were highly dispersed.
Figure 5.
TEM images and particle size distributions of (a) Pt/Zn@NPCNs, (b) Pt/Fe@NPCNs, (c) Pt/Cu@NPCNs, (d) Pt/Ni@NPCNs, (e) Pt/Zr@NPCNs, (f) Pt/Mn@NPCNs, and (g) Pt/Ce@NPCNs.
Figure 6.
TEM mapping images of the Pt/Zr@NPCN catalyst.
2.7. XPS Analyses
The compositions and chemical states of the catalyst surfaces were assessed via the peak fitting of their XPS data (Figure 7). Three types of peaks were identified in the C 1s spectra, respectively, representing the sp2 hybrid graphite TYPE C (284.4 eV), the C in the sp2 hybrid C–N bond (about 286.1 eV), and the C in the sp2 hybrid C=N bond (about 288.5 eV).57 Three types of oxygen on the catalyst surface were confirmed by O 1s spectra: lattice oxygen (529–530 eV), chemisorption oxygen (531.3–531.9 eV), and hydroxyl oxygen (532.7–533.5 eV). As can be seen from Figure S4, there is a deviation of each lattice oxygen for each catalyst, which may be related to the properties of the different metals. Deconvolution of the N 1s spectra suggested the formation of pyridinic-N (398.9 eV) and quaternary-N (400.6 eV).58 The relevant article reported that pyridinic-N can reduce the energy barrier for the adsorption energy barrier of reactants on adjacent carbon atoms, accelerate the rate-limiting first electron transfer process, and thus significantly improve the catalytic activity of the carbon surface. In addition, the quaternary-N in the graphene structure can lead to an uneven electron distribution and significantly improve the catalytic activity of the carbon surface. The introduction of N into the catalyst can enhance the interaction between Pt NPs and carbon support, making it easier to transfer electrons from N atoms to Pt NPs, and these electron-rich Pt particles are more active in glycerol oxidation.59
Figure 7.
XPS spectra of Pt/M@NPCN catalysts.
Pt existed in two forms of Pt0 and Pt2+ in all catalysts,44,60 together with the binding energy values of the Pt 4f7/2 and 4f5/2 peaks. Typically, a gap of ca. 1.4 BE is attributed to Pt0 (71.0 eV) and Pt2+ (72.4 eV), but the charge states and chemical potentials of metal nanoparticles are affected by the oxide support. We can see that there is a certain deviation of Pt2+ and Pt0 binding energies among all catalysts, indicating a higher electronic density of Pt due to charge transfer from the corresponding oxide. For the Pt/Fe@NPCN and Pt/Ce@NPCN catalysts, the spectrum obtained is composed of two photoelectron peaks characteristic of Pt0 at binding energies of 70.60 eV (Pt 4f7/2) and 73.90 eV (Pt 4f5/2) and two photoelectron peaks characteristic of the Pt oxidic form at binding energies of 71.32 eV (Pt 4f7/2) and 74.58 eV (Pt 4f5/2). These indicate that the PtCo/MCM-41(200) catalyst exhibits a greater degree of charge transfer.37,61 The Pt 4f7/2 data are summarized in Table 1 and indicate that the proportion of Pt0 in all catalysts except for Pt/Ce@NPCNs was 70%.
The peaks related to the various transition metals were fitted by a peak separation process, and the resulting binding energies were compared with standard values to ascertain the valence states. Mn on the catalyst surface was found to be present in the form of Mn2+ and Mn4+,62 while Zr was in the form of Zr4+.63 The Ce 3d spectrum showed that both Ce3+ and Ce4+ were present.64,65 Fe was primarily in the form of Fe2+ and Fe3+,66 while both Ni0 and Ni2+ were found on the Ni-based sample67 and Cu was mainly in the elemental form.68
2.8. Evaluation of the Catalytic Activity
2.8.1. Pt/M@NPCNs
The Pt/M@NPCN catalysts were used to promote the oxidation of glycerol, and the results are presented in Table 2. With the exception of Pt/Ni@NPCNs and Pt/Zr@NPCNs, these materials exhibited little or no catalytic activity. The main product obtained from Pt/Ni@NPCNs, Pt/Zr@NPCNs, Pt/Mn@NPCNs, and Pt/Ce@NPCNs was GLYA, while Pt/Zn@NPCNs gave primarily DHA. Pt/Ni@NPCNs and Pt/Zr@NPCNs provided the highest conversion values (70.1 and 81.6%) and GLYA selectivity of 63.3 and 63.1%, respectively.
Table 2. Data from the Catalytic Oxidation of Glycerol by the Pt/M@NPCN Catalystsa.
| selectivity
(%) |
||||||
|---|---|---|---|---|---|---|
| catalyst | conv. (%) | OA | LA | GLYA | DHA | TA |
| Pt/Ni@NPCNs | 70.10 | 1.10 | 10.00 | 63.30 | 20.40 | 5.10 |
| Pt/Zr@NPCNs | 63.10 | - | 4.30 | 81.60 | 14.10 | - |
| Pt/Mn@NPCNs | 13.30 | 4.70 | 28.10 | 48.40 | 18.80 | - |
| Pt/Ce@NPCNs | 12.70 | - | 3.10 | 71.30 | 25.30 | - |
| Pt/Zn@NPCNs | 0.40 | 1.30 | 22.40 | 26.30 | 50.00 | - |
| Pt/Cu@NPCNs | - | - | - | - | - | - |
| Pt/Fe@NPCNs | - | - | - | - | - | - |
Reaction conditions: glycerol in water (0.3 M, 20 mL), glycerol: Pt = 500:1 mol mol–1, 333 K, 6 h, P(O2) = 10 bar.
