Abstract
Background and Objectives
The specificity of novel blood biomarkers for multiple sclerosis (MS)–related neurodegeneration is unclear because neurodegeneration also occurs during normal aging. To understand which aspects of neurodegeneration the serum biomarkers neurofilament light (sNfL), serum glial fibrillary acidic protein (sGFAP), and serum contactin-1 (sCNTN1) reflect, we here explore their cross-sectional association with disability outcome measures and MRI volumes in a unique cohort of people with MS (PwMS) of the same age.
Methods
sNfL, sGFAP (both singe-molecule array technology) and sCNTN1 (Luminex) were measured in serum samples of 288 PwMS and 125 healthy controls (HCs) of the Project Y cohort, a population-based cross-sectional study of PwMS born in the Netherlands in 1966 and age-matched HC.
Results
sNfL (9.83 pg/mL [interquartile range {IQR}: 7.8–12.0]) and sGFAP (63.7 pg/mL [IQR: 48.5–84.5]) were higher in PwMS compared with HC (sNfL: 8.8 pg/mL [IQR: 7.0–10.5]; sGFAP: 51.7 pg/mL [IQR: 40.1–68.3]) (p < 0.001), whereas contactin-1 (7,461.3 pg/mL [IQR: 5,951.8–9,488.6]) did not significantly differ between PwMS compared with HC (7,891.2 pg/mL [IQR: 6,120.0–10,265.8]) (p = 0.068). sNfL and sGFAP levels were 1.2-fold higher in secondary progressive patients (SPMS) compared with relapsing remitting patients (p = 0.009 and p = 0.043). Stratified by MS subtype, no relations were seen for CNTN1, whereas sNfL and sGFAP correlated with the Expanded Disability Status Scale (ρ = 0.43 and ρ = 0.39), Nine-Hole Peg Test, Timed 25-Foot Walk Test, and Symbol Digit Modalities Test (average ρ = 0.38) only in patients with SPMS. Parallel to these clinical findings, correlations were only found for sNfL and sGFAP with MRI volumes. The strongest correlations were observed between sNfL and thalamic volume (ρ = −0.52) and between sGFAP with deep gray matter volume (ρ = − 0.56) in primary progressive patients.
Discussion
In our cohort of patients of the same age, we report consistent correlations of sNfL and sGFAP with a range of metrics, especially in progressive MS, whereas contactin-1 was not related to clinical or MRI measures. This demonstrates the potential of sNfL and sGFAP as complementary biomarkers of neurodegeneration, reflected by disability, in progressive MS.
Multiple sclerosis (MS) is an inflammatory demyelinating and neurodegenerative disease of the CNS, accompanied by an unpredictable risk of disability.1 Although inflammatory demyelinating white matter lesions are associated with relapses in people with MS (PwMS), neurodegeneration has been considered as the driving force of disability progression.2,3 Although neurodegeneration progresses more rapidly in PwMS compared with healthy controls (HCs), neurodegeneration also occurs during physiologic aging with a mean rate of brain atrophy of −0.3% per year in HC.3-5
Body fluid biomarkers have been subject of research in an attempt to quantify, monitor, and predict neurodegeneration.6 Therapeutic options targeting neurodegeneration and herewith disease progression especially for progressive PwMS are still scarce, and therefore, particularly for progressive MS, there is an urgent need to develop novel treatments.7,8 The identification of reliable body fluid biomarkers for neurodegeneration could therefore facilitate trial design by providing new outcome measures.
Three candidate CSF and blood biomarkers for neurodegeneration in MS are neurofilament light (NfL) and the less well characterized but promising biomarkers glial fibrillary acidic protein (GFAP) and contactin-1 (CNTN1). NfL, GFAP, and CNTN1 are biomarkers of interest because they reflect different aspects of CNS-related pathophysiologic processes and can be reliably measured in CSF and blood.9 NfL, a cytoskeletal protein exclusively found in neurons, has consistently been shown to be a biomarker for active inflammation-induced axonal damage and treatment response in MS.10 Although several studies have reported that serum NfL levels obtained at baseline are predictive of disability and brain volume change later in the disease course,11-13 its utility as a marker of the different CNS-related pathophysiologic processes resulting in neurodegeneration—reflected by disability—in progressive MS has yet to be confirmed.7,8
The second biomarker of interest, GFAP, is a primary component of the intermediate filaments found in the astrocyte cytoskeleton and is a marker of astrocyte activation.14 Previous studies reported higher GFAP concentrations in progressive PwMS compared with relapsing remitting (RR) patients with MS and GFAP correlated with disease severity defined by clinical and MRI metrics, especially in the progressive subtypes.15-17 CNTN1, a cell adhesion molecule expressed in paranodal axonal domains, mediates neuron-glia communication in central myelin and is therefore essential for CNS myelination.18 It is hypothesized to be reduced when myelin degenerates. We recently observed reduced CSF and serum CNTN1 concentrations in patients with RRMS and secondary progressive (SP) MS compared with HC.19,20
One of the most important hurdles when studying NfL, GFAP, and CNTN1 as MS biomarkers is their correlation with age, because concentrations and brain volumes change because of physiologic aging.8,21-23 Studies have demonstrated several solutions to bypass the problem of age as confounding factor, such as using age-dependent percentile categories with HC as a reference group. Because disease progression in MS is strongly related to disease duration, which in turn is strongly related to patients' age, controlling for age has its drawbacks.21 To date, it remains unclear how specific novel blood biomarkers are for MS-related neurodegeneration, independent of age.
Therefore, we investigate a well-characterized birth year cohort of 288 PwMS all born in 1966 in the Netherlands and 125 age matched HC.24 The aim of this study was to explore the cross-sectional association between serum NfL, GFAP, and CNTN1 and (1) disability outcome measures and (2) brain and spinal cord volumes to investigate which aspects of neurodegeneration these 3 candidate markers reflect, independent of age.
Methods
Study Population
Our study population consisted of PwMS and HC from the cohort study Project Y (Netherlands Trial Register NL6362), a population-based cross-sectional birth year cohort of PwMS and HC, which aimed to include all PwMS (as defined by the 2017 McDonald Criteria)25 born in the Netherlands in 1966. In addition, 125 age and sex-matched HC born in between 1965 and 1967 in the Netherlands were included. All participants with available serum samples were selected for the purpose of this study (total sampled population n = 414; PwMS n = 289; HC n = 125). The study design has been previously described in detail.26
Standard Protocol Approvals, Registrations, and Patient Consents
The Project Y protocol was approved by the Medical Ethical Committee of the Amsterdam UMC, location VUMC. Written informed consent was obtained from all participants at inclusion.
