Abstract
Background
The prognostic role of decongestion‐related change of cardiac morphology and in particular right heart function has not been investigated comprehensively in AHF patients.
Methods and results
This prospective observational single‐centre study included consecutive patients hospitalized for treatment of AHF with reduced, mildly‐reduced or preserved left ventricular ejection fraction (LVEF). Comprehensive transthoracic echocardiography at admission and discharge assessed decongestion‐related change of cardiac function and morphology. The combined endpoint of 1 year all‐cause mortality and cardiovascular rehospitalization explored the prognostic importance of decongestion‐related change. The 176 study participants were 83 years old [74–87] and 54% were men. Fifty one (29%) had rLVEF, 65 (37%) mrLVEF, and 60 (34%) pLVEF. The proportion of de novo or worsening chronic HF was not different between LVEF groups. HF aetiology and cardiovascular risk factors were equally distributed across all groups except for a higher BMI in the pLVEF group. Decongestion equally reduced body weight, heart rate, systolic and diastolic blood pressure, tricuspid regurgitation gradient, and inferior vena cava diameter across all groups (P < 0.004 for all). Decongestion‐related increase in TAPSE independent of the LVEF was associated with improvement of right‐ventricular‐pulmonary artery coupling and a lower incidence of the combined outcome in the Cox proportional hazard risk analysis (unadjusted HR 0.50 95% CI 0.33–0.78, P = 0.002; adjusted HR 0.46 95% CI: 0.33–0.78, P = 0.001).
Conclusions
Decongestion‐related increase in TAPSE and recovery of RV/pulmonary artery coupling was observed across all LVEF groups and associated with a risk reduction for the combined endpoint highlighting the important prognostic role of right heart recovery after an AHF episode.
Keywords: Decongestion, Acute heart failure, Right heart, Left heart systolic and diastolic function
Introduction
Decongestion remains the principal initial therapeutic goal in AHF patients irrespective of the particular precipitant of this perilous clinical condition. 1 , 2 The current pathophysiological concept of decongestion suggests improvement of LV contractile function, decrease of mitral valve regurgitation severity, reduction of pulmonary congestion, and decrease of dyspnoea. 3 Further evidence indicates that decongestion decreases right ventricular (RV) preload and reduces the diameter of the inferior vena cava (IVC) 4 with attenuation of hepatic and renal congestion. 5
The prognostic role of decongestion‐related change of echocardiographic parameters has not been comprehensively investigated so far, and in particular, it remains unknown whether improvement of right heart function is associated with outcome. Right heart function is an established predictor of survival in stable chronic HF patients with rLVEF, mrLVEF and pLVEF. 6 , 7 Furthermore, coupling of the RV with the pulmonary circulation as assessed by the echocardiographic TAPSE/PASP ratio is an important determinant of survival with more severe heart failure. 8 In AHF, many patients present clinical signs of right heart failure; therefore, we hypothesized that reestablishment of right heart function should be of prognostic importance across all LVEF subgroups.
In this observational study, we used transthoracic echocardiography to investigate decongestion‐related change in cardiac morphology and function in consecutive patients hospitalized for AHF treatment. Decongestion‐related change was first investigated in the total cohort because of our intention to identify a common underlying pathophysiology of prognostic importance and, in a second step, in the different LVEF subgroups. The prognostic relevance of decongestion‐related echocardiographic change was then explored using the combined endpoint of 1 year all‐cause mortality and first cardiovascular hospitalization after discharge from index hospitalization.
Methods
Study population
This prospective observational single‐centre study recruited a total of 221 patients based on the following inclusion criteria (i) age ≥18 years; (ii) emergency hospitalization for AHF treatment; (iii) transthoracic echocardiography performed within the first 12 h of admission; (iv) written consent. Excluded were patients presenting with signs of AHF in the context of acute STEMI or NSTEMI, complex congenital heart disease, acute pulmonary embolism, pregnancy, exacerbation of obstructive pulmonary disease, metabolic, toxic or infectious disorder, or comorbidity considered to reduce survival time to <1 year. Study inclusion was considered after mutual consensus of AHF diagnosis by the emergency medical physician and the consulting cardiologist. In case of disagreement, a heart failure specialist was called in. If a patient was admitted more than once during the follow‐up period of 1 year after discharge, only the data from the first cardiovascular admission were considered. Heart failure treatment in the acute phase followed the current HF guidelines of the ESC and clinical judgement determined readiness for discharge.
The study protocol is in accordance with the Declaration of Helsinki and was approved by the local ethics committee (CER Vaud 120/15). All participants provided informed consent. Anonymized data will be made publicly available.
Acquisition of anthropometric, biological, and clinical data
Anthropometric, biological, clinical admission, and discharge data as well as medical history were collected prospectively from the individual patient's electronic health report at the Lausanne University Hospital. Data accuracy was confirmed by revisiting all patients data revealing 99.7% accuracy. Comprehensive transthoracic echocardiography was performed within the first 12 h of admission, and the second one was executed upon discharge. Images were acquired on Vivid E9 machines (GE Healthcare) by board certified cardiologists. LVEF was quantitatively assessed using the biplane Simpson method; the severity of valvular regurgitations was graded using a multiparametric assessment in accordance with the European Association of Cardiovascular Imaging. 9 Global longitudinal strain analysis was performed offline using EchoPac software (GE Medical Systems).
Types of heart failure
Participants with established HF history were classified as chronic HF; patients without prior HF diagnosis were considered as de novo HF. The LVEF in the admission echocardiography grouped study participants into AHF with pLVEF (LVEF ≥ 50%), with mrLVEF (LVEF 40–49%), and rLVEF (LVEF < 40%). 10
Phenotype of acute heart failure
AHF was categorized into (i) cardiogenic shock if systolic blood pressure was <90 mmHg and oliguria <0.5 mL/kg/h was present for ≥6 h; (ii) pulmonary oedema if the chest X‐ray showed alveolar oedema and oxygen saturation was <90% (without supplemental oxygen). (iii) Left HF was diagnosed if dyspnoea or tachycardia were present with pulmonary congestion or interstitial oedema in chest X‐ray. (iv) Right HF was diagnosed when jugular venous pressure or liver size were increased and peripheral oedema was present. (v) Hypertension emergency was identified by high blood pressure (≥180/≥100 mmHg) with symptoms of dyspnoea, tachycardia, and radiological findings of pulmonary congestion or oedema. 11
Study outcomes
Primary outcome was decongestion‐related change in cardiac morphology and function parameters between admission and discharge as assessed by transthoracic echocardiography. Secondary outcome was the combined endpoint including 1 year all‐cause mortality (ACM) and first cardiovascular rehospitalization after discharge. The endpoint was assessed at 3 and 12 months after discharge and this stepped temporal analysis was chosen in order to differentiate between short‐term and mid‐term effects.
