Abstract
Background
Post-COVID syndrome (PCS) affects millions of people worldwide, causing a multitude of symptoms and impairing quality of life months or even years after acute COVID-19. A prothrombotic state has been suggested; however, underlying mechanisms remain to be elucidated.
Objectives
To investigate thrombogenicity in PCS using a microfluidic assay, linking microthrombi, thrombin generation, and the von Willebrand factor (VWF):a Disintegrin and Metalloproteinase with a Thrombospondin Type 1 motif, member 13 (ADAMTS13) axis.
Methods
Citrated blood was perfused through microfluidic channels coated with collagen or an antibody against the VWF A3 domain, and thrombogenicity was monitored in real time. Thrombin generation assays were performed and α(2)-antiplasmin, VWF, and ADAMTS13 activity levels were also measured.
Results
We investigated thrombogenicity in a cohort of 21 patients with PCS with a median time following symptoms onset of 23 months using a dynamic microfluidic assay. Our data show a significant increase in platelet binding on both collagen and anti-VWF A3 in patients with PCS compared with that in controls, which positively correlated with VWF antigen (Ag) levels, the VWF(Ag):ADAMTS13 ratio (on anti-VWF A3), and inversely correlated with ADAMTS13 activity (on collagen). Thrombi forming on collagen presented different geometries in patients with PCS vs controls, with significantly increased thrombi area mainly attributable to thrombi length in the patient group. Thrombi length positively correlated with VWF(Ag):ADAMTS13 ratio and thrombin generation assay results, which were increased in 55.5% of patients. α(2)-Antiplasmin levels were normal in 89.5% of patients.
Conclusion
Together, these data present a dynamic assay to investigate the prothrombotic state in PCS, which may help unravel the mechanisms involved and/or establish new therapeutic strategies for this condition.
Keywords: long COVID, microfluidics, post-COVID syndrome, thrombosis, von Willebrand factor
1. Introduction
The COVID-19 pandemic has been a challenging global public health issue. A proportion of patients continue to experience ongoing symptoms after acute infection, including, but not limited to fatigue, shortness of breath, difficulty concentrating, chest pain, anxiety, and depression [1,2]. Post-COVID syndrome (PCS) is defined by the National Institute for Health and Care Excellence as signs and symptoms that develop during or following SARS-CoV-2 infection, continue for more than 12 weeks, and are not explained by an alternative diagnosis [3]. More than 22 million people have tested positive for COVID-19 in the United Kingdom, with 2 million people reporting long-term symptoms for at least 12 weeks (1.4 million), 1 year (826 000), or more than 2 years (376 000), at the time of writing this article [4]. More than 60% of people with PCS have ongoing symptoms 6 months after acute infection, with an average of 13.79 symptoms per person, thus severely impacting their quality of life [5,6].
A prothrombotic state secondary to acute SARS-CoV-2 is well documented [[7], [8], [9]], and the pathogenesis of PCS has been considered a persistence of a hypercoagulable state. Posthospitalized COVID-19 patients had increased thrombin-generating capacity alongside decreased plasma fibrinolytic capacity 4 months after discharge [9]. Increased endothelin-1 levels and sustained inflammation were also reported 3 months after acute COVID-19 [10]. Persistently raised D-dimers have been found in convalescing COVID-19 patients who were not hospitalized [9,11]. Furthermore, Pretorius et al. [12] reported the presence of platelet hyperactivation and “anomalous (amyloid) microclots” in PCS platelet-poor plasma, having subsequently proposed triple therapy anticoagulation as a potential treatment in PCS [13]. We and others also observed an increased von Willebrand factor antigen (VWF[Ag]):ADAMTS13 ratio, which was associated with impaired exercise capacity in patients with PCS [14,15]. The mechanisms underlying the different symptoms observed in patients with PCS are, however, unclear and, therefore, warrant further research.
In this study, we investigated the prothrombotic state in patients with PCS using a dynamic, microfluidic assay and analyzing platelet accumulation, thrombi formation, and geometries in real time. We further assessed the VWF(Ag):ADAMTS13 axis, thrombin generation, and α2-antiplasmin levels to identify whether these abnormalities were also confirmed in a small PCS cohort but with a much longer period since symptom onset than previously reported.