Serum NfL, GFAP, and CNTN-1 Analyses
All examinations were performed during a 1-day study visit. Blood was collected via standard venipuncture, centrifuged within 2 hours at 1200g, 10 minutes room temperature, and serum aliquots were stored at −80°C until analysis. All analyses were performed at the neurochemistry laboratory of the Department of Clinical Chemistry (Amsterdam UMC, location VUmc). A single-molecule array assay was used to measure serum biomarkers neurofilament light (sNfL) and serum glial fibrillary acidic protein (sGFAP) levels on a HD-X analyser (Quanterix, Billerica, MA) according to the manufacturer's instructions, as described previously.27 For CNTN1 measurements an in-house validated Luminex assay was used, which is described in detail elsewhere.20 Serum GFAP and CNTN1 concentrations were measured in duplicate and laboratory personnel was blinded to the clinical data. The intra-assay coefficients of variation (CV) of sGFAP (average 5.0%) and serum contactin-1 (sCNTN1) (average 2.7%) were well below the accepted threshold of <20% and <15%, respectively. The inter-assay CV was calculated using the average CV of low, medium, and high concentrations; the inter-assay CV of NfL (7.0%) sGFAP (average 8.6%) and CNTN1 (average 8.7%) were below the accepted threshold of <20% and <15%, respectively.
MRI Scans
Patients were scanned on a 3T full body MRI scanner (whereas; GE, Milwaukee, WI), including three-dimensional (3D) T1-weighted fast-spoiled gradient echo for volumetric measurements and 3D-fluid-attenuated inversion recovery for lesion detection. Normalized brain volumes were measured on lesion-filled T1 images using FAST (part of SIENAX) and FIRST to segment total gray matter and deep gray matter (NDGMV) including thalamic volume, respectively. Normalized total brain volume (NBV) and normalized white matter volume (NWMV) were also measured with SIENAX. Normalized cerebellar gray matter volumes were calculated using a previously described method.28 All brain volumes were normalized for head size using the V-scaling factor of SIENAX. Mean upper cervical cord area (MUCCA) was measured on lesion-filled 3DT1 images using SCT-DeepSeg. Full information on the MRI protocol and scan parameters have been described previously.24
Clinical Assessment
Clinical assessments were performed in all PwMS during the 1-day study visit and included, among others, a comprehensive interview regarding (MS) disease history, Expanded Disability Status Scale (EDSS), the Timed 25-Foot Walk test (T25FWT), the Nine-Hole Peg Test (9HPT) and the Symbol Digit Modalities Test (SDMT).24 Data on disease duration, total amount of relapses throughout the disease course, disease-modifying therapy (DMT), and time to EDSS ≥6 were collected from the interview and by reviewing the patients' medical record. The T25FWT was performed twice, and the 9HPT twice for each hand during the study visit; the average time of the trials was used in the analyses. According to international guidelines, inability to perform the T25FWT or 9HPT was scored as 180 seconds and 300 seconds, respectively. All patients unable to perform the T25FWT (n = 21) or 9HPT (n = 8) were excluded from correlation analyses. For group comparisons, PwMS were classified into 4 different EDSS groups: 0–2.5 (n = 77); 3.0–4.5 (n = 142); 5.0–6.5 (n = 51), and ≥7.0 (n = 18).
Statistical Analyses
All statistical analyses were performed using the Statistical Package for Social Science (SPSS) version 22 (SPSS, Chicago, IL). Normality of distribution was assessed using histograms and normality plots. SNfL, sCNTN1, and sGFAP were non-normally distributed and therefore ln-transformed.
Group Differences
First, group comparisons were performed between PwMS vs HC and between MS subtypes using general linear models, correcting for body mass index (BMI), sex, disease duration, and DMT duration, unless stated otherwise. No significant changes in sNfL, sGFAP, and sCNTN1 levels were found between smokers and nonsmokers, and the analyses were therefore not corrected for smoking. A subanalysis was performed to further investigate the ability of the blood biomarkers to discriminate between MS subtypes, excluding PwMS on the borderline of RRMS and PwMS on the borderline of secondary progressive MS (SPMS) (EDSS = 4.0 and EDSS = 4.5). A p-value for group comparisons ≤0.05 was considered as statistically significant after Bonferroni correction, where applicable.
Correlation Analysis
Furthermore, the relationship of sNfL, sGFAP, and sCNTN1 with other variables such as BMI, disease duration, disability measures (EDSS, T25FWT, 9HPT), and volumetric MRI measures was studied using Spearman correlations. Here, a p-value ≤0.01 was considered as statistically significant because multiple correlations were performed.
Logistic Regression
To investigate whether blood biomarkers have the same predictive value as MRI measures for EDSS 6 and to assess whether blood biomarkers and MRI measures are independently associated with the milestone of EDSS 6, logistic regression analysis were performed using 4 steps. First, each blood biomarker was separately fed into a logistic regression including sex, BMI, DMT use, and disease duration as standard covariates. Second, to assess whether blood biomarkers independently predicted EDSS 6, the logistic regression was repeated including all blood biomarkers which significantly predicted EDSS 6 in step 1.
Third, a model was created with MRI measures only using backward selection with a removal p-value of 0.10. Finally, predictor variables were fed into separate blocks of variables, consisting of a blood biomarker block (sNfL, sGFAP, and sCNTN1), a brain volume block (white matter volume, cortical gray matter volume, deep gray matter volume, lesion volume, and cerebellar gray matter volume), and a spinal cord block (mean upper cervical cord area), also including sex, BMI, DMT use, and disease duration as standard covariates. Using backward selection with a removal p-value of 0.10, significant variables from each block were then entered in the subsequent block until all variables remaining in the model were statistically significant (p < 0.05).
Data Availability
Anonymized data supporting the findings of this study are available from the corresponding author for the purpose of research only, upon reasonable request.
