Seasonal influenza vaccination is established as important infection prevention measure, especially among highly exposed healthcare workers (HCWs) [1]. Coadministration with the third dose of COVID-19 vaccine could be an efficient strategy protecting HCWs from two major viral respiratory infections [2–4]. To date, the humoral immunogenicity and side-effects of a coadministered third COVID-19 and a seasonal quadrivalent influenza vaccine are still unclear, and the available data is limited in transferability to the general public [5–7]. This preference-based non-randomised controlled study examines the antibody-mediated immunogenicity and vaccine-related side-effects of mRNA-based COVID-19 and seasonal influenza vaccine coadministration in HCWs.
Short abstract
Coadministration of seasonal quadrivalent influenza and COVID-19 booster vaccination is safe and does not increase vaccine-related side-effects, but may limit anti-SARS-CoV-2 antibody formation https://bit.ly/3uKFUie
To the Editor:
Seasonal influenza vaccination is established as important infection prevention measure, especially among highly exposed healthcare workers (HCWs) [1]. Coadministration with the third dose of COVID-19 vaccine could be an efficient strategy protecting HCWs from two major viral respiratory infections [2–4]. To date, the humoral immunogenicity and side-effects of a coadministered third COVID-19 and a seasonal quadrivalent influenza vaccine are still unclear, and the available data is limited in transferability to the general public [5–7]. This preference-based non-randomised controlled study examines the antibody-mediated immunogenicity and vaccine-related side-effects of mRNA-based COVID-19 and seasonal influenza vaccine coadministration in HCWs.
1231 participants of the CoVacSer study with two doses of BNT162b2mRNA (30 µg mRNA each) as basic COVID-19 immunisation received a third dose of mRNA-based COVID-19 vaccine administering BNT162b2mRNA (30 µg mRNA) or mRNA-1273 (50 µg mRNA) depending on age and vaccine availability. HCWs could opt for a simultaneous influenza vaccine coadministration (Influvac Tetra vaccine 2021/2022) injected intramuscularly into the opposite arm. HCWs without coadministration received either no seasonal influenza vaccination or at least 14 days apart from the COVID-19 booster. Convalescent HCWs having passed a PCR-confirmed SARS-CoV-2 infection were excluded.
Pseudonymised serum blood samples combined with a questionnaire were collected from 1 October 2021 to 31 January 2022 between 14 and 90 days after the COVID-19 vaccination. Anti-SARS-CoV-2-spike IgG levels were obtained using the SERION ELISA agile SARS-CoV-2 IgG (SERION diagnostics, Wuerzburg, Germany) [8].
The statistical analyses were performed with R (version 3.1.2) on logarithmised anti-SARS-CoV-2-spike IgG concentrations based on a multiple linear regression analysis. The parameters including potential influencing factors were estimated using a generalised least squares fit (R package nlme) [9, 10]. Statistical subgroup differences of the independent categorical variables were calculated with the estimated marginal means. Using the Tukey test statistics, pairwise post hoc tests were performed to expose statistically significant differences. The emmeans package was used to estimate marginal means and to calculate pairwise differences [11]. Investigating the role of side-effects, pairwise Fisher exact tests were performed. To correct against multiple testing, p-values were adjusted using the Benjamini–Yekutielie procedure [12]. Only adjusted p-values below a significance level of 0.05 were considered statistically significant.
The study protocol was approved by the ethics committee of the University of Wuerzburg in accordance with the Declaration of Helsinki (file number 79/21).
20.2% (249/1231) of all participants opted for coadministration, whereas 90.4% (225/249) received BNT162b2mRNA and 9.6% (24/249) mRNA-1273 as COVID-19 booster vaccine. 79.8% (982/1231) of all participants were vaccinated with a mRNA-based COVID-19 vaccine only. Among them, 78.5% (771/982) received BNT162b2mRNA, 21.5% (211/982) mRNA-1273. Except differences in the gender distribution (24.0% male in the coadministration versus 15.7% male in the control group) no significant differences could be assessed comparing the coadministration and the control group regarding age, weight, height, smoking, immune deficiency, interval between vaccine doses and sampling, and the median baseline anti-SARS-CoV-2-spike IgG levels (coadministration group: 138.1 binding antibody units (BAU)·mL−1, control group: 133.1 BAU·mL−1).
Obtained anti-SARS-CoV-2-spike IgG levels ranged from 239.1 to 18 541.5 BAU·mL−1 (median 1605.0 BAU·mL−1; interquartile range (IQR) 1078.0–2504.7 BAU·mL−1) in the coadministration group. In the control cohort, anti-SARS-CoV-2-spike IgG levels from 17.7 to 21 287.9 BAU·mL−1 were determined (median 2150.2 BAU·mL−1; IQR 1341.1–3242.3 BAU·mL−1). Median anti-SARS-CoV-2-spike IgG levels were 34.0% higher in the control group (p<0.01) (figure 1).