2. Methods
2.1. Collection of clinical data
Citrate-anticoagulated blood was collected from patients with PCS attending a post-COVID clinic between January and April 2022 as part of their routine analysis. In 21 anonymized cases, residual blood was used for an extended hemostasis profile. The patients with PCS included in this study had symptoms for >3 months. Forty-five age-matched control samples were collected from healthy volunteers providing informed consent (Medical Research Ethics Committee Numbers 08/H0810/54 and 08/H0716/72), who had COVID-19 or were vaccinated against COVID-19 in the past 2 years but experienced no post-COVID symptoms. Patients and controls who had taken anticoagulant/antiplatelet drugs before sample collection were excluded. Access to clinical data for all patients seen in the post-COVID service was approved by the data access committee on September 20, 2020. This was for service evaluation.
2.2. Laboratory assays
Thrombin generation assay (TGA) was performed using a Ceveron TGA RC Low Kit (Pathway Diagnostics) [16] and area under the curve (AUC) was reported (normal range: 1236-2945 nM). α(2)-Antiplasmin levels were analyzed using the BIOPHEN α(2)-antiplasmin kit (LRT, part number 220502) on the Sysmex CS2500 analyzer following the manufacturer’s instructions (normal range: 75%-135%).
For the microfluidic assay, fresh human blood samples were analyzed on VenaFluoro8+ microchips (Cellix), coated overnight, at 4°C, as previously described [17,18], with either collagen type I (Horm:100 μg/mL), an antibody against VWF A3 domain (82D6A3: 86 μg/mL) [19] or VWF (Haemate P: 2 μM), and blocked with phosphate-buffered saline 1% (w/v) bovine serum albumin. Citrated blood was labeled with DiOC6 (2.5 μM) and perfused through the channels at 1800/s for 3 minutes, using a nanopump (Mirus-Evo/Cellix) within 2.5 hours of collection. Platelet accumulation was recorded using an inverted fluorescent microscope (Zeiss) and a QImage camera.
2.3. Data analysis
Images from across the channels were analyzed to establish platelet surface coverage, thrombi geometry (area, length, width), and thrombi numbers. Data were quantified using software developed in-house, in a standardized, automated manner to avoid biased interpretation. Statistical analysis was performed in GraphPad Prism 9, using unpaired Student’s t-test and Pearson correlation, following Shapiro–Wilk normality test. A p value of <.05 was considered significant.
3. Results and Discussion
3.1. Clinical data
Twenty-one PCS cases, symptomatic for a median of 23 months (range: 13-25 months) were analyzed, of whom 81% were female, with a median age of 46 years (range: 28-70 years). Only 1 patient was hospitalized with acute COVID-19. Approximately 95.2% of the patients reported fatigue, 90.5% reported breathlessness, 81% had difficulty concentrating and memory problems, and 52.4% had chest pain. The median number of symptoms was 8 (range: 5-16 symptoms).
Previous studies report an increase in thrombin generation, with significantly lower lag times in PCS [20]. In 10 of 18 (55.5%) patients from our study, the AUC was outside the normal range (PCS range: 2597.5-3502.1 nM) and 8 of 18 (44.4%) patients had a lag time ≤ 2 minutes, suggesting an increase in thrombin generation in half of our cohort (Table ). Being in line with the literature, this increase is considered clinically significant and could contribute to the hypercoagulable state of these patients. Seventeen of 19 (89.5%) patients had normal levels of α2-antiplasmin (Table, range: 103.8-137.1). This is in contrast with previous findings by Pretorius et al. [12], who reported an increase in the α2-antiplasmin levels. As fibrinogen was reported to increase in acute COVID-19 [21], we also assessed whether this occurs in PCS. Six of 19 (31.6%) patients had levels above the normal laboratory range (range: 2.145 g/L) (Table). However, the increases in fibrinogen levels were only marginal and not considered clinically significant.
Table.
