Abstract
Background
Dietary diversity score has long been recognized as a key component of diets quality balances for healthy life status. However, diets with more variety of food items might increase calorie intake and body weight, which, in turn leads to central obesity (waist circumference).Therefore, this study aims to determine the prevalence of metabolic syndrome among dietary diversity score groups, and its associated factors among adults in the urban community of Jimma, Southwest Ethiopia.
Methods
A total of 915 adults aged ≥ 18 years were randomly recruited in this cross-sectional study.The study was undertaken from June 17, 2019, up to July 27, 2019. To this end, the collected data were entered to Epi Data 3.1 and analysed using and SPSS 25 version. What’s more, a multivariable logistic regression was used to assess associated factors of the unrecognized metabolic syndrome; adjusted odds ratio (AOR) with its corresponding 95% CI, at P-value ≤ 0.05.
Results
The occurrence of metabolic syndrome was 14.4%, and it is more prevalent in females, 11.15% than males, and 3.25%. The most prevalent components of the metabolic syndrome were low level of high-density lipoprotein, elevated level of triacylglycerol, and waist circumferences. Even though metabolic syndrome is not significantly associated with any of the dietary diversity score groups, its prevalence distribution varies among the groups (6.6% in middle, 5.8% in high and 1.9% in low dietary diversity groups). With potential confounders adjusted, by 75% female was significantly associated with the occurrence of metabolic syndrome than male (102 vs. 29, AOR = 0.25 at 95%CI: 0.15–0.40, P = 0.001). Whereas, age ≥ 35 years old (104 vs. 27, AOR = 2.91 at 95%CI:1.78–4.86,P = 0.001), large family size > 5 (65 vs. 10,AOR = 2.43 95% CI: 1.10–5.36, P = 0.03), overweight and obesity (121 vs. 10, AOR = 6.97, 95% CI: 4.50 –10.83, P = 0.005), elevated total cholesterol (103 vs. 28,AOR = 2.46, 95% CI: 1.47–4.11, P = 0.001), and consuming ( spices, condemns and beverages) ≥ 4 days per week (79 vs. 52, AOR = 0.52, 95% CI:0.33 –0.82, P = 0.005) were positively associated with the prevalence of metabolic syndrome as compared to their counterparts.
Conclusion
Unrecognized metabolic syndrome was relatively high in the study community. The prevalence of metabolic syndrome varied among dietary diversity groups. But any of the dietary diversity scoring categories was not significantly associated with the occurrence of metabolic syndrome. Thus, awareness needs to be made to practice healthy diet and regular physical activity to maintaining normal body weight. Moreover, early screening of metabolic syndrome should be promoted.
Keywords: Metabolic syndrome, Lipid profile, Dietary diversity, Ethiopia
Background
Even though there is an ongoing international debate on MetS’s definitions and existence, there are many official definitions of it [1, 2]. For the current study purpose, the authors used the definition of International Diabetic Federation (IDF). According to the new IDF definition, MetS is defined as central obesity (defined by waist circumference) plus any two of the risk factors: raised triglyceride, low high-density lipoprotein (HDL) cholesterol, raised blood pressure, and/or raised fasting plasma glucose level [3]. Since, MetS is the result of the highly complex interplay among several risk factors; a single etiology cannot be assigned to define it [1, 2]. For this reason, MetS is defined by various organizations and scholars, the IDF [3], Executive Summary of the Third Report of the national cholesterol Education program(NCEP) expert panel discussion [4], and World health organization [5–7].
Based on the IDF criteria, up to 25% of the world’s adults have MetS [3]. This is particularly common on adults who are lower levels of obesity in Asians compared to Europeans. After considering the difficulties in recognizing pooled criteria that are relevant through all ethnicities for MetS, the IDF suggests as using a new set of criteria per ethnic/racial specific cut-offs. Moreover, the IDF also recommends using European criteria, for where there are limited data in sub-Saharan Africa countries [3].
Furthermore, MetS is a metabolic derangement associated with the increased risk of Cardiovascular Diseases (CVDs) and Diabetes Mellitus (DM). Likewise, MetS is characterized by a special pattern of reversible major risk factor for Type 2 Diabetes Mellitus (T2DM) and CVDs. Plus, to this, MetS is not a disease by itself; it is a set of undesirable condition rooted in one’s poor lifestyle. It is also associated with the increased prevalence of obesity [1, 3, 8].
On top of this, MetS is a group of clinical and biological abnormalities with a global prevalence of 20–25% in the adult population [9]. According to a joint interim statement,in Japan, MetS’s prevalence has been reported to range from 18.5% to 37.3% in men and 4.4% to 12.8% in women [10, 11]. Besides, the magnitude of MetS among T2DM patients in Tigray, North Ethiopia was 51.5% and it’s associated with overweight, and obesity has been increasing in sub-Saharan African countries [12, 13].
According to the WHO, Chronic Non-communicable Diseases (NCDs) related to obesity will exceed that of infectious diseases in Sub-Saharan Africa by 2030 [14, 15]. Among NCDs, MetS is the commonest consequence of malnutrition [16]. In recent years, economic development and urbanization have rapidly changed the dietary habits shifts to processed food, which raise great concern about obesity and other health associated outcomes [17].
However, a study conducted in Southwest China showed that people with medium and high DDS were at higher risk of general and central obesity than people with less DDS. According to this study high DDS was associated with excessive energy intake calories induced obesity. In line with this, public health messages about DDS emphasis only on widely recommended for health with less stress [18]. On the contrary, there is study showed that higher DDS had an inverse association with MetS and some of its features. Therefore, a higher DDS might be associated with a lower possibility of having some metabolic disorders [19].
