Abstract
Purpose
Group B streptococcus (GBS) remains a leading cause of invasive disease, mainly sepsis and meningitis, in infants < 3 months of age and of mortality among neonates. This study, a major component of the European DEVANI project (Design of a Vaccine Against Neonatal Infections) describes clinical and important microbiological characteristics of neonatal GBS diseases. It quantifies the rate of antenatal screening and intrapartum antibiotic prophylaxis among cases and identifies risk factors associated with an adverse outcome.
Methods
Clinical and microbiological data from 153 invasive neonatal cases (82 early-onset [EOD], 71 late-onset disease [LOD] cases) were collected in eight European countries from mid-2008 to end-2010.
Results
Respiratory distress was the most frequent clinical sign at onset of EOD, while meningitis is found in > 30% of LOD. The study revealed that 59% of mothers of EOD cases had not received antenatal screening, whilst GBS was detected in 48.5% of screened cases. Meningitis was associated with an adverse outcome in LOD cases, while prematurity and the presence of cardiocirculatory symptoms were associated with an adverse outcome in EOD cases. Capsular-polysaccharide type III was the most frequent in both EOD and LOD cases with regional differences in the clonal complex distribution.
Conclusions
Standardizing recommendations related to neonatal GBS disease and increasing compliance might improve clinical care and the prevention of GBS EOD. But even full adherence to antenatal screening would miss a relevant number of EOD cases, thus, the most promising prophylactic approach against GBS EOD and LOD would be a vaccine for maternal immunization.
Keywords: Group B streptococcus, Streptococcus agalactiae, Neonatal infection, Early-onset disease, Late-onset disease, Group B streptococcal vaccine
Introduction
Streptococcus agalactiae, also termed Group B streptococcus (GBS), remains one of the leading causative agents of invasive bacterial infections in infants and contributes substantially to neonatal morbidity and mortality globally [1]. When occurring during the first 6 days of life, the infection is termed early-onset disease (EOD), and between days 7 and 89, late-onset disease (LOD). In EOD cases, infected neonates rapidly develop bacteremia with or without sepsis and/or pneumonia and less commonly meningitis. In LOD cases, neonates are usually bacteremic without focus and often develop meningitis [2, 3]. The main risk factor for EOD is maternal rectovaginal colonization with GBS at the end of the pregnancy; additional established risk factors include prolonged rupture of membranes (ROM) before delivery (> 18 h), intrapartum fever (≥ 38 °C), GBS bacteriuria during the current pregnancy, a previous infant with GBS disease, and preterm delivery at less than 37 weeks of gestation [4]. Risk factors and the transmission route for LOD are less well established; the GBS may be acquired from the mother or from environmental sources [5, 6].
With the implementation of prevention strategies in many countries, the incidence of infant GBS disease globally has declined to an estimated 0.49/1,000 live births, with a probable underestimation in developing countries [7, 8]. The incidence in Europe is similar, with important regional differences [5, 7, 9–11]. The EOD incidence has been successfully reduced by universal antenatal screening and appropriate intrapartum antibiotic prophylaxis (IAP) programs for colonized pregnant women [7]. These include a standardized culture-based detection of GBS in a vaginal-rectal swab in late pregnancy (between 35 and 38 weeks, depending on national guidelines) combined with intravenous administration of antibiotics during labor to GBS-positive women. Alternatively, a risk factor-based approach for IAP is implemented in some countries [4]. National guidelines in Europe propose different strategies, though efforts are being made to standardize these recommendations [12]. Whatever the recommended strategy is, these measures have failed to substantially reduce the incidence of LOD [13].
GBS express a type-specific capsular polysaccharide (CPS), one of the most important GBS virulence factors [14]. To date, ten antigenically distinct types have been described (Ia, Ib, II to IX) [15]. GBS isolates can be further characterized by detection of surface proteins such as the pili, which are also known virulence factors [14, 16]. Furthermore, multi-locus sequence typing (MLST) characterizes the isolates into distinct sequence types (ST) clustered into clonal complexes (CC), which may provide information on the pathogenicity of the isolates. CC17, as an example, represents a hyper-virulent cluster of clones that is increasingly found in LOD cases and frequently causes meningitis, while also containing an emerging sub-lineage of multi-drug resistant isolates [17–19]. Usually, GBS isolates are considered uniformly susceptible to penicillin with the exception of very few invasive isolates initially described in Japan and later in the USA presenting reduced susceptibility to penicillin (PenRGBS) and other β-lactam antibiotics [20]. Today, in Japan, these isolates have increased up to 14% and higher. More recently, rare cases of PenRGBS have been reported from Canada, China, England, Germany, Italy, Korea [21]. More widespread is the increased resistance to erythromycin and clindamycin [17, 21, 22] and emerging resistance to fluoroquinolones [21].
