Abstract
Background
It has been shown that active exposure to tobacco is associated with adverse pregnancy outcomes including, but not limited to, intrauterine fetal death, reduced fetal weight, and higher risk of preterm birth. We want to investigate these effects in a high-income country.
Methods
This cross-sectional study examined 20,843 pregnant women who delivered over 10 years at the Maternity Hospital of the Centre Hospitalier Universitaire Vaudois (CHUV) in Lausanne, Switzerland. The objective was to evaluate a dose–response relationship between daily cigarette use during pregnancy and possible adverse perinatal outcomes. The social and clinical characteristics as well as obstetric and neonatal outcomes were compared between the smoking and the non-smoking groups. Adjusted odds ratios (aOR) and trend analyses (ptrend) were calculated.
Results
Nineteen thousand five hundred fifty-four pregnant women met the inclusion criteria and 2,714 (13.9%) of them were smokers. Even after adjusting for confounding factors, smoking during pregnancy was associated with preterm birth, birthweight < 2500 g, intrauterine growth restriction, neonatal respiratory and gastrointestinal diseases, transfer to the neonatal intensive care unit, and neonatal intensive care unit admissions > 7 days. Intrauterine death and neonatal infection were associated with heavy smoking (≥ 20 cigarettes/day). Smoking appeared to be a protective factor for pre-eclampsia and umbilical cord arterial pH below 7.1. A significant trend (ptrend < 0.05) was identified for preterm birth, intrauterine growth restriction, birthweight < 2500 g, umbilical cord arterial pH below 7.1, transfers to our neonatal intensive care unit, and neonatal intensive care unit admissions more than 7 days.
Conclusion
Cigarette smoking is associated with several adverse perinatal outcomes of pregnancy with a dose-dependent effect.
Keywords: Smoking during pregnancy, Perinatal outcomes, Preterm birth, Birthweight, Intrauterine growth restriction
Background
Among adults, the consequences of cigarette use are well known and can lead to cardiovascular, pulmonary, and oncological diseases as well as other chronic illnesses [1]. These negative health consequences are remote in time and therefore do not always cause sufficient immediate concern to motivate smoking cessation, especially in younger individuals [2]. The number of smokers worldwide in 2019 was estimated to be 1.14 billion, corresponding to 7.69 million deaths and 200 million DALYs (Disability Adjusted Life Years). Globally, the proportion of smokers is much lower among women with 6.62% of female individuals identified as smokers compared to 32.7% of male individuals. However, this proportion is considerably higher among women in high-income countries with 17.6% of women compared to 26.9% of men identifying as smokers [3].
There is evidence that women are more likely to discontinue cigarette use during their pregnancy [4]. The global prevalence of smoking during pregnancy is estimated to be 1.7% [5]. This proportion, also evaluated in 2018, is significantly higher in high-income countries, reaching 7.2% in the USA [6] and 8.1% in Europe [5]. These numbers should be interpreted cautiously as up to 25% of pregnant women with cigarette use prior to pregnancy incorrectly indicated that they ceased smoking during pregnancy [7]. Pregnant women with a lower level of education and those who experience an unplanned pregnancy have a higher prevalence of smoking and a lower probability of quitting [8, 9].
The effects of smoking during pregnancy have been the subject of numerous studies and have been associated with many adverse perinatal outcomes. Specifically active exposure to tobacco has been shown to be associated with a dose–response relationship to adverse outcomes such as preterm birth (birth before 37 weeks of pregnancy) [10–12], reduced birth weight [13, 14], with the reduction in fetal measurements occurring after the first trimester [15], and transfer to a neonatal intensive care unit [16]. Smoking has also been associated in a dose-dependent manner with an increased risk of intrauterine fetal death [17–20]. In contrast to adverse outcomes cited, smoking has been identified to be a protective factor against pre-eclampsia [21, 22]. Regarding the neonatal impact, smoking during pregnancy can alter fetal lung development and lead to respiratory problems [23, 24]. Long term, fetal exposure to smoking during pregnancy can result in more frequent development of gastrointestinal pathologies [25].
In summary, many studies have already investigated adverse obstetric and neonatal outcomes [26, 27]. However, not all of them included a large sample from a single center or adjusted their results to account for potential confounding factors. In addition, many studies have focused only on a single adverse outcome. For example, Soneji et al. focused their study on prematurity [12], and Larsen et al. focused mainly on birth weight [13]. If we take the main studies found in the literature that focused on several outcomes, Ratnasiri et al. did not focus on neonatal outcomes and did not evaluate a potential dose–response [28]. Finally, the well conducted research of Li et al. did not focus on several key outcomes including the risk of pre-eclampsia or neonatal infections, pulmonary pathologies, or gastrointestinal pathologies and did not evaluate a potential dose–response as well [29].
For all these reasons, we firstly aimed to assess multiple obstetric and neonatal outcomes associated with cigarette smoking during pregnancy within a single and large Swiss obstetric cohort with prospectively collected data. Some have already been studied, others not. Secondly, we want to evaluate a potential dose–response relationship between the quantity of cigarette use and adverse outcomes.