The catalytic results indicate that the Pt/Zr@NPCN catalyst had better catalytic performance than other Pt/M@NPCN catalysts. According to the results of the BET surface area and pore size analyses, the BET surface area and pore structure affect the catalytic activity of catalytic oxidation of glycerol. Compared with the other samples, Pt/Zr@NPCNs had the largest specific surface area and a larger pore volume, indicating that the incorporation of transition metals affected the specific surface area by modifying the structure of the support and in turn the catalytic activity of catalytic oxidation of glycerol. As discussed in previous research, the basic sites contained in the supports may accelerate the oxidation of alcohols.44,69 According to the results of Section 2.4, the Pt/Zr@NPCN catalyst generated intense peaks at 380 °C, showing that this catalyst was moderately alkaline and had more alkaline sites, which may also contribute to the conversion of glycerol, but we believe that this may not be the main reason. The TEM and XPS results indicate that the small size of Pt nanoparticles and the interaction between Pt nanoparticles and the support influence the catalytic activity. For the Pt/Zr@NPCN catalyst, the particle size distributions of Pt nanoparticles were smaller than those of the other catalysts. Also, the binding energy of Pt0 is the lowest, which is coincident with the conversion of glycerol. This difference can be attributed to the interaction between the two metals because the N atoms would be expected to provide electrons to Pt nanoparticles.
According to the above result, the excellent catalytic performance of the Pt/Zr@NPCN catalyst can be attributed to three factors. First, the high surface nitrogen concentration of this sample enhanced the transfer of electrons to both Pt nanoparticles and adsorbed oxygen, thus accelerating the activation of molecular oxygen. This effect also promoted the conversion of surface Pt active sites to more active sites to improve the activity of the catalyst. Second, the Pt/Zr@NPCN catalyst had a higher specific surface area than the other catalysts and thus was able to provide more active sites. Third, the Pt nanoparticles on this specimen were smaller.
2.8.2. Effect of Reaction Conditions on the Oxidation of Glycerol
2.8.2.1. Effect of Zr Concentration on the Oxidation of Glycerol
The Pt/Zr@NPCN catalyst, which showed the highest selectivity for GLYA, was used to study the effect of varying the modified metal concentration on the catalytic oxidation of glycerol. Samples were synthesized while varying the amount of Zr in the support, but by keeping all other conditions unchanged and heating all of the precursors at 800 °C, the supported Pt catalyst was prepared by the same method; these materials are referred to herein as Pt/Zr@NPCN-X, where X represents the Zr concentration. The results of these experiments are shown in Table S1.
It is evident that increasing the amount of Zr in the support initially increased and then decreased the conversion of glycerol. The Pt/Zr@NPCN-1.5 had the best catalytic activity, giving a glycerol conversion of 68.6%. In contrast, the selectivity of all catalysts for GLYA basically remained unchanged. XRD analyses (Figure 8) demonstrated that each of these samples contained tetragonal ZrO2 (PDF# 37-1484) and monoclinic ZrO2 (PDF# 79-1767). The intensity of the tetragonal phase characteristic diffraction peak is greatly affected by the Zr content, and the intensity of the ZrO2 peak increased and then decreased with the increase of Zr content. This effect was related to the greatly enhanced crystallinity resulting from the increase in the ZrO2 content, which reached a maximum at a Zr level of 1.5%. The CO2-TPD data presented in Figure 9 demonstrate that each catalyst generated a peak representing moderately alkaline sites, with the peak intensity first increasing and then decreasing with increasing Zr content. The peak intensity reached a maximum at a Zr concentration of 1.5%, indicating that the Pt/Zr@NPCN-1.5 catalyst had many surface alkaline sites. Meanwhile, there are CO2-TPD and NH3-TPD of Pt/Zr@NPCNs (Figure S5). It is evident that there is a moderate alkaline desorption peak at 300–500 °C and a strong acid desorption peak at 450–550 °C. Among them, most of the basic sites play a leading role, which is helpful in improving the catalytic activity.
Figure 8.

XRD patterns of the Pt/Zr@NPCN-X catalysts.
Figure 9.

CO2-TPD data for the Pt/Zr@NPCN-X catalysts.
The SEM images in Figure S5 indicate that increases in the amount of Zr changed the morphology of the catalyst, with the 1.5% specimen exhibiting a flakelike structure. Observations at higher magnification showed a high concentration of pellets and pores evenly distributed on the catalyst surface. With an increase in the Zr content, the pellet and pore size on the catalyst surface gradually increased and became larger, which was consistent with BET data (Table S1).
XPS characterization of the Pt/Zr@NPCN-X catalysts (Figure S6) demonstrated that the Pt in these materials was present as Pt0 and Pt2+. Increases in the Zr content also negatively shifted the Pt0 binding energy, confirming that the surface electron density of the Pt was increased. The N 1s spectra of the Pt@NPCN-X catalysts were analyzed, and two peaks corresponding to two different types of N were obtained at binding energies of 398 and 400 eV. These peaks were attributed to pyridinic- and graphitic-N, respectively, indicating that the high-temperature treatment of these materials generated graphite. The binding energy values were positively shifted as the Zr concentration was increased, demonstrating that nitrogen provided electrons to the metal nanoparticles and thus enhanced the interaction between these nanoparticles and the support. Fitting of the O 1s spectra indicated three types of oxygen on the surfaces of the specimens: lattice oxygen (Olatt: 529–530.0 eV), chemisorbed oxygen (Oads: 531.3–531.9 eV), and hydroxyl oxygen (Ohly: 532.7–533.5 eV). The Olatt binding energy in the case of the Pt/Zr@NPCN-1.5 catalyst was significantly higher than those observed for the other catalysts, suggesting that O interacted strongly with the other elements in the material. The original Zr 3d5/2 binding energy of 182.7 eV was shifted to the right with an increase in the Zr content in the support, possibly because of electron transfer between Pt and Zr.70
The characterization data indicating four different catalyst structures and the varying interaction between Pt and the support can possibly be attributed to the synergistic effect of a monoclinic ZrO2 phase and a tetragonal phase. The tetragonal phase promoted glycerin oxidation. However, increasing the amount of Zr in the catalyst reduced the specific surface area and produced less alkaline specimens with fewer active sites and lower activity.