Results
Patient Characteristics
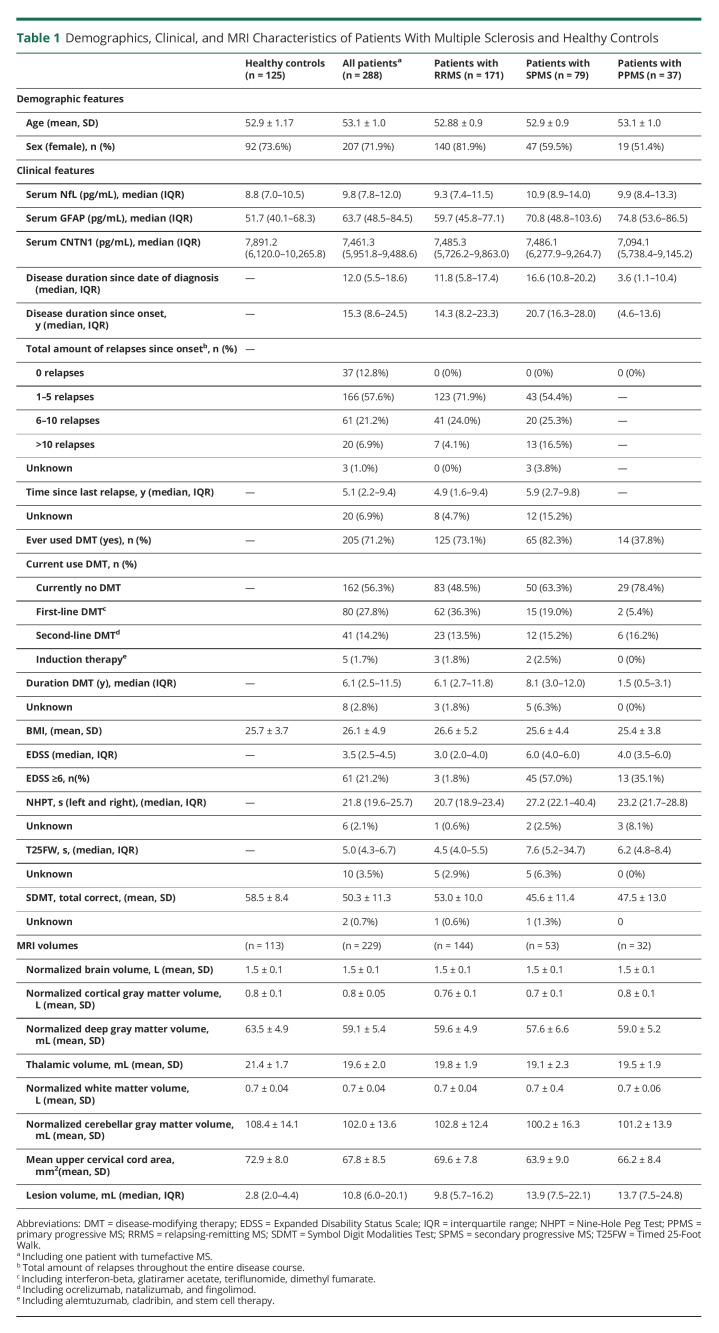
The study population consisted of 289 PwMS of the same age and 125 age-matched HC. One patient with MS, with a medical history of seizures, had a seizure during the study visit. Owing to extreme values of sNfL (273.9 pg/mL) and sGFAP (299.5 pg/mL), most likely caused by the seizure, this participant was left out of all analyses. Therefore, a total of 288 PwMS and 125 HC were included; details of the study population, including MRI data (n = 342; PwMS n = 229; HC n = 113), are provided in table 1. Disease duration since symptom onset significantly differed between all phenotypes (RRMS: 14.3; SPMS: 20.7; PPMS: 8.1, median years).
Table 1.
Demographics, Clinical, and MRI Characteristics of Patients With Multiple Sclerosis and Healthy Controls
SNfL–sGFAP–sCNTN1 in People With MS and Healthy Controls
Serum NfL, GFAP, and CNTN1 correlated weakly with BMI in all PwMS (ρ = −0.208, ρ = −0.158 and ρ = −0.178, all p < 0.01, respectively); subsequent analyses were therefore corrected for BMI. Because sex differences were only found in CNTN1 concentrations (lower in men vs women), comparisons of CNTN1 levels between groups were therefore corrected for sex and BMI. Median sNfL, sGFAP, and sCNTN1 concentrations did not significantly differ between PwMS using DMT (either first line or second line) and adjusting for DMT (yes/no) did not significantly change the results. Both serum NfL (9.8 pg/mL [interquartile range {IQR}: 7.8–12.0]) and GFAP (63.7 pg/mL [IQR: 48.5–84.5]) were higher in PwMS compared with age-matched HCs (sNfL: 8.8 pg/mL [IQR: 7.0–10.5]; sGFAP: 51.7 pg/mL [IQR: 40.1–68.3]) (all p < 0.001). sCNTN1 levels were lower in PwMS (7,461.3 pg/mL [IQR: 5,951.8–9,488.6]) compared with HCs (7,891.2 pg/mL [IQR: 6,120.0–10,265.8] (p = 0.048, sex adjusted), but this was not significant anymore after correcting for both sex and BMI (p = 0.068).
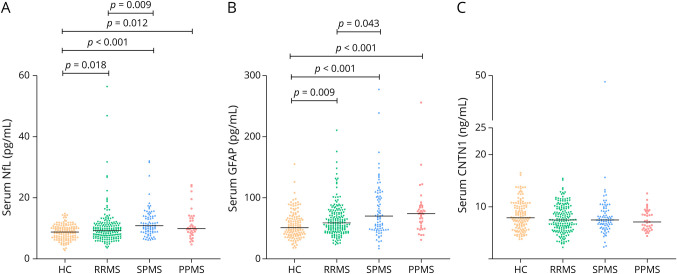
Biomarker levels stratified by MS type are shown in Figure 1. sNfL and sGFAP differed among the MS types and HCs, whereas no difference was found in sCNTN1 levels. A post hoc analysis indicated that both sNfL and sGFAP concentrations were higher in SPMS (sNfL:10.90 pg/mL [IQR: 8.9–14.0]; sGFAP: 70.8 pg/mL [IQR: 48.8–103.6]) than in RRMS (sNfL: 9.3 pg/mL [IQR: 7.4–11.5]; GFAP: 59.7 pg/mL [IQR: 45.8–77.1]) (p = 0.009 and p = 0.043, respectively). After separately analyzing MS types and adjusting for the effect of BMI, disease duration, and DMT use, the difference in sNfL levels between patients with RRMS and SPMS remained significant (p = 0.046); sGFAP was higher in patients with SPMS compared with patients with RRMS but did not reach statistical significance (p = 0.061). By excluding PwMS with EDSS = 4.0 and EDSS = 4.5, the median difference in sNfL levels between patients with RRMS and patients with SPMS changed slightly from 1.6 pg/mL (including all PwMS) to 1.9 pg/mL (excluding PwMS on the borderline of RRMS and SPMS); however, IQRs were still overlapping (RRMS: 9.3 pg/mL [IQR: 7.4–11.5]; SPMS: 11.2 pg/mL [IQR: 9.1–14.5], p = 0.021). Regarding sGFAP, the median difference between RRMS and SPMS changed from 11.1 pg/mL (including all PwMS] to 15.3 pg/mL [excluding PwMS on the borderline of RRMS and SPMS]) and reached statistical significance (RRMS: 58.2 pg/mL [IQR: 46.1–77.9]; SPMS: 73.5 pg/mL [IQR: 49.0–110.1], p = 0.036).