Methods
This cross-sectional study utilized our obstetrical database at the Maternity Hospital of the Centre Hospitalier Universitaire Vaudois (CHUV) in Lausanne, Switzerland, where 20,843 pregnant women gave birth between 1997 and 2006. Data available in this database include demographic, labor, and delivery information, as well as maternal and neonatal outcomes.
All information regarding patient health and pregnancy was collected at the time of admission to the hospital, with the majority occurring at the time of admission for delivery or, for some, at the time of admission to the antepartum unit in the case of complicated pregnancies. A medical history was taken for each patient presenting to the hospital by the obstetrical care provider. If urgent care was required, the history was postponed to an appropriate time during the hospitalization. Our computer system did not permit closure of a patient file that did not include all the mandatory information, including smoking habits. This information was collected verbally with the following question: "Do you smoke cigarettes daily?" with a dichotomous “yes/no” answer. If the answer was “yes”, the number corresponding to the current consumption was then requested by the computer system. The number of cigarettes consumed thus represents usage in the late third trimester, and does not take into account variation of smoking during pregnancy.
Regarding neonatal data, all information was added to our database at the end of the stay by the neonatologists and/or the obstetricians. All women whose records contained all the data needed for our study were included regardless of mode of delivery. The exclusion criteria were as follows: women under 18 years of age or women with multiple pregnancies. The quality of this database of prospectively collected data has already been described elsewhere (cross-check congruent data in 98.2–99.8% of cases) [30].
The following social and clinical characteristics were extracted from the database: daily cigarette use, maternal age, country of birth, marital status, parity, previous pregnancy loss, education, professional status, health insurance, and the presence of significant psychosocial difficulties. The latter was defined as pregnant women referred for a dedicated indication for consultation associated with challenging psychosocial circumstances (psychiatric pathologies, alcohol or drug abuse, etc.…). We assessed the following obstetric and neonatal outcomes: delivery mode, pre-eclampsia, intrauterine death, neonatal death, preterm birth, intrauterine growth restriction, birthweight, umbilical cord arterial pH, APGAR score at 5 min, neonatal infection, hypoglycemia, cerebral hemorrhage or convulsion, jaundice, neonatal anemia, respiratory diseases (including pulmonary infection, pneumothorax, apnea, and hyaline membrane disease), gastrointestinal diseases (including feeding difficulties, occlusive syndrome, digestive hemorrhage, necrotizing enterocolitis, diarrhea, and vomiting), transfers to our neonatal intensive care unit, and neonatal intensive care unit admissions longer than 7 days.
The social and clinical characteristics, as well as the obstetric and neonatal outcomes, were compared between the smoking and non-smoking pregnant women. For the same comparisons, the group of smoking pregnant women was also divided into 3 subgroups according to their daily cigarette usage (< 10/day, ≥ 10/day, and ≥ 20/day). The p-value for each clinical and social characteristic, comparing smokers and non-smokers, was calculated using a Chi-squared test. Logistic regression models to assess the association between smoking and obstetric and neonatal outcomes were built and odds ratios were calculated (aOR), adjusted for maternal age, country of birth, marital status, parity, previous pregnancy loss, education, professional status, psychosocial difficulties and insurance. For some outcomes, such as birth weight, intrauterine growth restriction, umbilical cord arterial pH, APGAR score at 5 min, respiratory diseases, gastrointestinal diseases, neonatal infection, hypoglycemia, cerebral hemorrhage or convulsion, jaundice, neonatal anemia, and transfers to or stay in our neonatal intensive care unit, the odds ratios were also adjusted for the gestational age as these outcomes can occur more frequently in preterm neonates. For the calculation of adjusted estimators in multivariate logistic regression models, the baseline variables that significantly differed between both the groups (confounders) or those that are known risk factors for adverse outcomes were included in the models.
Finally, trend analyses (ptrend) were also calculated, using the Cochran-Armittage test, for all the outcomes examined to evaluate a potential dose–response relationship according to the number of daily cigarettes consumed.
Statistical analyses were performed using STATA 16 (Stata Corporation, College Station, USA).
The study was carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). This study was approved by the local IRB (Ethical Commission of the Canton of Vaud, Switzerland, protocol no. 101/08).
Results
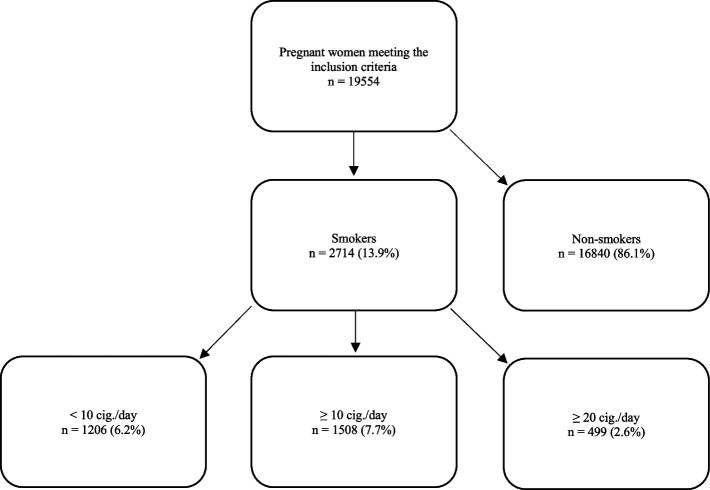
Over a period of 10 years, 19.554 pregnant women met the inclusion criteria. Among them, 16,840 (86.1%) identified as non-smokers and 2,714 (13.9%) identified as smokers (Fig. 1).
