Abstract
Background
Lynch syndrome (LS) is a cancer predisposition syndrome affecting more than 1 in every 300 individuals worldwide. Clinical genetic testing for LS can be life-saving but is complicated by the heavy burden of variants of uncertain significance (VUS), especially missense changes.
Result
To address this challenge, we leverage a multiplexed analysis of variant effect (MAVE) map covering >94% of the 17,746 possible missense variants in the key LS gene MSH2. To establish this map’s utility in large-scale variant reclassification, we overlay it on clinical databases of >15,000 individuals with LS gene variants uncovered during clinical genetic testing. We validate these functional measurements in a cohort of individuals with paired tumor-normal test results and find that MAVE-based function scores agree with the clinical interpretation for every one of the MSH2 missense variants with an available classification. We use these scores to attempt reclassification for 682 unique missense VUS, among which 34 scored as deleterious by our function map, in line with previously published rates for other cancer predisposition genes. Combining functional data and other evidence, ten missense VUS are reclassified as pathogenic/likely pathogenic, and another 497 could be moved to benign/likely benign. Finally, we apply these functional scores to paired tumor-normal genetic tests and identify a subset of patients with biallelic somatic loss of function, reflecting a sporadic Lynch-like Syndrome with distinct implications for treatment and relatives’ risk.
Conclusion
This study demonstrates how high-throughput functional assays can empower scalable VUS resolution and prospectively generate strong evidence for variant classification.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13059-022-02839-z.
Background
Identification of a pathogenic variant in a familial cancer risk gene can inform treatment and prevention strategies for patients and their blood relatives. As a prominent example of a common and broadly screened cancer risk syndrome, Lynch syndrome (LS) affects nearly 1 in 300 individuals worldwide [1, 2] and is primarily associated with colorectal and endometrial cancers. Heterozygous carriers’ risk for these cancers approaches ~80 and ~60%, respectively [3, 4], with onset decades earlier on average compared to sporadic cases [5].
LS is caused by inherited defects in any of four key DNA mismatch repair (MMR) factors: MSH2, MLH1, MSH6, and PMS2. These genes are included on most cancer gene panel tests, and it is standard of care to screen them for pathogenic germline variants when their loss is observed in tumors by histology or by tests for microsatellite instability, the mutational consequence of MMR loss. Increased carrier screening in LS holds great potential for reducing risk: it is estimated that the large majority of individuals who carry an LS variant go undetected at present [6], in part because many of them lack the clear family history needed to meet current genetic testing criteria [7, 8].
Despite decades of clinical screening and functional studies of MMR genes, upwards of one-third of the variants identified during clinical genetic evaluation for Lynch syndrome cannot be classified and remain as variants of uncertain significance, or VUS [9, 10]. The eventual reclassification rate for VUS in LS and other hereditary cancer genes is modest, reaching only ~25% [11, 12]. The difficulty of resolving these VUS stands as one of the most persistent barriers to the utility of broader genetic testing for LS genes [13].
As a group, missense variants are particularly challenging to interpret as most are individually rare (i.e., population minor allele frequencies ≤10−4), and they can have functional defects resulting from diverse molecular mechanisms [14]. Over 94% (n=8614) of the LS gene missense variants in the NCBI ClinVar database [15] remain as VUS or have conflicting interpretations, reflecting both the volume of VUS’ discovery and the challenge of their classification. Even when tumor molecular testing is available, it may not resolve these variants’ effects—for example, missense variants can retain protein staining by immunohistochemical testing despite causing mismatch repair deficiency, a source of false negatives that could potentially limit access to immunotherapy [16].
Functionally testing individual missense VUSs in experimental model systems is time- and labor-intensive but can provide key evidence to guide their classification. New approaches (collectively termed multiplex assays of variant effect, or MAVEs) have enabled the systematic testing of many variants at a time [17, 18]. Promisingly, benchmarking comparisons have shown that MAVEs can accurately recapitulate standing classifications from sources such as ClinVar [19, 20]. However, despite the recent proliferation of MAVE studies, practical examples of their use in clinical variant interpretation have been scarce. One recent study [21] evaluated MAVE scores’ predictive accuracy for variants found during genetic testing in three cancer-associated genes (BRCA1, TP53, and PTEN). Under recently proposed guidelines [22, 23], the authors were able to reclassify just over half (176/324) of the VUSs that had functional information across the three genes. In another recent study, the same BRCA1 MAVE scores [24] were intersected with variants discovered during exome sequencing of an unselected healthcare cohort, and an association between MAVE-predicted loss-of-function variants and breast and ovarian cancer diagnoses was observed [25].
Here, we set out to use MAVE-based function maps to facilitate variant classification in Lynch syndrome. We combined a recent MAVE [26] covering 16,749 missense variants in the key Lynch syndrome gene MSH2 with a clinical dataset containing 15,520 patients with an LS gene variant. We validated the MSH2 MAVE data across 47 previously classified missense variants and found that it meets the established threshold for ‘strong’ functional evidence [27]. Critically, during validation, we excluded any variants for which the standing classification relied upon functional data, thus avoiding the risk of circularity inherent in validating one functional assay using another. We then applied these MAVE scores to 682 standing MSH2 missense VUS, formally reclassifying 10 to pathogenic/likely pathogenic, and showing that another 497 could be moved to benign/likely benign upon reassessment. Leveraging the detailed, individual-level clinical information in this cohort, we demonstrate that missense MSH2 variants with abnormal MAVE function scores are associated with elevated colorectal and endometrial cancer risk. Finally, going beyond germline variant interpretation, we demonstrate that MAVE scores can uncover loss-of-function somatic ‘second hits’ as well as biallelic mutations with the availability of tumor DNA tests.
Results
Clinical validation of MSH2 function map
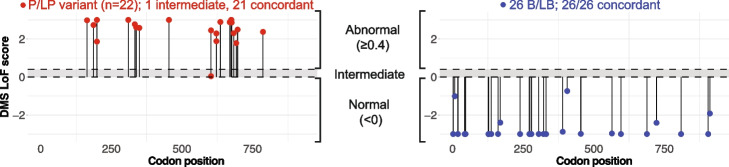
To establish the clinical validity of multiplexed analyses of variant effect (MAVEs) for interpreting and reclassifying variants in Lynch syndrome, we intersected loss-of-function (LoF) scores from a deep mutational scan of MSH2 [26] with a clinical database of 1604 individuals with MMR gene variants detected by paired tumor and germline testing. To gauge the strength of evidence provided by our functional data, we curated a list of MSH2 missense variants previously classified as pathogenic/likely pathogenic (n=22) or benign/likely benign (n=26) as known controls. Crucially, to avoid the circularity of validating one functional assay using classifications that relied upon other mechanistically similar assays, we included only variants for which clinical interpretation could be reached without the use of any prior functional evidence (e.g., only using population frequency, family history, or tumor characteristics).