Figure 1. Levels of Serum Neurofilament Light (NfL) (A), Serum Glial Fibrillary Acidic Protein (GFAP) (B), and Serum Contactin-1 (CNTN1) (C) in Healthy Controls and Patients With Multiple Sclerosis.
Each dot in the scatter box-plot represents a sample. p-values were calculated with a general linear model (BMI and/or sex adjusted), followed by post hoc analyses, Bonferroni corrected. BMI = body mass index
Relation to Disease Duration and Clinical Disease Activity
Only sNfL correlated weakly with disease duration (ρ = 0.124, p = 0.040) in the whole MS group but did not reach the statistical threshold of p < 0.01. No correlations between serum NfL, GFAP, and CNTN1, and time since last relapse was observed in patients with RR onset. Moreover, patients with a clinical relapse (n = 7) within 3 months before sampling had no significantly different levels of all 3 biomarkers compared with patients in remission (n = 164) (data not shown). Three of the 7 PwMS with a clinical relapse received methylprednisolone IV, of whom only one patient had a higher NfL level (47.0 pg/mL) compared with the median of all PwMS (9.8 pg/mL).
Relation to EDSS
After correcting for sex, BMI, disease duration, and DMT duration, concentrations of both sNfL and sGFAP significantly differed between the EDSS subgroups (Figure 2), which was driven by patients in the 2 highest EDSS groups. No difference in sNfL or sGFAP concentrations were found between patients with EDSS 0–2.5 and EDSS 3.0–4.5.
Figure 2. Serum Neurofilament Light (NfL) (A), Serum Glial Fibrillary Acidic Protein (GFAP) (B), and Serum Contactin-1 (CNTN1) (C) in Patients With Multiple Sclerosis With Expanded Disability Status Scale (EDSS) 0–2.5 (n = 77); 3.0–4.5 (n = 142); 5.0–6.5 (n = 51) and ≥7.0 (n = 18).
The figure shows median and 95% confidence interval of all biomarker levels.
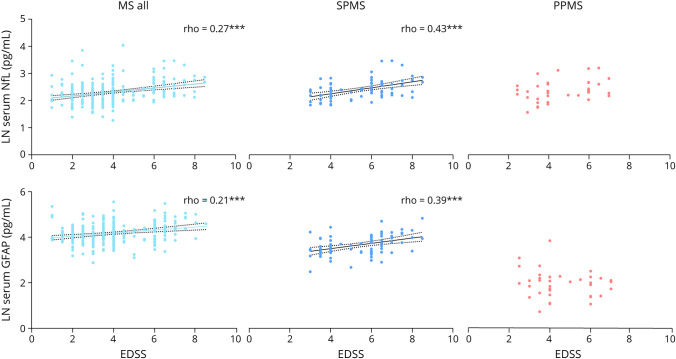
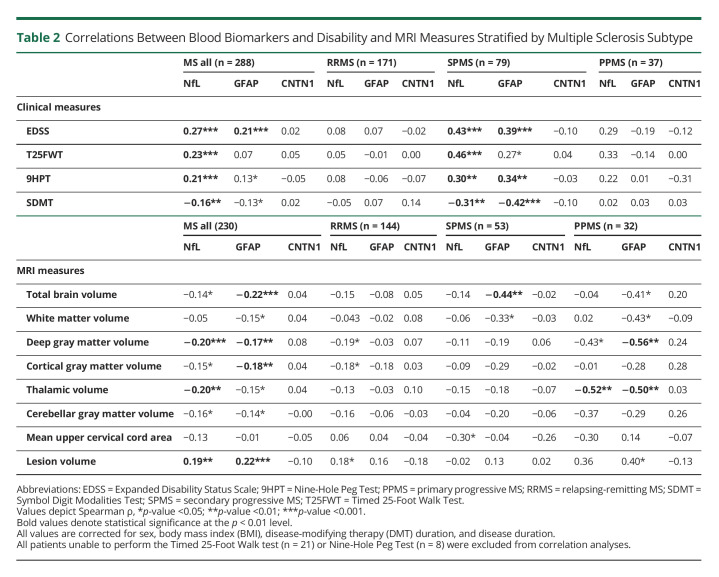
In addition, there was a significant correlation between sNfL and EDSS (ρ = 0.27, p < 0.001) and between sGFAP and EDSS (ρ = 0.21, p < 0.001) across the whole MS group (Figure 3), whereas serum CNTN1 levels did not correlate with EDSS. When stratifying by MS type, sNfL and sGFAP were only correlated to EDSS in the SPMS subtype (ρ = 0.43 and ρ = 0.39, p < 0.001, respectively). sCNTN1 negatively correlated with EDSS in the subgroup (n = 44) of patients with SPMS with EDSS ≥6.0 (ρ = −0.33, p = 0.004). Correlations with all disability outcome measures are listed in Table 2.
Figure 3. Spearman Correlation (ρ) of Serum Neurofilament Light (NfL) and Serum Glial Fibrillary Acidic Protein (GFAP) With the Expanded Disability Status Scale (EDSS) in People With MS, Patients With Secondary Progressive MS (SPMS), and Patients With Primary Progressive MS (PPMS).
Correlations between both biomarkers and EDSS were strongest in Patients with SPMS, whereas no significant correlations were found in patients with PPMS. Values depict Spearman ρ, ***p-value <0.001. Dashed lines indicate the 95% confidence bands. SPMS = secondary progressive patients.
Table 2.
Correlations Between Blood Biomarkers and Disability and MRI Measures Stratified by Multiple Sclerosis Subtype
Relation to Other Disability Outcome Measures
Significant correlations between sNfL and 9HPT, T25FW, and SDMT were found in the whole MS group (Table 2). Similar to EDSS correlations, the 9HPT, T25FW and SDMT only correlated with sNfL and sGFAP in patients with SPMS after subtype stratification, with the strongest correlation between sNfL and T25FW (ρ = 0.46, p < 0.001) and sGFAP and SDMT (ρ = −0.42, p < 0.001).
