Abstract
Aims
Hypoxia‐inducible factor‐prolyl hydroxylase (HIF‐PH) inhibitors have been developed for the treatment of renal anaemia; however, no study has evaluated the safety and efficacy of HIF‐PH inhibitors in patients with heart failure (HF). This study was designed to evaluate the safety and efficacy of daprodustat, a HIF‐PH inhibitor, in patients with HF and renal anaemia.
Methods and results
We designed a pilot, multi‐centre, open‐label, randomized controlled study, in which 50 patients with HF complicated with chronic kidney disease and anaemia will be randomized 1:1 to either the daprodustat or control group at seven sites in Japan. Study entry requires New York Heart Association Class II HF symptoms or a history of hospitalization due to HF, an estimated glomerular filtration rate of <60 mL/min/1.73 m2, and a haemoglobin level of 7.5 to <11.0 g/dl. Patients randomized to the daprodustat group will be treated with oral daprodustat, and the dose will be uptitrated according to the changes in the haemoglobin level from previous visits. In this study, we will evaluate the impact of HIF‐PH inhibitors on cardiac function using advanced cardiovascular imaging modalities, including cardiac magnetic resonance imaging. The primary outcome is the haemoglobin level at 16 weeks of randomization, and all adverse events will be recorded and evaluated for any association with daprodustat treatment.
Conclusion
Considering the hypothetical upside and downside of using HIF‐PH inhibitors in anaemic patients with HF and chronic kidney disease, and because there are virtually no safe and effective treatments for patients with anaemia not caused by iron deficiency, our study results will contribute significantly to this field.
Keywords: Anaemia, Chronic kidney disease, Daprodustat, Heart failure, HIF‐PH inhibitor, Study protocol
1. Introduction
The number of patients with heart failure (HF) is increasing worldwide, and it is an important global health issue. Anaemia is a major co‐morbidity in patients with HF. 1 Previous studies have shown that anaemia is present in 35% of patients with HF and is associated with a poor prognosis in both acute and chronic HF. 2 , 3 The pathophysiology of anaemia in HF is multifactorial, but one of the major causes is renal anaemia, which results due to a relative decrease in erythropoietin (EPO) production. Previous studies have shown that renal failure is a common co‐morbidity in patients with HF, and the mortality rate is higher in patients with coexisting anaemia and renal failure. 4 Erythropoiesis‐stimulating agents (ESAs) are widely used for the treatment of renal anaemia and their efficacy has been well proven; however, previous studies have demonstrated no clinical benefits. 5 , 6 Therefore, there is practically no approved treatment for patients with HF and anaemia that is not attributable to an iron deficiency.
Recently, hypoxia‐inducible factors (HIFs) have been identified as key peptides involved in the regulation of EPO synthesis in the kidney. 7 Under the hypoxic condition, mainly HIF‐2α subunit is activated and subsequently contributes to increasing EPO production. 8 However, under conditions of sufficient oxygen, the newly translated HIF‐1α protein from the mRNA undergoes hydroxylation at the proline residues, which results in hydroxylated‐proline residue‐dependent ubiquitination by the von‐Hippel Lindau system and transport to the proteasome, the intracellular protein destruction machinery, for degradation. In this context, HIF‐prolyl hydroxylase (HIF‐PH) inhibitors have been developed for the treatment of renal anaemia. HIF‐PH inhibitors block the activity of PH, which degrades HIF alpha (HIFα) and consequently stimulates the synthesis of endogenous EPO. Moreover, HIF‐PH inhibitors have been demonstrated to increase iron availability by directly increasing mobilization and absorption and indirectly decreasing serum hepcidin levels in patients with chronic kidney disease (CKD). 9 , 10 In patients with HF, abnormally increased hepcidin production may cause impaired iron utilization, leading to anaemia even though not all studies support this hypothesis 11 From this perspective, HIF‐PH inhibitors may be an ideal and effective therapeutic option for patients with HF. Moreover, some additional favourable cardiovascular effects of HIF‐PH inhibitors have been reported. 8 , 12 , 13 , 14 However, as anaemia in patients with HF is multifactorial and is not caused by renal dysfunction alone, it is unclear whether HIF‐PH inhibitors are as effective and safe in patients with HF as in patients without HF. Therefore, we designed and initiated a pilot study aimed at evaluating the efficacy and safety of daprodustat, a HIF‐PH inhibitor, in patients with HF and renal anaemia.