A further point is that earlier studies have documented adult lifestyles like smoking cigarette, drinking alcohol, less physical exercise, and consuming unhealthy dietary habits as the risk factors for the development of MetS [20–22]. But, there are limited data on the occurrence of MetS and its risk factors among apparently healthy communities in Ethiopia. Therefore, this study attempted to detect the distribution of unrecognized MetS varied in DDS groups and its associated factors among adults in the urban community of Jimma, southwest Ethiopia.
Materials and methods
Study setting and period
Urban community-based cross-sectional study was conducted among adults aged greater or equal to 18 years. Data were collected from June 17 to July 27, 2019. It was conducted in Jimma town, Southwest Ethiopia.
Population, sample size and sampling technique
All adults who have been living in Jimma town for at least six months before data collection randomly recruited for the purpose of this study. The sample size was determined using the single population proportion formula considering 17.9% of prevalence of MetS in Addis Ababa, Ethiopia [22], 95% confidence interval (CI), 3% margin of error, 10% non-response rate, and 1.5 design effect.
10% of non-respondents rate = 62.8
1.5 design effect of 628 = 942
Final sample size = 1005.
A multi-stage sampling technique was employed, where from 17 Kebeles (the lowest administrative structure in Ethiopia) in the town; six Kebeles were purposively selected by considering the settlement cluster, as the district sampling unit. Then, the sample size number of adults was allocated into the selected Kebeles by considering population proportion to the total number of households in each Kebeles. Then, households in each Kebele were selected by systematic sampling technique and the lottery method was used to select the study participants where more than one eligible adult have been living in the same household.
Data tool development and collection techniques
Data were collected using WHO STEPS questionnaire [23] adapted to the local context based on the study objective. The questionnaire was first adopted and written in English, translated into Amharic and Afaan-Oromo by experts, and then translated back into English by a panel of professionals. A four-day training of the contents of the questionnaire, data collection techniques, and ethical concern of human research was provided to the research interviewers prior to the commencement of the study. The questionnaire was pretested in Agaro town of Jimma zone which has similar characteristics with the study area before the initiation of the study, to check the flow of the questionnaires, measure the length of time required for interviews, feasibility, and the clarity of the language used. Data were collected by six trained BSc nurses, along with six health extension workers permanently works at each kebeles.,. All interviews and measurements were done with close supervision of the research team.
Socio-demographic and behavioral characteristics
Socio-demographic and behavioral characteristics were collected through face-to-face detail interviews using open-ended questionnaires. WHO guideline-recommended on physical activities as all adults should undertake 150–300 min of moderate-intensity, or 75–150 min of vigorous-intensity physical activity, per week. Therefore, as if the study participant’s physical activity trend fitting with the WHO recommendation categorized as physically active and did not fit categorized as physically inactive. Similarly, the sedentary behavior of the study participants was measured by interviewing about the time spent sitting during a typical week and their responses were classified as categories of sedentary (< 4 h/day) and not sedentary (≥ 4 h/day) [24]. Likewise, khat chewing and tobacco using behavior were defined as self-reported currently chewing of khat and practicing of smoked tobacco or smokeless tobacco products, respectively. Current alcohol users are defined as people who drink alcohol more than one day per week [25].
Anthropometric measurements
In this step, height, weight, waist, and hip are needed to calculate body mass index (BMI), waist circumference (WC), waist to hip ratio. Therefore, the height of the study participants was measured to the nearest 0.1 cm using an adjustable portable stadiometer with the subjects positioned at the Frankfurt Plane, the four points (Heel, Calf, Buttocks, and Shoulder) touching the vertical stand and their shoes were taken off. Before starting the measurements, the stadiometer was checked using calibration rods. Likewise, Weight was measured with a digital scale (Model 871, Seca, Germany) accurate to 100 g with the subjects wearing very light clothes and shoes taken off. The validity of the scale was checked using an object of a known weight every morning and between the measurements. After BMI was calculated by weight (kg)/height (meter)2 performed, BMI of the study participants was categorized as underweight (< 18.5 kg/m2), normal [18.5–24.9 kg/m2], overweight [25–29.9 kg/m2] and obese (≥ 30 kg/m2) [26, 27]. Similarly, WC was measured at the middle between the lowest costal margin at the mid-clavicular line and the anterior superior iliac spine using a fixed tension tape meter without any pressure to the body surface and then measurements were recorded to the nearest 0.1 cm. The waist-to-hip ratio was calculated as WC divided by hip circumference. According to the WHO recommendation, WC values > 94 cm for men and > 80 cm for women were considered as high [28]. Therefore, as per the WHO, the study participants were grouped as high and low WC.
Blood pressure measurement
Blood pressure (BP) was measured in a sitting position from the right arm. It was measured in triplicate using an Aneroid Sphygmomanometer with small, medium, and large handcuff size [29], as fit to the subjects take rest for a minimum of five minutes. Then, two more successive measurements within five minutes apart were done. As per the WHO recommendation, the mean of systolic and diastolic blood pressures was considered for analysis. Accordingly, participants were considered as prehypertensive as if systolic BP was 120–139 and diastolic BP 80–89 mmHg, and hypertensive as if systolic BP ≥ 140 mmHg and/or diastolic BP ≥ 90 mmHg [30].
Biochemical measurements
A five Milliliter venous blood sample was taken in a sitting position according to the standard protocol from each study participant and centrifuged within 30–45 min of collection. The blood Sample was used to determine either random or fasting blood glucose (12–14 h fasting) levels or lipid profiles (HDL, LDL, TG, and TC). Blood glucose was measured by using a glucometer soon sample drowns and measured in the laboratory too. Blood serum was carried out in ABX Pentra 400 Automated Chemistry Machine (Horiba ABX SAS, 34,184 Montpellier, France) at Jimma Medical Center (JMC) Clinical chemistry core laboratory to determine serum lipid profiles and glucose. But Low-Density Lipoprotein(LDL) level was calculated by using the Freidwald formula [31]. After analysis, TC ≥ 200 mg/dl and < 200 mg/dl were considered as high and normal respectively. Likewise, LDL was considered as optimal when LDL < 100 mg/dl but elevated as LDL ≥ 100 mg/dl. Similarly, TG level was considered as normal when TG < 150 mg/dl and HDL was considered as desirable/normal if it is > 40 mg/dl for men and > 50 mg/dl for women, otherwise it was categorized as low.