Relation to Brain Volumes
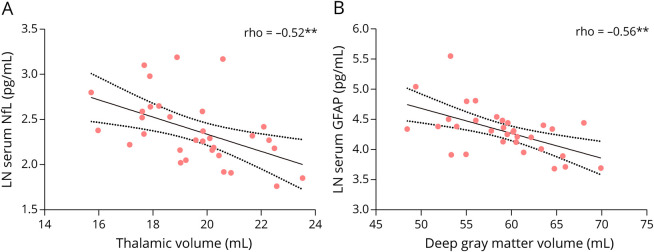
SNfL levels correlated weakly (range ρ = −0.20–0.19) with NDGMV, thalamic volume, and lesion volume in the whole MS group (Table 2). SGFAP correlated weakly (range ρ = −0.17–0.22) with NBV, NDGMV, cortical gray matter volume (NCGMV), and lesion volume in all PwMS. For both sNfL and sGFAP, strong correlations were observed with thalamic volume (ρ = −0.52, p = 0.004; ρ = −0.50, p = 0.007, respectively) in patients with PPMS (Figure 4).
Figure 4. Spearman Correlation (ρ) of Serum Neurofilament Light (NfL) (A) and Serum Glial Fibrillary Acidic Protein (GFAP) (B) With Thalamic Volume (A) and Deep Gray Matter Volume (B) in Patients With Primary Progressive MS (PPMS), Respectively.
Values depict Spearman ρ, **p-value <0.01. Dashed lines indicate the 95% confidence bands.
Moreover, sGFAP correlated with NDGMV in patients with PPMS (ρ = −0.56, p = 0.002) (Figure 4). In patients with SPMS, a significant correlation was found between serum GFAP levels and NBV (ρ = −0.43, p < 0.003). Furthermore, sNFL correlated with MUCCA (ρ = −0.294, p = 0.045), however the significance level was above the threshold of p < 0.01. In patients with RRMS, no correlation between all blood biomarkers and MRI measures were observed with p < 0.01. CNTN1 did not correlate with MRI parameters in any group. Finally, blood biomarkers did not correlate to brain volumes in HC.
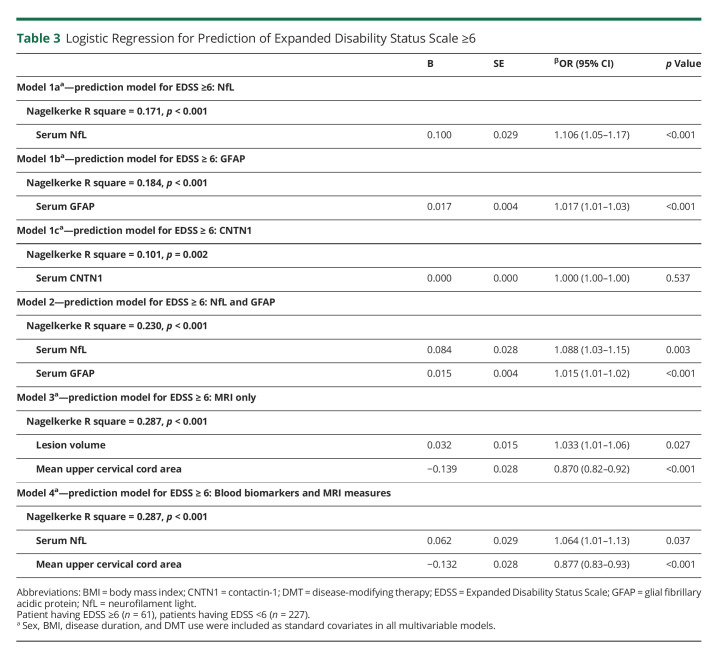
Logistic Regression: EDSS ≥6
In addition, we identified which combinations of blood and radiologic biomarkers best related to EDSS ≥6. First, 3 separate regression analyses showed that higher sNfL and sGFAP concentrations were significantly associated with higher odds of EDSS 6, but not CNTN1 (Table 3). A 10-unit (pg/mL) increase in sNfL corresponded to an odds ratio of 2.739 of having EDSS ≥6, whereas a 10-unit (pg/mL) increase in GFAP corresponded to an odds ratio of 1.184 of having EDSS ≥6. Next, we found that sNfL and sGFAP were both independently related to EDSS 6 (Nagelkerke R2 = 0.230), with an explained variance in the same range as the model including MRI measures only (Nagelkerke R2 = 0.287, only including lesion volume and MUCCA). Finally, the logistic regression analysis was repeated using brain volumes, MUCCA, and blood biomarkers as separate blocks. The final model indicated that increased sNfL levels and atrophy of the upper cervical cord best related to EDSS 6 (Nagelkerke R2 = 0.287, p < 0.001) (Table 3).
Table 3.
Logistic Regression for Prediction of Expanded Disability Status Scale ≥6
Discussion
This study investigated the value of serum NfL, GFAP, and CNTN1 levels to determine neurodegeneration in MS, using a population-based birth year cohort of PwMS and age-matched HC. Increased sNfL and sGFAP levels were related to worse disability measures and lower deep gray matter volumes in PwMS. However, CNTN1 was not related to disability outcome measures and brain volumes. The correlation of sNfL and GFAP with the EDSS, T25FWT, 9HPT, and SDMT was most consistently found in patients with SPMS.
Furthermore, both sNfL and sGFAP levels correlated with deep gray matter volume and thalamic volume in patients with PPMS. In patients with SPMS, sNfL and sGFAP correlated with total brain volume. Overall, our findings illustrate the potential of sNfL and sGFAP as biomarkers of different CNS related pathophysiologic processes resulting in neurodegeneration, reflected by disability, in progressive disease.
Patients of the Project Y cohort were all born in 1966 and thus are nearly of the same age. This allowed us to both study the effect of disease duration and control for disease duration, bypassing the problem of collinearity between age and disease duration.29-31 Because of this, we were able to assess biomarker concentrations as a result of MS-specific axonal damage and astrocyte activation, which is crucial because age has a well-known effect on NfL, GFAP, and CNTN1 concentrations. Aging PwMS have a substantial loss of brain volume and differ from their younger equivalents in the extent of neurodegeneration.32 These seemingly accelerated aging processes show a high degree of heterogeneity among PwMS, which influences the interpretation of biomarkers.32 Therefore, our data add valuable new information to the question whether sNfL, sGFAP, and sCNTN1 are specific for MS-related neurodegeneration.