Our functional measurements agreed with the clinical interpretation for all 47 of the 47 variants that scored as functionally abnormal or normal (Fig. 1, and Additional file 1: Table S1), with one pathogenic variant scoring in the intermediate range. This resulted in a strength of evidence, as quantified by the OddsPath score [28], of 24.9 for abnormal LoF scores, and 0.043 for neutral scores. Following recommendations for application of the functional evidence criterion using the ACMG/AMP variant interpretation framework [27], MSH2 LoF scores ≥ 0.4 can therefore be used as ‘strong evidence’ in support of variant pathogenicity (PS3 evidence code), while LoF scores < 0 can be conversely be used a ‘strong evidence’ against pathogenicity (BS3 evidence code).
Fig. 1.
Validation of MSH2 function scores across 48 previously classified MSH2 missense variants. LoF scores for known pathogenic/likely pathogenic (red, at left) and known benign/likely benign variants (blue, at right) are plotted against codon position. Gray shaded interval denotes intermediate score range
We next applied the MSH2 missense function scores to a larger panel of individuals (n=13,916) for whom germline-only testing had identified at least one germline Lynch syndrome gene variant. Among this cohort, 1937 individuals carried a scorable MSH2 missense variant. We focused first on the 32 distinct missense variants, carried by 108 individuals, which had been previously classified by the clinical laboratory as pathogenic or likely pathogenic. Of those, 31 had an abnormal function score. The lone exception was the missense variant NM_000251.3(MSH2):c.2020G>A (p.Gly674Ser), which was classified as likely pathogenic and has been shown to be partially deficient in ATP binding in vitro [29]; this variant may be a false negative in the functional assay. Of the 31 correctly identified pathogenic variants, 27/31 had an abnormal LoF score from deep mutational scanning, while 4/31 were predicted to be splice disruptive by SpliceAI, indicating that at least among known variants in MSH2, disruption at the level of protein function contributes a greater share of the pathogenic burden than splicing defects. In all, the MSH2 missense function scores achieved a recall of 96.9% in a validation dataset independent from the patient cohort used to derive the OddsPath score.
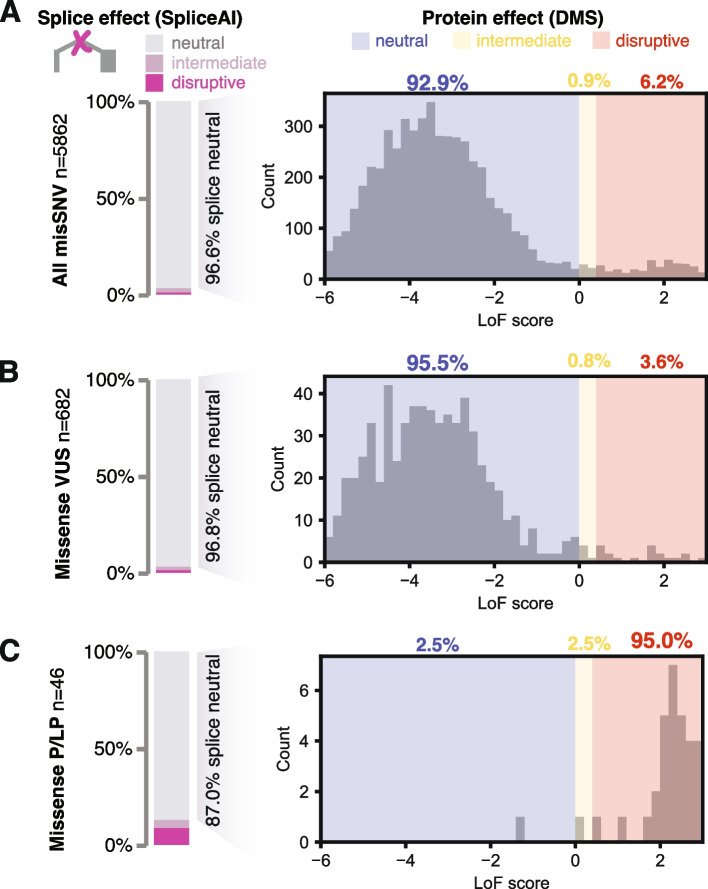
For the overwhelming majority of MSH2 missense carriers in this cohort, the variants carried were VUS (1829 individuals, 94.4%). Of the 682 unique such missense VUS, 5.0% scored as functionally abnormal: 24 by DMS LoF score and another 10 by SpliceAI (with another 17 in the intermediate range by either measure; Fig. 2). Thus, loss of function was modestly depleted among these extant variants, relative to the 5130 missense SNVs not observed in this study, among which 6.8% were abnormal (298 and 53 each by DMS and SpliceAI, respectively; P=0.048, two-sided binomial test). The depletion of functionally deleterious variants among standing missense VUSs likely reflects the ongoing removal of those with sufficient lines of evidence to be classified as pathogenic.
Fig. 2.
Function scores and variant reclassification for MSH2 missense variants, for A all single nucleotide missense variants, B missense VUSs, and C missense variants previously classified as pathogenic or likely pathogenic, including those used for validation. For each group of variants, splicing status was scored by SpliceAI (bar charts at left), and for splice-neutral variants (SpliceAI score<0.2), a histogram of LoF scores from deep mutational scanning are displayed to the right
VUS reclassification
We next pursued clinical variant reclassification for MSH2 missense variants with abnormal DMS LoF scores, adding functional evidence codes in support of their pathogenicity. Of the 24 such variants, 14 variants had scores exceeding (i.e., more abnormal than) the lowest score in the P/LP validation set (LoF score ≥1.7), and we added PS3 (strong evidence) codes for each of these. After adding this evidence, ten of these VUSs met criteria to be reclassified as pathogenic or likely pathogenic (Table 1). Consistent with their causal, pathogenic role, nine of these ten exhibited MMR deficiency as shown by IHC and/or MSI testing. To be conservative, we did not pursue formal reclassification for variants with LoF scores between 0.4 and 1.7; while these were in the abnormal range, they scored below all the training variants used to establish the OddsPath score, and these could in principle be given a weaker evidence code (PS3_moderate) in the future. In sum, there were 14 remaining missense VUSs in the functionally abnormal range which require additional evidence for formal reclassification under the ACMG/AMP framework, including observation in additional cases, or co-occurrence with a somatic loss-of-function variant in the same gene. In the other direction, there were 635 patient missense VUSs (carried by 1772 individuals) which were functionally normal by both deep mutational scanning and SpliceAI prediction. We set out to determine what percentage could potentially be reclassified as benign/likely benign by adding a BS3 functional evidence code and found that 497 of these variants (78.2%) could be reclassified to B/LB with the addition of that evidence. Thus, with the addition of functional evidence, approximately three quarters of all standing MSH2 missense VUS in this large patient cohort could be newly classified (Fig. 3).
Table 1.