Fig. 1.
Classification of pregnant women according to the number of cigarettes consumed per day
The prevalence of pregnant women who reported cigarette use was higher among pregnant women of Swiss origin, single, divorced, or widowed, those who have had a previous spontaneous abortion, those with significant psychosocial difficulties, and nulliparous pregnant women (Table 1).
Table 1.
Participant characteristics
| Total | Smokers | Non-Smokers |
p-value (comparing smokers and non-smokers) |
|||
|---|---|---|---|---|---|---|
| (n) | (n) | (%) | (n) | (%) | ||
| Total | 19,554 | 2714 | 13.9 | 16,840 | 86.1 | |
| Maternal age | ||||||
| < 25 | 4040 | 732 | 18.1 | 3308 | 81.9 | < 0.001 |
| 26—30 | 5889 | 826 | 14 | 5063 | 86 | |
| 31—35 | 6156 | 739 | 12 | 5417 | 88 | |
| > 35 | 3469 | 417 | 12 | 3052 | 88 | |
| Origin | ||||||
| Swiss | 7307 | 1228 | 16.8 | 6079 | 83.2 | < 0.001 |
| Europe | 7479 | 1157 | 15.5 | 6322 | 84.5 | |
| Other | 4768 | 329 | 6.9 | 4439 | 93.1 | |
| Marital status | ||||||
| Married | 16,525 | 1975 | 12.0 | 14,550 | 88 | < 0.001 |
| Single/divorced/widowed | 3029 | 739 | 24.4 | 2290 | 75.6 | |
| Parity | ||||||
| Multiparous | 10,409 | 1387 | 13.3 | 9022 | 86.7 | 0.017 |
| Nulliparous | 9145 | 1327 | 14.5 | 7818 | 85.5 | |
| Pregnancy loss | ||||||
| No abortion | 13,434 | 1687 | 12.6 | 11,747 | 87.4 | < 0.001 |
| Previous abortion | 6120 | 1027 | 16.8 | 5093 | 83.2 | |
| Education | ||||||
| Non-academic studies | 18,016 | 2630 | 14.6 | 15,386 | 85.4 | < 0.001 |
| Academic studies | 1538 | 84 | 5.5 | 1454 | 94.5 | |
| Professional Status | ||||||
| Employed | 16,113 | 2194 | 13.6 | 13,919 | 86.4 | 0.021 |
| Unemployed | 3441 | 520 | 15.1 | 2921 | 84.9 | |
| Health Insurance | ||||||
| Minimal insurance | 18,804 | 2664 | 14.2 | 16,140 | 85.8 | < 0.001 |
| Private insurance | 750 | 50 | 6.7 | 700 | 93.3 | |
| Psychosocial difficulties | ||||||
| No | 19,158 | 2621 | 13.7 | 16,537 | 86.3 | < 0.001 |
| Yes | 396 | 93 | 23.5 | 303 | 76.5 | |
After adjustment for confounding factors, smoking during pregnancy was associated with preterm birth (aOR 1.16 [95%CI 1.03–1.31]), birthweight < 2500 g (aOR 1.78 [95%CI 1.53–2.08]), small for gestational age (aOR 1.83 [95%CI 1.64–2.05]), respiratory diseases (aOR 1.32 [95%CI 1.13–1.56]), gastrointestinal diseases (aOR 1.63 [95%CI 1.11–2.42]), transfers to the neonatal intensive care unit (aOR 1.44 [95%CI 1.26–1.63]), and neonatal intensive care unit admission > 7 days (aOR 1.64 [95%CI 1.42–1.90]). These associations were stronger in the groups of women with higher number of cigarettes consumed per day. Intrauterine death (aOR 1.98 [95%CI 1.01–3.89]) and neonatal infection (aOR 1.53 [95%CI 1.05–2.22]) were only associated with heavy smoking (≥ 20 cigarettes/day) but not with lower smoking exposure. In contrast, smoking appeared to be a protective factor for pre-eclampsia (aOR 0.62 [95%CI 0.44–0.88]) and umbilical cord arterial pH below 7.1 (aOR 0.65 [95%CI 0.50–0.86]). Rate of cesarean section, neonatal deaths and other neonatal outcomes such as an APGAR score below 7 at 5 min, hypoglycemia, cerebral hemorrhage or convulsion, jaundice, and neonatal anemia showed no significant differences between the smoking and the non-smoking groups (Table 2).
Table 2.