Even if efficient to prevent numerous EOD cases, current preventive strategies do not appear to be adequate for the expected control of GBS disease, therefore, additional efforts are urgently required. Vaccines for pregnant women are promising approaches to protect infants through placentally transferred antibodies; they have been successfully tested in early phase clinical studies [23–25]. Vaccine development programs require detailed microbiological characterization of circulating strains [26]. Current efforts are being made to develop conjugated CPS-based or protein-based (e.g., pili) vaccines, and to define correlates of seroprotection to facilitate the assessment of vaccine efficacy [27], which has been shown to be mediated mainly by opsonophagocytic antibodies [28].
The DEVANI (Design of a Vaccine Against Neonatal Infections) consortium was established in 2008 as a pan-European initiative to assess neonatal GBS disease burden in Europe, to provide clinical and microbiological information for vaccine design, and to improve laboratory performance in diagnosing GBS colonization and infection [29]. The DEVANI project also included an investigation of serological correlates of neonatal protection through the analysis of CPS- and pilus-specific immune responses in sera from 984 GBS colonized and 473 non-colonized pregnant women who delivered healthy neonate and in mothers of 153 infants with GBS disease [28]. From this cohort, Fabrini et al. demonstrated that IgG levels against CPS and pilus proteins were significantly higher in GBS colonized women delivering healthy neonates than in mothers of babies with GBS disease or non-colonized women. Here, we report on the clinical and microbiological characteristics of invasive neonatal GBS infections along with frequencies of maternal risk factors of 153 neonatal cases collected over a 2.5-year acquisition period in eight European countries.
Methods
Study design and participating countries
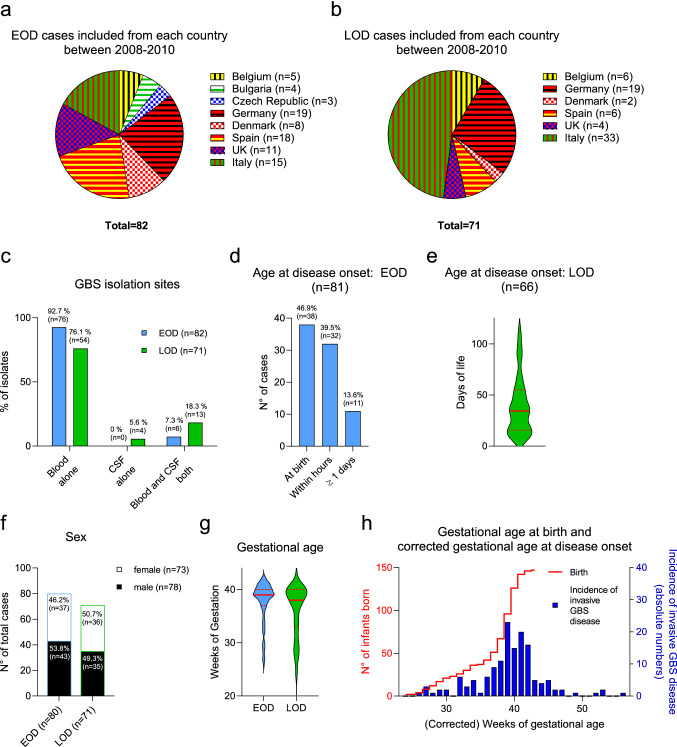
The DEVANI study design has been widely described by Rodriguez-Granger et al. [29]. In summary, for the description of invasive neonatal disease, clinical characteristics and GBS isolates from cases have been collected between mid-2008 and end-2010 in eight European countries (Belgium, Bulgaria, Czech Republic, Denmark, Germany, Italy, Spain and the UK). The study has been approved by local ethics committees of each participating institution, and informed consent for study participation has been obtained from the legal caregivers. According to the estimated number of births per participating institution, the expected number of eligible cases of GBS invasive disease ranged from 100 to 200. The number of reported cases from each participating country is shown in Fig. 1a, b. Overall, 82 EOD cases and 71 LOD cases were included in the study.