Based on the American Diabetes Association (ADA), Diabetes Mellitus (DM) is considered as positive (diabetic) with criteria of fasting blood glucose of ≥ 126 mg/dl and/or random blood glucose of ≥ 200 mg/dl. But considered as pre-diabetic with criteria of RBG: 100 mg/dl ≤ FBG ≤ 126 mg/dl and RBG: 140 mg/dl ≤ RBG < 199 mg/dl [32, 3].
Metabolic syndrome measurement
Following the IDF criteria, study subjects were classified as MetS if participants had central obesity (defined as a waist circumference of ≥ 95 cm for men and ≥ 80 cm women plus two of any of the following risk factors: (1) raised TG level (≥ 150 mg/dL) or specific treatment for this lipid abnormality; (2) reduced HDL-C(< 40 mg/dL in males and < 50 mg/dL in females) or specific treatment for this lipid abnormality; (3) raised blood pressure (systolic BP ≥ 130 or diastolic BP ≥ 85 mmHg) or treatment of previously diagnosed hypertension; (4) raised FBG (≥ 100 mg/dL) or previously diagnosed with type 2 diabetes [7], and study subjects categorize as normal if participants had either normal central obesity or any two of the two risk factors values in the normal range [3].
Unrecognized metabolic syndrome in this study supposed to be operationalized as the study participants existing in community without confirmed positive MetS and its components before this study.
Dietary diversity score and survey
Dietary diversity score was based on 24-h recall of the study participants consumption of food groups within the past 24-h using the Dietary individual or Women Dietary diversity Guidelines [33].
Trained interviewers from Jimma Medical Center (JMC) paired with local health extension workers visited the selected adult/s from the selected households to collect and register the information on food consumption using the 24-h dietary recalls.
Based on the guideline, all food items (16) were categorized into 9 groups which were grains (including cereals, tubers, and roots), vegetables, fruits, meat (including pork, beef, poultry, and organs), beans (including beans, nuts, and seeds), eggs, fish (including seafood, freshwater fish and aquatic products), dairy (including milk and products), and oil (including animal and vegetable oil). If a participant consumed any food item from any of the above-mentioned categories, he/she would get one point in that food category. If not, he/she would be scored zero. Consuming different foods from the same category would assume not count repeatedly. The total score would be the sum scores of the nine food groups and the maximum score could go up to 9. Therefore, for this study purpose, the DDS was categorized into low (1-3), medium (1-3), and high (1-3), [34].
Data processing and analysis
The data were checked for completeness, coded and entered into EPI data 3.1, and exported to SPSS 25 version for the analysis. Descriptive analysis was explored using frequency and percentage for categorical variables. Continuous variables were expressed as mean ± SD. Normality was checked for continuous variables and transformation was made for data that are not normally distributed. Bivariate and multivariate logistic regression analysis was used to evaluate the differences in the distribution of categorical variables for the study groups. P-value < 0.2 was used as a cut-off to include variables for the multivariate binary logistic regression model. The result of the OR was used for interpretation of the strength of prediction of the independent variables to the dependent outcome. For all statistical significance tests, the cut-off value set was P < 0.05 with CI of 95%.
Results
Participants’ socio-demographic and behavioral characteristics
A total of 915 adults aged ≥ 18 years old were participated in the study. Nearly half, 439 (48%), of the respondents were females. The mean age of the respondents was 38.4 ± 13.5 years whereas the minimum and maximum ages were 19 and 88 years, respectively. Among the total study participants, 72 (7.9%), 153 (16.7%) and 321(35.1%), have been currently smoking cigarettes, drinking alcohol, and chewing khat, respectively. Around 604(66.0%) of the study participants did not practice physical exercises as per WHO recommended and 531(58.0%) represented under sedentary life. Nearly, most of the study participants, 722(78.9%) often have been using vegetable oil in meal preparation. These socio-demographic and behavioral Characteristic of the respondents are presented in (Table 1).
Table 1.
Socio-Demographic and Behavioral Characteristic among Adults in Urban Community of Jimma, Southwest Ethiopia 2019 (N = 915)
| Variable | Category | Frequency | Percent |
|---|---|---|---|
| Sex |
Female Male |
439 476 |
47.9 52.1 |
| Age group(yrs) |
<35 ≥35 |
403 512 |
44.0 56.0 |
| Marital status |
Single Married Divorced Widowed |
30 654 131 100 |
14.2 71.5 14.3 10.0 |
| Occupation |
Unemployed Employed Private work |
316 316 283 |
34.5 34.5 31.0 |
| Family size |
1–2 3–5 >5 |
151 483 281 |
16.5 52.8 30.7 |
| Annual income (Ethiopia Birr) |
≤10,000 10,001–49,999 ≥50,000 |
374 446 95 |
40.9 48.7 10.4 |
| Currently Smoking | Yes | 72 | 7.9 |
| No | 843 | 92.1 | |
| Currently drinking alcohol | Yes | 153 | 16.7 |
| No | 762 | 83.3 | |
| Currently khat chewing | Yes | 321 | 35.1 |
| No | 594 | 64.9 | |
| Type of oil or fat used in meal preparation |
Vegetable oil Butter, margarine |
722 193 |
78.9 21.1 |
| Level of physical activity | Insufficient | 604 | 66.0 |
| Sufficient | 311 | 34.0 | |
| Sedentary life |
<4 hours ≥4 hours |
384 531 |
42.0 58.0 |
Anthropometric and biochemical measurements
More than one-fourth, 255(27.8%), of the participants have been overweight and obese. And 194(21.2%) of the respondents have been existing with confirmed diagnosed hypertension for this study purpose. On the other hand, 672(73.4%) had a high west-to hip ratio and 126 (13.8%) of the participants have had more than 200 mg/dl total cholesterol. In this study, Mets prevalence was 131(14.3%) with a higher prevalence in females, 102 (11.14%), than males. Moreover, low HDL 455 (49.7%), elevated LDL 311(34.0%), elevated TAG 255(27.9%) and Central obesity 217 (23.7%) were the high to low prevalent component of the MetS (Table 2).