Hemostasis profile in patients with post-COVID syndrome.
| Patient with PCS (no.) | VWF antigen (RR 0.5-1.6 IU/dL) | ADAMTS13 act. (RR 60-146 IU/dL) | VWF(Ag):ADAMTS13 | tLag (min) | tPeak (min) | AUC nM (RR 1236-2945) | α2-Antiplasmin (RR 75%-135%) | Fbg g/L (RR 1.5-4.0) g/L (RR 1.5-4.0) |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.85 | 80.7 | 1.05 | 1.4 | 5.2 | 3502.1 | 122 | 4.25 |
| 2 | 1.04 | 112.4 | 0.93 | 2.3 | 8.3 | 3281.6 | 131 | 3.54 |
| 3 | 3.36 | 87.7 | 3.83 | 2.4 | 8.2 | 3507.2 | 122.3 | 4.59 |
| 4 | 1.18 | 104.5 | 1.13 | 1.9 | 8.3 | 2954.9 | 123.5 | 2.14 |
| 5 | 1.83 | 87.2 | 2.10 | 2.4 | 7.3 | 3060.4 | 135.3 | 3.96 |
| 6 | 1.24 | 95.9 | 1.29 | 2.2 | 8.1 | 3314 | 114.1 | 4.15 |
| 7 | 1.27 | 104.7 | 1.21 | 1.9 | 7.5 | 2873.7 | 103.8 | 2.17 |
| 8 | 1.48 | 106.5 | 1.39 | 2.6 | 7.9 | 3127.4 | 114.6 | 3.39 |
| 9 | 1.16 | 117 | 0.99 | 1.5 | 5.6 | 3113.7 | 116 | 2.36 |
| 10 | 0.87 | 95 | 0.92 | 2.3 | 8 | 2918 | 128.7 | 3.13 |
| 11 | 1.43 | 96 | 1.50 | 1.5 | 5.9 | 2895.4 | 105.3 | 2.62 |
| 12 | 1.37 | 93.7 | 1.46 | 2.6 | 8.2 | 3190.3 | 120.7 | 4.47 |
| 13 | 1.46 | 120.7 | 1.21 | 2.1 | 7.3 | 2697 | 124.9 | 3.32 |
| 14 | 1.8 | 87.1 | 2.07 | 2 | 6.3 | 3471.2 | 137.1 | 4.98 |
| 15 | 1.38 | 81.9 | 1.68 | 1.1 | 4.5 | 2398.5 | 126.9 | 3.07 |
| 16 | 1.87 | 113.2 | 1.65 | 2.3 | 7.9 | 2970.9 | 118.9 | 2.8 |
| 17 | 1.8 | 91.8 | 1.96 | 2 | 7.5 | 3159.1 | 113.5 | 3.96 |
| 18 | 1.85 | 93.1 | 1.99 | 2.2 | 7.9 | 2597.5 | 119.4 | 5 |
| 19 | 1.21 | 84.3 | 1.44 | 114.5 | 2.62 | |||
| 20 | 0.67 | 98.5 | 0.68 | |||||
| 21 | 1.07 | 125 | 0.86 |
Twenty-one residual blood samples from patients with PCS were analyzed for multiple hemostatic parameters – VWF antigen levels, ADAMTS13 activity, VWF(Ag):ADAMTS13 ratio, thrombin generation assay (lag time – tLag, time to peak – tPeak and area under the curve – AUC), α(2)-antiplasmin levels, and fibrinogen (Fbg) levels. Values in bold fall outside the normal range. For VWF(Ag):ADAMTS13 ratio, values > 1.5 are in bold.
Fbg, fibrinogen; PCS, post-COVID syndrome; RR, reported range; tLag, lag time; tPeak, time to peak; VWF, von Willebrand factor.
Importantly, as we previously reported an increased VWF(Ag):ADAMTS13 ratio in PCS [14], this was also measured. Eight of 21 (38.08%) patients had a VWF(Ag):ADAMTS13 ratio ≥ 1.5 (Table), suggesting that this parameter might also play a significant role in the thrombogenic aspect of PCS.