FIGURE 1.
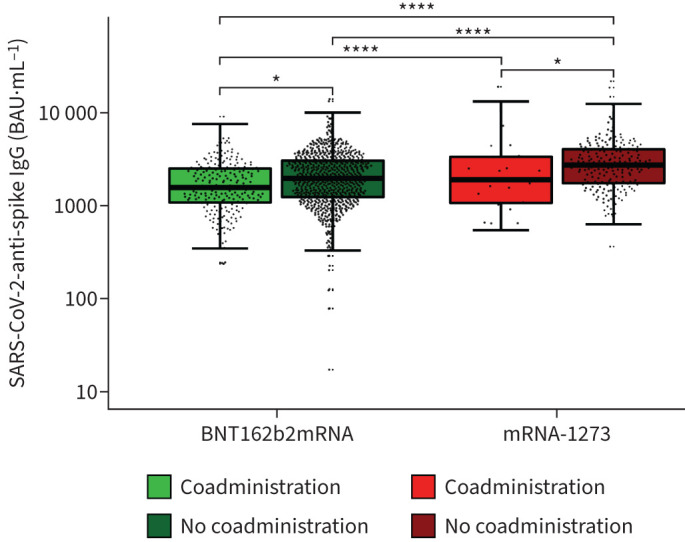
Anti-SARS-CoV-2-spike IgG concentrations after third dose of COVID-19 vaccine with or without influenza vaccine coadministration, separated for COVID-19 booster vaccine (BNT162b2mRNA or mRNA-1273), logarithmically scaled. ****: p<0.0001 *: p<0.05. BAU: binding antibody units.
In the case of BNT162b2mRNA coadministration, the median anti-SARS-CoV-2-spike IgG was 1560.7 BAU·mL−1 (IQR 1078.0–2493.6 BAU·mL−1), compared to 1955.1 BAU·mL−1 (IQR 1234.6–3022.9 BAU·mL−1) in BNT162b2mRNA-only vaccinated. For mRNA-1273 coadministration, a median of 1891.1 BAU·mL−1 (IQR 1068.2–3324.0 BAU·mL−1) was observed, in contrast to 2709.8 BAU·mL−1 (IQR 1735.4–4001.2 BAU·mL−1) for mRNA-1273-only vaccinated. Anti-SARS-CoV-2-spike IgG concentrations were significantly higher without coadministration, for BNT162b2mRNA (+25.3%; p=0.02) and mRNA-1273 (+43.3%; p=0.02).
Independent of influenza vaccine coadministration, mRNA-1273 induced significantly higher anti-SARS-CoV-2-spike IgG concentrations than BNT162b2mRNA (p<0.0001) (figure 1).
81.1% (202/249) of the coadministered participants reported vaccine-related side-effects compared to 87.3% (857/982) in the control cohort. 59.8% (149/249) of the coadministered participants suffered from local reactions, 37.3% (93/249) from headache, 34.1% (85/249) from muscle pain, 26.1% (65/249) from fever and/or chills, 47.8% (119/249) from fatigue, and 12.9% (32/249) from other complaints. Among the control group, 62.0% (609/982) reported local reactions, 43.3% (425/982) headache, 41.8% (410/982) muscle pain, 29.5% (290/982) fever and/or chills, 52.6% (517/982) fatigue, and 15.1% (148/982) other complaints after vaccination. Numerically, all queried side-effects were less common in the coadministration group without any statistical significance (p>0.05).
However, median anti-SARS-CoV-2-spike IgG levels were lower by more than 25% in the case of coadministration for both mRNA-based vaccines, possibly due to parallel stimulation of the immune system. These findings are in line with decreased anti-SARS-CoV-2 antibody titres observed in individuals receiving a combined protein subunit COVID-19 and influenza vaccine compared to a protein subunit COVID-19 vaccine without an influenza component [6]. The clinical consequences of the impaired anti-SARS-CoV-2-spike IgG levels are still unclear, as the anti-SARS-CoV-2-spike IgG level required for reliable protection against SARS-CoV-2 infection and severe COVID-19 remains to be determined [13]. The limited immune response may explain the absence of increased side-effects despite the enhanced immunisation stimulus also observed before [5–7].
It is possible that an adapted and augmented COVID-19 vaccine dosage in case of influenza vaccine coadministration might compensate for the lower levels of anti-SARS-CoV-2-spike IgG after coadministration. The comparison of the higher-dosed mRNA-1273 (50 µg) COVID-19 booster vaccine administration with BNT162b2mRNA (30 µg) confirms this assumption, by displaying similar anti-SARS-CoV-2-spike IgG levels in the BNT162b2mRNA-only vaccinated control cohort and the mRNA-1273 coadministered.