NfL is correlated with many clinical and radiologic measures in RRMS and is considered as a well-established marker of disease activity (i.e., axonal damage induced by acute inflammation) and treatment response. However, the relationship between NfL and neurodegeneration, reflected by disability, in progressive disease is less clear.7,33,34 Although NfL levels are associated with future brain atrophy in progressive PwMS, the results from studies reporting correlations between NfL and disability (progression) are inconsistent.7,10,35-38 In our cohort, we report consistent correlations between NfL and disability in patients with SPMS but not in patients with RRMS, providing support for the hypothesis that NfL is also relevant in patients with less acute inflammatory activity. Furthermore, our research showed significant higher sNfL levels in patients with SPMS compared with patients with RRMS, which persisted after adjustment for several confounders including disease duration and DMT use.
Our finding that GFAP is increased in progressive patients compared with HC and patients with RRMS is in accordance with other studies.15,16,36,39 Contrary to previous studies, we also observed higher GFAP levels in patients with RRMS vs HC, which might be explained by the higher median EDSS of our RRMS group.15,36 Of note, the IQR of the EDSS overlapped in both patients with RRMS and SPMS which may have limited the possibility to distinguish between RRMS and SPMS. The correlation we found between sGFAP and clinical disability in progressive MS reflects the results of an earlier study, which also reported correlations in progressive MS, but not in patients with RRMS.15 This suggests a prominent role of astrocyte proliferation and activation in progressive MS, which is further supported by our observation that serum GFAP and NfL were independently associated with advanced disability, indicative of seemingly partially independent processes of both late neuroaxonal loss and astrogliosis. Although more correlations were found between sGFAP and clinical and radiologic measures compared with sNfL, we here suggest a complementary role for sGFAP and sNfL, because sNfL and sGFAP were independently associated with advanced disability.
In our study, lower sCNTN1 levels were related to patients having EDSS ≥6.0. Because CNTN1 is mostly expressed in myelinated axons, we speculate that this could be because of a significant reduction in axonal density, which is most pronounced in advanced disease.19 Although we reported a trend for lower CNTN1 concentrations in PwMS compared with HC, no statistical difference was found when adjusting for BMI and sex. Subsequently, we found no difference in CNTN1 levels across MS subtypes, which is in contrast to other studies reporting decreased levels of CNTN1 in either CSF19,40 or serum20 of patients with RRMS compared with HC. Differences may be because of the higher but homogeneous median age of our cohort (53 years) and the associated decrease in active inflammation compared with earlier work. This would imply that CNTN1 is mostly a marker in early stages of MS.
To date, relatively few studies have correlated sNfL, sGFAP, and sCNTN1 with various regional brain volume structures in a well-defined and homogeneous MS cohort. We found the strongest correlations between sGFAP, sNFL, and NDGMV, whereas no significant correlations were found between sNfL and NWMV. This is in line with a previous study, which demonstrated that the associations of sNfL levels and future atrophy were specifically driven by changes within (deep) gray matter regions.41 Axonal loss within cortico-subcortical connections and hence network disconnection are considered the primary driving force behind deep gray matter atrophy, which is especially severe in progressive patients and might therefore be reflected by NfL.42 Astrocyte activation, reflected by GFAP, might contribute to damage in gray matter structures via chronic activation of the CNS innate immune system, which could result in an inhibition of remyelination and/or axonal mitochondrial dysfunction.43 Nevertheless, earlier studies have also showed the ability of NfL to predict WM atrophy, a result which we cannot confirm in our cohort.44,45
Remarkably, the multivariable logistic regression model with sNfL and sGFAP had an explained variance in the same range as the model including MRI measures only. This suggests that the easily accessed biomarkers NfL and GFAP may have comparable accuracy with neuroimaging biomarkers when assessing advanced disability. Moreover, we demonstrated that sNfL and MUCCA independently predicted EDSS 6, where sNfL replaced lesion volumes, possibly indicating that sufficient information on neuroinflammation and axonal damage can be gained from sNfL alone. Compared with such (lesional) neuroimaging markers, NfL is a less costly biomarker, is well-standardized, and measures ongoing neuroaxonal damage more directly.26 Future research will have to elucidate whether NfL and GFAP may complement neuroimaging biomarkers or whether these blood biomarkers could be used as a standalone or triaging quantitative marker for the extent of neurodegeneration. Based on our findings, sNfL and sGFAP could be valuable biomarkers in clinical trials addressing progressive disease at group level but may have less additional value in clinical decisions at the individual level, given the overlap between concentrations in PwMS vs HC and across the MS subtypes.
The results of our study cannot be interpreted without considering the following limitations. Owing to the cross-sectional nature of the study design, the temporal evolution of all biomarkers could not be studied. Longitudinal studies with large samples of progressive PwMS are necessary to test the temporal relation with disability progression while filtering out the effects of chronological age. Toward clinical implementation, further studies are required to evaluate GFAP and CNTN1 concentrations in early and more active disease and their response to treatment. In addition, our research design did not allow us to differentiate the effects on biomarkers between axonal loss because of (chronic) inflammation and because of inactive processes, a distinction which will be important to understand disease dynamics.
Because current therapeutic options for progressive PwMS remain scarce, it is crucial to find reliable, easy-accessible and low-cost biomarkers for quantifying MS-related neuronal loss. Data of our cohort highlight that sNfL and sGFAP are related to disability and deep gray matter volumes especially in progressive disease, implicating that NfL and GFAP are valuable complementary biomarkers in clinical practice and clinical trials for evaluating neurodegeneration in progressive disease. By using a well-defined cohort of PwMS with the same age, we have demonstrated that NfL and GFAP are complementary and that both reflect neurodegenerative processes, independent of age.
Acknowledgment
The authors would like to extend their gratitude and acknowledgements to all study participants of the Project Y. Furthermore, the authors wish to thank the research assistants and all study team members for their time and energy spent on this project.
Glossary
- 3D
three-dimensional
- 9HPT
Nine-Hole Peg Test
- BMI
body mass index
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- HC
healthy control
- IQR
interquartile range
- MUCCA
mean upper cervical cord area
- NBV
normalized total brain volume
- NWMV
normalized white matter volume
- NDGMV
total gray matter and deep gray matter
- PwMS
people with MS
- RRMS
relapsing-remitting MS
- sGFAP
serum glial fibrillary acidic protein
- sNfL
serum biomarkers neurofilament light
- sCNTN1
serum contactin-1
- SDMT
Symbol Digit Modalities Test
- SPMS
secondary progressive patients
- T25FWT
Timed 25-Foot Walk test
Appendix. Authors
Study Funding
This study was supported by the VriendenLoterij, Dutch MS Research Foundation, Mission Summit, and VUmc Foundation.