Statistical analysis
All statistical analyses were performed with Stata® 16.0 (StataCorp, College Station, Texas, USA). Continuous variables were presented as medians with interquartile ranges (IQR) and categorical variables as absolute numbers and percentages. Comparisons of baseline characteristics between the three LVEF groups were made with Kruskal–Wallis test and χ 2 test, respectively. Admission and discharge parameters were compared with Wilcoxon matched‐pairs signed‐rank test for continuous variables, and two‐sample test of proportion for categorical variables. Bivariate logistic regression analysis was used to explore the echocardiographic determinants associated with the secondary outcome. Finally, the incidence of the combined secondary endpoint ACM and first rehospitalization in the patients with and without RV function improvement during decongestion was compared using Cox regression analysis, both unadjusted and adjusted for significant baseline characteristics and the HF subtype, and represented as adjusted Kaplan–Meier survival curves. A two‐sided P‐value <0.05 was considered statistically significant.
Results
Study population
A total of 221 AHF patients were included; a total of 45 patients were excluded from final analysis for missing discharge echocardiography. Among them, 31 patients were referred to peripheral hospitals, 8 patients died during hospitalization, and 6 refused discharge echocardiography (Supporting Information, Figure S1 ). The first echocardiographic assessment was performed early upon admission, with a median delay of 6.6 [4.1–11.9] hours. Baseline clinical characteristics, in particular NT‐proBNP levels, were not different between these 45 patients and the 176 study participants remaining in the final analysis (Supporting Information, Table S1 ).
Baseline demographic, clinical, and echocardiographic characteristics
In the study cohort, 29% of AHF patients had rLVEF (n = 51), 37% mrLVEF (n = 65), and 34% pLVEF (n = 60). Patients were 83 [74–87] years old and 54% were men; neither median age nor the proportion of male gender was different between groups. The BMI was higher in pLVEF patients when compared with mrLVEF or rLVEF groups (P = 0.0002). Haemodynamic parameters, baseline rhythm, co‐morbidity load, HF aetiology, and the prevalence of de novo HF were not different between groups (Table 1 ).
Table 1.
Baseline demographic and clinical characteristics
| Clinical characteristics | Unit | All | AHF with rLVEF | AHF with mrLVEF | AHF with pLVEF | P |
|---|---|---|---|---|---|---|
| n = 176 | n = 51 (29%) | n = 65 (37%) | n = 60 (34%) | |||
| Age | years | 83 [74–87] | 84 [75–87] | 83 [75–88] | 80 [73–86] | 0.25 |
| Male gender | n (%) | 95 (54%) | 22 (43%) | 39 (60%) | 34 (57%) | 0.18 |
| Height | cm | 166 [160–172] | 165 [156–170] | 165 [160–170] | 168 [162–174] | 0.08 |
| Weight | kg | 74 [62–86] | 63 [53–83] | 73 [65–81] | 84 [68–101] | 0.0001 |
| Body mass index | kg/m2 | 26.8 [23.1–31.6] | 23.8 [20.7–27.4] | 27.0 [23.6–29.4] | 29.0 [24.6–35.2] | 0.0002 |
| Heart rate | b.p.m. | 86 [73–102] | 87 [77–106] | 84 [73–102] | 83 [70–97] | 0.39 |
| Systolic blood pressure | mmHg | 133 [119–150] | 134 [118–150] | 133 [118–145] | 131 [119–157] | 0.57 |
| Diastolic blood pressure | mmHg | 75 [63–88] | 77 [67–90] | 73 [62–84] | 76 [62–89] | 0.45 |
| Rhythm at admission | 0.87 | |||||
| Sinus | n (%) | 89 (51%) | 24 (47%) | 32 (49%) | 33 (55%) | |
| AF/Flutter | n (%) | 74 (42%) | 22 (43%) | 29 (45%) | 23 (38%) | |
| V pacing | n (%) | 13 (7%) | 5 (10%) | 4 (6%) | 4 (7%) | |
| Hypertension | n (%) | 143 (81%) | 39 (76%) | 52 (80%) | 52 (87%) | 0.38 |
| Diabetes | n (%) | 65 (37%) | 16 (32%) | 31 (48%) | 18 (30%) | 0.09 |
| Current smoker | n (%) | 20 (11%) | 6 (12%) | 6 (9%) | 8 (13%) | 0.80 |
| COPD | n (%) | 37 (21%) | 10 (20%) | 13 (20%) | 14 (23%) | 0.87 |
| De novo HF | n (%) | 76 (43%) | 21 (41%) | 24 (37%) | 31 (52%) | 0.24 |
| Known cardiomyopathy | ||||||
| Ischaemic | n (%) | 78 (44%) | 20 (39%) | 32 (49%) | 26 (43%) | 0.57 |
| Valvular | n (%) | 69 (39%) | 18 (35%) | 29 (45%) | 22 (37%) | 0.56 |
| Dilated | n (%) | 22 (13%) | 6 (12%) | 11 (17%) | 8 (8%) | 0.36 |
| Pacemaker | n (%) | 15 (9%) | 4 (8%) | 4 (6%) | 7 (12%) | 0.52 |
COPD, chronic obstructive pulmonary disease.
The acute heart failure clinical phenotype at admission
The clinical AHF phenotype presentation did not vary largely between groups except for the right heart failure phenotype, which was more prevalent in AHF patients with pLVEF (P = 0.003) (Table 2 ).
Table 2.
Baseline clinical phenotype of acute decompensation
| Clinical presentation | Unit | All | AHF with rLVEF | AHF with mrLVEF | AHF with pLVEF | P |
|---|---|---|---|---|---|---|
| n = 175 | n = 51 (29%) | n = 64 (37%) | n = 60 (34%) | |||
| Cardiogenic shock | n (%) | 2 (1%) | 0 | 1 (1%) | 1 (2%) | 0.66 |
| Pulmonary oedema | n (%) | 20 (11%) | 5 (10%) | 9 (14%) | 6 (10%) | 0.73 |
| Left heart failure | n (%) | 152 (86%) | 45 (88%) | 55 (85%) | 52 (87%) | 0.85 |
| Right heart failure | n (%) | 109 (62%) | 30 (59%) | 32 (49%) | 77 (78%) | 0.003 |
| HT emergency | n (%) | 10 (6%) | 2 (4%) | 5 (8%) | 3 (5%) | 0.66 |
Biological parameters at admission
AHF patients with rLVEF presented with a lower leucocytes count (P = 0.01); the haemoglobin levels were by trend higher in rLVEF (P = 0.06). The NT‐proBNP level was highest in rLVEF patients (P = 0.0001). Sodium, potassium, creatinine, and thrombocyte levels were not different between groups (Table 3 ).
Table 3.