The limitations of the study were that data were collected as non-randomised preference-based controlled trial without evaluation of the reasons for the individual decision. As the study was restricted to the third COVID-19 vaccination and participants were excluded after a PCR-confirmed SARS-CoV-2 infection, the data may differ in hybrid-immunised individuals and for coadministration with a fourth dose of COVID-19 vaccine. SARS-CoV-2 infections without diagnosis or merely diagnosed by lateral flow test may have occurred and influenced antibody titres in the study population. The study population was predominantly female (82.5%) reflecting the current gender distribution among HCWs in Germany (75.5% female) [14]. Male gender was slightly overrepresented in the coadministration group and previous work shows lower anti-SARS-CoV-2-spike IgG levels in males [15]. However, the multiple regression analysis modelling reveals that the group differences cannot be explained by gender distribution. Baseline anti-SARS-CoV-2-spike IgG levels were available for 94.8% (1167/1231) of the participants, showing no statistically significant differences between the groups. A subgroup analysis including these participants confirmed the significant lower levels in the coadministration versus control group, as well as after the booster vaccination with BNT162b2mRNA versus mRNA-1273. Antibody responses were assessed quantitatively while neutralisation capacity was not examined. Vaccine-related side-effects were assessed using self-report questionnaires. The influenza-specific humoral immune response was not assessed and should be considered in future studies containing an “influenza-vaccinated only” group. Due to vaccine availability and participants’ individual reasons, COVID-19 booster vaccinations were administered with either BNT162b2mRNA or mRNA-1273 representing a point-of-care scenario.
In summary, coadministration significantly limits anti-SARS-CoV-2-spike IgG levels, in particular for mRNA-1273 but also for BNT162b2mRNA, and will not jeopardise public healthcare capacities due to increased sick leave. These aspects will support the development of public health recommendations for coadministration of COVID-19 and influenza vaccines in anticipation of the imminent infection waves in the coming winter season [1, 2, 4]. The possibility of coadministration could increase vaccination rates among highly exposed HCWs [3].
Shareable PDF
Acknowledgements
We thank the technical assistants of the serological diagnostic laboratory for sharing their laboratory, and especially for their help and advice.
Footnotes
Data sharing statement: The reproducible script of all statistical analyses can be accessed at https://github.com/AlexGa/Immunogenicity-and-safety-of-coadministration-of-COVID-19-and-influenza-vaccination. Additional data that underlie the results reported in this article, after de-identification (text, tables, figures, and appendices) as well as the study protocol, statistical analysis plan, and analytic code is made available to researchers who provide a methodologically sound proposal to achieve aims in the approved proposal on request to the corresponding author.
Author contributions: All authors had unlimited access to all data. I. Wagenhäuser, J. Reusch, A. Gabel, N. Petri and M. Krone take responsibility for the integrity of the data and the accuracy of the data analysis. Conception and design: T-T. Lâm, G. Almanzar, M. Prelog, A. Frey, A. Schubert-Unkmeir, L. Dölken, S. Frantz, O. Kurzai, U. Vogel, N. Petri and M. Krone. Anti-SARS-CoV-2-spike IgG concentration determination: I. Wagenhäuser and J. Reusch. Trial management: I. Wagenhäuser, J. Reusch, A. Höhn, N. Petri and M. Krone. Statistical analysis: I. Wagenhäuser, J. Reusch, A. Gabel, L.B. Krone, N. Petri and M. Krone. Obtained funding: O. Kurzai and U. Vogel. First draft of the manuscript: I. Wagenhäuser, J. Reusch, A. Gabel, N. Petri and M. Krone. Reviewing and modifying the manuscript and approving its final version: A. Höhn, T-T. Lâm, G. Almanzar, M. Prelog, L.B. Krone, A. Frey, A. Schubert-Unkmeir, L. Dölken, S. Frantz and O. Kurzai. U. Vogel had a major contribution to the conception and design of the study as well as in obtaining funding, supervising and supporting the users. As he passed away during the review process, he was not able to review and approve the final version of the manuscript.
Conflict of interest: M. Prelog received honoraria for presentations at scientific meetings from Abbvie, Chugai-Roche, GSK, Janssen, Novartis, MSD, Pfizer, Sanofi and SOBI, received honoraria for advisory boards from Abbvie, GSK, Janssen, Novartis, Pfizer and BioNTech, received travel scholarships from Chugai-Roche, GSK, Novartis and Pfizer, received financial support for conduction of investigator-initiated research from Baxter, Chugai-Roche, GSK, Novartis, MSD, Pfizer and SOBI; M. Prelog declares to have no conflict of interest regarding the manuscript. M. Krone receives honoraria from GSK and Pfizer, outside the submitted work. All other authors declare no potential conflicts of interest.