3.2. Increased platelet binding in PCS
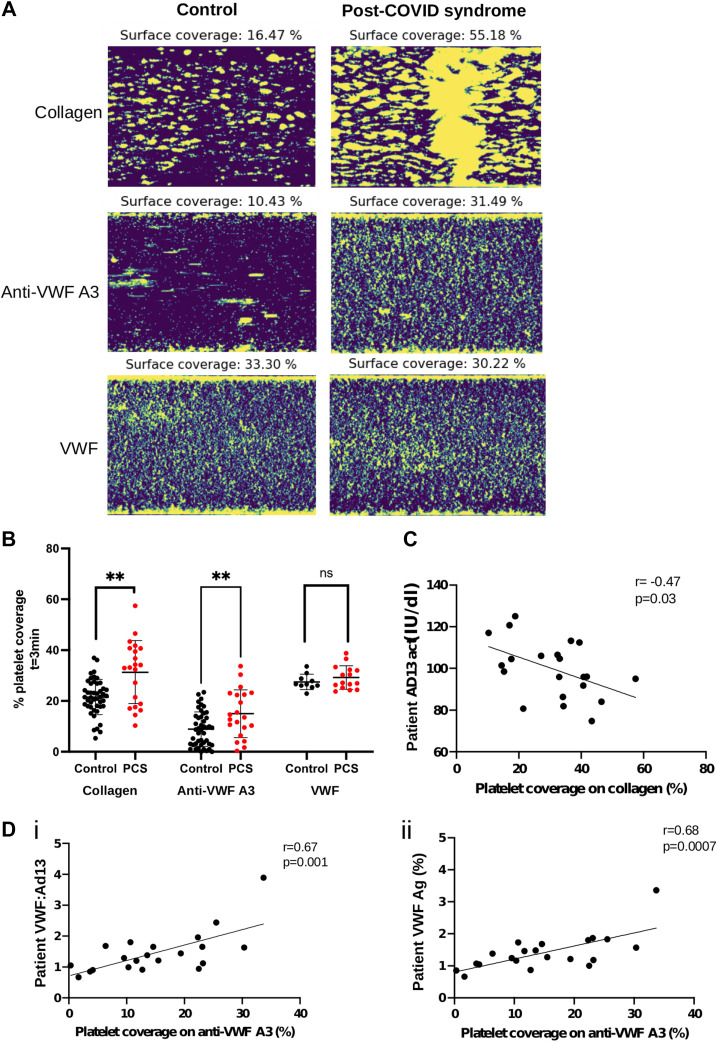
To explore the increase in VWF(Ag):ADAMTS13 ratio on thrombi formation/platelet binding in PCS, we set up a microfluidic assay using fresh human whole blood, whereby initial platelet recruitment to the channel surface was mediated by plasma VWF. Platelet binding was significantly increased in patients with PCS vs controls, on both anti-VWF A3 and collagen-coated channels (p < .01) but not on channels directly coated with VWF (Figure 1 A, B). This is in line with the hypothesis that raised VWF(Ag) levels in PCS plasma may play a role in the prothrombotic state of these patients, by promoting platelet accumulation.
Figure 1.
Platelet coverage in post-COVID syndrome. (A) Representative images (20× magnification) of platelets labelled with DiOC6 bound to collagen, anti-von Willebrand factor (VWF) A3, or VWF, after 3 minutes of flow at 1800/s in patients with post-COVID syndrome (PCS) (n = 21) vs healthy controls (n = 45). (B) Platelet surface coverage on microchannels coated with collagen, anti-VWF A3, or VWF, after 3 minutes of perfusing whole blood from healthy controls (black, n = 45) vs patients with PCS (red, n = 21 for collagen, anti-VWF A3, n = 15 for VWF-coated channels), at 1800/s. Data displayed as mean±SD and analyzed using unpaired Student’s t-test with Welch’s correction. ∗∗p < .01. (C) Correlation between platelet coverage on collagen and ADAMTS13 activity levels. Data analyzed using Pearson correlation coefficients. (D) Correlation between platelet coverage on anti-VWF A3 and VWF(Ag):ADAMTS13 ratio (i) or VWF antigen levels (ii). Data was analyzed using Pearson correlation coefficients.
Platelets formed microthrombi on collagen channels and small aggregates on anti-VWF A3 and VWF (Figure 1A). This is expected, as VWF-dependent platelet signaling via glycoprotein Ibα does not fully activate platelets but only “primes” them with limited activation of integrin αIIbβ3, which potentiates aggregation via fibrinogen [17]. Conversely, on collagen-coated channels, platelets initially captured by VWF can further directly interact with collagen, a known potent platelet agonist, via their glycoprotein VI and α2β1 receptors. This stabilizes platelet binding and can cause full platelet activation [22], interlinking glycoprotein VI- and glycoprotein Ib-dependent signaling [23]. Platelet coverage on anti-VWF A3 correlated with VWF(Ag) levels (r = 0.67, p = .001) and VWF(Ag):ADAMTS13 ratio (r = 0.68, p = .0007) (Figure 1D), whereas platelet coverage on collagen was not linked (data not shown). However, platelet coverage on collagen inversely correlated with ADAMTS13 activity (r = −0.47, p = .03) (Figure 1C). This suggests that platelet coverage on collagen is partly dependent on VWF levels but is perhaps also influenced by the additional direct interaction between platelets and collagen and by collagen-dependent platelet activation.