Association between obstetric and neonatal outcomes and smoking status in pregnant women as well as dose-dependent relationship with quantity of cigarettes
| Pre-eclampsia | Cesarean section | Preterm birth | IUGR | Birthweight < 2500 g | Intrauterine death | |||||||
| Cig./day | Prevalence % | aOR (95% CI) a | Prevalence % | aOR (95% CI) a | Prevalence % | aOR (95% CI) a | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) a |
| Non-smokers | 2.1 | 1 | 25.6 | 1 | 12.0 | 1 | 11.2 | 1 | 11.0 | 1 | 0.92 | 1 |
| Smokers | 1.3 | 0.62 (0.44–0.88) | 27.2 | 1.08 (0.99–1.19) | 14.2 | 1.16 (1.03–1.31) | 19.2 | 1.83 (1.64–2.05) | 16.7 | 1.78 (1.53–2.08) | 0.88 | 0.87 (0.56–1.36) |
| < 10 cig./day | 1.1 | 0.51 (0.29–0.90) | 26.5 | 1.15 (1.00–1.31) | 11.2 | 0.92 (0.77–1.12) | 16.9 | 1.57 (1.34–1.84) | 13.6 | 1.61 (1.28–2.01) | 0.41 | 0.43 (0.17–1.05) |
| ≥ 10 cig./day | 1.5 | 0.71 (0.46–1.09) | 27.7 | 1.15 (0.99–1.33) | 16.6 | 1.35 (1.17–1.57) | 21.0 | 2.06 (1.79–2.36) | 19.1 | 1.93 (1.60–2.33) | 1.26 | 1.21 (0.74–1.99) |
| ≥ 20 cig./day | 1.6 | 0.75 (0.36–1.53) | 28.1 | 1.14 (0.93–1.40)) | 17.6 | 1.43 (1.12–1.81) | 25.9 | 2.76 (2.24–3.41) | 25.3 | 3.42 (2.58–4.53) | 1.8 | 1.98 (1.01–3.89) |
| Neonatal death | Umbilical cord arterial pH < 7.1 | Apgar at 5 min < 7 | Neonatal infection | Hypoglycemia | Cerebral hemorrhage or convulsion | |||||||
| Prevalence % | aOR (95% CI) a | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | |
| Non-smokers | 0.8 | 1 | 3.6 | 1 | 4.9 | 1 | 4.1 | 1 | 3.5 | 1 | 0.9 | 1 |
| Smokers | 0.9 | 0.89 (0.56–1.42) | 2.3 | 0.65 (0.50–0.86) | 5.3 | 0.97 (0.81–1.19) | 4.5 | 1.04 (0.85–1.29) | 4.4 | 1.18 (0.96–1.48) | 0.8 | 0.75 (0.47–1.19) |
| < 10 cig./day | 0.6 | 0.73 (0.34–1.60) | 2.5 | 0.70 (0.48–1.02) | 4.8 | 1.01 (0.76–1.34) | 3.7 | 0.93 (0.68.1.28) | 3.5 | 1.06 (0.76–1.49) | 0.7 | 0.71 (0.35–1.47) |
| ≥ 10 cig./day | 1.1 | 0.99 (0.58–1.71) | 2.2 | 0.62 (0.43–0.88) | 5.7 | 0.95 (0.75–1.22) | 5.1 | 1.13 (0.88–1.46) | 5.0 | 1.27 (0.98–1.66) | 0.9 | 0.77 (0.44–1.36) |
| ≥ 20 cig./day | 1.4 | 1.25 (0.56–2.79) | 1.8 | 0.51 (0.26–0.99) | 6.4 | 1.04 (0.71–1.54) | 6.8 | 1.53 (1.05–2.22) | 5.8 | 1.45 (0.96–2.19) | 0.6 | 0.47 (0.15–1.50) |
| Jaundice | Neonatal anemia | Respiratory diseases c | Gastrointestinal diseases d | Transfers to NICU | Stay in NICU > 7 days | |||||||
| Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | Prevalence % | aOR (95% CI) b | |
| Non-smokers | 4.0 | 1 | 1.6 | 1 | 7.4 | 1 | 0.7 | 1 | 11.8 | 1 | 7.6 | 1 |
| Smokers | 4.5 | 1.09 (0.88–1.35) | 2.0 | 1.12 (0.82–1.54) | 10.1 | 1.32 (1.13–1.56) | 1.3 | 1.63 (1.11–2.42) | 16.5 | 1.44 (1.26–1.63) | 11.9 | 1.64 (1.42–1.90) |
| < 10 cig./day | 3.5 | 0.92 (0.66–1.29) | 1.3 | 0.88 (0.51–1.50) | 8.8 | 1.33 (1.05–1.69) | 1.1 | 1.54 (0.86–2.79) | 13.0 | 1.19 (0.98–1.45) | 7.8 | 1.11 (0.88–1.42) |
| ≥ 10 cig./day | 5.4 | 1.21 (0.94–1.56) | 2.5 | 1.28 (0.89–1.85) | 11.1 | 1.32 (1.08–1.61) | 1.5 | 1.69 (1.06–2.71) | 19.3 | 1.63 (1.39–1.91) | 15.2 | 2.07 (1.73–2.46) |
| ≥ 20 cig./day | 5.8 | 1.26 (0.83–1.90) | 2.8 | 1.36 (0.76–2.44) | 11.8 | 1.35 (0.98–1.88) | 0.8 | 0.84 (0.30–2.35) | 23.9 | 2.22 (1.74–2.83) | 20.2 | 3.04 (2.34–3.96) |
IUGR Intrauterine growth retardation, NICU Neonatal intensive care unit
a Adjusted odds ratios for maternal age, origin, marital status, parity, previous pregnancy loss, education, professional status, psychosocial difficulties and health insurance, 95% confidence interval
b Adjusted odds ratios for maternal age, origin, marital status, parity, previous pregnancy loss, education, professional status, psychosocial difficulties, health insurance and gestational age, 95% confidence interval
c Respiratory distress syndrome, pulmonary infection, pneumothorax, apnea, hyaline membrane disease
d Feeding difficulties, occlusive syndrome, digestive haemorrhage, necrotizing enterocolitis, diarrhea, vomiting