Fig. 1.
Case distribution across the eight European countries participating at the DEVANI study and characterization of the study cohort. a, b Number of included invasive GBS cases from each participating country. (a EOD cases. b LOD cases). c Isolation sites of invasive GBS strains in EOD and LOD cases. d, e Age at onset of disease of EOD, LOD and VLOD cases. The red lines in e. indicate the median with interquartile ranges. f Sex of EOD and LOD cases. g Gestational age at birth of EOD and LOD cases. The red lines indicate the median with interquartile ranges. h The reverse Kaplan–Meier curve in red indicates the gestational age at birth, the blue bar graphs represent the incidence during the respective corrected gestational age. Data from EOD and LOD are pooled in this graph. GBS Group B streptococcus, CSF Cerebrospinal fluid, EOD Early-onset disease, LOD Late-onset disease
Data collection and case definitions
Eligible cases were identified through daily surveillance according to the following inclusion criteria: GBS isolation from blood or cerebrospinal fluid (CSF) in infants aged 1–6 days (EOD) or ≥ 7 days up to 89 days (LOD) or ≥ 90 days (very late-onset disease [VLOD], included in the LOD groups). For each case, obstetrical information, clinical signs at onset of disease, paraclinical assessment of infection, microbiological characteristics of GBS strains and information on management and outcome were collected and saved in a secured online database according to the defined criteria of the DEVANI project. Infants were regarded as preterm if they were born earlier than 37 weeks of gestation.
Collection of GBS isolates and strain characterization
Cultures of clinical specimens collected from normally sterile sites, such as blood and CSF, were processed according to local microbiological procedures. All GBS isolates were sent to national central laboratories. GBS identification was confirmed by an agglutination method for the detection of the Lancefield group B antigen [30], stored and further characterized. Capsular serotyping was performed by standardized latex agglutination tests using the Strep-B latex kit (Statens Serum Institut, Denmark) [30, 31]. Multiplex polymerase chain reaction (PCR) assay was used to determine capsular genotype as described previously [32–34]. Repeated external quality assessments had been organized and ensured the accuracy of the results for the different typing methods [29]. Isolates were further characterized by MLST to identify the STs and CCs [18, 19].
Clinical and laboratory assessment
C-reactive protein (CRP) levels and chest X-rays were analyzed according to local standards. Clinical examinations were performed by local medical staff and reported using a standardized online tool, that was the DEVANI web database, by members of the local study groups. CRP values were considered significantly elevated above a threshold of 10 mg/l.
Statistical analysis of risk factors
The relative risk values were calculated by Graphpad prism 9 using the Koopman asymptotic score and Fisher’s exact test to determine the p-value. Two-tailed Fisher’s exact test was calculated with the online tool “Graphpad Quickcalcs” (https://www.graphpad.com/quickcalcs/). CRP values were compared using unpaired t-test. P-values below 0.05 were considered statistically significant.
Results
Clinical characteristics of neonatal invasive disease
Overall, 153 neonatal cases with invasive GBS disease were collected. 82 EOD cases were included in the study, all of which had a positive blood culture, while six patients (7.3%) additionally had GBS culture-positive meningitis (Fig. 1c). Sixty-six of the EOD cases (80.5%) were term neonates and the overall median gestational age was 39 weeks (Fig. 1g, h). Seventy neonates (86.4% of EOD cases) presented symptoms at birth or within the first day of life (Fig. 1d). Of the 71 LOD cases (including seven VLOD) enrolled in the study, 67 (94.4%) showed bloodstream infection, while 17 (23.9%) had GBS culture-positive meningitis, i.e., four patients (5.6%) had a positive GBS culture only from the CSF and 13 (18.3%) had both a positive blood and a positive CSF culture (Fig. 1c). Among the LOD cases, 69% were term infants while 22 (31.0%) were preterm infants, and the overall median gestational age is 38 weeks (Fig. 1g, h). The median age at disease-onset was 34 days, the maximum was 114 days (Fig. 1e). The sex ratio male/female was 1.16 for the EOD cases and 0.97 for the LOD cases (Fig. 1f).