Summary of reclassification for missense MSH2 germline VUS with abnormal LoF scores (n=24), with evidence codes applied for each variant
| Variant (in NM_000251.3) | Reclassification outcome | LOF Score (Jia et al. 2021) [26] | MAPP MMR (Chao et al. 2008) [30] | gnomAD frequency | LOF score evidence | Pop. database evidence | Clinical (MSI/IHC) | Computational evidence | Additional evidence |
|---|---|---|---|---|---|---|---|---|---|
| c.2083G>A (p.Val695Met) | Reclassified to LP | 4.511 | 20.15 | - | PS3 | PM2_P | PP4 | ||
| c.929T>G (p.Leu310Arg) | Reclassified to LP | 3.774 | 33.02 | - | PS3 | PM2_P | PP4_S | PP3 | PP1 |
| c.1568T>C (p.Phe523Ser) | Reclassified to LP | 3.547 | 10.09 | - | PS3 | PM2_P | PP4 | PM1 | |
| c.443T>A (p.Val148Glu) | Insufficient evidence | 3.239 | 4.13 | - | PS3 | PM2_P | PP4 | ||
| c.2011A>T (p.Asn671Tyr) | Reclassified to LP | 3.114 | 20.2 | - | PS3 | PM2_P | PS4_M | PP3 | PM1_P |
| c.1808A>T (p.Asp603Val) | Reclassified to LP | 2.999 | 22.25 | - | PS3 | PM2_P | PP4 | PP3 | |
| c.1067T>A (p.Ile356Lys) | Reclassified to LP | 2.518 | 14.66 | - | PS3 | PM2_P | PP4 | PP3 | |
| c.2030C>G (p.Thr677Arg) | Reclassified to LP | 2.469 | 37.76 | - | PS3 | PM2_P | PP4_M | PP3 | PM1 |
| c.2276G>T (p.Gly759Val) | Insufficient evidence | 2.215 | 29.36 | - | PS3 | PM2_P | PP3 | ||
| c.2281G>C (p.Gly761Arg) | Reclassified to LP | 2.193 | 40.89 | - | PS3 | PM2_P | PM1_P | ||
| c.2272G>T (p.Asp758Tyr) | Insufficient evidence | 1.955 | 15.52 | - | PS3 | PM2_P | |||
| c.2099C>A (p.Ala700Glu) | Reclassified to LP | 1.917 | 37.91 | - | PS3 | PM2_P | PP4 | ||
| c.2282G>T (p.Gly761Val) | Insufficient evidence | 1.785 | 9.19 | - | PS3 | PM2_P | |||
| c.1241T>C (p.Leu414Pro) | Reclassified to LP | 1.766 | 12.66 | - | PS3 | PM2_P | PP4 | PM1 | |
| c.2768T>A (p.Val923Glu) | Conflicting/insufficient evidence | 1.669 | n/a | - | PS3_moderatea | PM2_P | BS2_P | ||
| c.1654A>C (p.Thr552Pro) | Insufficient evidence | 1.61 | 10.84 | - | PS3_moderatea | PM2_P | PS4_M | ||
| c.1601G>C (p.Arg534Pro) | Insufficient evidence | 1.383 | 30.22 | - | PS3_moderatea | PM2_P | |||
| c.1703C>T (p.Thr568Ile) | Conflicting/insufficient evidence | 0.932 | 4.04 | - | PS3_moderatea | PM2_P | BP4 | ||
| c.1862G>C (p.Arg621Pro) | Insufficient evidence | 0.745 | 30.86 | - | PS3_moderatea | PM2_P | PP4 | ||
| c.2272G>C (p.Asp758His) | Insufficient evidence | 0.701 | 18.79 | - | PS3_moderatea | PM2_P | |||
| c.386C>G (p.Ser129Cys) | Conflicting/insufficient evidence | 0.574 | 1.84 | 2.35E−05 | PS3_moderatea | PP4 | BS2_P | ||
| c.925G>C (p.Ala309Pro) | Insufficient evidence | 0.528 | 7.68 | - | PS3_moderatea | PM2_P | PP3 | ||
| c.2132G>T (p.Arg711Leu) | Conflicting/insufficient evidence | 0.491 | 19.03 | - | PS3_moderatea | PM2_P | BS2_P | PP3 | |
| c.1571G>T (p.Arg524Leu) | Insufficient evidence | 0.449 | 17.81 | - | PS3_moderatea | PM2_P |
aPS3_moderate codes are applicable for variants with scores between 0.4 and 1.7, but their reclassification was not pursued here
Fig. 3.
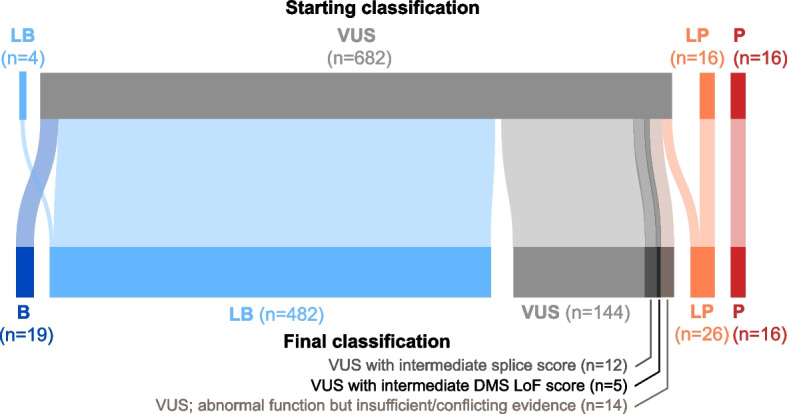
Reclassification outcomes for 718 MSH2 missense variants. Flow diagram showing starting and final variant classifications; in total, 74% of the missense VUSs have sufficient evidence to enable potential reclassification to benign (B), likely benign (LB), likely pathogenic (LP) or pathogenic (P). A subset of remaining VUSs had intermediate function scores (n=19) or had abnormal function scores but lacked sufficient lines of evidence (or had conflicting evidence) and so remain as VUS (n=12)
Cancer prevalence among LOF VUS missense carriers
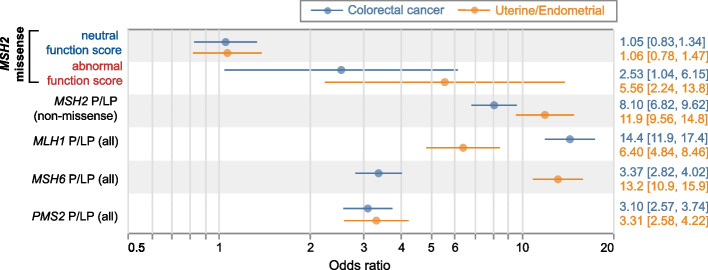
We next sought to compare the risk conferred by LOF MSH2 missense variants to that of established P/LP variants in MSH2 and other LS genes. In this patient cohort, LS-related cancer diagnoses were enriched relative to the general population, but far from completely prevalent: 13.6% of patients in this cohort had a CRC diagnosis, with higher rates in males (38.3%, n=2229) compared to females (8.9%, n=11,687), possibly reflecting broader inclusion criteria for genetic screening in women (e.g., for breast cancer). Uterine and endometrial cancers (UEC) were similarly prevalent to CRC, affecting 9.5% of females. Other cancers not primarily associated with Lynch syndrome were also prevalent in this cohort, affecting 49.6% of females and 40.7% of males. Gene by gene differences in penetrance closely mirrored those seen previously [2, 31]: P/LP variants in MLH1 and MSH2 were the most strongly associated with CRC (odds ratio=14.4 and 8.10, respectively), with lesser effects from MSH6 and PMS2 P/LP variants. As previously noted [5], uterine and endometrial cancers differed from colorectal cancer, with MSH6 (OR=13.2) and MSH2 (OR=11.9) emerging as the top risk factors, followed by MLH1 and PMS2. Separating MSH2 missense variants by their functional status, those with abnormal function scores (by DMS or SpliceAI) were significantly associated with both CRC (OR=2.53, 95%CI:[1.04, 6.15], P=0.04) and EC (OR=5.56, 95%CI:[2.24,13.8], P=2.2×10−4), though with smaller effects than truncating P/LP variants’ (Fig. 4). By contrast, MSH2 missense variants with neutral function scores did not contribute significant risk for CRC or EC (P≥0.67 for each), nor were they associated with other cancers (Additional file 2: Fig. S1). Thus, loss-of-function missense variants in MMR genes contribute measurable risk for LS-associated cancers, but may exhibit lower risk than their truncating counterparts, underscoring the challenge of their accurate classification.