2.8.2.2. Effect of Calcination Temperature on Glycerol Oxidation
In additional trials, the carbon material used as the support to prepare the Pt catalysts was calcined at 600, 700, 800, or 900 °C to generate materials referred to herein as Pt/Zr@NPCN-T, where T represents the calcination temperature. Pt/Zr@NPCN-T catalysts were applied to the oxidation of glycerol, and the results are provided in Table S2. These data demonstrate that the conversion of glycerol initially increased and then decreased with an increase in the calcination temperature. The Pt/Zr@NPCN-800 catalyst showed the highest activity and gave a conversion of 68.6%, which was significantly higher than those obtained using the Pt/Zr@NPCN-600 and Pt/Zr@NPCN-700 catalysts. When the temperature was higher than 800 °C, the catalytic activity of Pt/Zr@NPCN-900 was decreased, and the selectivity for GLYA was found to continually increase with an increase in the calcination temperature, but the rate of increase became slower at higher temperatures. ZrO2 in these materials was present as a tetragonal phase (PDF# 37-1484) following calcination at the lower temperatures of 600 and 700 °C, while processing at 800 °C produced monocline ZrO2 (PDF# 79-1767) together with tetragonal ZrO2. This change may have been a result of a variation in the crystal pattern of ZrO2 at higher temperatures. A further increase to 900 °C generated many hybrid ZrO2 peaks, while the peak related to tetragonal ZrO2 became much less intense and the monoclinic phase peak increased in intensity. A higher calcination temperature would be expected to favor better crystallization, but, because the particles may have grown slowly, pore expansion and decreases in the specific surface area could have occurred, which is in agreement with the specific surface area results (Table 2). The XRD data in Figure S8b indicate that the loading of Pt nanoparticles did not change the intensity of each diffraction peak, confirming that the Pt nanoparticles were evenly dispersed on the surface of the support.
2.8.2.3. Effect of Reaction Time on Glycerol Oxidation
The effects of reaction time on the conversion of glycerol and the selectivity for GLYA when using the Pt/Zr@NPCN-1.5 catalyst were investigated, and the results are shown in Figure 10. It is apparent that the glycerol conversion gradually increased with the extension of the reaction time, while the selectivity for GLYA remained almost unchanged after 6 h, meaning that 6 h was the most efficient reaction time.
Figure 10.

Conversion/selectivity values during the catalytic oxidation of glycerol by Pt/Zr@NPCNs as a function of time.
2.9. Recycling Performance of the Pt/Zr@NPCN Catalyst
The same Pt/Zr@NPCN-1.5 specimen was reused several times under nonalkaline conditions, and the results are presented in Figure 11. After using five times, the catalytic activity remained basically unchanged with the exception of a decrease from 68 to 50% conversion after the first use. In addition, the selectivity remained at approximately 75%, indicating that the catalyst showed good stability.
Figure 11.
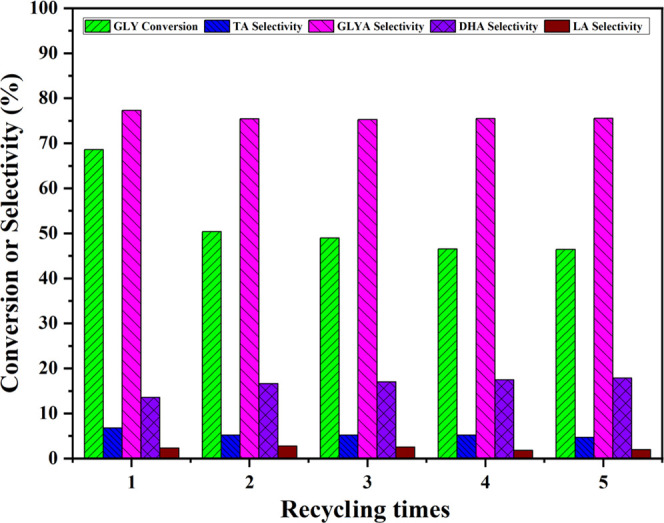
Performance of a Pt/Zr@NPCN-1.5 catalyst specimen upon repeated usage.
2.10. Catalytic Mechanism
Based on the product distributions in glycerol oxidation, the mechanism of glycerol oxidation over the Pt/Zr@NPCN catalyst under nonalkaline conditions is proposed in Scheme 1. The solid arrow line indicates that the reaction goes very fast, while the virtual arrow line reacts slowly. It can be seen that glycerol can easily be oxidized to produce GLAD; however, GLAD cannot be detected, probably due to the GLAD being rapidly oxidized to GLYA by OH oxidation and activation of the C–H bond. Meanwhile, accompanied by a possible simultaneous conversion of glycerol to DHA, DHA and GLYA are separately formed probably through two different pathways.71 The presence of TA and LA could be attributed to the accompanying side reaction of oxidation decarboxylation of GLYA, and then, TA could cleave the C–C bond to generate GLYCA. The as-formed GLYCA still undergoes the oxidation reaction to form OA. At the same time, DHA was further oxidized to HPYA and then could cleave the C–C bond to generate OA.
Scheme 1. Mechanism of Glycerol Oxidation over the Pt/Zr@NPCN Catalyst.