2. Study design
2.1. Study participants
Patients who meet all the following inclusion criteria and do not meet any of the exclusion criteria will be enrolled in the study; these criteria are presented in Table 1 . Patients with HF with New York Heart Association (NYHA) class II symptoms or a history of HF hospitalization, anaemia, and renal impairment will be included. We will exclude patients with NYHA class III/IV and those on or expected to be on haemodialysis or peritoneal dialysis within 6 months. In addition, those who have been treated with an ESA or have a history of active cancer or thromboembolic events will also be excluded.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
| 1. Symptomatic NYHA class II heart failure or a history of hospitalization due to heart failure |
| 2. Aged 20–90 years at the time of consent to participate |
| 3. eGFR of 〈60 ml/min/1.73 m2 at screening |
| 4. Patients without atrial fibrillation at screening: BNP > 100 pg/ml or NT‐proBNP >600 pg/ml; Patients with atrial fibrillation at screening: BNP > 150 pg/ml or NT‐proBNP >900 pg/ml |
| 5. Haemoglobin level of ≥7.5 and <11 g/dl at screening |
| 6. Ferritin level of ≥100 ng/ml and TSAT ≥20% at screening |
| 7. Folic acid and vitamin B12 levels above the lower limit of normal at screening |
| 8. Provided written consent to participate in the study |
| Exclusion criteria |
| 1. Severe heart failure (NYHA class III/IV) |
| 2. Haemodialysis or peritoneal dialysis at the time of consent, or scheduled within 6 months |
| 3. Treated with an ESA within 5 weeks before enrolment |
| 4. Red blood cell transfusion within 12 weeks before enrolment, or expected to occur during the study period |
| 5. A history of treatment with HIF‐PH inhibitors |
| 6. Anaemia due to reasons other than chronic kidney disease and current bleeding or bleeding within 3 months |
| 7. A history of sickle cell disease, myelodysplastic syndromes, myelofibrosis, haematologic tumours, myeloma, haemolytic anaemia, thalassaemia, or locus coeruleus tumour |
| 8. History of pulmonary hypertension or polycystic kidney disease |
| 9. Presence of a malignant tumour noted within 2 years, except for treated basal cell carcinoma of the skin, squamous cell carcinoma cured by resection, and intraepithelial carcinoma of the cervix |
| 10. History of acute myocardial infarction, cerebral infarction, deep vein thrombosis, or pulmonary thromboembolism within 48 weeks before enrolment |
| 11. Judged by the principal investigator to be inappropriate as a research subject |
Abbreviations: BNP, brain natriuretic peptide; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis‐stimulating agent; HIF‐PH, hypoxia‐inducible factor‐prolyl hydroxylase; NYHA, New York Heart Association; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TSAT, transferrin saturation
2.2. Randomization and blinding
Eligible patients who agree to be enrolled in this study will be randomized using a web‐based randomization system constructed by an independent third party, Mebix, Inc., in an open‐label fashion. Minimization randomization using baseline haemoglobin as an adjustment variable will be applied to ensure balance groups for baseline haemoglobin. Although no concealment will be performed for the study procedure, investigators who perform analysis of cardiovascular imaging (i.e. echocardiography and cardiac magnetic resonance imaging) in core laboratories are blinded for randomization until they finalize their analysis.
2.3. Study visits and follow‐up
Patients will be screened at their hospital visit and included after informed consent is obtained (Figure 1 ). Patients will be randomized in a 1:1 ratio to either the daprodustat or control group via a web‐based randomization system (Day 0). After randomization, patients will complete regular visits at 2 weeks (Visit 1), 4 weeks (Visit 2), 8 weeks (Visit 3), 12 weeks (Visit 4), and 16 weeks (Visit 5). Participants randomized to the daprodustat group will undergo automatic dose adjustments based on a prespecified dose‐adjustment algorithm (Table S1 ). The detailed schedules and evaluation items are presented in Table S2 . At these visits, efficacy endpoints and adverse events will be assessed, as along with patient symptoms and laboratory data. Additionally, echocardiography, electrocardiography, and Kansas City Cardiomyopathy Questionnaire total symptom score will be evaluated at Visit 5.