Table 2.
Physical and Biochemical Measurements Characteristic among Adults in Urban Community of Jimma, Southwest Ethiopia 2019 (N = 915)
| Variable | Category | Frequency | Percent |
|---|---|---|---|
| Anthropometry indices |
Body mass index Underweight Normal Overweight Obese |
||
| 135 | 14.8 | ||
| 525 | 57.4 | ||
| 177 | 19.3 | ||
| 78 | 8.5 | ||
|
Waist circumferences Normal Central obesity |
698 | 76.3 | |
| 217 | 23.7 | ||
| Waist to hip ratio | |||
| Normal | 243 | 26.6 | |
| Elevated | 672 | 73.4 | |
| Hypertension | Yes | 194 | 21.2 |
| No | 721 | 78.8 | |
| Lipid profiles |
Total cholesterol <200 mg/dl ≥200 mg/dl |
||
| 789 | 86.2 | ||
| 126 | 13.8 | ||
|
HDL cholesterol Low Normal |
|||
|
455 460 |
49.7 50.3 |
||
|
LDL cholesterol <100 mg/dl ≥100 mg/dl |
|||
| 604 | 66.0 | ||
| 311 | 34.0 | ||
|
Triacylglycerol <150 mg/dl ≥150 mg/dl |
660 | 72.1 | |
| 255 | 27.9 |
Metabolic syndrome (MetS) and dietary diversity score (DDS)
The frequency and percentage distribution of low, middle and high DDScatagories were represented by 114(12.5%), 447(48.8%) and (35,436.6%) respectively. The prevalance of the MetS remarkaibly varied among DDSgroups. Its prevalance was 60(6.6%) in middle, 53(5.8%) in high and [18] 1.9% in low DDS groups.The prevalence of MetS among the DDScategories patter is the same with non-MetS (Fig. 1).
Fig. 1.
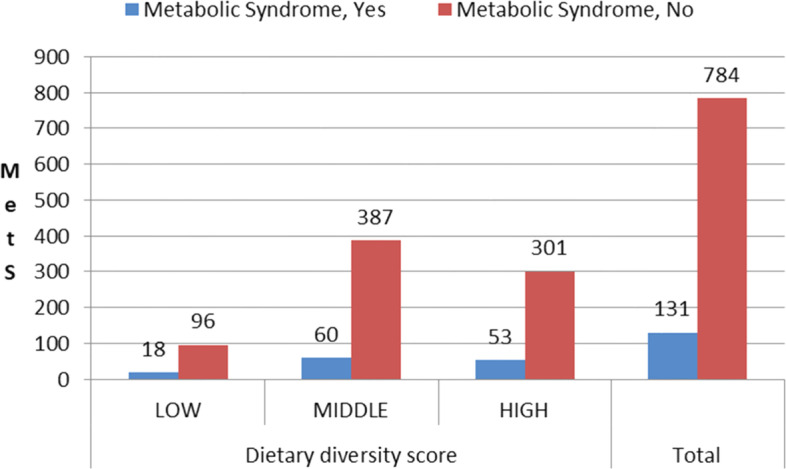
Metabolic Syndrome occurrences among DDS Group of Adults in Urban Community of Jimma, Southwest Ethiopia 2019(N = 915)
Factors associated with metabolic syndrome
Age, sex, occupation, level of physical activity, sedentary life, type of oil used for cooking, middle DDS,elevated cholesterol, large family size,current smoking of cigarette, current drink of alcohol, consuming (spices,condemns and beverages) more than 4 days per week, and BMI were included in multiple logistic regression for having P-value < 0.2. In multiple logistic regression only age > 35 years old, female, family size > 5, consuming (spices, condemns and beverages) more than 4 days per week, overweight and obese BMI and elevated cholesterol were significantly associated with metabolic syndrome at P-value < 0.05.
In the final model, by 75% female was significantly associated with the occurrence of metabolic syndrome than male (102 vs. 29, AOR = 0.25 at 95%CI: 0.15–0.40, P = 0.001). Whereas, age ≥ 35 years old (104 vs. 27, AOR = 2.91 at 95%CI:1.78–4.86, P = 0.001), large family size > 5 (65 vs. 10,AOR = 2.43 95% CI: 1.10 –5.36, P = 0.03), overweight and obesity (121 vs. 10, AOR = 6.97, 95% CI: 4.50 –10.83, P = 0.005), elevated total cholesterol (103 vs. 28,AOR = 2.46, 95% CI: 1.47–4.11, P = 0.001), and consuming ( spices, condemns and beverages) more than 4 days per week (79 vs. 52, AOR = 0.52, 95% CI:0.33 –0.82, P = 0.005) were positively associated with the prevalence of metabolic syndrome as compared to their counterparts (Table 3).
Table 3.