3. Conclusions
Transition-metal-modified NPCNs were generated at high temperatures and loaded with Pt using a colloidal deposition method. The morphological characteristics of these catalysts were studied using BET, XRD, SEM, XPS, CO2-TPD, and other techniques. These materials were then applied to the catalytic oxidation of glycerol under nonalkaline conditions. Among the seven catalysts, Pt/Ni@NPCNs and Pt/Zr@NPCNs showed the highest catalytic activity, with Pt/Zr@NPCNs exhibiting the best selectivity for GLYA. This excellent catalytic performance was attributed to the small Pt nanoparticles on these specimens, the interaction between the active Pt and the support, and the inherent properties of the support (meaning its high specific surface area, large pore volume, and small pore size). A Zr concentration of 1.5 wt %, a support calcination temperature of 800 °C, a reaction temperature of 60 °C, an oxygen pressure of 10 bar, and a reaction time of 6 h were considered optimal reaction conditions, meanwhile giving 68.8% glycerol conversion and selectivity for GLYA as high as 77.3%. The catalytic activity of Pt/Zr@NPCNs decreased after five reuse cycles, but the sample maintained good selectivity for GLYA. This work suggests that the development of MOF-derived materials may open up a way to improve the catalytic efficiency of many reactions under green and mild conditions.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant no. 21862001), the Foundation of Academic Backbone Talent Support Program of the North Minzu University (Grant no. 2019BGGZ12), the Innovative team for transforming waste cooking oil into clean energy and high-value-added chemicals (Grant no. 2022QCXTD03), the Ningxia low-grade resource high value utilization and environmental chemical integration technology innovation team project, China, and the Post-graduated Innovation Research Program of North Minzu University (Grant no. YCX 21143).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.2c05155.
XRD patterns of M-MOF precursors and M@NPCN samples (Figure S1); N2 adsorption isotherms of Pt/M@NPCN catalysts (Figure S2); aperture distribution curve of Pt/M@NPCNs (Figure S3); XPS spectra of O 1s for Pt/M@NPCNs (Figure S4); SEM images of the Pt/Zr@NPCN-X catalysts (Figure S5); XPS data obtained from the Pt/Zr@NPCN-x catalysts (Figure S6); CO2-TPD and NH3-TPD of the Pt/Zr@NPCN catalyst (Figure S7); XRD patterns of supports with and without Pt prepared at different calcination temperatures (Figure S8); specific surface areas, pore sizes, pore volumes, and glycerol oxidation performance data for Pt/Zr@NPCN-X specimens (Table S1); and effect of calcination temperature on glycerol oxidation using the Pt/Zr@NPCN-T catalyst (Table S2) (PDF)
Author Contributions
K.Y.: Project administration and writing—review and editing. Z.C.: Writing—original draft. L.J.: Data curation and formal analysis. L.H.: Visualization. Y.H.: Funding acquisition.
The authors declare no competing financial interest.
Notes
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Supplementary Material
References
- Walgode P. M.; Faria R. P.; Rodrigues A. E. A review of aerobic glycerol oxidation processes using heterogeneous catalysts: a sustainable pathway for the production of dihydroxyacetone. Catal. Rev. 2021, 63, 422–511. 10.1080/01614940.2020.1747253. [DOI] [Google Scholar]
- Talebian-Kiakalaieh A.; Amin N. A. S.; Najaafi N.; Tarighi S. A review on the catalytic acetalization of bio-renewable glycerol to fuel additives. Front. Chem. 2018, 6, 573. 10.3389/fchem.2018.00573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L.; Cheung C. S.; Ning Z. Effects of biodiesel-ethanol and biodiesel-butanol blends on the combustion, performance and emissions of a diesel engine. Energy 2018, 155, 957–970. 10.1016/j.energy.2018.05.049. [DOI] [Google Scholar]
- Behr A.; Eilting J.; Irawadi K.; Leschinski J.; Lindner F. Improved Utilisation of Renewable Resources: New Important Derivatives of Glycerol. Green Chem. 2008, 10, 13–30. 10.1039/B710561D. [DOI] [Google Scholar]
- He Z. Y.; Ning X. M.; Yang G. X.; Wang H. J.; Cao Y. H.; Peng F.; Yu H. Selective oxidation of glycerol over supported noble metal catalysts. Catal. Today 2021, 365, 162–171. 10.1016/j.cattod.2020.04.019. [DOI] [Google Scholar]
- Moreira R.; Bimbela F.; Gandía L. M.; Ferreira A.; Sánchez J. L.; Portugal A. Oxidative steam reforming of glycerol. A review. Renewable Sustainable Energy Rev. 2021, 148, 111299 10.1016/j.rser.2021.111299. [DOI] [Google Scholar]