3.3. Increased thrombi geometry parameters in PCS
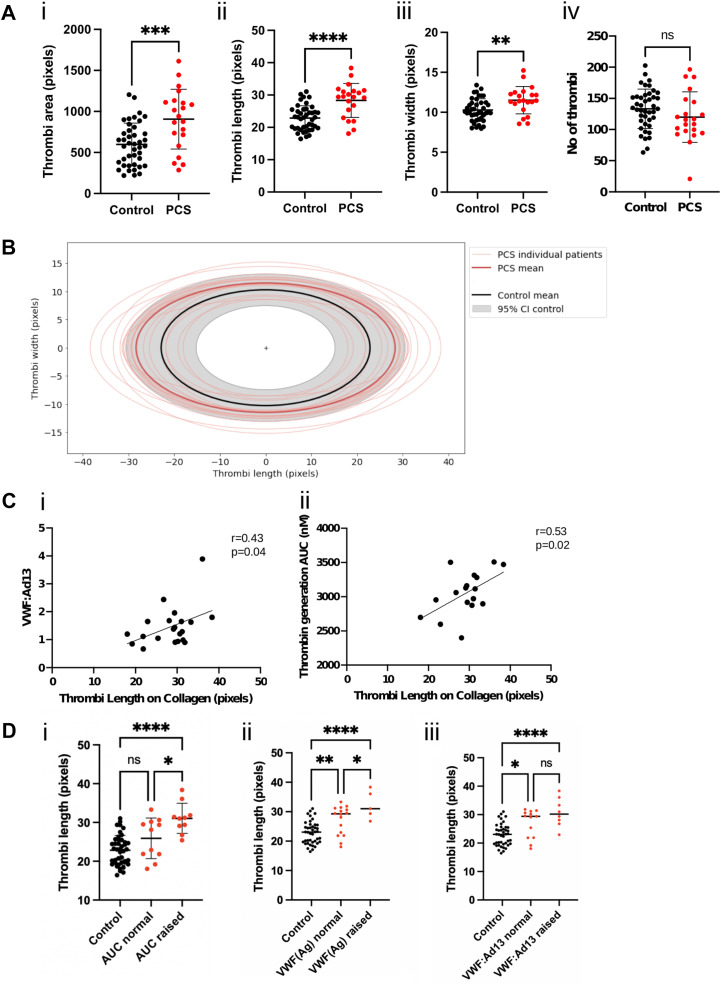
To further explore the effect of PCS on thrombi formation, we analyzed thrombi geometry on collagen-coated channels. There was no difference in thrombi numbers (Figure 2 A(iv)), but there was a significant increase in the thrombi area (Figure 2A(i), B), attributable to the length of the thrombi (Figure 2A(ii), B). This correlated with the AUC in TGA (r = 0.53, p = .02) (Figure 2C), suggesting the importance of thrombin generation for thrombi formation on collagen. Indeed, the difference in thrombi length between controls and PCS was primarily in patients with raised AUC (Figure 2D, p < .0001). Thrombi length on collagen also correlated with VWF(Ag):ADAMTS13 ratio (r = 0.43, p = .04) (Figure 2C, D), confirming a role for VWF in thrombogenesis.
Figure 2.
Thrombi geometry in post-COVID syndrome (PCS). (A) Thrombi geometry is represented as thrombi area (i), length (ii), width (iii), and the number of thrombi (iv) on collagen-coated channels in controls (black, n = 42) vs patients with PCS (red, n = 21). Data displayed as mean±SD and analyzed using unpaired Student’s t-test. ∗∗p < .01, ∗∗∗p < .001, ∗∗∗∗p < .0001. (B) Graphical representation of thrombi forming on collagen in patients with PCS (red) vs controls (gray). The mean length and width of thrombi are shown for individual patients/controls, with the mean thrombi geometry for all patients. The 95% confidence interval (CI) for controls is shown in the gray area. (C) Correlation analysis between thrombi length on collagen-coated channels and von Willebrand factor (VWF)(Ag):ADAMTS13 (i) or the area under the curve (AUC) in thrombin generation assay (ii). Data analyzed using Pearson correlation coefficients. (D) Difference in thrombi length in controls vs patients with normal/raised AUC (i), VWF(Ag) (ii), or VWF:ADAMTS13 (iii). Data analyzed using 1-way anova test. ∗p < .05, ∗∗∗∗p < .0001.