A significant dose–response relationship trend was identified between the number of daily cigarettes consumed and preterm birth (ptrend < 0.001), intrauterine growth restriction (ptrend < 0.001), birthweight < 2500 g (ptrend < 0.001), umbilical cord arterial pH below 7.1 (ptrend = 0.001), transfers to our neonatal intensive care unit (ptrend < 0.001), and neonatal intensive care unit admissions more than 7 days (ptrend < 0.001).
No trend was found for the other outcomes investigated: pre-eclampsia, increased rate of cesarean section, neonatal death, intrauterine death, APGAR score < 7 at 5 min, hypoglycemia, jaundice, neonatal anemia, neonatal infection, cerebral hemorrhage or convulsion, respiratory diseases, and gastrointestinal diseases.
Discussion
As our database includes a sample of pregnant women from the 1997 to 2006, this likely explains why the rate of pregnant individuals who identify as smokers, 13.9%, is higher than the rate described in statistics from 2018, which are estimated to be 8.1% in Europe [5] and 7.2% in the USA [6].
Cigarette smoking has an impact on pregnancy with several adverse perinatal outcomes. In our study, cigarette use was strongly associated with preterm birth, lower birthweight, intrauterine growth restriction, transfers to the neonatal intensive care unit, and neonatal intensive care unit admissions > 7 days. All of the above associations have a dose–response relationship, with significant trend values. Our results align with those found in the literature [10–14, 16]. Intrauterine death was associated with heavy cigarette consumption (≥ 20/day), while other studies attributed intrauterine death with lower tobacco consumption [17–19]. Finally, smoking during pregnancy can induce neonatal pulmonary and gastrointestinal pathologies. Heavy cigarette consumption (≥ 20/day) also increases the risk of neonatal infections.
The mechanisms by which tobacco smoking result in adverse perinatal outcomes are complex. They may occur as a result of disruption of fundamental processes such as proliferation, apoptosis, and invasion of the trophoblasts during placental development. Alteration of the vascularization and the metabolism of the placenta may also be a cause [31].
The association between neonatal gastrointestinal pathology and smoking during pregnancy, as well as the association with neonatal infections, has been little studied until now. As a comparison, it has been shown that adult smokers are themselves more susceptible to bacterial or viral infections than non-smokers which may be due to alteration of the structural, functional, and immunological functions of the host defenses [32, 33].
Smoking during pregnancy may, however, also still be a protective factor. Cigarette use during pregnancy has been shown to reduce the risk of pre-eclampsia [21, 22] as was also identified in our study. The protective role of smoking can be partially explained by the effects of carbon monoxide, one of the products of tobacco combustion. Carbon monoxide inhibits the placental production of anti-angiogenic proteins such as sFlt1 or sEng, which play a role in the pathogenesis of preeclampsia. However, the pathogenesis of pre-eclampsia remains complex and is still not fully understood [34]. It may be worth mentioning that Luque-Fernandez et al. have partially explained the paradoxical phenomenon of this protective effect by studying prevalent cases at birth rather than all incident cases in a pregnancy cohort, which results in selection bias [35]. In our study, tobacco smoking was also a protective factor against the risk of umbilical cord arterial pH below 7.1. This phenomenon has been little studied. However, we will qualify our results by comparing them with those of Zaigham et al. whose prospective-observational cohort study of 308 patients showed no significant differences in pH values between smokers and non-smokers [36].
Our results do not suggest a significant association for some outcomes such as an APGAR score below 7 at 5 min, hypoglycemia, cerebral hemorrhage or convulsion, jaundice, and neonatal anemia.