- Charisiou N. D.; Siakavelas G. I.; Papageridis K. N.; Motta D.; Dimitratos N.; Sebastian V.; Polychronopoulou K.; Goula M. A.; et al. The ect of noble metal (M: Ir, Pt, Pd) on M/Ce2O3--Al2O3 catalysts for hydrogen production via the steam reforming of glycerol. Catalysts 2020, 10, 790. 10.3390/catal10070790. [DOI] [Google Scholar]
- Charisiou N. D.; Siakavelas G. I.; Dou B.; Sebastian V.; Hinder S. J.; Baker M. A.; Polychronopoulou K.; Goula M. A. Nickel supported on AlCeO3 as a highly selective and stable catalyst for hydrogen production via the glycerol steam reforming reaction. Catalysts 2019, 9, 411. 10.3390/catal9050411. [DOI] [Google Scholar]
- Wu F. L.; Jiang H. F.; Zhu X. H.; Lu R.; Shi L.; Lu F. Effect of tungsten species on selective hydrogenolysis of glycerol to 1,3-propanediol. ChemSusChem 2021, 14, 569–581. 10.1002/cssc.202002405. [DOI] [PubMed] [Google Scholar]
- Mazarío J.; Concepción P.; Ventura M.; Domine M. E. Continuous catalytic process for the selective dehydration of glycerol over Cu-based mixed oxide. J. Catal. 2020, 385, 160–175. 10.1016/j.jcat.2020.03.010. [DOI] [Google Scholar]
- Vávra A.; Hajek M.; Kocian D. The influence of vegetable oils composition on separation of transesterification products, especially quality of glycerol. Renewable Energy 2021, 176, 262–268. 10.1016/j.renene.2021.05.050. [DOI] [Google Scholar]
- He S.; Muizebelt I.; Heeres A.; Schenk N. J.; Blees R.; Heeres H. J. Catalytic pyrolysis of crude glycerol over shaped ZSM-5/bentonite catalysts for bio-BTX synthesis. Appl. Catal., B 2018, 235, 45–55. 10.1016/j.apcatb.2018.04.047. [DOI] [Google Scholar]
- Katryniok B.; Hiroshi K.; Elzbieta S.; Jean-Sébastien G.; Pascal F.; Mickaël C.; Rémy D.; Naoki M.; Sébastien P.; Franck D. Selective catalytic oxidation of glycerol: perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. 10.1039/c1gc15320j. [DOI] [Google Scholar]
- Silvio C.; Paul M.; Peter J.; Ken G.; Graham J. H. Selective oxidation of glycerol to glyceric acid using a gold catalyst in aqueous sodium hydroxide. Chem. Commun. 2002, 7, 696–697. [DOI] [PubMed] [Google Scholar]
- Dou J.; Zhang B. W.; Liu H.; Hong J. D.; Yin S. M.; Huang Y. Z.; Xu R. Carbon supported Pt9Sn1 nanoparticles as an efficient nanocatalyst for glycerol oxidation. Appl. Catal., B 2016, 180, 78–85. 10.1016/j.apcatb.2015.06.007. [DOI] [Google Scholar]
- Yang L. H.; He T. Q.; Lai C. J.; Chen P.; Hou Z. Y. Selective oxidation of glycerol with oxygen in base-free solution over N-doped-carbon-supported Sb@PtSb2 hybrid. Chin. J. Catal. 2020, 41, 494–502. 10.1016/S1872-2067(19)63476-5. [DOI] [Google Scholar]
- Yan H.; Yao S.; Yin B.; Liang W.; Jin X.; Feng X.; Liu Y. B.; Chen X. B.; Yang C. H. Synergistic effects of bimetallic PtRu/MCM-41 nanocatalysts for glycerol oxidation in base-free medium: Structure and electronic coupling dependent activity. Appl. Catal., B 2019, 259, 118070 10.1016/j.apcatb.2019.118070. [DOI] [Google Scholar]
- Liu S. S.; Sun K. Q.; Xu B. Q. Specific selectivity of Au-catalyzed oxidation of glycerol and other C3-polyols in water without the presence of a base. ACS Catal. 2014, 4, 2226–2230. 10.1021/cs5005568. [DOI] [Google Scholar]
- Ke Y. H.; Wang X.; Qin H. Y.; Liu H.; Yuan H.; Liu; C L.; Dong W. S. Cu-Al composite oxides: a highly efficient support for the selective oxidation of glycerol to 1, 3-dihydroxyacetone. New J. Chem. 2020, 44, 18173–18184. 10.1039/D0NJ02967J. [DOI] [Google Scholar]
- An Z.; Ma H. H.; Han H. B.; Huang Z. Y.; Jiang Y. T.; Wang W. L.; Zhu Y. R.; Song H. Y.; Shu X.; Xiang X.; He J. Insights into the multiple synergies of supports in the selective oxidation of glycerol to dihydroxyacetone: layered double hydroxide supported Au. ACS Catal. 2020, 10, 12437–12453. 10.1021/acscatal.0c02844. [DOI] [Google Scholar]
- Wang Y. X.; Pu Y. F.; Yuan D. P.; Luo J.; Li F.; Xiao F. K.; Zhao N. Selective oxidation of glycerol to dihydroxyacetone over Au/CuxZr1-xOy catalysts in base-free conditions. ACS Appl. Mater. Interfaces 2019, 11, 44058–44068. 10.1021/acsami.9b12886. [DOI] [PubMed] [Google Scholar]
- Zhan T.; Liu W. B.; Teng J. J.; Yue C. C.; Li D. H.; Wang S. H.; Tan H. Selective oxidation of glycerol to tartronic acid over Pt/N-doped mesoporous carbon with extra framework magnesium catalysts under base-free condition. Chem. Commun. 2019, 55, 2620–2623. 10.1039/C8CC10273B. [DOI] [PubMed] [Google Scholar]