Figure 1.
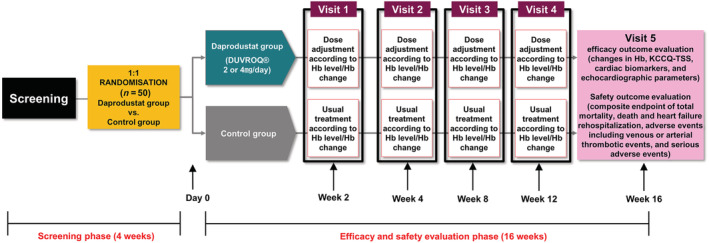
Study flowchart. Hb, haemoglobin; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score.
2.4. Primary and secondary outcomes
The primary endpoint of this study is the haemoglobin level at 16 weeks after randomization. The secondary endpoints, evaluated at 16 weeks after randomization, are listed in Table 2 . For the analysis of the primary endpoint, the difference in haemoglobin levels between the two groups after 16 weeks of randomization will be tested by an analysis of covariance using the baseline haemoglobin level as a covariate. This analysis will follow the intention‐to‐treat principle, and patients will be randomly assigned to treatment groups.
Table 2.
Primary and secondary outcomes
| Primary outcome |
| • Haemoglobin level at 16 weeks after randomization |
|
Secondary outcomes The following items at 16 weeks after randomization: |
| • Red blood cell transfusion status |
| • Number of patients with an increase of at least 5 points on the KCCQ‐TSS relative to baseline |
| • Blood tests: NT‐proBNP, high‐sensitivity troponin T, serum iron, transferrin, total iron binding capacity, transferrin saturation, ferritin, hepcidin, high‐sensitivity C‐reactive protein, total cholesterol, triglycerides, HDL cholesterol, LDL cholesterol, TNF‐α, IL‐1β, and VEGF (plasma), and angiopoietin |
| • Echocardiography: left and right ventricular function analysis, performed in the following cross sections, using M‐mode, colour Doppler, tissue Doppler, and strain analysis |
| • Cardiac MRI: left and right heart function, including strain analysis on cine MRI, native T1 abnormality, and extracellular volume fraction on T1 mapping |
Abbreviations: HDL, high density lipoprotein; IL‐1β, interleukin 1 beta; KCCQ‐TSS, Kansas City Cardiomyopathy Questionnaire total symptom score; LDL, low density lipoprotein; MRI, magnetic resonance imaging; NT‐proBNP, N‐terminal pro‐brain natriuretic peptide; TNF‐α, tumour necrosis factor alpha; VEGF, vascular endothelial growth factor.
2.5. Safety assessment
During the study, all safety information is recorded and collected for adverse events, including liver injury, gastrointestinal disorders, and thrombosis, as well as serious adverse events such as all‐cause mortality, fatal events, and adverse conditions requiring hospitalization or prolonged hospitalization, regardless of whether they are related to the investigational drug or protocol. When an adverse event is identified by the investigator, its severity and grade, the procedure performed, the outcome, and its relevance to the investigational drug are recorded and evaluated. The investigator will promptly report the occurrence of the adverse event to the study coordinators, who will immediately contact the principal investigator. The principal investigator will report to the authorized clinical research review committee within 15 days.
2.6. Sample size calculations
In a previous study, haemoglobin levels in patients with baseline levels of 7.5–11 g/dl increased after 4 weeks of daprodustat treatment by 0.47 ± 0.15 g/dl in the 2 mg daprodustat group and 1.07 ± 0.15 g/dl in the 5 mg daprodustat group. 15 As daprodustat is primarily adjusted to achieve a haemoglobin level of 11.5–12.5 g/dl, we estimated the post‐treatment difference between the two groups to be 0.75 g/dl at 16 weeks. Among patients enrolled in the JEDI‐AHF, a retrospective registry database of patients with HF requiring hospitalization at the Juntendo University Department of Cardiology, 16 haemoglobin levels were measured at two time points, with a time interval of 16 ± 2 weeks in 31 patients with HF who met the inclusion criteria of this study were evaluated. The standard deviations of the haemoglobin levels of the first and second measurements were 0.78 and 0.95 g/dl, respectively, and the correlation coefficient of these two measurements was 0.54. Conservatively setting the standard deviation and correlation coefficient to 1.0 g/dl and 0.5, respectively, we calculated a sample size of 44 patients for an analysis of covariance, using the baseline haemoglobin value as a covariate, with a power of 80% and a two‐sided significance level of 5%. Considering a 10% drop‐out rate, the final sample size was defined as 50 patients in total.