Baseline biological parameters
| Biological characteristics | Unit | All | AHF with rLVEF | AHF with mrLVEF | AHF with pLVEF | P |
|---|---|---|---|---|---|---|
| n = 175 | n = 51 (29%) | n = 65 (37%) | n = 60 (34%) | |||
| Haemoglobin | g/L | 123 [109–139] | 127 [111–141] | 117 [103–135] | 123 [112–140] | 0.06 |
| Leucocytes | g/L | 8.7 [7.0–11.7] | 7.7 [6.5–9.6] | 9.3 [7.4–12.7] | 8.9 [7.4–12.0] | 0.01 |
| Thrombocytes | g/L | 234 [186–307] | 221 [183–304] | 263 [210–318] | 213 [178–294] | 0.10 |
| RDW | % | 14.8 [13.7–16.5] | 14.8 [13.7–16.8] | 15.1 [13.7–16.8] | 14.4 [13.6–16.3] | 0.36 |
| Sodium | mmol/L | 139 [137–142] | 138 [135–141] | 140 [137–142] | 140 [138–142] | 0.08 |
| Potassium | mmol/L | 4.4 [3.9–4.8] | 4.3 [3.9–4.7] | 4.5 [4.0–4.9] | 4.3 [3.9–4.7] | 0.41 |
| Creatinine | μmol/L | 113 [87–149] | 101 [78–149] | 112 [91–149] | 115 [91–148] | 0.25 |
| NT‐proBNP | ng/L | 4,903 [2,365–10,689] | 8,693 [3,182–18,415] | 5,967 [3,590–10,727] | 3,218 [1,465–5,279] | 0.0001 |
RDW, red cell distribution width.
Changes of clinical parameters and heart failure drug treatment
Mean length of stay was 13 days [9–19] and not different between LVEF groups (pLVEF vs. mrLVEF vs. rLVEF: 13 [8–22] vs. 13 [9–20] vs. 13 [8–16] days, P = 0.68). Body weight decreased overall by −1.8 [−5.0 to 0] kg (Table 4 , P = 0.0001), and this decrease was not significantly different between groups (Supporting Information, Table S3 , P = 0.97). Discharge heart rate decreased in the entire study population by −10.5 [−27.5 to 0] b.p.m. (P = 0.0001) and more importantly in AHF with mrLVEF and rLVEF (Supporting Information, Table S3 , P = 0.04). Discharge systolic and diastolic blood pressure was lower when compared with admission (Table 4 ), and the decrease in blood pressure was not different between the LVEF groups (Supporting Information, Table S3 , P = 0.45). By trend, more patients were in sinus rhythm at discharge (107 vs. 89 patients, 0 = 0.053) (Table 4 , Supporting Information, Table S3 ).
Table 4.
Changes in clinical and echocardiographic parameters between admission and discharge
| Unit | All AHF types (n = 176) | P | |||
|---|---|---|---|---|---|
| Admis. | Discharge | Median difference | |||
| Weight | kg | 74 [62–86] | 72 [61–84] | −1.8 [−5.0–0] | 0.0001 |
| Heart rate | b.p.m. | 86 [73–102] | 74 [65–84] | −10.5 [−27.5–0] | 0.0001 |
| Systolic BP | mmHg | 133 [119–150] | 124 [108–139] | −8.5 [−28–5] | 0.0001 |
| Diastolic BP | mmHg | 75 [63–88] | 65 [55–74] | −10.5 [−21–0] | 0.0001 |
| Sinus rhythm | n (%) | 89 (51%) | 107 (61%) | +18 (10%) | 0.053 |
| LV EDV index | mL/m2 | 52 [39–74] | 53 [40–68] | 0.2 [−6.6–5.1] | 0.76 |
| LV ejection fraction | % | 45 [37–54] | 45 [36–56] | −0.1 [−1.7–2.6] | 0.95 |
| GLS | % | 10.3 [7.2–15.1] | 11.8 [8.1–15.0] | +0.4 [−1–2.1] | 0.02 |
| Mitral E velocity | cm/s | 100 [80–124] | 96 [74–117] | −3 [−20–7] | 0.001 |
| e′ velocity | cm/s | 6.5 [5.0–8.0] | 6.5 [5.8–8.0] | −0.35 [−1–1.1] | 0.44 |
| E/e′ ratio | 16.1 [12.0–20.0] | 14.6 [11.1–19.3] | −1.2 [−3.9–1.8] | 0.003 | |
| LA volume index | ml/m2 | 40 [31–51] | 43 [29–53] | +0.5 [−3.2–5.9] | 0.09 |
| RV basal diameter | mm | 41 [36–45] | 40 [34–44] | −1 [−4–2] | 0.007 |
| TAPSE | mm | 15 [12–19] | 15 [12–19] | 0 [−2–3] | 0.11 |
| TR gradient (n = 136) | mmHg | 40 [32–52] | 35 [28–44] | −5 [−13–2] | 0.0001 |
| Systolic PAP (n = 116) | mmHg | 52.5 [43–65] | 41 [35–52] | −8 [−18–1] | <0.0001 |
| TAPSE/PASP (=115) | mm/mmHg | 0.29 [0.20–0.40] | 0.36 [0.25–0.48] | +0.06 [−0.01–0.14] | <0.0001 |
| IVC diam. | mm | 22 [19–26] | 19 [15–23] | −2 [−6–0] | 0.0001 |
BP, blood pressure; b.p.m., beats per minute; GLS, global longitudinal strain; IVC, inferior vena cava; LA, left atrial; LVEDV, left ventricular enddiastolic volume; RV, right ventricle; TAPSE, tricuspid annulus plane systolic excursion; TR, tricuspid regurgitation.
At discharge, more study participants were on treatment with beta‐blockers (129 vs. 106; P = 0.009), MRA (47 vs. 25; P = 0.004), or loop diuretics (152 vs. 114; P = 0.0001) while thiazide diuretic treatment was less frequent when compared with baseline (14 vs. 34; P = 0.002) (Supporting Information, Table S4 ).
Change of echocardiographic parameters
Table 4 and Supporting Information, Table S3 show that significant decongestion‐related change of left ventricular end‐diastolic volume index or LVEF did not occur in the entire study population or in LVEF groups. However, global longitudinal strain improved in all study participants (Table 4 , P = 0.02) and significantly in patients with rLVEF or mrLVEF (Supporting Information, Table S3 ; P = 0.005, P = 0.02, respectively). The mitral E‐wave velocity decreased overall (Table 4 , P = 0.001) and significantly in the mrLVEF group (Supporting Information, Table S3 ; P = 0.006). The E/e′ ratio decreased in the entire study population (Table 4 , P = 0.003), and significantly in the rLVEF group (Supporting Information, Table S3 , P = 0.01). The basal RV diameter decreased in all study participants (Table 4 , P = 0.007) and significantly in the rLVEF group (Supporting Information, Table S3 , P = 0.007). There was no overall change in the TAPSE overall (Table 4 , P = 0.11) while a significant increase was observed in AHF with rLVEF (Supporting Information, Table S3 , P = 0.03). The tricuspid regurgitation gradient decreased with decongestion in the 136 study participants where the measure could be obtained (Table 4 , P = 0.0001) and in each LVEF group (Supporting Information, Table S3 , P < 0.009). The diameter of the inferior vena cava (IVC) decreased in all study participants (Table 4 , P = 0.0001) and in each LVEF group (Supporting Information, Table S3 , P < 0.0003).