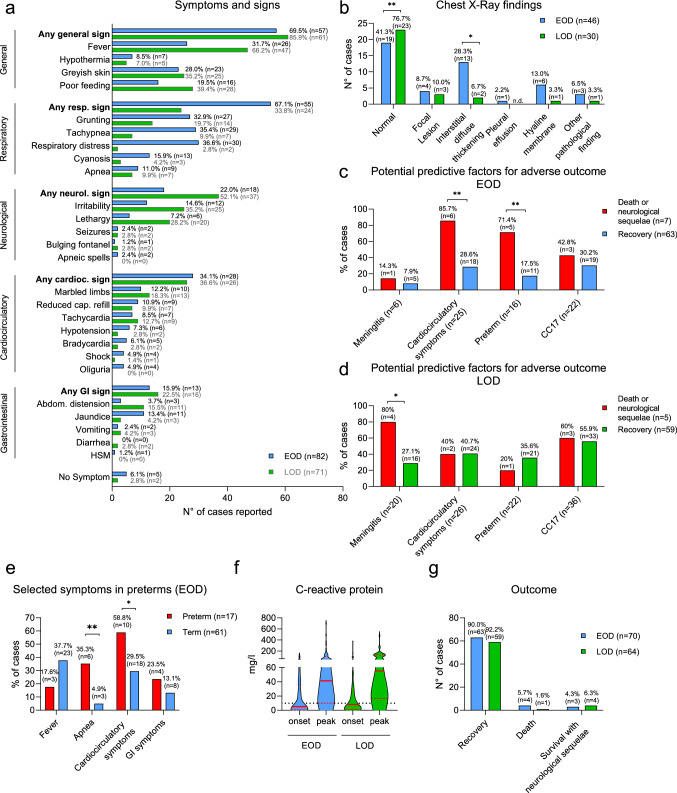
The clinical presentation differed between EOD and LOD cases (Fig. 2a). At onset, in EOD cases, respiratory symptoms were predominant (67.1%), while LOD cases more frequently presented with fever (66.2%) and neurological signs (52.1%). Cardiocirculatory and gastrointestinal (GI) symptoms were equally frequent in EOD and LOD cases (Fig. 2a). Chest X-rays were obtained from 46 (56.1%) of EOD cases and from 30 (42.2%) of LOD cases. As expected, EOD cases were more likely to have an abnormal X-ray finding than LOD cases (58.7% vs 23.3%, p = 0.0043), with “interstitial diffuse thickening” being the most common sign (Fig. 2b). Preterm neonates with EOD exhibited a different clinical presentation dominated by apnea (p = 0.0026) and cardiocirculatory symptoms (p = 0.0434) and a tendency to more GI symptoms and less fever than term neonates (Fig. 2e). CRP levels were determined in 73 EOD cases (89.0%) and 61 LOD cases (85.9%). Maximum CRP levels during the disease course ranged from < 3 to 701 mg/l (median = 41 mg/l, interquartile range [IQR] 10–102 mg/l) in EOD and from < 3 to 479 mg/l (median = 55 mg/l, IQR 17–133 mg/l) in LOD cases. CRP levels remained below the threshold value of 10 mg/l in 13 EOD and 7 LOD cases (Fig. 2f).
Fig. 2.
Clinical presentation and outcome. a Symptoms and signs of EOD and LOD cases, b Chest X-ray findings of EOD and LOD cases. P-values from “Fisher’s exact test” are as follows: “normal findings”: p = 0.0043; “interstitial diffuse thickening”: p = 0.0364; all other differences are not statistically significant. c Potential predictive factors for adverse outcomes in EOD cases. Statistical significance by “Fisher’s exact test” is reached for “cardiocirculatory symptoms” (p = 0.0055) and “preterm” (p = 0.0056). d Potential predictive factors for adverse outcomes in LOD cases. Statistical significance by “Fisher’s exact test” is reached for “Meningitis” (p = 0.0300). Meningitis is based on the clinical diagnosis, not the positive CSF culture. e Selected symptoms of preterm versus term EOD cases. P-values from “Fisher’s exact test” are as follows: “apnea”: p = 0.0026; “cardiocirculatory symptoms”: p = 0.0434. The other comparisons are not statistically significant. f Serum C-reactive protein levels at disease onset and maximal levels of EOD and LOD cases. The red lines indicate median values, the dashed red lines the IQRs. The black dotted line indicates a level of 10 mg/l, the threshold for this study. Differences between EOD and LOD are not significant (unpaired student’s t-test). g outcome of EOD and LOD cases. GBS Group B streptococcus, EOD Early-onset disease, LOD Late-onset disease, GI Gastrointestinal, HSM Hepatosplenomegaly, CC17 Clonal complex 17
10.0% (7/70) of EOD cases and 7.8% (5/64) of LOD cases had an unfavorable outcome, i.e., early neurological sequelae (during initial in-hospital treatment) or death (Fig. 2g). Potential predictive factors for an adverse outcome in EOD were the occurrence of cardiocirculatory symptoms (RR 3.00; CI 1.56–4.76) and prematurity (RR 4.09; CI 1.77–7.86) (Fig. 2c), while the diagnosis of meningitis or the detection of a CC17 strain were not significantly associated with an adverse outcome. Conversely, in LOD cases, meningitis was the only predictive factor for an adverse outcome (RR 2.95; CI 1.28–4.98), though 80% of meningitis cases recovered without early sequelae (Fig. 2d). Of note, this assessment was based on the clinical diagnosis of meningitis (n = 20), as opposed to a positive CSF culture (n = 17).