Together, these data present, for the first time, a dynamic assay showing a prothrombotic tendency in patients who have been suffering from PCS for approximately 2 years. Our results confirm a hypercoagulable state in patients with PCS related to an increase in VWF(Ag):ADAMTS13 ratio and thrombin generation but not in α2-antiplasmin levels. Investigating thrombogenicity in a larger cohort of patients using this microfluidic assay, VWF(Ag):ADAMTS13 axis and TGA, would be crucial in providing new mechanistic insights for the multitude of symptoms in PCS and for establishing therapeutic targets for this emerging condition.
Acknowledgments
This work was supported by Howard de Walden Estate and STIMULATE ICP.
Author contributions
A.C.-B. designed the research, performed the experiments, analyzed the data, and wrote the manuscript. A.K. provided patient samples, analyzed the data, and wrote the manuscript. R.d.G. designed the research and reviewed the manuscript. B.D. performed experiments and analyzed the data. M.H. and T.H. designed the research, collected patient samples, and reviewed the manuscript. L.C.P. collected patient samples and reviewed the manuscript. B.E. collected patient samples. R.S. developed the analysis software. K.V. provided the anti-VWF A3 antibody (82D6A3) and reviewed the manuscript. D.S. performed experiments and reviewed the manuscript. M.S. designed the research, analyzed data, and reviewed the manuscript.
Declaration of competing interest
The authors have no conflict of interest to disclose.
Footnotes
Funding information This work was supported by Howard de Walden Estate and STIMULATE-ICP. This work is part funded by the National Institute for Health and Care Research (NIHR) (STIMULATE-ICP, COV-LT2-0043: MH and TH). The views expressed in this publication are those of the author(s) and not necessarily those of NIHR or The Department of Health and Social Care.
Manuscript handled by: Keith Neeves
Final decision: Keith Neeves, 24 October 2022
The online version contains supplementary material available at https://doi.org/10.1016/j.jtha.2022.10.013.
Supplementary material
References
- 1.Carfì A., Bernabei R., Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence (NICE) COVID-19 rapid guideline: managing the long-term effects of COVID-19. National Institute for Health and Care Excellence (NICE); London: 2020. [PubMed] [Google Scholar]
- 4.Office for National Statistics . 2022. ONS prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6may2022. Accessed May 6, 2022. [Google Scholar]
- 5.Davis H.E., Assaf G.S., McCorkell L., Wei H., Low R.J., Re'em Y., Redfield S., Austin J.P., Akrami A. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jennings G., Monaghan A., Xue F., Mockler D., Romero-Ortuño R. A systematic review of persistent symptoms and residual abnormal functioning following acute COVID-19: ongoing symptomatic phase vs. post-COVID-19 syndrome. J Clin Med. 2021;10:5913. doi: 10.3390/jcm10245913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abou-Ismail M.Y., Diamond A., Kapoor S., Arafah Y., Nayak L. The hypercoagulable state in COVID-19: incidence, pathophysiology, and management. Thromb Res. 2020;194:101–115. doi: 10.1016/j.thromres.2020.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F., Vanstapel A., Werlein C., Stark H., Tzankov A., Li W.W., Li V.W., Mentzer S.J., Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Meijenfeldt F.A., Havervall S., Adelmeijer J., Lundström A., Magnusson M., Mackman N., Thalin C., Lisman T. Sustained prothrombotic changes in COVID-19 patients 4 months after hospital discharge. Blood Adv. 2021;5:756–759. doi: 10.1182/bloodadvances.2020003968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Willems L.H., Nagy M., Ten Cate H., Spronk H.M.H., Groh L.A., Leentjens J., Janssen N.A.F., Netea M.G., Thijssen D.H.J., Hannink G., van Petersen A.S., Warlé M.C. Sustained inflammation, coagulation activation and elevated endothelin-1 levels without macrovascular dysfunction at 3 months after COVID-19. Thromb Res. 2022;209:106–114. doi: 10.1016/j.thromres.2021.