MR was absent/mild/moderate/severe in 14%/62%/18%/6% at admission and absent/mild/moderate/severe in 15%/64%/16%/5% at discharge. With unloading, MR severity remained unchanged in 78%, improved by 1 class in 13% and improved by 2 classes in 1%. MR worsened by 1 class in 8%.
Combined endpoint of all‐cause mortality and cardiovascular rehospitalization
The combined endpoint of 1 year all‐cause mortality and cardiovascular rehospitalization occurred in 87 patients (49%) (Figure 1 A ; Supporting Information, Table S5 ). The Cox proportional hazard risk analysis did not show a significant difference in the risk for the combined endpoint when comparing pLVEF, mrLVEF, and rLVEF patients (pLVEF: HR 0.68 95% CI 0.39–1.21, P = 0.19; mrLVEF: HR 1.38, 95% CI 0.83–2.3, P = 0.22; with the rLVEF group serving as the reference) (Figure 1 ). Heart failure treatment was not associated with the combined outcome.
Figure 1.
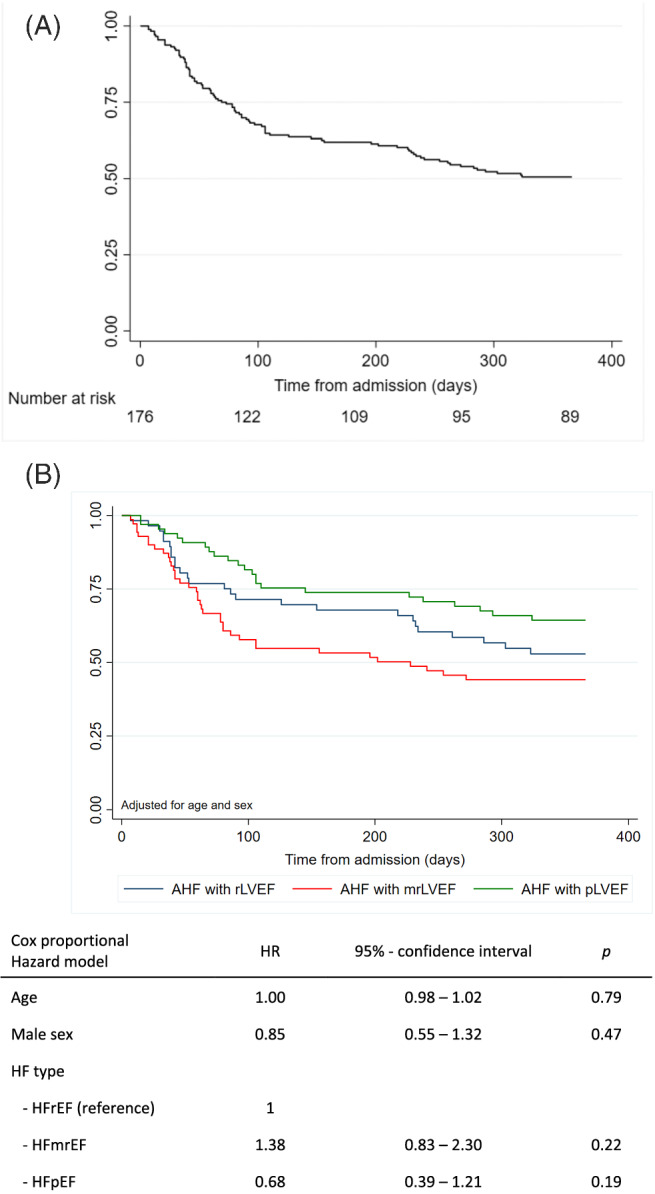
(A) Combined endpoint of death or cardiovascular hospitalization in all study participants. (B) Combined endpoint of death or cardiovascular readmission of study participants with reduced LVEF, mid‐range LVEF, or preserved LVEF.
Prognostic value of relative change in echocardiographic parameters between admission and discharge
Decongestion‐related increase in TAPSE >1 mm was observed in 86 (49%) patients and increase in TAPSE with decongestion was associated with a lower odds ratio for the incidence of the combined endpoint at 3 and 12 months in bivariate analysis (OR = 0.92; 95% CI 0.86–0.98, P = 0.025, and OR = 0.93; 95%‐CI 0.87–0.98, P = 0.036, respectively), and this relation remained significant after adjustment for age, sex, and body surface area (Table 5 , P = 0.018 and P = 0.041, respectively). The correlation between TAPSE and the combined endpoint was likewise maintained at 3 months after adjustment for change in MR class (3 months: OR = 0.92; 95%‐CI 0.85–0.99, P = 0.025; 12 months: OR 0.93; 95%‐CI 0.87–0.99, P = 0.046). The proportion of study participants increasing the TAPSE was similar in all LVEF groups (pLVEF vs. mrLVEF vs. rLVEF: 46% vs. 52% vs. 51%, P = 0.79). In the 86 patients with TAPSE increase, the median augmentation was 3.5 [2–6] mm vs. −2 [−4 to 0] mm in the rest of the cohort. Furthermore, we observed that the increase of the TAPSE was higher in study participants with 1 year survival and without cardiovascular rehospitalization when compared with patients with 1 year survival and 1 or more readmissions (change in TAPSE 1.2 ± 4.3 mm vs. TAPSE 0.6 ± 4.4 mm or −1.5 ± 5.5 mm; P = 0.03; P for trend = 0.008) (Supporting Information, Figure S2 ).
Table 5.