Fig. 4.
Cancer associations by variant type. Association between colorectal cancer (blue) or uterine/endometrial cancer (female) and missense variants in MSH2 (missense, separated by DMS+SpliceAI function score), or P/LP variants in other Lynch syndrome genes; odds ratios shown from logistic regression
Joint annotation of germline and somatic variants
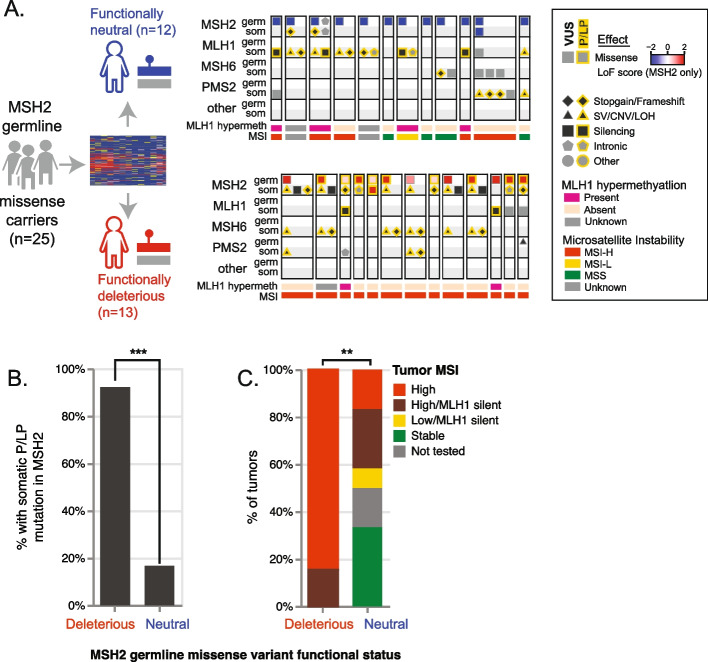
We next sought to apply these functional measures to jointly interpret germline and somatic mutations in MSH2. Most cases of Lynch syndrome follow a ‘two hit’ model, with one inherited loss-of-function variant followed by a second somatic mutation disrupting the remaining copy. Therefore, it is expected that in a cohort including individuals with LS, pathogenic somatic ‘second hits’ in MSH2 would be more common among those individuals who inherited a ‘first hit’ loss-of-function variant in the same gene. We tested this within the paired tumor-normal cohort (n=1604 individuals), among the 25 individuals for whom the sole germline finding was a missense MSH2 variant (Fig. 5 and Additional file 3: Table S2). DMS scores indicated 13 of these 25 germline variants are functionally deleterious, constituting pathogenic inherited ‘first hits’. Among these 13 carriers, 12 (92.4%) had a P/LP somatic ‘second hit’ in MSH2, or a structural variant in the upstream gene EPCAM (which causes epigenetic silencing of MSH2) [32, 33]. By contrast, among the other 12 individuals who inherited a single MSH2 missense variant scored as neutral by DMS, only two (16.7%) had a P/LP somatic mutation in MSH2, a significantly lower prevalence (P=0.00021, Fisher’s exact test).
Fig. 5.
Joint analysis of germline and tumor mutations. A Patterns of germline and somatic mutations among LS genes, among germline carriers of MSH2 missense variants, separated into those scored as functionally neutral (top) or deleterious (bottom) by deep mutational scanning LoF score. B Fraction of individuals with a somatic P/LP mutation in MSH2, by MSH2 germline missense functional status. C Tumor microsatellite status, by MSH2 germline missense variant functional status. ***, P<0.001; **, P<0.01
We next examined these functional measures’ association with tumor microsatellite instability (MSI), a hallmark of MMR deficiency. By genotyping at microsatellite markers, tumors can be rated as microsatellite stable (MSS), MSI-low, or MSI-high, with distinct implications for treatment and prognosis [34]. We observed that MSI was universal among patients with MSH2 missense alleles that had abnormal function scores in our map, reflecting their functional disruptiveness. After excluding individuals with MLH1 promoter hypermethylation (an independent somatic epigenetic mechanism sufficient to cause MMR deficiency and MSI), tumors from all 11 of 11 individuals who carried a germline MSH2 missense variant deemed LoF by DMS were MSI-high, whereas only 2/6 individuals with a germline functionally neutral missense MSH2 variant and without MLH1 hypermethylation had MSI-high or MSI-low tumors (P=0.006), and both of those two cases could be explained by biallelic somatic mutations in other LS genes (in one, NM_000535.7(PMS2):c.1687C>T (p.Arg563Ter) + LOH; in the other, NM_000249.4(MLH1):c.676C>T (p.Arg226Ter) + LOH). The ability of the MSH2 function map to identify germline variants associated with pathogenic somatic second hits, and MSI demonstrates how patterns of tumor mutation can support germline variant classification [35].