Bivariate Regression Analysis to Identity Risk Factors Associated with Unrecognized MetS among Adults in Urban Community of Jimma, Southwest Ethiopia 2019(N = 915)
| Variables | Metabolic syndrome | COR,95%CI | AOR,95%CI | |
|---|---|---|---|---|
| Yes | No | |||
| Sex of the participants | ||||
|
Female Male |
102 | 337 | 1 | 1 |
| 29 | 447 | 0.21 (0.14–0.33) | 0.25(0.15–0.40)** | |
| Age | ||||
|
< 35 years ≥ 35 years |
27 | 376 | 1 | 1 |
| 104 | 408 | 3.55(2.27–5.54) | 2.9(1.78–4.86)** | |
| Family size | ||||
| (1, 2) | 10 | 141 | 1 | 1 |
| (3-5) | 56 | 427 | 2.193(1.10–4.38) | 1.91(0.88–4.10) |
| (> 5) | 65 | 216 | 3.51(1.73–7.10) | 2.43(1.10–5.36) * |
| Using (Spices, condiments, beverages) | ||||
| No | 52 | 260 | 1 | 1 |
| Yes | 79 | 574 | 0.556(0.38–0.82) | 0.52(0.33–0.82)** |
| BMI | ||||
| ≤ 24.99 kg/m2 | 10 | 650 | 1 | 1 |
| ≥ 25 kg/m2 | 121 | 134 | 8.60(5.71–12.96) | 6.97(4.50–10.83)** |
| Total cholesterol | ||||
| < 200 mg/dl | 28 | 761 | 1 | 1 |
| ≥ 200 mg/dl | 103 | 23 | 3.23(2.07–5.01) | 2.46(1.47–4.11)** |
**P = 0.001; *P = 0.05; BMI Body mass Index
Discussion
Nowadays, the prevalence of MetS has increased worldwide and it is considered as a major public health problem [35–37]. Hence, this study attempted to detect the magnitude of MetS and its variation with the DDS among adults in the urban community of Jimma, Southwest Ethiopia.
The main finding of this study indicates that MetS and its associated factors is existing as a major health concern of adults who were unaware of the disorder in the study community. In the current study, the prevalance of the MetS remarkably varied among DDS groups. Even though larger prevalence of the MetS has been observed in middle and higher DDS as compared with low DDS, the association is not statically significant. In addition to that, the pattern of variations of MetS as compared with non-MetS among DDS catagories near similar (Fig. 1). Contrary to this, other studies revealed as the high dietary diversity intake is favorably associated with MetS [18, 34, and 38] and it’s inversely associated with MetS and some of its features [19].
Finally, sex (female), advancing age, participants who have been consuming (spices, condemns and beverages) more than 4 days per week, having larger family size, hypercholesterolemia, being overweight and obese were independently increase the odds of metabolic syndrome (Table 3).
What more, the magnitude of MetS among the study participants was 14.3% which is near to the finding obtained from working adults in Addis Ababa, Ethiopia (17.9%) [22], also the result lies within the range of 12–86 from sub-Saharan Africa study [12]. Indeed, it is higher than on the result found from residents of Mizan-Aman town of southwest Ethiopia (9.6%.) and NCDs in Ethiopia (4.4%) [39, 40]. But, the current result indicated low prevalent rate as compared with the study conducted among adult urban dwellers of northern Ethiopia (21.8%). This is more verified by results from systematic review and meta-analysis on Ethiopia population (27.92%), on type T2DM patients in Ethiopia (57%), on adults of across European countries (24%) and UK (32%), people with T2DM of Gahanna (68.6%), Nigerians with type T2DM (68.7%) and on T2DM patients in Kerman of Iran (64.9%) [41–47]. This difference is due to differences in study subjects, setting, sample size, methods, socio-economic status, ethnicity, lifestyle, cut-off differences, the criteria used to define MetS, and other contributing factors for the higher province of MetS.
A further point is that, the odd of MetS three times higher in adults whose age was greater than or equal to 35 years than less. This may be due to age-related physiological processes and environmental factors altered. For example, gradual decrease in the basal metabolic rate, dyslipidemia, stress-induced hypercortisolism, hypogonadism, decreased growth hormone secretion, concomitant insulin resistance, and abdominal fat deposition, changes of the functioning of beta cells and other environmental and physiological factors, may trigger a genetic expression of MetS which becomes more prominent with biological maturation [48, 49].
By the same token, the prevalence of MetS was higher in females than males. The result of the higher prevalence of MetS among aged and female participants agreed with previous other related studies [9, 12, 23, 46, 47]. Higher MetS in females than males might be due to the use of hormonal oral contraceptives that can decrease insulin sensitivity, glucose tolerance, increase blood pressure and increase weight gain; menopause promotes a change in body fat distribution to increase central adiposity [50]. Additionally, less proportion of females were involved in regular physical exercise than males in this study, which might have its own contribution to the observed higher MetS prevalence among them. Others justification is that in women; low HDL cholesterol, elevated BMI, increased waist circumference and hyperglycemia were significantly larger contributors to the MetS as compared with male [51].
In the current study, the odd of MetS was seven times higher among participants', BMI ≥ of 25 kg/m2(include overweight and obese) as compared to those, BMI ≤ of 24.99 kg/m2) (include normal and underweight). This is higher with studies done in Ethiopia, Gahanna, Nigeria, and Iran [42, 44–46]. This is due to obesity which rises in countries like Ethiopia experiencing domestic nutrition transition from a primarily plant-based diet to meat and processed food diet associated with weight gain and chronic illnesses and the types of body fat distribution are still the core aspects of insulin resistance which links to MetS. Furthermore, in the current study, for participants with hypercholesterolemia (≥ 200 mg/dl), the odd of MetS was 2.46 times higher than the normal one. This finding is in line with the higher cholesterol synthesis and cholesterol excretion as neutral sterols in patients with MetS than in healthy populations [52].