2.7. Ethics and dissemination
This is a multicentre, open‐label, randomized controlled trial designed to evaluate the clinical benefits and safety of daprodustat in patients with chronic HF and renal anaemia. The study has been registered at the jRCT (registration no. jRCT1051210196) and will be conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Good Clinical Practice guidelines. The ethical and safety aspects of the study plan and protocol, and informed consent process have been approved by the certified review board of Kobe City Medical Center General Hospital (No. tk2199) on February 9 and seven participating university and community hospitals in Japan, in advance of the study commencement. All participants will provide written informed consent before being enrolled in the study. This study is self‐funded and does not use any funds from any particular company. The study will be monitored by an independent data safety monitoring committee.
2.8. Patient and public involvement statement
It was not feasible or appropriate to involve patients or members of the public in the design, planning, or conduct of the planned research.
3. Discussion
Anaemia is independently associated with increased mortality and hospitalization in patients with both HF and reduced and preserved ejection fraction, 17 , 18 and the coexistence of CKD has been observed to increase mortality by 1.5‐fold. 19 ESAs have been recommended to treat anaemia in patients with CKD; however, the optimal treatment for anaemia in patients with HF and CKD has yet to be determined, considering the findings of previous studies that examined the clinical implication of ESAs on anaemia in patients with HF.
The RED‐HF trial was a double‐blind placebo‐controlled trial that randomized 2,278 patients with NYHA class II to IV HF, left ventricular ejection fraction of ≤40%, and mild to moderate anaemia (haemoglobin, 9.0–12.0 g/dl) receiving guideline‐recommended HF therapy to receive either darbepoetin alfa (to achieve a haemoglobin target of 13 g/dl) or placebo.66 This study was not confined to those with CKD, and more than half of the patients had an estimated glomerular filtration rate of <60 ml/min/1.73 m2. After a median follow‐up of 28 months, darbepoetin did not affect the primary composite outcome of death resulting from any cause or hospitalization for worsening HF, and significantly, more thromboembolic adverse events were observed in the darbepoetin group than in the placebo group. Moreover, treatment with darbepoetin alfa was associated with a significantly higher incidence of ischaemic stroke and embolic/thrombotic events than that with the placebo. Based on these findings, the American College of Cardiology Foundation/American Heart Association guidelines provided Class III recommendations and the European Society of Cardiology clearly stated that treatment with an ESA is not recommended for anaemia in patients with HF. Likewise, the Japanese Circulation Society guidelines recommend only red blood cell transfusion with class IIb recommendations, while oral iron supplementation and ESAs are classified as class III for the treatment of anaemia in patients with HF. 20 Nevertheless, the use of an ESA is commonly accepted in the treatment of anaemic HF with concomitant CKD, as there are no other treatment options, particularly in patients without an iron metabolism disorder.
Recently, HIFs have been identified as key peptides involved in the regulation of EPO synthesis in the kidney. 7 HIF activation leads to improved anaemia through EPO production, as well as improved iron uptake and utilization; however, it is rapidly degraded by HIF‐PH under normal oxygen conditions. Novel and orally active HIF‐PH inhibitors that stabilize HIF and increase its activity by preventing its degradation have recently been developed. Their efficacy has already been tested in several randomized, double‐blind, placebo‐controlled studies, demonstrating improvements in anaemia in patients with non–end‐stage and end‐stage renal diseases. However, their safety and efficacy have not been evaluated in patients with HF.