- Dusselier M.; Pieter V. W.; Annelies D.; Ekaterina M.; Bert F. S. Lactic acid as a platform chemical in the biobased economy: the role of chemocatalysis. Energy Environ. Sci. 2013, 6, 1415–1442. 10.1039/c3ee00069a. [DOI] [Google Scholar]
- Li S.; Deng W. P.; Li Y. Y.; Zhang Q. H.; Wang Y. Catalytic conversion of cellulose-based biomass and glycerol to lactic acid. J. Energy Chem. 2019, 32, 138–151. 10.1016/j.jechem.2018.07.012. [DOI] [Google Scholar]
- Xiu Z. X.; Wang H. Y.; Cai C. L.; Li C. Z.; Yan L.; Wang C. G.; Li W. Z.; Xin H. S.; Zhu C. H.; Zhang Q.; et al. Ultrafast glycerol conversion to lactic acid over magnetically recoverable Ni-NiOx@C catalysts. Ind. Eng. Chem. Res. 2020, 59, 9912–9925. 10.1021/acs.iecr.0c01145. [DOI] [Google Scholar]
- Gil S.; Nicolás C.; Amaya R.; José L. V.; Luz S. S. Optimization of the synthesis procedure of microparticles containing gold for the selective oxidation of glycerol. Appl. Catal., A 2014, 472, 11–20. 10.1016/j.apcata.2013.12.008. [DOI] [Google Scholar]
- Yuan Z. F.; Gao Z. K.; Xu B. Q. Acid-base property of the supporting material controls the selectivity of Au catalyst for glycerol oxidation in base-free water. Chin. J. Catal. 2015, 36, 1543–1551. 10.1016/S1872-2067(15)60936-6. [DOI] [Google Scholar]
- Villa A.; Sebastiano C.; Khaled M H. M.; Nikolaos D.; Floriana V.; Maela M.; Wilm J.; Michael B.; Graham J. H.; Laura P. Tailoring the selectivity of glycerol oxidation by tuning the acid-base properties of Au catalysts. Catal. Sci. Technol. 2015, 5, 1126–1132. 10.1039/C4CY01246A. [DOI] [Google Scholar]
- Porta F.; Laura P. Selective oxidation of glycerol to sodium glycerate with gold-on-carbon catalyst: an insight into reaction selectivity. J. Catal. 2004, 224, 397–403. 10.1016/j.jcat.2004.03.009. [DOI] [Google Scholar]
- Yan H.; Qin H. S.; Feng X.; Jin X.; Liang W.; Sheng N.; Zhu C.; Wang H. M.; Yin B.; Liu Y. B.; et al. Synergistic Pt/MgO/SBA-15 nanocatalysts for glycerol oxidation in base-free medium: Catalyst design and mechanistic study. J. Catal. 2019, 370, 434–446. 10.1016/j.jcat.2019.01.015. [DOI] [Google Scholar]
- Tang T.; Wang Y. N.; Dong W. S.; Liu C. L.; Xu C. L. Reusable and active Pt@Co-NC catalysts for oxidation of glycerol. Renewable Energy 2020, 153, 472–479. 10.1016/j.renene.2020.02.029. [DOI] [Google Scholar]
- Zhang X. Q.; Zhou D.; Wang X. J.; Zhou J.; Li J. F.; Zhang M. K.; Shen Y. H.; Chu H. B.; Qu Y. Q. Overcoming the deactivation of Pt/CNT by introducing CeO2 for selective base-free glycerol-to-glyceric acid oxidation. ACS Catal. 2020, 10, 3832–3837. 10.1021/acscatal.9b05559. [DOI] [Google Scholar]
- El Roz A.; Pascal F.; Franck D.; Mickael C. Glycerol to glyceraldehyde oxidation reaction over Pt-based catalysts under base-free conditions. Front. Chem. 2019, 7, 156. 10.3389/fchem.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. S.; Qi P. Y.; Chen J.; Yuan Y. Z. Platinum nanoparticles supported on N-doped carbon nanotubes for the selective oxidation of glycerol to glyceric acid in a base-free aqueous solution. RSC Adv. 2015, 5, 31566–31574. 10.1039/C5RA02112J. [DOI] [Google Scholar]
- Tsuji A.; Kasanneni T. V. R.; Shun N.; Atsushi T.; Kohki E. Selective oxidation of glycerol by using a hydrotalcite-supported platinum catalyst under atmospheric oxygen pressure in water. ChemSusChem 2011, 4, 542. 10.1002/cssc.201000359. [DOI] [PubMed] [Google Scholar]
- Liang D.; Gao J.; Sun H.; Chen P.; Hou Z. Y.; Zheng X. M. Selective oxidation of glycerol with oxygen in a base-free aqueous solution over MWNTs supported Pt catalysts. Applied Catal., B 2011, 106, 423–432. 10.1016/j.apcatb.2011.05.050. [DOI] [Google Scholar]
- Yan H.; Qin H. S.; Liang W.; Jin X.; Zhang Y. S.; Feng X.; Liu Y. B.; Chen X. B.; Yang C. H. Enhanced performances of bimetallic PtCo/MCM-41 catalysts for glycerol oxidation in base-free medium. Catal. Sci. Technol. 2019, 9, 4909–4919. [Google Scholar]
- Han Z. Y.; Xie R. F.; Song Y. H.; Fan G. L.; Yang L.; Li F. Efficient and stable platinum nanocatalysts supported over Ca-doped ZnAl2O4 spinels for base-free selective oxidation of glycerol to glyceric acid. Mol. Catal. 2019, 477, 110559 10.1016/j.mcat.2019.110559. [DOI] [Google Scholar]
- Villa A.; Vindigni F.; Manzoli M.; Nikolaos D.; Floriana V.; Maela M.; Wilm J.; Michael B.; Graham J. H.; Laura P. Tailoring the selectivity of glycerol oxidation by tuning the acid–base properties of Au catalysts. Catal. Sci. Technol. 2015, 5, 1126–1132. 10.1039/C4CY01246A. [DOI] [Google Scholar]