With the proportion of pregnant smokers estimated to be 8.1% in Europe [5] and 7.2% in the USA [6] in 2018, it is clear that there is still much to be done in terms of prevention. Although low tobacco consumption is associated with less severe outcomes than heavy consumption, it is important to inform pregnant women that even at low doses, smoking has consequences for the fetus, in addition to the consequences on their own health. Effective interventions for smoking cessation during pregnancy include regular interval counseling and the provision of nicotine replacement therapy to patients who do not respond to counseling only [37]. The use of incentives to motivate smoking cessation also showed encouraging results [38].
The strength of our study is the analysis of multiple prospectively collected outcomes within a single, large cohort. It confirms the different outcomes studied separately in the literature but also demonstrated a dose–response effect, which has not been systematically evaluated [10–29].
Our research also contains some weaknesses. First, we did not assess a possible change in smoking during pregnancy and we also did not include the occasional smokers. This constitutes an information bias. By using a large available database, which was not designed specifically for this research, we were also unable to utilize a standardized questionnaire to assess cigarette consumption. Second, we did not assess passive smoking or secondhand exposure, which may also affect the fetus [39]. Furthermore, we did not take into account certain factors that could be confounding, such as alcohol or cannabis use [40, 41]. Information regarding other factors, such as comorbidities or concomitant medication use were not available and therefore were also not taken into account.
In addition, it is important to mention that some odds ratio confidence intervals are wide, especially for the subgroup of “ ≥ 20 cig/day”. This may be explained by the fact that this subgroup only includes 499 patients out of 19,554 patients. We thus acknowledge that some of the comparisons are underpowered, and therefore the lack of statistically significant relationships for some of the comparisons may not necessarily indicate that there is no relationship. Since the associations found in our study might be underestimated due to patients underreporting their consumption, this “ > 20 cig/day” group might represent the true impact of smoking during pregnancy. Indeed, about 24% of pregnant smokers stop smoking during pregnancy and up to 25% of pregnant smokers also misreport their actual tobacco consumption. This represents a possible classification bias.
We can also mention the lack of generalizability due to a localized sample. Finally, during the time period of our study (1997–2006), obstetrical management may have altered. This potential change was not taken into account as a covariate. Also, the rate of smoking in pregnancy has been declining [5]. Within the Swiss population, the latest existing data to our knowledge includes the years 2011–2016. The proportion of pregnant smokers during this time was estimated to be 6.8%, showing a decrease in consumption since the data collected for our research [42]. Although the estimate of association may hold, many characteristics of women in the study may not hold.
Conclusion
Cigarette smoking during pregnancy is associated with several adverse perinatal outcomes. This relationship is often dose-dependent, as with preterm birth, birthweight < 2500 g, intrauterine growth restriction, transfers to neonatal intensive care unit, and neonatal intensive care unit admissions more than 7 days. Prevention among women must be further emphasized, as some adverse outcomes could be avoided by a smoke-free pregnancy.
Acknowledgements
We thank all midwives and doctors who computerized obstetrical data used in this study. Their involvement was essential to allow this study.
Authors’ contributions
BT handled the literature review as well as the writing of the manuscript. DB took care of the project development, the data collection, and the data analysis. JC also participated in the project development. Finally, CC was responsible for the manuscript's critical reviewing. The authors read and approved the final manuscript.
Funding
This work was supported by the research fund in obstetrics and gynecology of the University Hospital of Lausanne, Switzerland. The funding sources had no role in the study design, data collection, data analysis or the interpretation thereof, or in writing the report.
Availability of data and materials
The datasets analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). This study was approved by the local IRB (Ethical Commission of the Canton of Vaud, Switzerland, protocol no. 101/08). Informed consent was obtained from all subjects and/or their legal guardian.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta (GA): Centers for Disease Control and Prevention (US); 2014. (Reports of the Surgeon General). Available from: http://www.ncbi.nlm.nih.gov/books/NBK179276/. [Cited 22 Dec 2021]. [PubMed]