Prognostic value of absolute change in echocardiographic parameters between admission and discharge for mortality and cardiovascular rehospitalisation
| Bivariate analysis | Unit | Mortality and rehospitalization at 3 months | Mortality and rehospitalization at 12 months | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% confidence interval | P | OR | 95% confidence interval | P | ||
| LV EDV index | per mL/m2 | 1.01 | 0.98–1.03 | 0.56 | 1.02 | 0.99–1.05 | 0.07 |
| LV ejection fraction | per 1% | 0.98 | 0.94–1.02 | 0.32 | 0.99 | 0.95–1.02 | 0.47 |
| Global longitudinal strain | per 1% | 1.00 | 0.91–1.10 | 0.97 | 0.96 | 0.88–1.04 | 0.31 |
| Mitral E velocity | per 1 cm/s | 1.00 | 0.99–1.01 | 0.60 | 0.99 | 0.98–1.01 | 0.30 |
| e′ velocity | per 1 cm/s | 1.01 | 0.85–1.21 | 0.88 | 1.05 | 0.89–1.23 | 0.56 |
| E/e′ ratio | per 1 unit | 0.98 | 0.93–1.03 | 0.42 | 0.96 | 0.92–1.01 | 0.12 |
| LA volume index | per 1 mL/m2 | 1.03 | 0.99–1.06 | 0.44 | 1.00 | 0.97–1.03 | 0.95 |
| RV basal diameter | per 1 mm | 1.04 | 0.98–1.10 | 0.19 | 1.02 | 0.97–1.08 | 0.38 |
| TAPSE | per 1 mm | 0.92 | 0.86–0.98 | 0.025 | 0.93 | 0.87–0.99 | 0.036 |
| TR gradient (n = 136) | per 1 mmHg | 1.02 | 1.00–1.05 | 0.10 | 1.00 | 0.98–1.03 | 0.89 |
| Systolic PAPs (n = 116) | per 1 mmHg | 1.02 | 0.99–1.05 | 0.11 | 1.00 | 0.98–1.03 | 0.78 |
| TAPSE/PASP (n = 115) | mm/mmHg | 0.29 | 0.001–0.55 | 0.02 | 0.12 | 0.01–1.38 | 0.09 |
| IVC diameter | mm | 1.04 | 0.97–1.10 | 0.29 | 1.04 | 0.98–1.11 | 0.17 |
After adjustment for age, sex, and body surface area, the correlations between change in TAPSE and outcome remained significant both à 3 months (OR = 0.92 [0.85–0.98], P = 0.018) and 12 months (OR = 0.93 [0.87–0.99], P = 0.041).
FAC, fractional area change; IVC, inferior vena cava; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; TAPSE, tricuspid annulus plane systolic excursion.
Among patients where PASP could be measured, an increase in TAPSE was associated with an increase in TAPSE/PASP ratio from 0.23 [0.18–0.36] to 0.41 [0.26–0.52] (P < 0.0001). And, a TAPSE/PASP ratio was associated with a lower odds ratio at 3 months and by trend at 12 months in those study participants with measurable PASP (Table 5 ; OR 0.29, 95% CI 0.001–0.55, P = 0.02; and 0 = 0.09, respectively). Conversely, patients with no increase in TAPSE also had no change in the TAPSE/PASP ratio (0.31 [0.23–0. 0.42] to 0.31 [0.23–0.44] (P = 0.39).
The 3 months combined outcome occurred in 21% of patients with decongestion‐related TAPSE improvement and in 41% in patients without TAPSE improvement (P = 0.005). This difference remained significant after 12 months (39% vs. 60%, respectively; P = 0.004) (Supporting Information, Table S5 ). The Cox proportional hazard risk analysis confirmed a significantly lower risk for the combined endpoint in patients with decongestion‐related improvement of the TAPSE both in the unadjusted analysis (HR 0.51 95% CI: 0.33–0.78, P = 0.002) and after adjustment for age, sex, ischaemic heart disease, NT‐proBNP, LV end‐diastolic volume index, LV mass index, LVEF and LA volume index (HR 0.46 95% CI: 0.29–0.74, P = 0.001) (Figure 2 ).
Figure 2.
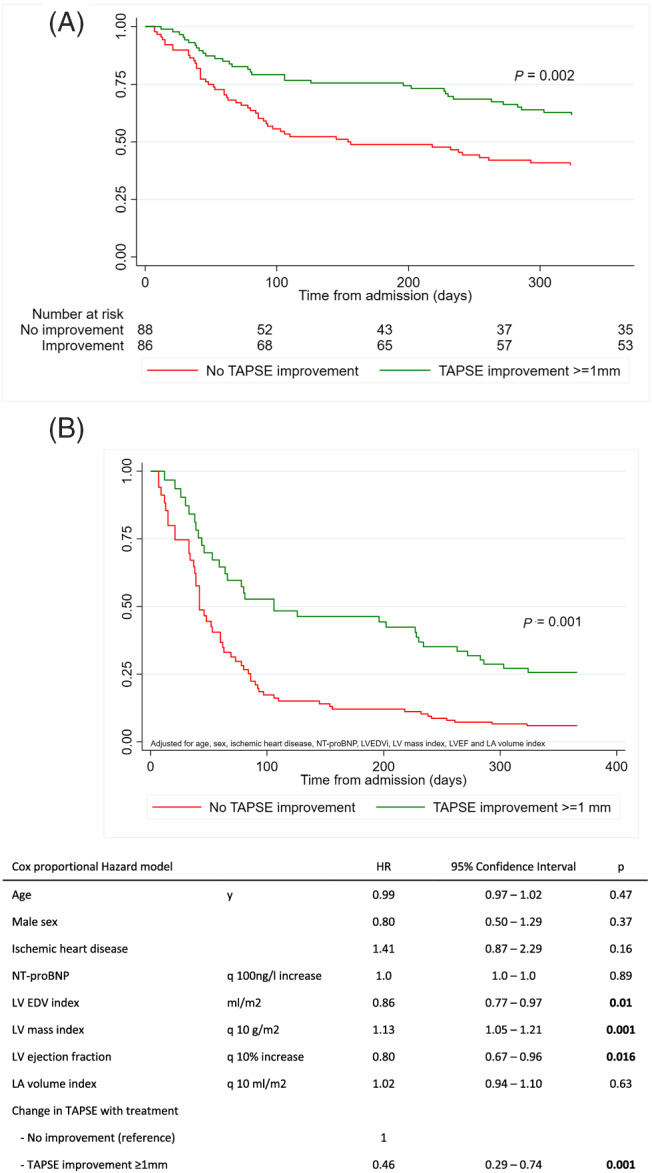
(A) Combined endpoint of death or cardiovascular readmission of study participants with or without improvement in TAPSE with acute treatment (unadjusted). (B) Combined endpoint of death or cardiovascular readmission of study participants with or without improvement in TAPSE with acute treatment (adjusted).
Discussion
Decongestion resulted in the entire study population and across all LVEF groups in a uniform reduction of body weight, blood pressure and in a decrease of the circulatory volume‐dependent echocardiographic parameters tricuspid regurgitation gradient, inferior vena cava diameter, and mitral E‐wave velocity. Decongestion resulted furthermore in almost half of all study participants in increase of the TAPSE and, in parallel, of the TAPSE/PASP ratio indicating improvement of RV‐pulmonary arterial coupling. These changes occurred across all LVEF groups and were associated with a significant improvement in prognosis.