- Zhou J.; Hu J. H.; Zhang X. Q.; Li J. F.; Jiang K. H.; Liu Y. J.; Zhao; G H.; Wang X. J.; Chu H. B. Facet effect of Pt nanocrystals on catalytical properties toward glycerol oxidation reaction. J. Catal. 2020, 381, 434–442. 10.1016/j.jcat.2019.11.019. [DOI] [Google Scholar]
- Gao J.; Liang D.; Chen P.; Hou Z. Y.; Zheng X. M. Oxidation of Glycerol with Oxygen in a Base-free Aqueous Solution over Pt/AC and Pt/MWNTs Catalysts. Catal. Lett. 2009, 130, 185–191. 10.1007/s10562-009-9849-6. [DOI] [Google Scholar]
- Zhang M.; Shi J.; Sun Y. Y.; Ning W. S.; Hou Z. Y. Selective oxidation of glycerol over nitrogen-doped carbon nanotubes supported platinum catalyst in base-free solution. Catal. Commun. 2015, 70, 72–76. 10.1016/j.catcom.2015.08.002. [DOI] [Google Scholar]
- Yang L. H.; Li X. W.; Sun Y. Y.; Yue; L H.; Fu X. Y.; Lu X. Y.; Hou Z. Y. Selective oxidation of glycerol in base-free conditions over N-doped carbon film coated carbon supported Pt catalysts. Catal. Commun. 2017, 101, 107–110. 10.1016/j.catcom.2017.08.008. [DOI] [Google Scholar]
- Wang F. F.; Shao S.; Liu C. L.; Xu C. L.; Yang R. Z.; Dong W. S. Selective oxidation of glycerol over Pt supported on mesoporous carbon nitride in base-free aqueous solution. Chem. Eng. J. 2015, 264, 336–343. 10.1016/j.cej.2014.11.115. [DOI] [Google Scholar]
- Zhan T.; Liu W. B.; Teng J. J.; Yue C. C.; Li D. H.; Wang S. H.; Tan H. Selective oxidation of glycerol to tartronic acid over Pt/N-doped mesoporous carbon with extra framework magnesium catalysts under base-free conditions. Chem. Commun. 2019, 55, 2620–2623. 10.1039/C8CC10273B. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Astruc D. State of the art and prospects in Metal–Organic Framework (MOF)-based and MOF-derived nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. 10.1021/acs.chemrev.9b00223. [DOI] [PubMed] [Google Scholar]
- Kim M.; Fernando J. F. S.; Wang J.; Nanjundan A. K.; Ashok K. N.; Na J.; Jongbeom N.; Hossain M. S. A.; Md S. A. H.; Nara H.; Hiroki N.; Martin D.; Darren M.; Sugahara Y.; Yoshiyuki S.; Golberg D.; Dmitri G. Efficient lithium-ion storage using a heterostructured porous carbon framework and its in situ transmission electron microscopy study. Chem. Commun. 2022, 58, 863–866. 10.1039/D1CC05298E. [DOI] [PubMed] [Google Scholar]
- Kim M.; Xu X. T.; Xin R. J.; Jacob E.; Aditya A.; Jeonghun K.; Teahoon P.; Ashok K. N.; Waleed A. E.; Jin W. Y.; et al. KOH-activated hollow ZIF-8 derived porous carbon: nanoarchitectured control for upgraded capacitive deionization and supercapacitor. ACS Appl. Mater. Interfaces 2021, 13, 52034–52043. 10.1021/acsami.1c09107. [DOI] [PubMed] [Google Scholar]
- Chen Y. Z.; Zhang R.; Jiao L.; Jiang H. L. Metal–Organic Framework-derived porous materials for catalysis. Coord. Chem. Rev. 2018, 362, 1–23. 10.1016/j.ccr.2018.02.008. [DOI] [Google Scholar]
- Zhu Q. L.; Xia W.; Akita T.; Zou R. Q.; Xu Q. Metal–Organic Framework–derived honeycomb-like open porous nanostructures as precious-metal-free catalysts for highly efficient oxygen electroreduction. Adv. Mater. 2016, 28, 6391–6398. 10.1002/adma.201600979. [DOI] [PubMed] [Google Scholar]
- Cao W.; Luo W. H.; Ge H. G.; Su Y.; Wang A. Q.; Zhang T. UiO-66 derived Ru/ZrO2@C as a highly stable catalyst for hydrogenation of levulinic acid to γ-valerolactone. Green Chem. 2017, 19, 2201–2211. 10.1039/C7GC00512A. [DOI] [Google Scholar]
- Kim M.; Park T.; Wang C.; Tang J.; Lim H.; Hossain M. S. A.; Md S. A. H.; Konarova M.; Muxina K.; Yi J. W.; Jin W. Y.; Na J.; Jongbeom N.; Kim J.; Jeonghun K. Tailored nanoarchitecturing of microporous ZIF-8 to hierarchically porous double-shell carbons and their intrinsic electrochemical property. ACS Appl. Mater. Interfaces 2020, 12, 34065–34073. 10.1021/acsami.0c07467. [DOI] [PubMed] [Google Scholar]
- Kim M.; Fernando J. F. S.; Li Z.; Azhar A.; Aditya A.; Xin R. J.; Darren M.; Ashok K. N.; Dmitri V. G.; Yusuke Y.; et al. Ultra-stable sodium ion storage of biomass porous carbon derived from sugarcane. Chem. Eng. J. 2022, 445, 136344 10.1016/j.cej.2022.136344. [DOI] [Google Scholar]
- Wang Y.; Zhang J.; Zhang Q. Y. Preparation and adsorption properties of ZIF-8 based porous carbon. Chem. Ind. Eng. Prog. 2017, 36, 299–304. [Google Scholar]
- Yan J.; Liu J. P.; Fan Z. J.; Wei T.; Zhang L. J. High-performance supercapacitor electrodes based on highly corrugated graphene sheets. Carbon 2012, 50, 2179–2188. 10.1016/j.carbon.2012.01.028. [DOI] [Google Scholar]