Somatic mutations also contribute to the variant interpretation burden, particularly for heavily mutated MMR-deficient tumors. Indeed, among the 437 individuals for which paired testing revealed at least one somatic MSH2 mutation, most (382, 87.4%) had more than one somatic mutation in a tested gene, and nearly half (182, 47.6%) had multiple somatic mutations in MSH2 alone. We focused on the 84 individuals who carried at least one missense somatic MSH2 variant, comparing the 46 carrying at least one somatic missense MSH2 variant we predict to be functionally disruptive, and the 38 for whom these somatic missense variants were exclusively functionally neutral and who did not carry any other somatic P/LP MSH2 mutations (Fig. 6 and Additional file 4: Table S3). Notably, among the latter (carriers of exclusively functionally neutral somatic MSH2 variants), additional somatic mutations in other LS genes (i.e., MSH6, MLH1, PMS2) were found in all 38 tumors (100%). By contrast, when at least one functionally disruptive MSH2 missense somatic mutation was found, somatic mutations in other LS genes were significantly less common (30 of 46 tumors; Fisher’s exact P=2.70×10−5). Similarly, MLH1 promoter hypermethylation was present in nearly a third of the tumors for which the only somatic MSH2 mutations were functionally neutral missense (9 of the 28 tumors in which MLH1 was assayed, 32.1%) but nearly absent among tumors with at least one somatic missense MSH2 mutation deemed LoF by DMS/SpliceAI (1 of 38, 2.6%; Fisher’s exact P=0.0013). MAVE measurements can thus identify functionally disruptive somatic mutations driving tumor MMR deficiency even in the absence of an inherited loss-of-function variant.
Fig. 6.
Mutational patterns in patients with somatic MSH2 missense variants. Tumor and germline mutations in LS genes are shown in patients who carry functionally neutral somatic MSH2 missense variants (upper track, n=38), or those with a functionally abnormal somatic MSH2 variant (lower track, n=46). Mutations and tumor characteristics are denoted as in Fig. 5A
Discussion
As MAVE function maps are put into practice for clinical variant interpretation, an important prerequisite is to assess their predictive value for disease risk and clinical phenotypes. Here we did so in the context of MSH2, a key DNA repair factor underyling Lynch syndrome. We supplemented protein-level MAVE effect measurements with deep learning-based splicing effect predictions [36], to newly reclassify over 74% of the 682 missense variants of uncertain significance (VUS) encountered in MSH2 in a cohort of tens of thousands of individuals with genetic testing results.
In particular, the reclassification of 10 variants to pathogenic/likely pathogenic newly enabled the return of definitive genetic diagnoses. Reclassification of these variants as pathogenic has critical clinical implications for these patients and family members who also inherited them, such as more frequent colonoscopies, risk-reducing surgeries to avoid gynecologic cancers, initiation of esophagogastroduodenoscopy for upper GI cancer surveillance and additional cancer screening recommendations not necessarily performed for the general population. Going forward, these MAVE-based function scores are now integrated into the variant interpretation process at clinical genetic testing laboratories and will assist with classification of newly observed rare MSH2 missense variants.
Our study leverages several unique features of this large cohort. Firstly, to validate the MSH2 MAVE, we selected a set of control variants for which the classification stands without including prior functional evidence, that is, based upon orthogonal features such as recurrence, tumor characteristics, and co-segregation with early-onset cancer. This avoids the risk of validating a MAVE in part by prior functional evidence from existing assays, which despite being lower-throughput, may be mechanistically similar and highly correlated with the MAVE.
For many genes, culling the training set to remove such variants may not be practical—obtaining a sufficient number of control variants is emerging as a key rate limiting step for many MAVEs; at least 11 control variants are needed to reach a ‘moderate’ strength of evidence [27]. This challenge is highlighted by a recent effort to reclassify variants in PTEN using MAVE data [21], which was hampered by the limited number (n=2) of known benign variants. In many cases, filtering variants for this or other criteria may not even be possible: public-facing databases such as ClinVar are often the primary source for these controls, and to protect privacy, they do not provide individual-level clinical or demographic data.
We used per-individual clinical information to explore the association between loss of function, as indicated by MAVE scores, and cancer prevalence. We observed that MSH2 missense variants with abnormal function identified by MAVE were associated with significantly elevated risk for LS-associated colorectal and uterine/endometrial cancers. Notably, these associations were weaker than those observed for P/LP variants that were not missense (i.e., truncating frameshift or stop-gain variants). Thus, functionally abnormal MSH2 missense variants as a group may be less penetrant than their truncating counterparts, while still being measurably pathogenic within the population of individuals selected for germline cancer testing.
In contrast, another recent study of MMR gene variant carriers found no difference in the incidence of LS-related cancers between carriers of MSH2 missense P/LP and truncating variants [37]. A key difference, however, was that the missense variants included in that study were restricted to those with standing P/LP classifications, which may reflect a particularly severe subset. Thus, the addition of MAVE-based functional data may have captured missense variants with intermediate functional defects which confer a moderate level of risk. An important future direction will be to replicate this analysis in an unselected population, as has recently been done for BRCA1 [25], and to model polygenic risk as a potential modifier [38].
To date, applications of MAVE data have largely focused on germline variants. Here, we demonstrated how MAVEs can also support joint analyses of germline and somatic mutations, leveraging a clinical database of 1604 individuals with paired tumor-normal tests. As expected under Knudson’s two-hit hypothesis [39], among individuals who inherited an MSH2 missense variant, pathogenic somatic ‘second hits’ in MSH2 were significantly more common when the MAVE data indicated the germline variant was functionally disruptive as compared to normal. In addition, we identified 29 individuals whose cancers had double MSH2 somatic mutations with at least one of the mutations identified as functionally deleterious by MAVE. Excluding an inherited predisposition as the cause for these individuals’ MMRd tumors has the potential to prevent unnecessary screenings for their blood relatives.
The large majority of MSH2 missense VUS considered here are functionally normal and do not provide a basis for a positive diagnosis. Nevertheless, in the context of an affected individual, they may still be partially informative by suggesting that ‘causal’ variant(s) may reside at a different locus. Alternatively, they may suggest a different molecular mechanism, including somatic mutation, epigenetic silencing (e.g., MLH1 promoter hypermethylation or loss of MSH2 expression secondary to EPCAM 3’ deletions), or structural variants [40, 41], some of which are challenging to detect by standard pipelines. Indeed, we observed that germline carriers of functionally normal MSH2 missense variants had much higher rates of MLH1 disruption by silencing and/or somatic mutation (Fig. 5) relative to carriers of disruptive MSH2 missense variants. Likewise, individuals with functionally normal somatic missense mutations in MSH2 had a much higher rate of somatic disruption of the other three primary LS factors (Fig. 6). As MAVE function maps become more broadly available, they may allow previously suspected VUS to be ruled out, offering an opportunity to identify previously obscured functional variants elsewhere.
A limitation of this study is that the MAVE function scores used here were derived from a cDNA-based deep mutational scan and so do not capture splice disruptive effects, which although in the minority relative to protein-disruptive variants, may still account for a substantial number of cases for LS [42, 43]. These effects can be obtained experimentally with other MAVE approaches such as saturation genome editing [24] or saturation prime editing [44], or directly measured by massively parallel splicing assays [45–47]. For the purposes of this study, we used predictions from SpliceAI [36], a deep learning-based splicing effect predictor which has been shown to be highly accurate [48].
Conclusions
As gene panel and exome sequencing are increasingly utilized in the clinical setting for a variety of indications, there is an opportunity to leverage the massive scale of MAVE experiments to prospectively generate functional evidence for as yet unseen variants. Here we established the validity of MAVE-based functional evidence for missense variant classification in the Lynch syndrome gene MSH2, and demonstrated how these functional measures can support resolution of standing missense VUS. With proper clinical validation, it appears promising that MAVE data may soon play a primary role in identifying patients who may not have otherwise come to clinical attention, but could benefit from additional monitoring based upon their genetic risk.