Study population
The present cohort is noteworthy for its overall old age (median age of 83 years). This old age is compatible with AHF registries recruiting within in the second decade of this millennium (77–82 years) 12 , 13 , 14 while patients were younger (70–80 years) in respective registries recruiting in the first decade. 15 Older age of heart failure patients has been related to longer survival with modern heart failure treatment as well as higher life expectancy. 16 Old age furthermore explains the high proportion of de novo HF patients reported in the present study and elsewhere 17 because of the high incidence of de novo heart failure in this group of the population. 18
In contrast, the proportion of AHF patients with mrLVEF was in the present study higher (37%) when compared with other contemporary AHF registries (13–24%). 19 This may relate to the monocentric study design, the relatively low number of study participants, or the catchment area of the study centre with its high portion of people originating from Southern Europe or Northern Africa, where HF with mrLVEF is more prevalent. 20 Demographic, clinical, and biological characteristics of the present mrLVEF group compared with mrLVEF groups reported elsewhere. 20 , 21 The comparatively high number of AHF patients with mrLVEF provided therefore the opportunity to compare decongestion‐related change of cardiac function and morphology between LVEF groups of equal size.
Classification into AHF patients with reduced, mid‐range and preserved left ventricular ejection fraction
The separation into three LVEF groups refers to the classification of chronic stable HF into HF patients with rLVEF, mrLVEF, or pLVEF. The proportion of AHF patients with either worsening chronic HF or de novo HF was similar in each LVEF group in accordance with other reports. 12 , 13 , 17 Furthermore, the distribution of common prime precipitants of AHF 10 did not differ between LVEF groups. In addition, the clinical phenotype was similar between the LVEF groups with the exception of the right heart failure phenotype, which was more prevalent in AHF patients with preserved LVEF when compared with mrLVEF and rLVEF (78 vs. 49 vs. 59%). This observation is surprising considering the higher prevalence of significant tricuspid regurgitation (≥2 + grade) in AHF patients with rLVEF (pLVEF vs. mrLVEF vs. rLVEF: 22% vs. 15% vs. 39%, P = 0.01). However, afterload‐dependance of the RV is exaggerated in HF patients with pLVEF 7 which can explain the higher prevalence of this AHF phenotype in the pLVEF group.
Decongestion‐related changes of the left heart
Decongestion resulted in an average weight loss of 1.8 kg, corresponding to the weight loss of 2.3 kg reported from the ASCEND‐HF trial. 22 In the present study, body weight decrease was similar across all LVEF groups indicating similar extent of decongestion across all groups. Decongestion also went along with overall blood pressure decrease in all study participants without significant differences between the LVEF groups.
Nonetheless, no change of either LVEF or LV end‐diastolic volume was observed between admission and discharge echocardiography. Decongestion, however, went along with an overall increase in GLS in the entire study population and this increase was significant in the subgroups of AHF patients with rLVEF or mrLVEF. This improvement is not unexpected because both LV preload and blood pressure are key determinants of GLS 23 , 24 and these parameters were significantly improved with decongestion. GLS improvement in the rLVEF and the mrLVEF group may also explain the improvement of diastolic function in these patients because diastolic function is not only determined by active LV relaxation but also depends on restoring forces and lengthening load. 25 In the pLVEF group, however, no significant increase in GLS was observed, possibly because baseline GLS was only mildly reduced in this subgroup 26 and echocardiography may have missed subtle change. Absence of larger change of the GLS may also explain why diastolic function parameters did not change with decongestion of pLVEF patients.
In the present study, no significant decongestion‐related change of LA size was observed as reported elsewhere. 4 However, a more recent report had indicated decongestion‐related decrease of LA size in AHF patients with rLVEF 27 but these patients were of younger age (64 years vs. 83 years in the present study). In addition, 32% had a history of atrial fibrillation but were not in atrial fibrillation at inclusion while 42% patients of the present study had atrial fibrillation at admission and were substantially older. Both age and atrial fibrillation are acknowledged risk factors for increased atrial fibrosis 28 and stiffening the left atrium, which might explain the heterogeneity in decongestion‐related change of LA size.
Decongestion‐related right heart changes
The right heart is exquisitely sensitive to changes in preload and afterload rendering it susceptible to decompensation‐induced change of volume and function. 1 , 2 Decongestion went in the present study along with reduction of body weight, tricuspid regurgitation gradient, and inferior vena cava diameter as reported elsewhere. 1 , 2 , 3 , 4 Furthermore, there was a corresponding decrease of basal RV diameter, which is a fair echocardiographic surrogate parameter of RV volume. 29 However, only half of the study participants improved the TAPSE suggesting that decongestion‐related increase of the TAPSE distinguishes AHF patients recovering from decompensation. In fact, improvement of the TAPSE was in the present study associated with a lower 1 year incidence of the combined endpoint underlining the important role of RV contractile function for the short and mid‐term prognosis after an AHF episode. The prognostic role of RV contractile function has already been described in the general population where a 1 mm decrease of the TAPSE is associated with increased risk of cardiovascular mortality. 30 Further evidence of a predictive role of the TAPSE derives from another AHF cohort showing that a normal TAPSE in the baseline echocardiography is associated with improved outcome. 13 Therefore, the finding that improvement of the TAPSE is associated with better prognosis integrates into the body of existing evidence.
Haemodynamic studies in pulmonary hypertension have shown that an increase of pulmonary arterial load first increases RV contractility to preserve right ventriculo‐arterial coupling. However, progression of pulmonary hypertension results in RV‐PA decoupling with increase of RV dilatation and decrease RV stroke volume. The TAPSE/PASP correlates strongly with the invasively measured end‐systolic RV elastance/PA elastance (Ees/Ea) ratio, the gold standard metric of intrinsic RV contractility and RV afterload 30 proving the TAPSE/PASP as a valid non‐invasive surrogate parameter for RV‐PA coupling. Secondary pulmonary hypertension is a frequent complication of worsening heart failure, and a low TAPSE/PASP ratio predicts worse outcome in stable chronic HF patients with rLVEF or pLVEF. 8 In the present study, decongestion‐related improvement of TAPSE/PASP ratio was associated with a lower the incidence of the combined endpoint during the first 3 months after discharge and by trend at 12 months. In addition, improvement of the TAPSE/PASP ratio resulted in a median value corresponding to the value that has been associated with a good prognosis in stable chronic HFrEF patients. 8
Critical evaluation
Limitations of this study are the relatively small number of study participants and the loss to follow‐up of 14% of patients. This drop‐out might introduce a bias however these patients were not different with respect to admission parameters when compared with participants remaining in the study. Last not least, similar drop‐out rates were also reported from other studies in AHF such as the EVEREST trial investigating the effect of tolvaptan in AHF. 31
The study participants did not receive diuretic treatment alone but had introduction or increase of standard heart failure treatment with vasodilators and beta‐blockers too. Therefore, we cannot exclude that drug‐related decrease of heart rate and blood pressure have contributed to the observed changes in left and right heart function. In this context, simultaneous invasive measure of right heart parameters could have provided complementary information in particular because TAPSE is a load‐dependent parameter. In addition, we have to account for the old age of the patients, which may explain findings that differ when compared with younger AHF patient cohorts.