Microbiological characteristics of GBS isolates from neonatal invasive disease
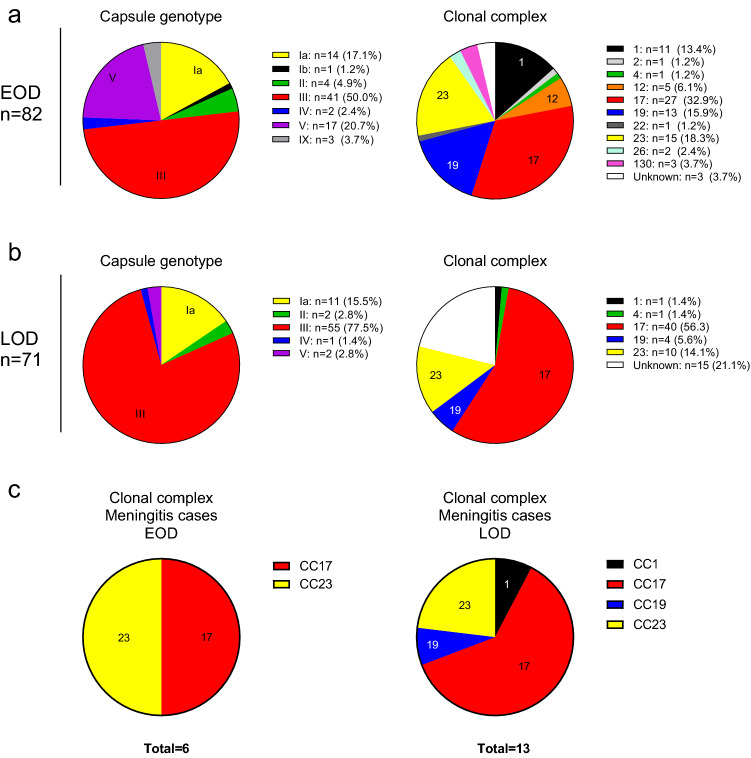
A CPS type assessed by latex agglutination and genotyping was assigned to all isolates. For EOD, the distribution showed a predominance of type III, V and Ia (50.0%, 20.7% and 17.1%), followed by types II, IX, IV and Ib (Fig. 3a). For LOD cases, type III was predominant with 77.5%, followed by types Ia (15.5%), V, II and IV (Fig. 3b). The distribution and in vitro expression of the three pilus variants assessed in 146 out of 153 neonatal strains had been previously reported [28]. MLST data are available for 79/82 (96.3%) of EOD and for 56/71 (78.9%) of LOD isolates. It revealed a heterogenous CC distribution in EOD cases, with 32.9% of the isolates clustering in CC17 while, overall, ten different CCs were detectable (Fig. 3a). CC17 was found only in CPS type III isolates and accounted for 27/39 (69.2%) of the CPS type III EOD cases for which MLST data were available. In LOD, CC17 is responsible for 40/43 (93.0%) CPS type III cases, i.e., 71.4% of all LOD cases for which MLST data were available (Fig. 3b). Thus, CC17 (as does CPS III) represented a significantly higher proportion among LOD (71.4%) than EOD (34.2%) cases (rate ratio [RR] 2.09; confidence interval [CI] 1.49–2.99). The prevalence of CC17 in EOD cases differed substantially between different European countries, ranging (within countries with more than four reported cases) from 18% in the UK to 67% in Italy. The same was observed among LOD cases, the prevalence of CC17 ranged from 40% in Spain to 86% in Italy. Only five different CCs were found among the LOD isolates (Fig. 3b). CC17 strains caused 50% of EOD and 62% of LOD meningitis cases (Fig. 3c).