Methods
Patient population
Clinical information and genetic variants were obtained for patients found to carry at least one variant in any of the four major Lynch syndrome genes (MSH2, MLH1, MSH6, PMS2) during multi-gene panel testing for cancer predisposition at Ambry Genetics before December 14, 2020. We obtained data for 13,916 LS gene variant carriers who underwent germline-only testing, and another 1604 patients who underwent paired tumor-germline testing at Ambry Genetics before August 31, 2020.
Functional annotation of MSH2 missense variants
Each MSH2 missense variant was annotated with two function scores: the loss-of-function (LoF) scores from a recent deep mutational scan [26] which measures impact upon MSH2 protein function, and SpliceAI deltaMax scores [36], a computational estimate of the probability of splicing disruption. Variants with an LoF score ≥ 0.4 or a SpliceAI deltaMax score ≥0.5 were considered deleterious; those with LoF scores between 0 and 0.4 or deltaMax between 0.2 and 0.5 were considered intermediate, while those with LoF scores < 0 and deltaMax < 0.2 were considered functionally neutral.
Variant classification
Patient variant classification was performed at Ambry Genetics using a point-based implementation [49] of ACMG/AMP variant classification guidelines [23, 50], assigning each variant into one of five tiers: pathogenic (P), likely pathogenic (LP), uncertain significance (VUS), likely benign (LB) or benign (B). To validate the MSH2 missense function scores, we used previously classified MSH2 missense variants from the paired (tumor-normal) dataset. To avoid redundant application of evidence, we used only those variants which had sufficient evidence to be classified as benign/likely benign and pathogenic/likely pathogenic without use of any prior functional data. Function scores’ strength of evidence for or against pathogenicity was quantified using the Oddspath score [28]. Additionally, structural evidence was assessed using a standard structural modelling protocol and energies of destabilization compared to nearby informative variants and identification of impacted motifs [51, 52].
Cancer association
For analysis of cancer prevalence among LS variant carriers, patients were categorized by variant classification(s) and gene(s) affected. For analysis of MSH2 VUS carriers, we excluded individuals who also carried a pathogenic or likely pathogenic (P/LP) variant in a non-Lynch syndrome gene, while individuals with both an MSH2 VUS and a P/LP variant in MLH1, MSH6, or PMS2 were considered as carriers for those respective genes and were excluded from MSH2 VUS association tests. Logistic regression models were fit using the python statsmodels package version 0.12.2 [53] using cancer diagnosis as the response variable and, as features, each individual’s carrier status for the following categories of variants, each encoded as zero or one: (1) MSH2 missense with deleterious function score, (2) MSH2 missense with neutral function score, (3) MSH2 other P/LP, (4) MLH1 any P/LP, (5) MSH6 any P/LP, and (6) PMS2 any P/LP. Models were fit separately for colorectal cancer and uterine/endometrial cancer (in the latter case, including only females).
Supplementary Information
Additional file 1: Table S1. Validation variants used to calculate OddsPath score.
Additional file 2: Figure S1. Associations with other cancers, by variant type.
Additional file 3: Table S2. Variants identified by paired tumor-normal test, among patients with a single germline MSH2 variant.
Additional file 4: Table S3. Tumor and germline mutations in individuals with MSH2 somatic tumor mutations.
Acknowledgements
We thank Matthew Varga and Min-Sun Park of Ambry Genetics for support of the structural investigation of these variants, and members of the Kitzman lab for helpful comments.
Review history
The review history is available as Additional file 5.
Peer review information
Anahita Bishop was the primary editor of this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Authors’ contributions
F.H. and R.K. abstracted clinical information. A.S., F.H., A.C., C.S., and J.O.K. analyzed the data. A.S., F.H., R.K., and J.O.K. wrote the manuscript. All author(s) read and approved the final manuscript.
Authors’ information
Twitter handles: @rachidkaram (Rachid Karam), @jacobkitzman (Jacob O. Kitzman)
Funding
This work was supported by the National Institute of General Medical Sciences (R01GM129123 to J.O.K.).
Availability of data and materials
MSH2 loss-of-function scores are available at MaveDB (https://www.mavedb.org) under accession urn:mavedb:00000050-a and are also provided as Table S4 in the published MSH2 deep mutational scan [26]. Underlying sequencing counts are available from NCBI GEO under accession GSE162130 [26].
MMR gene variants and clinical interpretation are deposited in NCBI ClinVar: https://www.ncbi.nlm.nih.gov/clinvar/?gr=0&term=(((msh2%5BGene%20Name%5D)%20OR%20msh6%5BGene%20Name%5D)%20OR%20mlh1%5BGene%20Name%5D)%20OR%20pms2%5BGene%20Name%5D
SpliceAI software: https://github.com/Illumina/SpliceAI [36].
Declarations
Ethics approval and consent to participate
Data collection and sharing procedures were reviewed by the Western IRB and University of Michigan Medical School IRB (study HUM00220511) and declared exempt from human subject regulations. All experimental methods comply with the Helsinki Declaration.
Consent for publication
Not applicable.