Conclusions
Decongestion was associated with an improvement in right ventricular function as measured by the TAPSE in almost half of the patients, irrespective of the LVEF category. TAPSE increase went along with decrease of the incidence of the combined primary endpoint both at 3 months and at 1 year while improvement of RV‐pulmonary artery coupling was independently associated with a lower incidence of the combined endpoint at 3 months. This highlights the pathophysiological and prognostic importance of improvement of right heart function with decongestion and provides new outcome parameters for evaluation of the individual patient prognosis and therapeutic strategies in acute heart failure.
Conflict of interest
All authors have nothing to disclose.
Funding
This study was supported by the Schweizerische Herzstiftung in 2017.
Supporting information
Figure S1. Flow‐chart of enrollment and FU.
Figure S2. Change of TAPSE with decongestion during index hospitalization in 1‐year survivors with no, 1 or more cardiovascular rehospitalizations within 1 year after discharge.
During the 1‐year follow‐up, 50.6% had no readmission, 17.6% had 1 readmission, 11.9% had >1 readmissions, and 19.9% died.
Table S1. comparison of baseline characteristics in patients with and without discharge echocardiography.
Table S2. Baseline echocardiographic characteristics.
Table S3. Changes in clinical and echocardiographic parameters between admission and discharge, by type of AHF.
Table S4. Baseline and discharge medical treatment.
Table S5. secondary endpoint – cardiovascular outcome data.
Acknowledgements
We thank Nathalie Lauriers, RN, and Sandrine Salzmann, RN, for their support in recruiting the study participants.
Hullin, R. , Tzimas, G. , Barras, N. , Abdurashidova, T. , Soborun, N. , Aur, S. , Regamey, J. , Hugelshofer, S. , Lu, H. , Crisinel, V. , Daux, A. , Vinet, E. , Mekoa‐Mbarga, S. J.‐R. , Kirsch, M. , Müller, O. , Hugli, O. , and Monney, P. (2022) Decongestion improving right heart function ameliorates prognosis after an acute heart failure episode. ESC Heart Failure, 9: 3814–3824. 10.1002/ehf2.14077.
References
- 1. Ambrosy AP, Pang PS, Khan S, Konstam MA, Fonarow GC, Traver B, Maggioni AP, Cook T, Swedberg K, Burnett JC Jr, Grinfeld L, Udelson JE, Zannad F, Gheorghiade M. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: Findings from the EVEREST trial. Eur Heart J. 2013; 34: 835–843. [DOI] [PubMed] [Google Scholar]
- 2. Arrigo M, Jessup M, Mullens W, Reza N, Shah AM, Siwa K, Mebazaa A. Acute heart failure. Nat Rev Cardiol. 2020; 6: 16–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mentz RJ, O'Connor CM. Pathophysiology and clinical evaluation of acute heart failure. Nat Rev Cardiol. 2015; 13: 28–35. [DOI] [PubMed] [Google Scholar]
- 4. Akiyama E, Cinotti R, Cerlinskaite K, van Aelst LNL, Arrigo M, Placido R, Chouihed T, Girerd N, Zannad F, Rossignol P, Badoz M, Launay J‐M, Gayat E, Cohen‐Solal A, Lam CSP, Testani J, Mullens W, Cotter G, Seronde M‐F, Mebaaza A. Improved cardiac and venous pressures during hospital stay in patients with acute heart failure: An echocardiography and biomarkers study. ESC Heart Fail. 2020; 7: 996–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pellicori P, Carubelli V, Zhang J, Castiello T, Sherwi N, Clark AL, Cleland JG. IVC diameter in patients with chronic heart failure: Relationships and prognostic significance. JACC Cardiovasc Imaging. 2013; 6: 16–28. [DOI] [PubMed] [Google Scholar]
- 6. Ghio S, Guazzi M, Scardovi AB, Klersy C, Clemenza F, Carluccio E, Temporelli PL, Rossi A, Faggiano P, Traversi E, Vriz O, Fl D. Different correlates but similar prognostic implications for right ventricular dysfunction in heart failure patients with reduced or preserved ejection fraction. Eur J Heart Fail. 2017; 19: 873–879. [DOI] [PubMed] [Google Scholar]
- 7. Melenovsky V, Hwang SJ, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J. 2014; 35: 3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schmeisser A, Rauwolf T, Groschek T, Fischbach K, Kropf S, Luani B, Tanev I, Hansen M, Meissler S, Schäfer K, Stendijk P, Braun‐Dullaeus RC. Predictors and prognosis of right ventricular function in pulmonary hypertension due to heart failure with reduced ejection fraction. ESC Heart Fail. 2021; 8: 2968–2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lancelotti P, Tribouilloy C, Hagendorff A, Popescu BA, Edvardsen T, Pierard LA, Badano L, Zamorano JL. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European association of cardiovascular imaging. Eur Heart J. 2013; 14: 611–644. [DOI] [PubMed] [Google Scholar]
- 10. McDonagh T, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leuro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK, ESC Scientific Document Group . 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 11. Nieminen MS, Brutsaert D, Dickstein K, Drexler H, Follath F, Harjola V‐P, Hochadel M, Komajda M, Lassus J, Lopez‐Sendon JL, Ponikowski P, Tavazzi L, on behalf of the EuroHeart Survey Investigators . EuroHeart failure survey II (EHFS II): A survey on hospitalized acute heart failure patients: Description of population. Eur Heart J 2006; 27:2725–2736. [DOI] [PubMed] [Google Scholar]
- 12. Yaku H, Ozasa N, Morimoto T, Inuzuka Y, Tamaki Y, Yamamoto E, Yoshikawa Y, Kitai T, Taniguchi R, Iguchi M, Kato M, Takahashi M, Jinnai T, Ikeda T, Nagao K, Kawai T, Komasa A, Nishikawa R, Kawase Y, Morinaga T, Su K, Kawato M, Sasaki K, Toyofuku M, Furukawa Y, Nakagawa Y, Ando K, Kadota K, Shizuta S, Ono K, Sato Y, Kuwahara K, Kato T, Kimura T, on behalf of the KCHF Study Investigators . Demographics, management, and in‐hospital outcome of hospitalized acute heart failure patients in contemporary real clinical practice in Japan. Circ J 2018; 82:2811–2819. [DOI] [PubMed] [Google Scholar]
- 13. Palazzuoli A, Ruocco G, Evangelist I, De Vivo O, Nuti R, Ghio S. Prognostic significance of an early echocardiographic evaluation of right ventricular dimension and function in acute heart failure. J Card Fail. 2020; 26: 813–820. [DOI] [PubMed] [Google Scholar]