Support statement: This study was funded by the Federal Ministry for Education and Science (BMBF) through a grant provided to the University Hospital of Wuerzburg by the Network University Medicine on COVID-19 (B-FAST, grant number 01KX2021) as well as by the Free State of Bavaria with COVID-research funds provided to the University of Wuerzburg, Germany. N. Petri is supported by the German Research Foundation (DFG) funded scholarship UNION CVD. The study was initiated by the investigators. The sponsoring institutions had no function in study design, data collection, analysis, and interpretation of data as well as in writing of the manuscript. All authors had unlimited access to all data. I. Wagenhäuser, J. Reusch, A. Gabel, M. Krone and N. Petri had the final responsibility for the decision to submit for publication. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Larrauri A, Prosenc Trilar K. Preparing for an influenza season 2021/22 with a likely co-circulation of influenza virus and SARS-CoV-2. Euro Surveill 2021; 26: 2100975. doi: 10.2807/1560-7917.ES.2021.26.41.2100975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giordano G, Blanchini F, Bruno R, et al. Modelling the COVID-19 epidemic and implementation of population-wide interventions in Italy. Nat Med 2020; 26: 855–860. doi: 10.1038/s41591-020-0883-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielicki JA, Duval X, Gobat N, et al. Monitoring approaches for health-care workers during the COVID-19 pandemic. Lancet Infect Dis 2020; 20: e261–e267. doi: 10.1016/S1473-3099(20)30458-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chotpitayasunondh T, Fischer TK, Heraud JM, et al. Influenza and COVID-19: what does co-existence mean? Influenza Other Respir Viruses 2021; 15: 407–412. doi: 10.1111/irv.12824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izikson R, Bolduc D, Bourron JS, et al. Safety and immunogenicity of a high-dose quadrivalent influenza vaccine administered concomitantly with a third dose of the mRNA-1273 SARS-CoV-2 vaccine in adults aged ≥65 years: a phase 2, randomised, open-label study. Lancet Respir Med 2022; 10: 392–402. doi: 10.1016/S2213-2600(21)00557-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toback S, Galiza E, Cosgrove C, et al. Safety, immunogenicity, and efficacy of a COVID-19 vaccine (NVX-CoV2373) co-administered with seasonal influenza vaccines: an exploratory substudy of a randomised, observer-blinded, placebo-controlled, phase 3 trial. Lancet Respir Med 2022; 10: 167–179. doi: 10.1016/S2213-2600(21)00409-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lazarus R, Baos S, Cappel-Porter H, et al. Safety and immunogenicity of concomitant administration of COVID-19 vaccines (ChAdOx1 or BNT162b2) with seasonal influenza vaccines in adults in the UK (ComFluCOV): a multicentre, randomised, controlled, phase 4 trial. Lancet 2021; 398: 2277–2287. doi: 10.1016/S0140-6736(21)02329-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krone M, Gütling J, Wagener J, et al. Performance of three SARS-CoV-2 immunoassays, three rapid lateral flow tests, and a novel bead-based affinity surrogate test for the detection of SARS-CoV-2 antibodies in human serum. J Clin Microbiol 2021; 59: e0031921. doi: 10.1128/JCM.00319-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinheiro J, Bates D, R Core Team . nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-157 (2022). https://CRAN.R-project.org/package=nlme
- 10.Pinheiro JC, Bates DM. Mixed-Effects Models in S and S-PLUS. New York, Springer, 2000. doi: 10.1007/b98882 [DOI] [Google Scholar]
- 11.Lenth RV. emmeans: Estimated Marginal Means, aka Least-Squares Means. R package version 1.7.0 (2021). https://CRAN.R-project.org/package=emmeans
- 12.Benjamini, Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Annal Stat 2001; 29: 1165–1188. doi: 10.1214/aos/1013699998 [DOI] [Google Scholar]
- 13.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med 2021; 27: 1205–1211. doi: 10.1038/s41591-021-01377-8 [DOI] [PubMed] [Google Scholar]
- 14.Statistisches Bundesamt (StBA) : Health Personnel: Germany, Years, Facilities, Sex. Date last accessed: 24 May 2022. www-genesis.destatis.de/genesis/online?sequenz=tabelleErgebnis&selectionname=23621-0001&language=en#abreadcrumb
- 15.Barin B, Kasap U, Selçuk F, et al. Comparison of SARS-CoV-2 anti-spike receptor binding domain IgG antibody responses after CoronaVac, BNT162b2, ChAdOx1 COVID-19 vaccines, and a single booster dose: a prospective, longitudinal population-based study. Lancet Microbe 2022; 3: e274–e283. doi: 10.1016/S2666-5247(21)00305-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01390-2022.Shareable (982.5KB, pdf)