Competing interests
F.H., A.C., and R.K. are employees of Ambry Genetics. J.O.K. serves as a scientific advisor to MyOme, Inc. The authors declare that there are no further competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Win AK, Jenkins MA, Dowty JG, Antoniou AC, Lee A, Giles GG, et al. Prevalence and penetrance of major genes and polygenes for colorectal cancer. Cancer Epidemiol Biomark Prev. 2017;26:404–412. doi: 10.1158/1055-9965.EPI-16-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haraldsdottir S, Rafnar T, Frankel WL, Einarsdottir S, Sigurdsson A, Hampel H, et al. Comprehensive population-wide analysis of Lynch syndrome in Iceland reveals founder mutations in MSH6 and PMS2. Nat Commun. 2017;8:14755. doi: 10.1038/ncomms14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jasperson KW, Tuohy TM, Neklason DW, Burt RW. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044–2058. doi: 10.1053/j.gastro.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira L, Balaguer F, Lindor N, de la Chapelle A, Hampel H, Aaltonen LA, et al. Identification of Lynch syndrome among patients with colorectal cancer. JAMA. 2012;308:1555–1565. doi: 10.1001/jama.2012.13088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez-Valentin M, Sampson JR, Seppälä TT, ten Broeke SW, Plazzer J-P, Nakken S, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the Prospective Lynch Syndrome Database. Genet Med. 2019;22:15–25. doi: 10.1038/s41436-019-0596-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampel H, de la Chapelle A. The search for unaffected individuals with Lynch syndrome: do the ends justify the means? Cancer Prev Res. 2011;4:1–5. doi: 10.1158/1940-6207.CAPR-10-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sjursen W, Haukanes BI, Grindedal EM, Aarset H, Stormorken A, Engebretsen LF, et al. Current clinical criteria for Lynch syndrome are not sensitive enough to identify MSH6 mutation carriers. J Med Genet. 2010;47:579–585. doi: 10.1136/jmg.2010.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.LaDuca H, Polley EC, Yussuf A, Hoang L, Gutierrez S, Hart SN, et al. A clinical guide to hereditary cancer panel testing: evaluation of gene-specific cancer associations and sensitivity of genetic testing criteria in a cohort of 165,000 high-risk patients. Genet Med. 2020;22:407–415. doi: 10.1038/s41436-019-0633-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sijmons RH, Greenblatt MS, Genuardi M. Gene variants of unknown clinical significance in Lynch syndrome. An introduction for clinicians. Familial Cancer. 2013;12:181–187. doi: 10.1007/s10689-013-9629-8. [DOI] [PubMed] [Google Scholar]
- 10.Tricarico R, Kasela M, Mareni C, Thompson BA, Drouet A, Staderini L, et al. Assessment of the InSiGHT Interpretation Criteria for the Clinical Classification of 24 MLH1 and MSH2 Gene Variants. Hum Mutat. 2017;38:64–77. doi: 10.1002/humu.23117. [DOI] [PubMed] [Google Scholar]
- 11.Mersch J, Brown N, Pirzadeh-Miller S, Mundt E, Cox HC, Brown K, et al. Prevalence of variant reclassification following hereditary cancer genetic testing. JAMA. 2018;320:1266–1274. doi: 10.1001/jama.2018.13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Welsh JL, Hoskin TL, Day CN, Thomas AS, Cogswell JA, Couch FJ, et al. Clinical decision-making in patients with variant of uncertain significance in BRCA1 or BRCA2 Genes. Ann Surg Oncol. 2017;24:3067–3072. doi: 10.1245/s10434-017-5959-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hampel H, Yurgelun MB. Point/counterpoint: is it time for universal germline genetic testing for all GI cancers? J Clin Oncol. 2022:JCO2102764. [DOI] [PubMed]
- 14.Backwell L, Marsh JA. Diverse molecular mechanisms underlying pathogenic protein mutations: beyond the loss-of-function paradigm. Annu Rev Genomics Hum Genet. 2022. 10.1146/annurev-genom-111221-103208. [DOI] [PubMed]
- 15.Landrum MJ, Lee JM, Benson M, Brown GR, Chao C, Chitipiralla S, et al. ClinVar: improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2018;46:D1062–D1067. doi: 10.1093/nar/gkx1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hechtman JF, Rana S, Middha S, Stadler ZK, Latham A, Benayed R, et al. Retained mismatch repair protein expression occurs in approximately 6% of microsatellite instability-high cancers and is associated with missense mutations in mismatch repair genes. Mod Pathol. 2020;33:871–879. doi: 10.1038/s41379-019-0414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weile J, Roth FP. Multiplexed assays of variant effects contribute to a growing genotype-phenotype atlas. Hum Genet. 2018;137:665–678. doi: 10.1007/s00439-018-1916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Starita LM, Ahituv N, Dunham MJ, Kitzman JO, Roth FP, Seelig G, et al. Variant interpretation: functional assays to the rescue. Am J Hum Genet. 2017;101:315–325. doi: 10.1016/j.ajhg.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Livesey BJ, Marsh JA. Using deep mutational scanning to benchmark variant effect predictors and identify disease mutations. Mol Syst Biol. 2020;16:e9380. doi: 10.15252/msb.20199380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cubuk C, Garrett A, Choi S, King L, Loveday C, Torr B, et al. Clinical likelihood ratios and balanced accuracy for 44 in silico tools against multiple large-scale functional assays of cancer susceptibility genes. Genet Med. 2021. 10.1038/s41436-021-01265-z. [DOI] [PMC free article] [PubMed]
- 21.Fayer S, Horton C, Dines JN, Rubin AF, Richardson ME, McGoldrick K, et al. Closing the gap: Systematic integration of multiplexed functional data resolves variants of uncertain significance in BRCA1, TP53, and PTEN. Am J Hum Genet. 2021;108:2248–2258. doi: 10.1016/j.ajhg.2021.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gelman H, Dines JN, Berg J, Berger AH, Brnich S, Hisama FM, et al. Recommendations for the collection and use of multiplexed functional data for clinical variant interpretation. Genome Med. 2019;11:85. doi: 10.1186/s13073-019-0698-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brnich SE, Rivera-Muñoz EA, Berg JS. Quantifying the potential of functional evidence to reclassify variants of uncertain significance in the categorical and Bayesian interpretation frameworks. Hum Mutat. 2018;39:1531–1541. doi: 10.1002/humu.23609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Findlay GM, Daza RM, Martin B, Zhang MD, Leith AP, Gasperini M, et al. Accurate classification of BRCA1 variants with saturation genome editing. Nature. 2018;562:217–222. doi: 10.1038/s41586-018-0461-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiabor Barrett KM, Masnick M, Hatchell KE, Savatt JM, Banet N, Buchanan A, et al. Clinical validation of genomic functional screen data: analysis of observed BRCA1 variants in an unselected population cohort. HGG Adv. 2022;3:100086. doi: 10.1016/j.xhgg.2022.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia X, Burugula BB, Chen V, Lemons RM, Jayakody S, Maksutova M, et al. Massively parallel functional testing of MSH2 missense variants conferring Lynch syndrome risk. Am J Hum Genet. 2021;108:163–175. doi: 10.1016/j.ajhg.2020.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brnich SE, Abou Tayoun AN, Couch FJ, Cutting GR, Greenblatt MS, Heinen CD, et al. Recommendations for application of the functional evidence PS3/BS3 criterion using the ACMG/AMP sequence variant interpretation framework. Genome Med. 2019;12:3. doi: 10.1186/s13073-019-0690-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tavtigian SV, Greenblatt MS, Harrison SM, Nussbaum RL, Prabhu SA, Boucher KM, et al. Modeling the ACMG/AMP variant classification guidelines as a Bayesian classification framework. Genet Med. 2018;20:1054–1060. doi: 10.1038/gim.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heinen CD, Wilson T, Mazurek A, Berardini M, Butz C, Fishel R. HNPCC mutations in hMSH2 result in reduced hMSH2-hMSH6 molecular switch functions. Cancer Cell. 2002;1:469–478. doi: 10.1016/S1535-6108(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 30.Chao EC, Velasquez JL, Witherspoon MS, Rozek LS, Peel D, Ng P, Gruber SB, Watson P, Rennert G, Anton-Culver H, Lynch H, Lipkin SM. Accurate classification of MLH1/MSH2 missense variants with multivariate analysis of protein polymorphisms-mismatch repair (MAPP-MMR). Hum Mutat. 2008;29(6):852-60. 10.1002/humu.20735. [DOI] [PubMed]