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend L., Fogarty H., Dyer A., Martin-Loeches I., Bannan C., Nadarajan P., Bergin C., O'Farrelly C., Conlon N., Bourke N.M., Ward S.E., Byrne M., Ryan K., O'Connell N., O'Sullivan J.M., Ni Cheallaigh C., O'Donnell J.S. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J Thromb Haemost. 2021;19:1064–1070. doi: 10.1111/jth.15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pretorius E., Vlok M., Venter C., Bezuidenhout J.A., Laubscher G.J., Steenkamp J., Kell D.B. Persistent clotting protein pathology in long COVID/post-acute sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc Diabetol. 2021;20:172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kell D.B., Laubscher G.J., Pretorius E. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. BiochemJ. 2022;479:537–559. doi: 10.1042/BCJ20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prasannan N., Heightman M., Hillman T., Wall E., Bell R., Kessler A., Neave L., Doyle A.J., Devaraj A., Singh D., Dehbi H.-M., Scully M. Impaired exercise capacity in post-COVID syndrome: the role of VWF-ADAMTS13 axis. Blood Adv. 2022;6:4041–4048. doi: 10.1182/bloodadvances.2021006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancini I., Baronciani L., Artoni A., Colpani P., Biganzoli M., Cozzi G., Novembrino C., Boscolo Anzoletti M., De Zan V., Pagliari M.T., Gualtierotti R., Aliberti S., Panigada M., Grasselli G., Blasi F., Peyvandi F. The ADAMTS13-von Willebrand factor axis in COVID-19 patients. J Thromb Haemost. 2021;19:513–521. doi: 10.1111/jth.15191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kintigh J., Monagle P., Ignjatovic V. A review of commercially available thrombin generation assays. Res Pract Thromb Haemost. 2018;2:42–48. doi: 10.1002/rth2.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Constantinescu-Bercu A., Grassi L., Frontini M., Salles C., II, Woollard K., Crawley J.T. Activated α(IIb)β(3) on platelets mediates flow-dependent NETosis via SLC44A2. eLife. 2020;9 doi: 10.7554/eLife.53353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alwan F., Vendramin C., Budde U., Liesner R., Taylor A., Thomas M., Lämmle B., Scully M. Assessing thrombogenesis and treatment response in congenital thrombotic thrombocytopenic purpura. eJHaem. 2021;2:188–195. doi: 10.1002/jha2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staelens S., Hadders M.A., Vauterin S., Platteau C., De Maeyer M., Vanhoorelbeke K., Huizinga E.G., Deckmyn H. Paratope determination of the antithrombotic antibody 82D6A3 based on the crystal structure of its complex with the von Willebrand factor A3-domain. J Biol Chem. 2006;281:2225–2231. doi: 10.1074/jbc.M508191200. [DOI] [PubMed] [Google Scholar]
- 20.Fogarty H., Townsend L., Morrin H., Ahmad A., Comerford C., Karampini E., Englert H., Byrne M., Bergin C., O'Sullivan J.M., Martin-Loeches I., Nadarajan P., Bannan C., Mallon P.W., Curley G.F., Preston R.J.S., Rehill A.M., McGonagle D., Ni Cheallaigh C., Baker R.I., et al. Persistent endotheliopathy in the pathogenesis of long COVID syndrome. J Thromb Haemost. 2021;19:2546–2553. doi: 10.1111/jth.15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Di Micco P., Russo V., Carannante N., Imparato M., Cardillo G., Lodigiani C. Prognostic value of fibrinogen among COVID-19 patients admitted to an emergency department: an Italian cohort study. J Clin Med. 2020;9 doi: 10.3390/jcm9124134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemetson K.J., Clemetson J.M. Platelet collagen receptors. Thromb Haemost. 2001;86:189–197. [PubMed] [Google Scholar]
- 23.Constantinescu-Bercu A., Wang Y.A., Woollard K.J., Mangin P., Vanhoorelbeke K., Crawley J.T., Salles C., II The GPIbα intracellular tail – role in transducing VWF- and collagen/GPVI-mediated signaling. Haematologica. 2021;107:933–946. doi: 10.3324/haematol.2020.278242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.