From a mechanistic point of view, there is a rationale for hypothesizing HIF‐PH inhibitors as an optimal treatment for anaemia in patients with HF concomitant with CKD. First, HIF‐PH inhibitors can increase haemoglobin levels, with relatively low levels of EPO. 21 The required circulating EPO level to increase the haemoglobin level is significantly higher in patients with CKD than in patients without CKD because of the shortened red cell survival in CKD, necessitating a high‐dose ESA. 22 Furthermore, given that HF is associated with chronic inflammatory status, which is strongly associated with ESA hyporesponsiveness, 23 patients with HF and concomitant with CKD may require a much higher dose of ESA to achieve a circulating EPO level high enough to subsequently increase the haemoglobin level compared with that in patients with CKD alone. However, several observational studies have revealed that high‐dose ESA is associated with increased mortality in patients with CKD. 24 , 25 , 26 , 27 For instance, a sub‐analysis of the Correction of Haemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial that included non‐dialysis patients with CKD demonstrated that a high dose of epoetin alpha, not the haemoglobin level, was correlated with the risk of adverse events, including HF. 27 Furthermore, higher EPO levels are associated with a poor prognosis in patients with HF. 28 In contrast, HIF‐PH inhibitors are able to increase haemoglobin levels with a much lower level of circulating EPO than that with ESAs, 21 independent of baseline inflammatory status. 8
Second, HIF‐PH inhibitors improve iron metabolism without increasing adverse events. 29 One of the important mechanisms contributing to anaemia in patients with HF and CKD is functional iron deficiency, which may be mediated by increased hepcidin production in the liver, preventing the release of iron from macrophages to circulating transferrin and inhibiting intestinal iron absorption. 11 As HIF‐PH inhibitors decrease hepcidin levels in patients with anaemia and CKD, 29 , 30 , 31 it is highly likely that HIF‐PH inhibitors can improve iron uptake even in patients with HF, independent of its effect on EPO production, and contribute to efficient anaemia correction. This implies that although high‐dose oral iron administration alone has no clinical benefit in patients with HF, 32 its co‐administration with HIF‐PH inhibitors may be an option to improve iron deficiency in this population. In a phase II clinical trial of roxadustat, patients who were supplemented with oral and intravenous iron had similar response rates to these two treatments (in contrast to what has been previously described in studies on ESAs), whereas patients receiving intravenous iron typically had better responses. 9 Moreover, as HIF‐PH inhibitors can be administered orally and offer more physiological treatments for functional iron deficiency than exogenous intravenous iron supplementation, they can be a preferable treatment for patients with HF. Moreover, this is advantageous considering that the intravenous administration of iron may be associated with some adverse events.
Lastly, HIF‐PH inhibitors may improve cardiac function independent of EPO production or iron metabolism regulation. Hypoxia is a major component of ischaemic disease. Moreover, HIF is induced to a variable extent in ischaemic tissues and activates a range of responses that protect cells from hypoxic damage and promote re‐oxygenation and repair, 33 , 34 which may contribute to the improvement of cardiovascular function. Previous studies have demonstrated additional beneficial effects independent of EPO production or iron metabolism regulation, such as the ability to alleviate ischaemic injury and improve heart function, vascular pathology, and diabetic nephropathy. 8 , 13 , 14 In addition, we previously demonstrated that HIF inhibition leads to a transition from cardiac hypertrophy to HF. 12 Thus, stabilizing HIF may directly improve cardiac function and, subsequently, HF status.
However, there are some concerns regarding the clinical application of HIF‐PH inhibitors to treat anaemia in patients with HF. For instance, it has been observed that chronic inflammation, which is a pathophysiological background in HF, directly reduces EPO production and erythropoiesis in the bone marrow, which may reduce the impact of HIF‐PH inhibitors on haemoglobin levels. 35 In addition, the overexpression of either HIF‐1α or HIF‐2α may be associated with the development of cardiomyopathy. 36 , 37 , 38 In this study, we will evaluate the impact of HIF‐PH inhibitors on cardiac function using advanced cardiovascular imaging modalities, including cardiac magnetic resonance imaging.
Considering the hypothetical upside and downside of applying HIF‐PH inhibitors to anaemic patients with HF and CKD, we designed and initiated a pilot study intended to evaluate the efficacy and safety of daprodustat in patients with HF and renal anaemia. Because there are virtually no safe and effective treatments for patients with anaemia not caused by iron deficiency, our study results will contribute significantly to this field.
Supporting information
Table S1. Dose‐adjustment algorithm of daprodustat.