In general, the findings of the present study showed that MetS is major prevalent with its risk factors in the community among adults in Ethiopia. Early identification of MetS and its risk factors among adults is of great importance since MetS suggest increased risk of morbidities such as CVD, decreased quality of life, increased health care cost, as well as mortality. Therefore, NCDs treating center must strengthen appropriate and targeted prevention strategies such as encouraging people to adopt dietary modification and physical activity which are reported to reduce incidence and progression of MetS [53]. Moreover, there should be a more frequent screening of adults in the community for MetS components and risk factors prior to full blow development of MetS.
The Strengths of this study include appraising the prevalence of MetS occurrences varied under DDS groups and its predictors at the community level and providing a baseline for future studies to early monitor MetS and risk factors as well as in health planning and managements strategies. This stud has also the limitations relating to the cross-sectional study design of which provides a snapshot of the burden at a particular moment in time. Additionally, the study also designed to include only adults who were ≥ 18 years which it was not included all age groups and epidemiological representing areas; therefore, it’s miss national representative. Since DDS is not quantified to calories and nutrient contents, it is not in concluding position to indicate the causes and effect relationship with MetS, It is also limited to show the health-related outcome in long term impacts and economic consequences of MetS among adults living in the community in Ethiopia.
Conclusion
Unrecognized MetS and its components were prevalent among adults in the study community. Moreover, sex (female), advancing age, participants who have been consuming (spices, condemns and beverages) ≥ 4 days per week, having larger family size, hypercholesterolemia, being overweight and obese were independently increase the odds of MetS. Even if higher occurrence of MetS in middle and high DDS as compared with low DDS categories, their association was statistically not significant. Thus, awareness needs to be created among the community to practice regular physical activity and maintaining normal body weight. Additionally, screening of MetS should be promoted for early detection, prevention, and treatment.
Acknowledgements
Jimma University is greatly acknowledged for the financial support of the study. Then, we would like to extend our appreciation to supervisors, data collectors, and study participants for their cooperation, participation, and willingness.
Abbreviations
- MetS
Metabolic Syndrome
- DDS
Dietary Diversity Score
- NCEP ATP III
National Cholesterol Education Program’s Adult Treatment Panel III
- AOR
Adjusted Odds Ratio
- BMI
Body Mass Index
- BP
Blood Pressure
- CI
Confidence Interval
- COR
Crude Odds Ratio
- DM
Diabetes Mellitus
- T2DM
Type 2 Diabetes Mellitus
- FBS
Fasting Blood Sugar
- HDL
High Density Lipoprotein
- IDF
International Diabetes Federation
- JMC
Jimma Medical Center
- LDL
Low-Density Lipoprotein
- NCD
Non-Communicable Diseases
- RBS
Random Blood Sugar
- TC
Total Cholesterol
- TAG
Triacylglycerol
- WC
Waist Circumference
- WHO
World Health Organization
Authors’ contributions
All authors made substantial contributions to conception and design, acquisition of data, analysis, and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the contents of the article.
Funding
Staff research project funded by Postgraduate and Research Directorate of the Institute of Health, Jimma University.
Availability of data and materials
The data sets generated and or analysed during the current study are not publicly available due to personal data protection legislation but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical clearance was obtained from Jimma University, Institute of Health Institutional Review Board. A letter of support was obtained from Jimma town and communicated to the selected study Kebele administrations. After well introduced the aim and goal of the study to participants, written informed consents were obtained from each study participant. All the participants’ information was kept confidential using a coding system. Participants identified with cases were advised and linked to nearby health facilities for further investigation and management. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Belay Zawdie, Email: bellzolla2000@gmail.com.
Temamen Tesfaye, Email: tekanetesfu@gmail.com.
Solomon Berhanu Moges, Email: solo.berhanu@gmail.com.
Yonas Tesfaye, Email: yonastesfaye71@yahoo.com.
Ayantu Kebede, Email: ayukebede2013@gmail.com.
Mulualem Tadesse, Email: mulualemt.tadesse@gmail.com.
Esayas Kebede Gudina, Email: esakgd@gmail.com.
Lelisa Sena Dadi, Email: lelisajitu@gmail.com.
Dessalegn Tamiru, Email: dessalegn97@gmail.com.
Tefera Belachew Lemma, Email: teferabelachew2@gmail.com.
References
- 1.Thaman. Ghay Richa, Arorra Greeti P. Metabolic Syndrome: Definition and Pathophysiology-the discussion goes on! Journal of Physiology and Pharmacology Advances. 2013;3:48–56. doi: 10.5455/jppa.20130317071355. [DOI] [Google Scholar]
- 2.Kahn R, Buse J, Ferrannini E, Stern M. The metabolic syndrome. Lancet. 2005;366(9501):1921–1924. doi: 10.1016/S0140-6736(05)67778-1. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation. Recommendations for Managing Type 2 Diabetes in Primary Care, 2017. https://www.idf.org/managing-type2-diabetes.