- 14. Abdurashidova T, Monney P, Tzimas G, Soborun N, Regamey J, Daux A, Barras N, Kirsch M, Müller M, Hullin R. Non‐severe aortic regurgitation in reases short‐term mortality in acute heart failure with preserved ejection fraction. ESC Heart Fail. 2020; 7: 3901–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ambrosy AP, Fonarow GC, Butler J, Chioncel O, Greene SJ, Vaduganathan M, Nodari S, Lam CSP, Sato N, Shah AN, Gheorghiade M. The global health and economic burden of hospitalizations for heart failure. J Am Coll Cardiol. 2014; 63: 1123–1133. [DOI] [PubMed] [Google Scholar]
- 16. Jones NR, Roalfe AK, Adoki I, Hobbs FDR, Taylor CJ. Survival of patients with chronic heart failure in the community: A systematic review and meta‐analysis. Eur J Heart Fail. 2019; 21: 1306–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Butt JA, Fosbol EL, Gerds TA, Andersson C, McMurray JJ, Petrie MC, Gustafsson F, Madelaire C, Lund Kristensen S, Gislason GH, Torp‐Pedersen C, Kober L, Schou M. Readmission and death in patients admitted with new‐onset versus worsening of chronic heart failure: Insights from a nationwide cohort. Eur J Heart Fail. 2020; 22: 1777–1785. [DOI] [PubMed] [Google Scholar]
- 18. Shah AM, Claggett B, Loehr LR, Chang PP, Matsushita K, Kitzman D, Konety S, Kucharska‐Newton A, Sueta CA, Mosley TH, Wright JD, Coresh J, Heiss G, Folsom AR, Solomon SD. Heart failure stages among older adults in the community. The atherosclerosis risk in communities study. Circulation. 2017; 135: 224–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah K, Haolin X, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction. J Am Coll Cardiol. 2017; 70: 2476–2486. [DOI] [PubMed] [Google Scholar]
- 20. Chioncel O, Lainscak M, Seferovic P, Anker SD, Crespo‐Leiro MG, Harjola V‐K, Parissis J, Laroche C, Piepoli MF, Fonseca C, Mebazaa A, Lund L, Ambrosio GA, Coats AJ, Ferrari R, Ruschitza F, Maggioni AP, Filippatos G. Epidemiology and 1‐year outcomes in patients with chronic heart failure and preserved, mid‐range and reduced ejection fraction: An analysis from the ESC heart failure long‐term registry. Eur J Heart Fail. 2017; 19: 1574–1585. [DOI] [PubMed] [Google Scholar]
- 21. Hsu JJ, Ziaeian B, Fonarow GC. Heart failure with mid‐range (borderline) ejection fraction: Clinical implications and future directions. JACC Heart Fail. 2017; 5: 763–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ambrosy AP, Cerbin LP, Armstrong PW, Butler J, Coles A, DeVore AD, Dunlap ME, Ezekowitz JA, Felker M, Fudim M, Greene SJ, Hernandez AF, O'Connor CM, Schulte P, Starling RC, Teerlink JR, Voors AA, Mentz RJ. Body weight change during and after hospitalization for acute heart failure: Patient characteristics, markers of congestion, and outcomes. JACC Heart Fail. 2017; 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aalen J, Storsten P, Remme EW, Sirnes PA, Gjesdal O, Larsen CK, Koongsgard E, Boe E, Skulstad H, Hisdal J, Smiseth OA. Afterload sensitivity in patients with left bundle branch block. JACC Cardiovasc Imaging. 2019; 12: 967–977. [DOI] [PubMed] [Google Scholar]
- 24. Stokke TM, Hasselberg NE, Smedsrud MK, Sarvari SI, Haugaa KH, Smiseth OA, Edvarsen T, Remme EW. Geometry as a confounder when assessing ventricular systolic function: Comparison between ejection fraction and strain. J Am Coll Cardiol. 2017; 70: 942–954. [DOI] [PubMed] [Google Scholar]
- 25. Hasselberg NE, Haugaa KH, Sarvari SI, Gullestad L, Andreassen AK, Smiseth OA, Edvarsen TH. Left ventricular global longitudinal strain is associated with exercise capacity in failing heart hearts with preserved and reduced ejection fraction. Eur Heart J Cardiovasc Imaging. 2015; 16: 217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park JJ, Park J‐B, Park J‐H, Cho G‐Y. Global longitudinal strain to predict mortality in acute heart failure. J Am Coll Cardiol. 2018; 71: 1947–1957. [DOI] [PubMed] [Google Scholar]
- 27. Deferm S, Martens P, Verbrugge FH, Bertrand PB, Dauw J, Verhaert D, Dupont M, Vandervoort PM, Mullens W. LA mechanics in decompensated heart failure. J Am Coll Cardiol Img. 2020; 13: 1107–1115. [DOI] [PubMed] [Google Scholar]
- 28. Marrouche NF, Wilber D, Hindricks G, Jais G, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, Deneke T, Duytschaever M, Neumann T, Mansour M, Mahnkopf C, Herweg B, Caoud E, Wissner E, Bansmann P, Brachmann J. Association of Atrial Tissue Fibrosis Identified by delayed enhancement MRI and atrial fibrillation catheter ablation. The DECAAF study. JAMA. 2014; 311: 498–506. [DOI] [PubMed] [Google Scholar]
- 29. Gorter TM, van Veldhuisen DJ, Bauersachs J, Borlaug BA, Celutkiene J, Coats AJS, Crespo‐Leiro MG, Guazzi M, Harjola V‐P, Heymans S, Hill L, Lainscak M, Lam CSP, Lund LH, Lyon AR, Mebazaa A, Mueller C, Paulus WJ, Pieske B, Piepoli MF, Ruschitzka F, Rutten FH, Seferovic PM, Solomon SD, Shah SJ, Triposkiadis F, Wachter R, Tschöpe C, de Boer RA. Right heart dysfunction and failure in heart failure with preserved ejection fraction: Mechanisms and management. Position statement on behalf of the heart failure Association of the European Society of cardiology. Eur J Heart Fail. 2018; 20: 16–37. [DOI] [PubMed] [Google Scholar]
- 30. Modin D, Mogelvang R, Madsen Andersen D, Biering‐Sorensen T. Right ventricular function evaluated by tricuspid annular plane excursion predicts cardiovascular death in the general population. J Am Heart Assoc. 2019; 8: e012197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Konstam MA, Gheorghiade M, Burnett JC Jr, Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, Zannad F, Cook T, Ouyang J, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome trial. JAMA. 2007; 297: 1319–1331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Flow‐chart of enrollment and FU.
Figure S2. Change of TAPSE with decongestion during index hospitalization in 1‐year survivors with no, 1 or more cardiovascular rehospitalizations within 1 year after discharge.
During the 1‐year follow‐up, 50.6% had no readmission, 17.6% had 1 readmission, 11.9% had >1 readmissions, and 19.9% died.
Table S1. comparison of baseline characteristics in patients with and without discharge echocardiography.
Table S2. Baseline echocardiographic characteristics.
Table S3. Changes in clinical and echocardiographic parameters between admission and discharge, by type of AHF.
Table S4. Baseline and discharge medical treatment.
Table S5. secondary endpoint – cardiovascular outcome data.