Disclosure
F.C. Loonstra, L.R.J. de Ruiter, B. Moraal, and E.M. Strijbis report no competing interests. M.M. Schoonheim serves on the editorial board of Neurology and Frontiers in Neurology, receives research support from the Dutch MS Research Foundation and Amsterdam Neuroscience, and has served as a consultant for or received research support and/or speaker honoraria from Atara Biotherapeutics, Biogen, Celgene, Genzyme, MedDay, and Merck. F. Barkhof: steering committee and iDMC member for Biogen, Merck, Roche, and EISAI; Consultant for Roche, Biogen, Merck, IXICO, Jansen, Combinostics. Research agreements with Novartis, Merck, Biogen, GE, Roche. Co-founder and shareholder of Queen Square Analytics LTD. B.M.J. Uitdehaag received consultancy fees from Biogen Idec, Genzyme, Merck Serono, Novartis, Roche, and Teva and Immunic Therapeutics. C. Teunissen has served on advisory boards for Roche, has received nonfinancial support in the form of research consumables from ADx NeuroSciences and Euroimmun, and has performed contract research or received grants from Probiodrug, Biogen, Esai, Toyama, Janssen Prevention Center, Boehringer, Axon Neuroscience, EIP Pharma, PeopleBio, Qunaterix, and Roche. J. Killestein has speaker relationships with Biogen, Genzyme, Merck, Novartis, Roche, Sanofi, and TEVA. Go to Neurology.org/NN for full disclosures.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372(9648):1502–1517. doi: 10.1016/s0140-6736(08)61620-7 [DOI] [PubMed] [Google Scholar]
- 2.Geurts JJ, Calabrese M, Fisher E, Rudick RA. Measurement and clinical effect of grey matter pathology in multiple sclerosis. Lancet Neurol. 2012;11(12):1082–1092. doi: 10.1016/S1474-4422(12)70230-2 [DOI] [PubMed] [Google Scholar]
- 3.Eshaghi A, Prados F, Brownlee WJ, et al. . Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210–222. doi: 10.1002/ana.25145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp. 2012;33(8):1987–2002. doi: 10.1002/hbm.21334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Stefano N, Giorgio A, Battaglini M, et al. . Assessing brain atrophy rates in a large population of untreated multiple sclerosis subtypes. Neurology. 2010;74(23):1868–1876. doi: 10.1212/WNL.0b013e3181e24136 [DOI] [PubMed] [Google Scholar]
- 6.Teunissen CE, Malekzadeh A, Leurs C, Bridel C, Killestein J. Body fluid biomarkers for multiple sclerosis--the long road to clinical application. Nat Rev Neurol. 2015;11(10):585–596. doi: 10.1038/nrneurol.2015.173 [DOI] [PubMed] [Google Scholar]
- 7.Williams T, Zetterberg H, Chataway J. Neurofilaments in progressive multiple sclerosis: a systematic review. J Neurol. 2021;268(9):3212–3222. doi: 10.1007/s00415-020-09917-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor R, Smith KE, Allegretta M, et al. . Serum neurofilament light as a biomarker in progressive multiple sclerosis. Neurology. 2020;95(10):436–444. doi: 10.1212/WNL.0000000000010346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Lierop Z, Verberk IMW, van Uffelen KWJ, et al. . Pre-analytical stability of serum biomarkers for neurological disease: neurofilament-light, glial fibrillary acidic protein and contactin-1. Clin Chem Lab Med. 2022;60(6):842–850. doi: 10.1515/cclm-2022-0007 [DOI] [PubMed] [Google Scholar]
- 10.Disanto G, Barro C, Benkert P, et al. . Serum Neurofilament light: a biomarker of neuronal damage in multiple sclerosis. Ann Neurol. 2017;81(6):857–870. doi: 10.1002/ana.24954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barro C, Benkert P, Disanto G, et al. . Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain. 2018;141(8):2382–2391. doi: 10.1093/brain/awy154 [DOI] [PubMed] [Google Scholar]
- 12.Thebault S, Abdoli M, Fereshtehnejad SM, Tessier D, Tabard-Cossa V, Freedman MS. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci Rep. 2020;10(1):10381. doi: 10.1038/s41598-020-67504-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Manouchehrinia A, Stridh P, Khademi M, et al. . Plasma neurofilament light levels are associated with risk of disability in multiple sclerosis. Neurology. 2020;94(23):e2457-e2467. doi: 10.1212/WNL.0000000000009571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosengren LE, Lycke J, Andersen O. Glial fibrillary acidic protein in CSF of multiple sclerosis patients: relation to neurological deficit. J Neurol Sci. 1995;133(1-2):61–65. doi: 10.1016/0022-510x(95)00152-r [DOI] [PubMed] [Google Scholar]
- 15.Abdelhak A, Huss A, Kassubek J, Tumani H, Otto M. Serum GFAP as a biomarker for disease severity in multiple sclerosis. Sci Rep. 2018;8(1):14798. doi: 10.1038/s41598-018-33158-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ayrignac X, Le Bars E, Duflos C, et al. . Serum GFAP in multiple sclerosis: correlation with disease type and MRI markers of disease severity. Sci Rep. 2020;10(1):10923. doi: 10.1038/s41598-020-67934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petzold A, Eikelenboom MJ, Gveric D, et al. . Markers for different glial cell responses in multiple sclerosis: clinical and pathological correlations. Brain. 2002;125(Pt 7):1462–1473. doi: 10.1093/brain/awf165 [DOI] [PubMed] [Google Scholar]
- 18.Colakoglu G, Bergstrom-Tyrberg U, Berglund EO, Ranscht B. Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc Natl Acad Sci U S A. 2014;111(3):E394–E403. doi: 10.1073/pnas.1313769110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chatterjee M, Koel-Simmelink MJ, Verberk IM, et al. . Contactin-1 and contactin-2 in cerebrospinal fluid as potential biomarkers for axonal domain dysfunction in multiple sclerosis. Mult Scler J Exp Transl Clin. 2018;4(4):2055217318819535. doi: 10.1177/2055217318819535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Lierop ZY, Wieske L, Koel-Simmelink MJ, et al. . Serum contactin-1 as a biomarker of long- term disease progression in natalizumab-treated multiple sclerosis. Mult Scler. 2021:13524585211010097. doi: 10.1177/13524585211010097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thebault S, Booth RA, Rush CA, MacLean H, Freedman MS. Serum neurofilament light chain measurement in MS: hurdles to clinical translation. Front Neurosci. 2021;15:654942. doi: 10.3389/fnins.2021.654942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porchet R, Probst A, Bouras C, Dráberová E, Dráber P, Riederer BM. Analysis of glial acidic fibrillary protein in the human entorhinal cortex during aging and in Alzheimer's disease. Proteomics. 2003;3(8):1476–1485. doi: 10.1002/pmic.200300456 [DOI] [PubMed] [Google Scholar]