Table S2. Items and schedule of observations and examinations.
Iso, T. , Matsue, Y. , Mizukami, A. , Tokano, T. , Isoda, K. , Suwa, S. , Miyauchi, K. , Yanagisawa, N. , Okumura, Y. , and Minamino, T. (2022) Daprodustat for anaemia in patients with heart failure and chronic kidney disease: A randomized controlled study. ESC Heart Failure, 9: 4291–4297. 10.1002/ehf2.14109.
Funding information: This research received no specific grant from any funding agency in the public, commercial or not‐for‐profit sectors.
Contributor Information
Yuya Matsue, Email: yuya8950@gmail.com.
Tohru Minamino, Email: t.minamino@juntendo.ac.jp.
References
- 1. Grote Beverborg N, van Veldhuisen DJ, van der Meer P. Anemia in heart failure: Still relevant? JACC Heart Fail. 2018; 6: 201–208. [DOI] [PubMed] [Google Scholar]
- 2. Yamauchi T, Sakata Y, Takada T, Nochioka K, Miura M, Tadaki S, Ushigome R, Sato K, Onose T, Tsuji K, Abe R, Takahashi J, Miyata S, Shimokawa H, on behalf of the CHART‐2 investigators . Prognostic impact of anemia in patients with chronic heart failure‐ with special reference to clinical background: Report from the CHART‐2 study. Circ J. 2015; 79: 1984–1993. [DOI] [PubMed] [Google Scholar]
- 3. Kajimoto K, Sato N, Takano T, investigators of the Acute Decompensated Heart Failure Syndromes r . Association between anemia, clinical features and outcome in patients hospitalized for acute heart failure syndromes. Eur Heart J Acute Cardiovasc Care. 2015; 4: 568–576. [DOI] [PubMed] [Google Scholar]
- 4. al‐Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol. 2001; 38: 955–962. [DOI] [PubMed] [Google Scholar]
- 5. Ghali JK, Anand IS, Abraham WT, Fonarow GC, Greenberg B, Krum H, Massie BM, Wasserman SM, Trotman ML, Sun Y, Knusel B, Armstrong P, Study of Anemia in Heart Failure Trial (STAMINA‐HeFT) Group . Randomized double‐blind trial of darbepoetin alfa in patients with symptomatic heart failure and anemia. Circulation. 2008; 117: 526–535. [DOI] [PubMed] [Google Scholar]
- 6. Swedberg K, Young JB, Anand IS, Cheng S, Desai AS, Diaz R, Maggioni AP, McMurray J, O'Connor C, Pfeffer MA, Solomon SD, Sun Y, Tendera M, van Veldhuisen D, RED‐HF Committees , RED‐HF Investigators . Treatment of anemia with darbepoetin alfa in systolic heart failure. N Engl J Med. 2013; 368: 1210–1219. [DOI] [PubMed] [Google Scholar]
- 7. Semenza GL, Wang GL. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol Cell Biol. 1992; 12: 5447–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coyne DW, Goldsmith D, Macdougall IC. New options for the anemia of chronic kidney disease. Kidney Int Suppl. 2017; 7: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Besarab A, Chernyavskaya E, Motylev I, Shutov E, Kumbar LM, Gurevich K, Chan DTM, Leong R, Poole L, Zhong M, Saikali KG, Franco M, Hemmerich S, Yu KHP, Neff TB. Roxadustat (FG‐4592): Correction of anemia in incident dialysis patients. J Am Soc Nephrol. 2016; 27: 1225–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nakanishi T, Kuragano T, Nanami M, Nagasawa Y, Hasuike Y. Misdistribution of iron and oxidative stress in chronic kidney disease. Free Radic Biol Med. 2019; 133: 248–253. [DOI] [PubMed] [Google Scholar]
- 11. Suzuki T, Hanawa H, Jiao S, Ohno Y, Hayashi Y, Yoshida K, Kashimura T, Obata H, Minamino T. Inappropriate expression of hepcidin by liver congestion contributes to anemia and relative iron deficiency. J Card Fail. 2014; 20: 268–277. [DOI] [PubMed] [Google Scholar]
- 12. Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M, Shimizu I, Asahara T, Hamada H, Tomita S, Molkentin JD, Zou Y, Komuro I. p53‐induced inhibition of Hif‐1 causes cardiac dysfunction during pressure overload. Nature. 2007; 446: 444–448. [DOI] [PubMed] [Google Scholar]