- 4.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (). Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA. 2001;285(19):2486–97. 10.1001/jama.285.19.2486. [DOI] [PubMed]
- 5.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 199 Alberti KG, Zimmet P, Shaw J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic medicine: a journal of the British Diabetic Association. 2006;23(5):469–80. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- 6.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 7.Han TS, Lean ME. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016; 5.doi: 10.1177/2048004016633371. [DOI] [PMC free article] [PubMed]
- 8.Tadewos A, Ambachew H, Assegu D. Pattern of Metabolic Syndrome in Relation to Gender among Type-II DM Patients in Hawassa University Comprehensive Specialized Hospital, Hawassa. Southern Ethiopia Health Science Journal. 2017;11:3. [Google Scholar]
- 9.Nagahama S, Kurotani K, Pham NM, Nanri A, Kuwahara K, Dan M, et al. Self-reported eating rate and metabolic syndrome in Japanese people: cross-sectional study. BMJ Open. 2014;4:e005241. doi: 10.1136/bmjopen-2014-005241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyatake N, Kawasaki Y, Nishikawa H, Takenami S, Numata T. Prevalence of metabolic syndrome in Okayama prefecture. Japan Intern Med. 2006;45(2):107–108. doi: 10.2169/internalmedicine.45.1509. [DOI] [PubMed] [Google Scholar]
- 11.Gebremeskel GG, Berhe KK, Belay DS, Kidanu BH, Negash AI, Gebreslasse KT, et al. Magnitude of metabolic syndrome and its associated factors among patients with type 2 diabetes mellitus in Ayder Comprehensive Specialized Hospital, Tigray, Ethiopia: a cross sectional study. BMC Res Notes. 2019;12(1):603. doi: 10.1186/s13104-019-4609-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okafor CI. The metabolic syndrome in Africa: Current trends. Indian J Endocrinol Metab. 2012;16(1):56–66. doi: 10.4103/2230-8210.91191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinaga M, Worku M, Yemane T, Tegene E, Wakayo T, Girma T, et al. Optimal cut-off for obesity and markers of metabolic syndrome for Ethiopian adults. Nutr J. 2018;17(1):109. doi: 10.1186/s12937-018-0416-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holmes MD, Dalal S, Volmink J, Adebamowo CA, Njelekela M, Fawzi WW, et al. Non- Communicable Diseases in Sub-Saharan Africa: The Case for Cohort Studies. PLoS Med. 2010;7(5):e1000244. 10.1371/journal.pmed.1000244. [DOI] [PMC free article] [PubMed]
- 15.Stanner SA, James S, Foster R. Cardiovascular Disease: Diet, Nutrition, Emerging Risk Factors, the launch of the BNF Task Force report. British Nutrition Foundation. 2005;30(4):392–6. 10.1111/j.1467-3010.2005.00529.x.
- 16.Azadbakht L, Fard NR, Karimi M, Baghaei MH, Surkan PJ, Rahimi M, et al. Effects of the Dietary Approaches to Stop Hypertension (DASH) eating plan on cardiovascular risks among type 2 diabetic patients: a randomized crossover clinical trial. Diabetes Care. 2011;34(1):55–57. doi: 10.2337/dc10-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox A, Feng W, Asal V. What is driving global obesity trends? Globalization or “modernization”? Global health. 2019;15(1):32. doi: 10.1186/s12992-019-0457-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azadbakht L, Mirmiran P, Azizi F. Dietary diversity score is favorably associated with the metabolic syndrome in Tehranian adults. Int J Obes (Lond) 2005;29(11):1361–13677. doi: 10.1038/sj.ijo.0803029. [DOI] [PubMed] [Google Scholar]
- 19.Gouda HN, Charlson F, Sorsdahl K, Ahmadzada S, Ferrari AJ, Erskine H, et al. Burden of non- communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375–e1387. doi: 10.1016/S2214-109X(19)30374-2. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf S, Hawken S, Ôunpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. The Lancet. 2004;364(9438):937–952. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 21.Baird J, Jacob C, Barker M, Fall CH, Hanson M, Harvey NC, et al. Developmental Origins of Health and Disease: A Life course Approach to the Prevention of Non-Communicable Diseases. Healthcare (Basel) 2017;5(1):14. doi: 10.3390/healthcare5010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran A, Gelaye B, Girma B, Lemma S, Berhane Y, Bekele T, et al. Prevalence of Metabolic Syndrome among Working Adults in Ethiopia. Int J Hypertens. 2011; Article ID: 193719.10.4061/2011/193719The [DOI] [PMC free article] [PubMed]
- 23.WHO Stepwise approach to Surveillance of non-communicable diseases (STEPS): non- communicable Diseases and Mental Health 20 Avenue Appia. [Internet]. 1211 Geneva 27; Switzerland. 2003[citedMay2,2020].Available from:https://www.who.int/ncd_surveillance/en/steps_framework_dec03.pdf.
- 24.WHO. Global Physical Activity Questionnaire (GPAQ) Analysis Guide [Internet]. . GenevaWorldOrganization2012: http://scholar.google.com/scholar?hl=en&btnG=Search&q=intitle:Global±Physical±Activity±Questionnaire±(GPAQ) ±Analysis±Guide#1)...(Heal).
- 25.Lasebikan V, Ola BA, Ayinde OO. Effectiveness of Alcohol, Smoking, and Substance Involvement Screening Test-Linked Brief Intervention on Harmful and Hazardous Alcohol Use in Nigerian Semirural Communities: A Non-Randomized Intervention Study. Front Psychiatry. 2017;8:50. doi: 10.3389/fpsyt.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mardolkar M, Mardolkar M. Int J Comput Sci Mob Comput. 2017;6(2):8–16. [Google Scholar]
- 27.Nuttall FQ. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr Today. 2015;50(3):117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.World Health Organization. Waist circumference and waist-hip ratio : report of a WHO expert consultation, Geneva, 8–11 December 2008. World Health Organization. 2011. https://apps.who.int/iris/handle/10665/44583.
- 29.Frese EM, Fick A, Sadowsky SH. Blood Pressure Measurement Guidelines for Physical Therapists. Cardiopulmonary Physical Therapy Journal. 2011;22(2):5–12. doi: 10.1097/01823246-201122020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42(6):1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 31.Hata YNK. Application of Friedewald’s LDL-cholesterol estimation formula to serum lipids in the Japanese population. Jpn Circ J. 1986;50(12):1191–2000. doi: 10.1253/jcj.50.1191. [DOI] [PubMed] [Google Scholar]
- 32.American DA. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kennedy G, Ballard T, Claude Dop M. Guidelines for measuring household and individual dietary diversity | Agrifood Economics | Food and Agriculture Organization of the United Nations (fao.org). 2011, Reprint on 2013; ISBN 978-92-5-106749-9.