Fig. 3.
Microbiological characteristics. a Isolates from EOD cases, capsule genotype and clonal complexes are indicated. b Isolates from LOD cases, capsule genotype and clonal complexes are indicated. c Distribution of clonal complexes of culture proven EOD or LOD meningitis cases. The absolute numbers for EOD cases (left panel) are 3 CC17 and 3 CC23. The absolute numbers for LOD cases (right panel) are 1 CC1, 8 CC17, 1 CC19 and 3 CC23. EOD Early-onset disease, LOD Late-onset disease, CC clonal complex
Risk factors for invasive neonatal disease
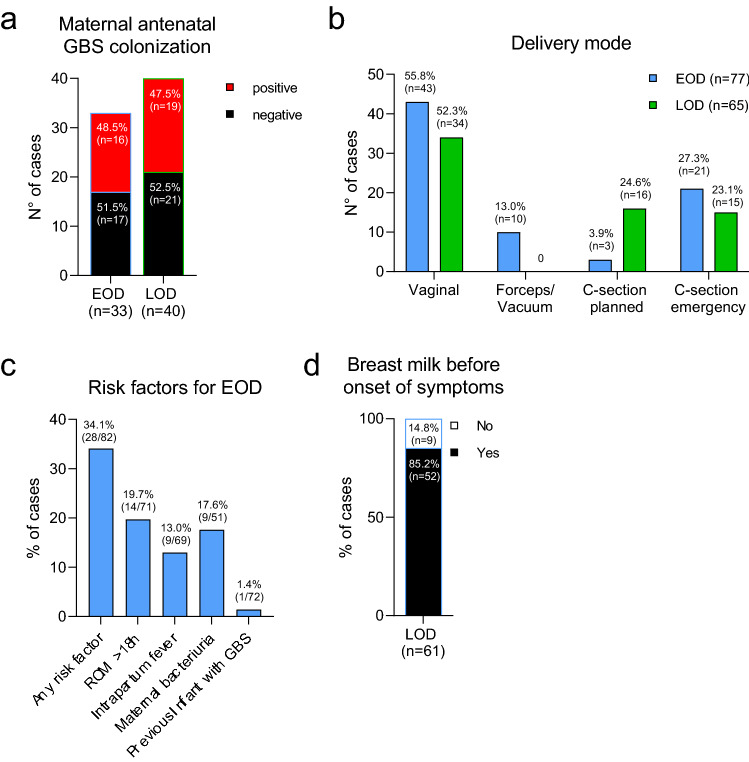
Across all cases (EOD and LOD), antenatal maternal GBS colonization status had been performed for 73 mothers (47.7%). In countries with recommendations for universal screening, among cases’ mothers, a selected population where preventive strategy failed, rates of screening cultures were 72.7% (8/11) in Belgium, 39.5% (15/38) in Germany, 66.7% (32/48) in Italy, 54.2% (13/24) in Spain and 33.3% (1/3) in Czech Republic. Reasons for the low reporting rate were not assessed but likely include at least premature delivery before timing of screening, lack of adherence to guidelines by health care providers, refused screening offers by pregnant women and incomplete data traceability. In countries without universal screening, GBS screening rates were 30% (3/10) in Denmark, 6.7% (1/15) in the UK and 0% (0/4) in Bulgaria. In 33 of the EOD cases (40.2%), antenatal maternal GBS colonization status had been assessed. Of those, 48.5% (n = 16) were positive (Fig. 4a). Of the 16 EOD cases with a positive antenatal screening result, only seven mothers (43.8%) had received IAP, and the first dose had been given less than four hours before birth in three cases, leaving a total of four mothers within our cohort of 82 EOD cases (4.9%) having received appropriate IAP (> 4 h). 19/39 LOD cases (47.5%) with antenatal screening results were born to mothers with confirmed positive GBS carrier status (Fig. 4a), four (21.1%) of whom received adequate antenatal IAP.
Fig. 4.