- 13. Young JM, Williams DR, Thompson AAR. Thin air, thick vessels: Historical and current perspectives on hypoxic pulmonary hypertension. Front Med (Lausanne). 2019; 6: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ghadge SK, Messner M, van Pham T, Doppelhammer M, Petry A, Görlach A, Husse B, Franz WM, Zaruba MM. Prolyl‐hydroxylase inhibition induces SDF‐1 associated with increased CXCR4+/CD11b+ subpopulations and cardiac repair. J Mol Med (Berl). 2017; 95: 825–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Holdstock L, Meadowcroft AM, Maier R, Johnson BM, Jones D, Rastogi A, Zeig S, Lepore JJ, Cobitz AR. Four‐week studies of Oral hypoxia‐inducible factor‐prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol. 2016; 27: 1234–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ishiwata S, Matsue Y, Nakamura Y, Dotare T, Sunayama T, Suda S, Yatsu S, Kato T, Hiki M, Kasai T, Minamino T. Clinical and prognostic values of urinary alpha1‐microglobulin as a tubular marker in acute heart failure. Int J Cardiol. 2021; 338: 115–120. [DOI] [PubMed] [Google Scholar]
- 17. Anand IS. Anemia and chronic heart failure implications and treatment options. J Am Coll Cardiol. 2008; 52: 501–511. [DOI] [PubMed] [Google Scholar]
- 18. Tang YD, Katz SD. The prevalence of anemia in chronic heart failure and its impact on the clinical outcomes. Heart Fail Rev. 2008; 13: 387–392. [DOI] [PubMed] [Google Scholar]
- 19. Herzog CA, Muster HA, Li S, Collins AJ. Impact of congestive heart failure, chronic kidney disease, and anemia on survival in the Medicare population. J Card Fail. 2004; 10: 467–472. [DOI] [PubMed] [Google Scholar]
- 20. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, Komuro I, Saiki Y, Saito Y, Sakata Y, Sato N, Sawa Y, Shiose A, Shimizu W, Shimokawa H, Seino Y, Node K, Higo T, Hirayama A, Makaya M, Masuyama T, Murohara T, Momomura SI, Yano M, Yamazaki K, Yamamoto K, Yoshikawa T, Yoshimura M, Akiyama M, Anzai T, Ishihara S, Inomata T, Imamura T, Iwasaki YK, Ohtani T, Onishi K, Kasai T, Kato M, Kawai M, Kinugasa Y, Kinugawa S, Kuratani T, Kobayashi S, Sakata Y, Tanaka A, Toda K, Noda T, Nochioka K, Hatano M, Hidaka T, Fujino T, Makita S, Yamaguchi O, Ikeda U, Kimura T, Kohsaka S, Kosuge M, Yamagishi M, Yamashina A, on behalf of the Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group . JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure‐ digest version. Circ J. 2019; 83: 2084–2184. [DOI] [PubMed] [Google Scholar]
- 21. Maxwell PH, Eckardt KU. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol. 2016; 12: 157–168. [DOI] [PubMed] [Google Scholar]
- 22. Sato Y, Mizuguchi T, Shigenaga S, Yoshikawa E, Chujo K, Minakuchi J, Kawashima S. Shortened red blood cell lifespan is related to the dose of erythropoiesis‐stimulating agents requirement in patients on hemodialysis. Ther Apher Dial. 2012; 16: 522–528. [DOI] [PubMed] [Google Scholar]
- 23. Shah HH, Uppal NN, Fishbane S. Inflammation and erythropoiesis‐stimulating agent Hyporesponsiveness: A critical connection. Kidney Med. 2020; 2: 245–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Solomon SD, Uno H, Lewis EF, Eckardt KU, Lin J, Burdmann EA, de Zeeuw D, Ivanovich P, Levey AS, Parfrey P, Remuzzi G, Singh AK, Toto R, Huang F, Rossert J, McMurray J, Pfeffer MA, Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) Investigators . Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010; 363: 1146–1155. [DOI] [PubMed] [Google Scholar]