- 2.West R. Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychol Health. 2017;32(8):1018–1036. doi: 10.1080/08870446.2017.1325890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reitsma MB, Flor LS, Mullany EC, Gupta V, Hay SI, Gakidou E. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019. Lancet Public Health. 2021;6(7):e472–e481. doi: 10.1016/S2468-2667(21)00102-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Munson ML. Births: final data for 2003. Natl Vital Stat Rep Cent Dis Control Prev Natl Cent Health Stat Natl Vital Stat Syst. 2005;54(2):1–116. [PubMed] [Google Scholar]
- 5.Lange S, Probst C, Rehm J, Popova S. National, regional, and global prevalence of smoking during pregnancy in the general population: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(7):e769–e776. doi: 10.1016/S2214-109X(18)30223-7. [DOI] [PubMed] [Google Scholar]
- 6.Drake P, Driscoll AK, Mathews TJ. Cigarette smoking during pregnancy: United States, 2016. NCHS Data Brief. 2018;305:1–8. [PubMed] [Google Scholar]
- 7.George L, Granath F, Johansson ALV, Cnattingius S. Self-reported nicotine exposure and plasma levels of cotinine in early and late pregnancy. Acta Obstet Gynecol Scand. 2006;85(11):1331–1337. doi: 10.1080/00016340600935433. [DOI] [PubMed] [Google Scholar]
- 8.Madureira J, Camelo A, Silva AI, Reis AT, Esteves F, Ribeiro AI, et al. The importance of socioeconomic position in smoking, cessation and environmental tobacco smoke exposure during pregnancy. Sci Rep. 2020;10(1):15584. doi: 10.1038/s41598-020-72298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flower A, Shawe J, Stephenson J, Doyle P. Pregnancy planning, smoking behaviour during pregnancy, and neonatal outcome: UK millennium cohort study. BMC Pregnancy Childbirth. 2013;13:238. doi: 10.1186/1471-2393-13-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diguisto C, Dochez V. Consequences of active cigarette smoking in pregnancy - CNGOF-SFT expert report and Guidelines on the management of smoking during pregnancy. Gynecol Obstet Fertil Senol. 2020;48(7–8):559–566. doi: 10.1016/j.gofs.2020.03.025. [DOI] [PubMed] [Google Scholar]
- 11.Moore E, Blatt K, Chen A, Van Hook J, DeFranco EA. Relationship of trimester-specific smoking patterns and risk of preterm birth. Am J Obstet Gynecol. 2016;215(1):109.e1–6. doi: 10.1016/j.ajog.2016.01.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soneji S, Beltrán-Sánchez H. Association of maternal cigarette smoking and smoking cessation with preterm Birth. JAMA Netw Open. 2019;2(4):e192514. doi: 10.1001/jamanetworkopen.2019.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larsen S, Haavaldsen C, Bjelland EK, Dypvik J, Jukic AM, Eskild A. Placental weight and birthweight: the relations with number of daily cigarettes and smoking cessation in pregnancy. A population study. Int J Epidemiol. 2018;47(4):1141–1150. doi: 10.1093/ije/dyy110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kataoka MC, Carvalheira APP, Ferrari AP, Malta MB, de Barros Leite Carvalhaes MA, de Lima Parada CMG. Smoking during pregnancy and harm reduction in birth weight: a cross-sectional study. BMC Pregnancy Childbirth. 2018;18(1):67. doi: 10.1186/s12884-018-1694-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abraham M, Alramadhan S, Iniguez C, Duijts L, Jaddoe VWV, Den Dekker HT, et al. A systematic review of maternal smoking during pregnancy and fetal measurements with meta-analysis. PLoS ONE. 2017;12(2):e0170946. doi: 10.1371/journal.pone.0170946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondracki AJ. Low birthweight in term singletons mediates the association between maternal smoking intensity exposure status and immediate neonatal intensive care unit admission: the E-value assessment. BMC Pregnancy Childbirth. 2020;20(1):341. doi: 10.1186/s12884-020-02981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anderson TM, Lavista Ferres JM, Ren SY, Moon RY, Goldstein RD, Ramirez JM, et al. Maternal smoking before and during pregnancy and the risk of sudden unexpected infant death. Pediatrics. 2019;143(4):e20183325. doi: 10.1542/peds.2018-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pineles BL, Hsu S, Park E, Samet JM. Systematic review and meta-analyses of perinatal death and maternal exposure to tobacco smoke during pregnancy. Am J Epidemiol. 2016;184(2):87–97. doi: 10.1093/aje/kwv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marufu TC, Ahankari A, Coleman T, Lewis S. Maternal smoking and the risk of still birth: systematic review and meta-analysis. BMC Public Health. 2015;15:239. doi: 10.1186/s12889-015-1552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flenady V, Koopmans L, Middleton P, Frøen JF, Smith GC, Gibbons K, et al. Major risk factors for stillbirth in high-income countries: a systematic review and meta-analysis. Lancet Lond Engl. 2011;377(9774):1331–1340. doi: 10.1016/S0140-6736(10)62233-7. [DOI] [PubMed] [Google Scholar]
- 21.England L, Zhang J. Smoking and risk of preeclampsia: a systematic review. Front Biosci J Virtual Libr. 2007;12:2471–2483. doi: 10.2741/2248. [DOI] [PubMed] [Google Scholar]