Maternal risk factors and GBS exposure. a Maternal GBS colonization status at the time of screening. b Delivery mode of EOD and LOD cases. c Frequency of established maternal risk factors for EOD cases. d Percentages of breast feeding before onset of symptoms of LOD cases. EOD Early-onset disease, LOD Late-onset disease, C-section Caesarean section, ROM Rupture of membrane
The delivery mode was vacuum/forceps-assisted in 10 EOD cases (13.0%); and 21 EOD cases (27.3%) required an emergency C-section (Fig. 4b). Established maternal risk factors for EOD included ROM (> 18 h) in 14 EOD cases (19.7%), and intrapartum fever (≥ 38 °C) in nine EOD cases (13.0%). GBS bacteriuria, a surrogate marker for heavy maternal colonization, was reported in 17.6% of EOD cases (n = 9). One mother (1.4%) had a previous infant with invasive GBS disease (Fig. 4c). Breastfeeding before disease onset was reported in 85.2% (52/61) of LOD cases (Fig. 4d).
Discussion
The present study reports on the clinical and microbiological characteristics, as well as risk factors and outcome in 153 infant patients with invasive GBS disease from eight different European countries, a cohort collected within the framework of the EU-funded DEVANI project.
Concordant with previous publications, GBS EOD presented shortly after birth with predominantly respiratory symptoms [35]. The time point of infection was indicative of intrapartum or in-utero GBS transmission from the colonized mother. At the time of the study, a screening-based strategy was recommended in Belgium, Czech Republic, Germany, Italy and Spain; nevertheless, mothers of numerous EOD cases had not been screened accordingly in these five countries, partly due to preterm birth before reaching the recommended time point for screening. Other reasons for the low reporting were likely lack of adherence to guidelines by health care providers, refused screening offers by pregnant women and incomplete data traceability. Indeed, poor adherence to existing recommendations has been identified as a factor limiting the effectiveness of antenatal screening [36]. Of note, this low adherence to recommendation does not apply to the general population, but reflects this selective population of infants with invasive GBS disease. On the other hand, a risk-based strategy was applied in the UK, Bulgaria and Denmark; however, a lower number of EOD cases’ mothers had had antenatal screening. In summary, in our study, less than half of EOD cases’ mothers (33/77) had an antenatal swab taken and of those, 48.5% were identified GBS-positive, which is concordant with previous studies [37, 38]. Concerning the presence of risk factors, the rate was even lower with 33% of parturients (to EOD cases) presenting with at least one risk factor, a frequently described limitation to this strategy of prevention [39, 40]. Thus, both strategies were likely to miss opportunities for preventing neonatal invasive cases. In addition, even with the knowledge of an existing positive screening result, IAP was not administered timely in a significant number of cases, a finding concordant with other publications [5, 6, 36, 38]. The reasons for this non-compliance had not been systematically analyzed but were potentially due to the limited time period between arrival in the hospital and birth. These considerations reflected some important limitations of both prevention strategies, emphasizing the necessity to more stringently adhere to the existing recommendations to improve the overall quality of care. They also highlighted the need to harmonize recommendations for prevention throughout Europe and for an alternative approach, i.e., maternal GBS immunization.
GBS LOD cases differed substantially concerning clinical presentation and microbiological characteristics. A high proportion of cases caused by GBS CC17 (71.4% of all sequence typable strains), known to be hypervirulent, was detected in our LOD cohort. This rate was lower than recently published findings from the USA [20], but similar to findings from France and China [41, 42]. More than half of the LOD cases showed neurological symptoms, confirming GBS LOD to be a neurotropic disease. Culture positive meningitis cases were not exclusively caused by CC17 strains, emphasizing the importance of considering different serotypes in the design of potential vaccines. The colonization rate in mothers of LOD cases, a known risk factor for invasive GBS disease, was roughly 50%, a rate similar to previously published findings [43], much higher than the ~ 20% in the general population [44].
Adverse outcomes are still repeatedly reported with invasive GBS disease despite adequate treatment, and the preterm cohort is especially at risk, both for developing invasive GBS disease and for unfavourable outcomes [45]. In our EOD cohort, this is mostly associated with cardiorespiratory failure. Death from LOD had occurred in only one case in our cohort, 3/20 cases with clinical meningitis (15%) had survived with neurological sequelae, representing a lower rate than previously reported after GBS meningitis [46]. However, no long-term follow-up data were available for our cohort. Overall, the mortality (5.7% in EOD and 1.6% in LOD) was lower than or similar to most published cohorts [41].