- Zhang P.; Sun F.; Xiang Z. H.; Shen Z. G.; Jimmy Y.; Cao D. P. ZIF-derived in situ nitrogen-doped porous carbons as efficient metal-free electrocatalysts for oxygen reduction reaction. Energy Environ. Sci. 2014, 7, 442–450. 10.1039/C3EE42799D. [DOI] [Google Scholar]
- Zhai Y. L.; Zhu C. Z.; Wang E.; Dong S. J. Energetic carbon-based hybrids: green and facile synthesis from soy milk and extraordinary electrocatalytic activity towards ORR. Nanoscale 2014, 6, 2964–2970. 10.1039/c3nr05357a. [DOI] [PubMed] [Google Scholar]
- Yan H.; Yao S.; Zhao S. M.; Liu M. Y.; Zhang W. X.; Zhou X.; Zhang G. Y.; Jin X.; Liu Y. B.; Feng X.; et al. Insight into the basic strength-dependent catalytic performance in aqueous phase oxidation of glycerol to glyceric acid. Chem. Eng. Sci. 2021, 230, 116191 10.1016/j.ces.2020.116191. [DOI] [Google Scholar]
- Wei J.; Hu Y. X.; Liang Y.; Kong B.; Zhang J.; Song C.; Bao Q. L.; George S.; Jiang S. P.; Wang H. T. Nitrogen-doped nanoporous carbon/graphene nano-sandwiches: synthesis and application for efficient oxygen reduction. Adv. Funct. Mater. 2015, 25, 5768–5777. 10.1002/adfm.201502311. [DOI] [Google Scholar]
- Yang L.; He T. Q.; Lai C. J.; Chen P.; Hou Z. Y. Selective oxidation of glycerol with oxygen in base-free solution over N-doped-carbon-supported Sb@ PtSb2 hybrid. Chin. J. Catal. 2020, 41, 494–502. 10.1016/S1872-2067(19)63476-5. [DOI] [Google Scholar]
- El Roz A.; Fongarland P.; Dumeignil F.; Capron M. Glycerol to glyceraldehyde oxidation reaction over Pt-based catalysts under base-free conditions. Front. Chem. 2019, 7, 156. 10.3389/fchem.2019.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao X. J.; Ma K. L.; Zou W. X.; He; S G.; An J. B.; Yang F. M.; Dong L. Influence of preparation methods on the physicochemical properties and catalytic performance of MnOx-CeO2 catalysts for NH3-SCR at low temperature. Chin. J. Catal. 2017, 38, 146–159. 10.1016/S1872-2067(16)62572-X. [DOI] [Google Scholar]
- Zhang X.; Qiao J.; Liu C.; Wang F. L.; Jiang Y. Y.; Cui P.; Wang Q.; Wang Z.; Wu L. L.; Liu J. R. A MOF-derived ZrO2/C nanocomposite for efficient electromagnetic wave absorption. Inorg. Chem. Front. 2020, 7, 385–393. 10.1039/C9QI01259A. [DOI] [Google Scholar]
- Kundakovic L.; Flytzani-Stephanopoulos M. Reduction characteristics of copper oxide in cerium and zirconium oxide systems. Appl. Catal., A 1998, 171, 13–29. 10.1016/S0926-860X(98)00056-8. [DOI] [Google Scholar]
- Purushothaman R. K. P.; van Haveren J.; van Es D. S.; Melian-Cabrera I.; Meeldijk J. D.; Heeres J. H. An efficient one pot conversion of glycerol to lactic acid using bimetallic gold-platinum catalysts on a nanocrystalline CeO2 support. Appl. Catal., B 2014, 147, 92–100. 10.1016/j.apcatb.2013.07.068. [DOI] [Google Scholar]
- Teng Y.; Wang X. D.; Liao J. F.; Li W. G.; Chen H. Y.; Dong Y. J.; Kuang D. B. Atomically thin defect-rich Fe–Mn–O hybrid nanosheets as high efficient electrocatalyst for water oxidation. Adv. Funct. Mater. 2018, 28, 1802463 10.1002/adfm.201802463. [DOI] [Google Scholar]
- Robert R.; Karolina C.; Karolina W. Modification of Ni/ZrO2 catalyst by selected rare earth metals as a promising way for increase in the efficiency of thermocatalytic conversion of lignocellulosic biomass to hydrogen-rich gas. Fuel 2020, 276, 118110 10.1016/j.fuel.2020.118110. [DOI] [Google Scholar]
- Tian L.; Zhang Y.; Ma Y.; Zhu X. D.; Tang B. Effect of Cu content and ion beam bombardment on microstructure and mechanical properties of Cr-Cu-N films. Surf. Coat. Technol. 2013, 228, S495–S498. 10.1016/j.surfcoat.2012.04.088. [DOI] [Google Scholar]
- Villa A.; Chan-Thaw C. E.; Veith G. M.; More K. L.; Ferri D.; Prati L. Au on nanosized NiO: a cooperative effect between Au and nanosized NiO in the base-free alcohol oxidation. ChemCatChem 2011, 3, 1612–1618. 10.1002/cctc.201100161. [DOI] [Google Scholar]
- Wang Y. X.; Yuan D. P.; Luo J.; Pu Y. F.; Li F.; Xiao F. K.; Zhao N. The effects of calcination temperature of support on Au/CuO-ZrO2 catalysts for oxidation of glycerol to dihydroxyacetone. J. Colloid Interface Sci. 2020, 560, 130–137. 10.1016/j.jcis.2019.10.017. [DOI] [PubMed] [Google Scholar]
- Zope B. N.; Hibbitts D. D.; Neurock M.; Davis R. J. Reactivity of the gold/water interface during selective oxidation catalysis. Science 2010, 330, 74–78. 10.1126/science.1195055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.