- 22.Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209(6):544.e1–544.e12. doi: 10.1016/j.ajog.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 23.McEvoy CT, Spindel ER. Pulmonary effects of maternal smoking on the fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr Respir Rev. 2017;21:27–33. doi: 10.1016/j.prrv.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gibbs K, Collaco JM, McGrath-Morrow SA. Impact of tobacco smoke and nicotine exposure on lung development. Chest. 2016;149(2):552–561. doi: 10.1378/chest.15-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karur O, Gutvirtz G, Wainstock T, Sheiner E. Maternal prenatal smoking and long-term gastrointestinal morbidity of the offspring: a population-based cohort analysis. Reprod Toxicol Elmsford N. 2021;103:133–138. doi: 10.1016/j.reprotox.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 26.Tran DT, Roberts CL, Havard A, Jorm LR. Linking birth records to hospital admission records enhances the identification of women who smoke during pregnancy. Aust N Z J Public Health. 2014;38(3):258–264. doi: 10.1111/1753-6405.12213. [DOI] [PubMed] [Google Scholar]
- 27.Avşar TS, McLeod H, Jackson L. Health outcomes of smoking during pregnancy and the postpartum period: an umbrella review. BMC Pregnancy Childbirth. 2021;21(1):254. doi: 10.1186/s12884-021-03729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ratnasiri AWG, Gordon L, Dieckmann RA, Lee HC, Parry SS, Arief VN, et al. Smoking during pregnancy and adverse birth and maternal outcomes in California, 2007 to 2016. Am J Perinatol. 2020;37(13):1364–1376. doi: 10.1055/s-0039-1693689. [DOI] [PubMed] [Google Scholar]
- 29.Li R, Lodge J, Flatley C, Kumar S. The burden of adverse obstetric and perinatal outcomes from maternal smoking in an Australian cohort. Aust N Z J Obstet Gynaecol. 2019;59(3):356–361. doi: 10.1111/ajo.12849. [DOI] [PubMed] [Google Scholar]
- 30.Baud D, Meyer S, Vial Y, Hohlfeld P, Achtari C. Pelvic floor dysfunction 6 years post-anal sphincter tear at the time of vaginal delivery. Int Urogynecology J. 2011;22(9):1127–1134. doi: 10.1007/s00192-011-1431-2. [DOI] [PubMed] [Google Scholar]
- 31.Morales-Prieto DM, Fuentes-Zacarías P, Murrieta-Coxca JM, Gutierrez-Samudio RN, Favaro RR, Fitzgerald JS, et al. Smoking for two- effects of tobacco consumption on placenta. Mol Aspects Med. 2021;87:101023. doi: 10.1016/j.mam.2021.101023. [DOI] [PubMed] [Google Scholar]
- 32.Jiang C, Chen Q, Xie M. Smoking increases the risk of infectious diseases: a narrative review. Tob Induc Dis. 2020;18:60. doi: 10.18332/tid/123845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bagaitkar J, Demuth DR, Scott DA. Tobacco use increases susceptibility to bacterial infection. Tob Induc Dis. 2008;4:12. doi: 10.1186/1617-9625-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karumanchi SA, Levine RJ. How does smoking reduce the risk of preeclampsia? Hypertension. 2010;55(5):1100–1101. doi: 10.1161/HYPERTENSIONAHA.109.148973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luque-Fernandez MA, Zoega H, Valdimarsdottir U, Williams MA. Deconstructing the smoking-preeclampsia paradox through a counterfactual framework. Eur J Epidemiol. 2016;31(6):613–623. doi: 10.1007/s10654-016-0139-5. [DOI] [PubMed] [Google Scholar]
- 36.Zaigham M, Helfer S, Kristensen KH, Isberg PE, Wiberg N. Maternal arterial blood gas values during delivery: Effect of mode of delivery, maternal characteristics, obstetric interventions and correlation to fetal umbilical cord blood. Acta Obstet Gynecol Scand. 2020;99(12):1674–1681. doi: 10.1111/aogs.13936. [DOI] [PubMed] [Google Scholar]
- 37.Diamanti A, Papadakis S, Schoretsaniti S, Rovina N, Vivilaki V, Gratziou C, et al. Smoking cessation in pregnancy: An update for maternity care practitioners. Tob Induc Dis. 2019;17:57. doi: 10.18332/tid/109906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leung LWS, Davies GA. Smoking Cessation Strategies in Pregnancy. J Obstet Gynaecol Can JOGC J Obstet Gynecol Can JOGC. 2015;37(9):791–797. doi: 10.1016/S1701-2163(15)30149-3. [DOI] [PubMed] [Google Scholar]
- 39.Crane JMG, Keough M, Murphy P, Burrage L, Hutchens D. Effects of environmental tobacco smoke on perinatal outcomes: a retrospective cohort study. BJOG Int J Obstet Gynaecol. 2011;118(7):865–871. doi: 10.1111/j.1471-0528.2011.02941.x. [DOI] [PubMed] [Google Scholar]
- 40.Popova S, Dozet D, O’Hanlon G, Temple V, Rehm J. Maternal alcohol use, adverse neonatal outcomes and pregnancy complications in British Columbia, Canada: a population-based study. BMC Pregnancy Childbirth. 2021;21(1):74. doi: 10.1186/s12884-021-03545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gunn JKL, Rosales CB, Center KE, Nuñez A, Gibson SJ, Christ C, et al. Prenatal exposure to cannabis and maternal and child health outcomes: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e009986. doi: 10.1136/bmjopen-2015-009986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gmel G, Notari L. Consommation d’alcool et de tabac pendant la grossesse en Suisse : évaluation de l’enquête du monitorage suisse des addictions 2011–2016. Addiction Suisse; 2018. Available from: https://www.bag.admin.ch/bag/fr/home/das-bag/publikationen/forschungsberichte/forschungsberichte-sucht/forschungsberichte-tabak.html#-497648074. [Cited 11 Nov 2022] .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analysed during the current study are available from the corresponding author on reasonable request.