- 31.Møller P, Seppälä TT, Bernstein I, Holinski-Feder E, Sala P, Gareth Evans D, et al. Cancer risk and survival in path_MMR carriers by gene and gender up to 75 years of age: a report from the Prospective Lynch Syndrome Database. Gut. 2018;67:1306–1316. doi: 10.1136/gutjnl-2017-314057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niessen RC, Hofstra RMW, Westers H, Ligtenberg MJL, Kooi K, Jager POJ, et al. Germline hypermethylation of MLH1 and EPCAM deletions are a frequent cause of Lynch syndrome. Genes Chromosom Cancer. 2009;48:737–744. doi: 10.1002/gcc.20678. [DOI] [PubMed] [Google Scholar]
- 33.Ligtenberg MJL, Kuiper RP, Chan TL, Goossens M, Hebeda KM, Voorendt M, et al. Heritable somatic methylation and inactivation of MSH2 in families with Lynch syndrome due to deletion of the 3’ exons of TACSTD1. Nat Genet. 2009;41:112–117. doi: 10.1038/ng.283. [DOI] [PubMed] [Google Scholar]
- 34.Boland CR, Thibodeau SN, Hamilton SR, Sidransky D, Eshleman JR, Burt RW, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 35.Shirts BH, Konnick EQ, Upham S, Walsh T, Ranola JMO, Jacobson AL, et al. Using somatic mutations from tumors to classify variants in mismatch repair genes. Am J Hum Genet. 2018;103:19–29. doi: 10.1016/j.ajhg.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaganathan K, Kyriazopoulou Panagiotopoulou S, McRae JF, Darbandi SF, Knowles D, Li YI, et al. Predicting splicing from primary sequence with deep learning. Cell. 2019;176:535–548.e24. doi: 10.1016/j.cell.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 37.Dominguez-Valentin M, Plazzer J-P, Sampson JR, Engel C, Aretz S, Jenkins MA, et al. No difference in penetrance between truncating and missense/aberrant splicing pathogenic variants in MLH1 and MSH2: a Prospective Lynch Syndrome Database Study. J Clin Med Res. 2021:10. 10.3390/jcm10132856. [DOI] [PMC free article] [PubMed]
- 38.Fahed AC, Wang M, Homburger JR, Patel AP, Bick AG, Neben CL, et al. Polygenic background modifies penetrance of monogenic variants for tier 1 genomic conditions. Nat Commun. 2020;11:3635. doi: 10.1038/s41467-020-17374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knudson AG., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971;68:820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rhees J, Arnold M, Boland CR. Inversion of exons 1-7 of the MSH2 gene is a frequent cause of unexplained Lynch syndrome in one local population. Familial Cancer. 2014;13:219–225. doi: 10.1007/s10689-013-9688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pritchard CC, Morrissey C, Kumar A, Zhang X, Smith C, Coleman I, et al. Complex MSH2 and MSH6 mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988. doi: 10.1038/ncomms5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rhine CL, Cygan KJ, Soemedi R, Maguire S, Murray MF, Monaghan SF, et al. Hereditary cancer genes are highly susceptible to splicing mutations. PLoS Genet. 2018;14:e1007231. doi: 10.1371/journal.pgen.1007231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morak M, Pineda M, Martins A, Gaildrat P, Tubeuf H, Drouet A, et al. Splicing analyses for variants in MMR genes: best practice recommendations from the European Mismatch Repair Working Group. Eur J Hum Genet. 2022:1–9. [DOI] [PMC free article] [PubMed]
- 44.Erwood S, Bily TMI, Lequyer J, Yan J, Gulati N, Brewer RA, et al. Saturation variant interpretation using CRISPR prime editing. Nat Biotechnol. 2022;40:885–895. doi: 10.1038/s41587-021-01201-1. [DOI] [PubMed] [Google Scholar]
- 45.Gergics P, Smith C, Bando H, Jorge AAL, Rockstroh-Lippold D, Vishnopolska SA, et al. High-throughput splicing assays identify missense and silent splice-disruptive POU1F1 variants underlying pituitary hormone deficiency. Am J Hum Genet. 2021;108:1526–1539. doi: 10.1016/j.ajhg.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adamson SI, Zhan L, Graveley BR. Vex-seq: high-throughput identification of the impact of genetic variation on pre-mRNA splicing efficiency. Genome Biol. 2018;19:71. doi: 10.1186/s13059-018-1437-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soemedi R, Cygan KJ, Rhine CL, Wang J, Bulacan C, Yang J, et al. Pathogenic variants that alter protein code often disrupt splicing. Nat Genet. 2017;49:848–855. doi: 10.1038/ng.3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rentzsch P, Schubach M, Shendure J, Kircher M. CADD-Splice-improving genome-wide variant effect prediction using deep learning-derived splice scores. Genome Med. 2021;13:31. doi: 10.1186/s13073-021-00835-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pesaran T, Karam R, Huether R, Li S, Farber-Katz S, Chamberlin A, et al. Beyond DNA: an integrated and functional approach for classifying germline variants in breast cancer genes. Int J Breast Cancer. 2016;2016:2469523. doi: 10.1155/2016/2469523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Martin S, Chamberlin A, Shinde DN, Hempel M, Strom TM, Schreiber A, et al. De novo variants in GRIA4 lead to intellectual disability with or without seizures and gait abnormalities. Am J Hum Genet. 2017;101:1013–1020. doi: 10.1016/j.ajhg.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sherrill JD, Kc K, Wang X, Wen T, Chamberlin A, Stucke EM, et al. Whole-exome sequencing uncovers oxidoreductases DHTKD1 and OGDHL as linkers between mitochondrial dysfunction and eosinophilic esophagitis. JCI Insight. 2018:3. 10.1172/jci.insight.99922. [DOI] [PMC free article] [PubMed]
- 53.Seabold S, Perktold J. Statsmodels: Econometric and statistical modeling with python. Proceedings of the 9th Python in Science Conference [Internet]. SciPy; 2010. Available from: https://conference.scipy.org/proceedings/scipy2010/seabold.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Validation variants used to calculate OddsPath score.
Additional file 2: Figure S1. Associations with other cancers, by variant type.
Additional file 3: Table S2. Variants identified by paired tumor-normal test, among patients with a single germline MSH2 variant.
Additional file 4: Table S3. Tumor and germline mutations in individuals with MSH2 somatic tumor mutations.
Data Availability Statement
MSH2 loss-of-function scores are available at MaveDB (https://www.mavedb.org) under accession urn:mavedb:00000050-a and are also provided as Table S4 in the published MSH2 deep mutational scan [26]. Underlying sequencing counts are available from NCBI GEO under accession GSE162130 [26].
MMR gene variants and clinical interpretation are deposited in NCBI ClinVar: https://www.ncbi.nlm.nih.gov/clinvar/?gr=0&term=(((msh2%5BGene%20Name%5D)%20OR%20msh6%5BGene%20Name%5D)%20OR%20mlh1%5BGene%20Name%5D)%20OR%20pms2%5BGene%20Name%5D
SpliceAI software: https://github.com/Illumina/SpliceAI [36].