- 34.Zhang Q, Chen X, Liu Z, Varma DS, Wan R, Zhao S. Diet diversity and nutritional status among adults in southwest China. PLoS ONE. 2017;12(2):e0172406. doi: 10.1371/journal.pone.0172406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo H, Gao X, Ma R, et al. Prevalence of Metabolic Syndrome and its Associated Factors among Multi-ethnic Adults in Rural Areas in Xinjiang, China. Sci Rep. 2017;7:17643. 10.1038/s41598-017-17870-5. [DOI] [PMC free article] [PubMed]
- 36.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gebreegziabiher G, Belachew T, Mehari K, Tamiru D. Magnitude and Associated Factors of Metabolic Syndrome among Adult Urban Dwellers of Northern Ethiopia. Diabetes Metab Syndr Obes. 2021;14:589–600. doi: 10.2147/DMSO.S287281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khamis AG, Ntwenya JE, Senkoro M, Mfinanga SG, Kreppel K, Mwanri AW, et al. Association between dietary diversity with overweight and obesity: A cross-sectional study conducted among pastoralists in Monduli District in Tanzania. PLoS ONE. 2021;16(1):e0244813. doi: 10.1371/journal.pone.0244813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kerie S, Menberu M, Geneto M. Metabolic syndrome among residents of Mizan-Aman town, South West Ethiopia, 2017: a cross sectional study. PLoS ONE. 2019;3:1. doi: 10.1371/journal.pone.0210969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shiferaw F, Letebo M, Misganaw A, et al. Non-communicable diseases in Ethiopia: disease burden, gaps in health care delivery and strategic directions. Ethiop J Health Dev. 2018;32:3. [Google Scholar]
- 41.Ambachew S, Endalamaw A, Worede A, Tegegne Y, Melku M, Biadgo B. The Prevalence of Metabolic Syndrome in Ethiopian Population: A Systematic Review and Meta-analysis. Journal of Obesity. 2020;2020:2701309. doi: 10.1155/2020/2701309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Birarra MK, Gelayee DA. Metabolic syndrome among type 2 diabetic patients in Ethiopia: a cross-sectional study. BMC Cardiovasc Disord. 2018;18(1):149. doi: 10.1186/s12872-018-0880-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scuteri A, Laurent S, Cucca F, Cockcroft J, Cunha PG, Manas LR, et al. Metabolic syndrome across Europe: different clusters of risk factors. Eur J Prev Cardiol. 2015;22(4):486–491. doi: 10.1177/2047487314525529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abagre TA. Metabolic syndrome among people with type 2 diabetes mellitus in two selected hospitals in the Brong Ahafo Region. Doctoral dissertation, University of Ghana.2016. URI: http://197.255.68.203/handle/123456789/21255.
- 45.Soyoye D, Adebayo O, Kolawole B, Ikem R. Prevalence and associated risks for metabolic syndrome in Nigerians with type 2 diabetes mellitus. Poster Presented at Society for Endocrinology BES 2013. Endocrine Abstracts 31 P237. 10.1530/endoabs.31.P237.
- 46.Foroozanfar Z, Najafpour H, Khanjani N, Bahrampour A, Ebrahimi H. The prevalence of metabolic syndrome according to diferent criteria and its associated factors in type 2 diabetic patients in Kerman. Iran Iran J Med Sci. 2015;40(6):522. [PMC free article] [PubMed] [Google Scholar]
- 47.Bentley-Lewis R, Koruda K, Seely EW. The metabolic syndrome in women. Nat Clin Pract Endocrinol Metab. 2007;3(10):696–704. doi: 10.1038/ncpendmet0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barzilay JI, Stein PK. Association of the metabolic syndrome with age-related, nonatherosclerotic, chronic medical conditions. Metab Syndr Relat Disord. 2011;9(5):327–335. doi: 10.1089/met.2011.0027. [DOI] [PubMed] [Google Scholar]
- 49.Kraja AT, Borecki IB, North K, Tang W, Myers RH, Hopkins PN, Arnett D, Corbett J, Adelman A, Province MA. Longitudinal and age trends of metabolic syndrome and its risk factors: the Family Heart Study. Nutr Metab (Lond) 2006;3:41. doi: 10.1186/1743-7075-3-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Huijgen R, Vissers MN, Defesche JC, Lansberg PJ, Kastelein JJ, Hutten BA. Familial hypercholesterolemia: current treatment and advances in management. Expert Rev Cardiovasc Ther. 2008;6(4):567–581. doi: 10.1586/14779072.6. [DOI] [PubMed] [Google Scholar]
- 51.Beigh SH, Jain S. Prevalence of metabolic syndrome and gender differences. Bioinformation. 2012;8(13):613–616. doi: 10.6026/97320630008613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Briones ER, Steiger DL, Palumbo PJ, O'Fallon WM, Langworthy AL, Zimmerman BR, Kottke BA. Sterol excretion and cholesterol absorption in diabetics and nondiabetics with and without hyperlipidemia. Am J Clin Nutr. 1986;44(3):353–361. doi: 10.1093/ajcn/44.3.353. [DOI] [PubMed] [Google Scholar]
- 53.Pitsavos C, Panagiotakos D, Weinem M, Stefanadis C. Diet, exercise, and the metabolic syndrome. Rev Diabet Stud. 2006;3(3):118. doi: 10.1900/RDS.2006.3.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets generated and or analysed during the current study are not publicly available due to personal data protection legislation but are available from the corresponding author on reasonable request.