- 23.Verkerke M, Hol EM, Middeldorp J. Physiological and pathological ageing of astrocytes in the human brain. Neurochem Res. 2021;46(10):2662–2675. doi: 10.1007/s11064-021-03256-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loonstra FC, De Ruiter LRJ, Doesburg D, et al. . Project Y: the search for clues explaining phenotype variability in MS. Mult Scler Relat Disord. 2022;57:10337. doi: 10.1016/j.msard.2021.103337 [DOI] [PubMed] [Google Scholar]
- 25.Thompson AJ, Banwell BL, Barkhof F, et al. . Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2 [DOI] [PubMed] [Google Scholar]
- 26.Leppert D, Kuhle J. Serum NfL levels should be used to monitor multiple sclerosis evolution—yes. Mult Scler. 2020;26(1):17–19. doi: 10.1177/1352458519872921 [DOI] [PubMed] [Google Scholar]
- 27.Verberk IMW, Thijssen E, Koelewijn J, et al. . Combination of plasma amyloid beta(1-42/1-40) and glial fibrillary acidic protein strongly associates with cerebral amyloid pathology. Alzheimers Res Ther. 2020;12(1):118. doi: 10.1186/s13195-020-00682-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eijlers AJC, Wink AM, Meijer KA, Douw L, Geurts JJG, Schoonheim MM. Reduced network dynamics on functional MRI signals cognitive impairment in multiple sclerosis. Radiology. 2019;292(2):449–457. doi: 10.1148/radiol.2019182623 [DOI] [PubMed] [Google Scholar]
- 29.von Wyl V, Decard BF, Benkert P, et al. . Influence of age at disease onset on future relapses and disability progression in patients with multiple sclerosis on immunomodulatory treatment. Eur J Neurol. 2020;27(6):1066–1075. doi: 10.1111/ene.14191 [DOI] [PubMed] [Google Scholar]
- 30.Tremlett H, Zhao Y, Joseph J, Devonshire V, Neurologists UC. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry. 2008;79(12):1368–1374. doi: 10.1136/jnnp.2008.145805 [DOI] [PubMed] [Google Scholar]
- 31.Scalfari A, Lederer C, Daumer M, Nicholas R, Ebers GC, Muraro PA. The relationship of age with the clinical phenotype in multiple sclerosis. Mult Scler. 2016;22(13):1750–1758. doi: 10.1177/1352458516630396 [DOI] [PubMed] [Google Scholar]
- 32.Vaughn CB, Jakimovski D, Kavak KS, et al. . Epidemiology and treatment of multiple sclerosis in elderly populations. Nat Rev Neurol. 2019;15(6):329–342. doi: 10.1038/s41582-019-0183-3 [DOI] [PubMed] [Google Scholar]
- 33.Bridel C, Leurs CE, van Lierop Z, et al. . Serum neurofilament light association with progression in natalizumab-treated patients with relapsing-remitting multiple sclerosis. Neurology. 2021;97(19):e1898-e1905. doi: 10.1212/wnl.0000000000012752 [DOI] [PubMed] [Google Scholar]
- 34.Gafson AR, Jiang X, Shen C, et al. . Serum neurofilament light and multiple sclerosis progression independent of acute inflammation. JAMA Netw Open. 2022;5(2):e2147588. doi: 10.1001/jamanetworkopen.2021.47588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferraro D, Guicciardi C, De Biasi S, et al. . Plasma neurofilaments correlate with disability in progressive multiple sclerosis patients. Acta Neurol Scand. 2020;141(1):16–21. doi: 10.1111/ane.13152 [DOI] [PubMed] [Google Scholar]
- 36.Hogel H, Rissanen E, Barro C, et al. . Serum glial fibrillary acidic protein correlates with multiple sclerosis disease severity. Mult Scler. 2020;26(2):210–219. doi: 10.1177/1352458518819380 [DOI] [PubMed] [Google Scholar]
- 37.Eikelenboom MJ, Petzold A, Lazeron RH, et al. . Multiple sclerosis: neurofilament light chain antibodies are correlated to cerebral atrophy. Neurology. 2003;60(2):219–223. doi: 10.1212/01.wnl.0000041496.58127.e3 [DOI] [PubMed] [Google Scholar]
- 38.Abdelhak A, Hottenrott T, Morenas-Rodríguez E, et al. . Glial activation markers in CSF and serum from patients with primary progressive multiple sclerosis: potential of serum GFAP as disease severity marker? Front Neurol. 2019;10:280. doi: 10.3389/fneur.2019.00280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun M, Liu N, Xie Q, et al. . A candidate biomarker of glial fibrillary acidic protein in CSF and blood in differentiating multiple sclerosis and its subtypes: a systematic review and meta-analysis. Mult Scler Relat Disord. 2021;51:102870. doi: 10.1016/j.msard.2021.102870 [DOI] [PubMed] [Google Scholar]
- 40.Kroksveen AC, Guldbrandsen A, Vedeler C, Myhr KM, Opsahl JA, Berven FS. Cerebrospinal fluid proteome comparison between multiple sclerosis patients and controls. Acta Neurol Scand Supplementum. 2012;(195):90–96. doi: 10.1111/ane.12029 [DOI] [PubMed] [Google Scholar]
- 41.Jakimovski D, Kuhle J, Ramanathan M, et al. . Serum neurofilament light chain levels associations with gray matter pathology: a 5-year longitudinal study. Ann Clin Transl Neurol. 2019;6(9):1757–1770. doi: 10.1002/acn3.50872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoonheim MM, Geurts JJG. What causes deep gray matter atrophy in multiple sclerosis? AJNR Am J Neuroradiol. 2019;40(1):107–108. doi: 10.3174/ajnr.A5942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Correale J, Farez MF. The role of astrocytes in multiple sclerosis progression. Front Neurol. 2015;6:180. doi: 10.3389/fneur.2015.00180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thebault S, Tessier DR, Lee H, et al. . High serum neurofilament light chain normalizes after hematopoietic stem cell transplantation for MS. Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e598. doi: 10.1212/NXI.0000000000000598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziemssen T, Arnold DL, Alvarez E, et al. . Prognostic value of serum neurofilament light chain for disease activity and worsening in patients with relapsing multiple sclerosis: results from the phase 3 ASCLEPIOS I and II trials. Front Immunol. 2022;13:852563. doi: 10.3389/fimmu.2022.852563 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data supporting the findings of this study are available from the corresponding author for the purpose of research only, upon reasonable request.