- 25. Bradbury BD, Wang O, Critchlow CW, Rothman KJ, Heagerty P, Keen M, Acquavella JF. Exploring relative mortality and epoetin alfa dose among hemodialysis patients. Am J Kidney Dis. 2008; 51: 62–70. [DOI] [PubMed] [Google Scholar]
- 26. Fishbane S, Besarab A. Mechanism of increased mortality risk with erythropoietin treatment to higher hemoglobin targets. Clin J Am Soc Nephrol. 2007; 2: 1274–1282. [DOI] [PubMed] [Google Scholar]
- 27. Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK. Secondary analysis of the CHOIR trial epoetin‐alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008; 74: 791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van der Meer P, Voors AA, Lipsic E, Smilde TD, van Gilst WH, van Veldhuisen DJ. Prognostic value of plasma erythropoietin on mortality in patients with chronic heart failure. J Am Coll Cardiol. 2004; 44: 63–67. [DOI] [PubMed] [Google Scholar]
- 29. Chen N, Hao C, Peng X, Lin H, Yin A, Hao L, Tao Y, Liang X, Liu Z, Xing C, Chen J, Luo L, Zuo L, Liao Y, Liu BC, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KHP. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med. 2019; 381: 1001–1010. [DOI] [PubMed] [Google Scholar]
- 30. Bonomini M, Del Vecchio L, Sirolli V, Locatelli F. New treatment approaches for the anemia of CKD. Am J Kidney Dis. 2016; 67: 133–142. [DOI] [PubMed] [Google Scholar]
- 31. Chen N, Hao C, Liu BC, Lin H, Wang C, Xing C, Liang X, Jiang G, Liu Z, Li X, Zuo L, Luo L, Wang J, Zhao MH, Liu Z, Cai GY, Hao L, Leong R, Wang C, Liu C, Neff T, Szczech L, Yu KHP. Roxadustat treatment for anemia in patients undergoing long‐term dialysis. N Engl J Med. 2019; 381: 1011–1022. [DOI] [PubMed] [Google Scholar]
- 32. Lewis GD, Malhotra R, Hernandez AF, McNulty SE, Smith A, Felker GM, Tang WHW, LaRue SJ, Redfield MM, Semigran MJ, Givertz MM, van Buren P, Whellan D, Anstrom KJ, Shah MR, Desvigne‐Nickens P, Butler J, Braunwald E, for the NHLBI Heart Failure Clinical Research Network . Effect of Oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF randomized clinical trial. JAMA. 2017; 317: 1958–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bishop T, Ratcliffe PJ. Signaling hypoxia by hypoxia‐inducible factor protein hydroxylases: A historical overview and future perspectives. Hypoxia (Auckl). 2014; 2: 197–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tekin D, Dursun AD, Xi L. Hypoxia inducible factor 1 (HIF‐1) and cardioprotection. Acta Pharmacol Sin. 2010; 31: 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Anand IS, Gupta P. Anemia and iron deficiency in heart failure: Current concepts and emerging therapies. Circulation. 2018; 138: 80–98. [DOI] [PubMed] [Google Scholar]
- 36. Kido M, du L, Sullivan CC, Li X, Deutsch R, Jamieson SW, Thistlethwaite PA. Hypoxia‐inducible factor 1‐alpha reduces infarction and attenuates progression of cardiac dysfunction after myocardial infarction in the mouse. J Am Coll Cardiol. 2005; 46: 2116–2124. [DOI] [PubMed] [Google Scholar]
- 37. Nickoloff BJ, Griffiths EM. Abnormal cutaneous topobiology: The molecular basis for dermatopathologic mononuclear cell patterns in inflammatory skin disease. J Invest Dermatol. 1990; 95: S128–S131. [DOI] [PubMed] [Google Scholar]
- 38. Moslehi J, Minamishima YA, Shi J, Neuberg D, Charytan DM, Padera RF, Signoretti S, Liao R, Kaelin WG Jr. Loss of hypoxia‐inducible factor prolyl hydroxylase activity in cardiomyocytes phenocopies ischemic cardiomyopathy. Circulation. 2010; 122: 1004–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Dose‐adjustment algorithm of daprodustat.
Table S2. Items and schedule of observations and examinations.