Microbiological characteristics showed a wide distribution between European countries, especially the frequency of isolates attributable to CC17 in LOD, which range from 40% in Spain to 86% in Italy. This is likely to impact on the disease course, e.g., the prevalence of meningitis in different countries. The reasons for this heterogeneity have not been assessed, but emphasize the importance to consider the regional seroprevalence distribution. Our data confirm the global trend that EOD cases decrease (due to prophylactic measures) and LOD cases increase. The reasons for the latter are unknown but might be due to delayed colonization in at-risk infants after maternal antibiotic treatment and due to an increased number of extremely low birth weight infants being cared for, a population at high risk for GBS LOD.
The study revealed relevant differences in clinical practice between the different participating countries such as the percentage of pregnancies with antenatal screening. Guidelines for antenatal GBS screening have not evolved significantly even if attempts towards a European consensus have been done but not yet concluded [12]. Therefore, it seems imperative to harmonize the recommendations to improve the quality of care and to assess effects of measures taken across Europe.
Limitations of our study include the lack of information on the actual incidence, as no steps were implemented to guarantee that every case was reported. Furthermore, the observational period extends only during inpatient care, thus probably missing the detection of neurological sequelae that frequently become apparent only later in life [46]. This study did not include a control group of healthy neonates, thus clinical characteristics cannot specifically be attributed to GBS disease and the specificity of maternal risk factors cannot be determined. The delay in reporting is attributable to difficulties in management of the dataset with single missing variables from different countries, as well as to changes in personal responsibilities within the consortium, and last but not least the work load caused by the COVID 19 pandemic. However, the clinical and microbiological findings in our very unique European cohort are still relevant to the situation of invasive GBS infections in neonates and young infants and reflect the spectrum of GBS disease nowadays. Indeed, our study includes data from eight European countries for which no (or very few) more recent epidemiological data have been published and where recommendations for prevention of GBS neonatal disease have not changed much.
Conclusions
This study describes the clinical and microbiological properties of 153 invasive infant GBS cases collected within the framework of the European DEVANI consortium. The originality of the study lies in the detailed description of clinical characteristics including specific features of premature infants. It identifies potential predictors of an adverse outcome, including cardiocirculatory symptoms and prematurity for EOD and meningitis for LOD. Microbiological characteristics were assessed via a harmonized protocol, ensuring comparability, and revealed important differences in strain prevalence throughout Europe.
The study emphasizes the importance of having standardized practical protocols and a better adherence for the prevention and care of invasive GBS disease. It also demonstrates that IAP indicated by a prevention strategy, either screening-based or risk factor-based, fails in a substantial number of cases across Europe. Therefore, additional efforts, such as a GBS vaccine for pregnant women along with standardized European guidelines, are imperative if we are to decrease the disease burden.
Acknowledgements
Other contributing members of the DEVANI (Design of a Vaccine Against Neonatal Infections) Study Group were: Petrunov B (Bulgaria); Krizova P (Czech Republic); Poulsen K (Denmark); Karstens L (Germany); Baldassarri L, Imperi M, Rigat F, Berardi A, Grandi G (Italy).
Authors’ contribution
FL wrote the first draft of the manuscript. Concept and design: RB, DM, PM, GO and JT. Acquisition of data: BA, AD, MdlRF, AE, MH, MKi, MKu, PM, JR-G and UBSSS. Investigation: BA, RB, RC, AD, MdlRF, AE, MKi, JK, MKu, DM, PM, DR, JR-G and UBSSS. Formal analysis: FL, MH, DM, IM, DR. Writing—review & editing: RB, AE, MH, DM, IM, PM, DR. Resources: DM, GO, DR, JT. Clinical project leader: GO. Scientific project leader: JT. All authors read and approved the final manuscript.
Funding
This work was supported by the European Commission Seventh Framework (Grant agreement number 200481) and by Novartis Vaccines Division and GlaxoSmithKline Biologicals SA (Novartis’ non-influenza vaccines business was acquired by the GSK group of companies on 2 March 2015) as part of the DEVANI program. Florens Lohrmann is recipient of an IMM-PACT stipend (DFG 413517907) and member of the Spemann Graduate School for Biology and Medicine.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
Financial interests: Margarit I, Maione D and Rinaudo D are employees of the GSK group of companies and hold shares in the GSK group of companies. Margarit I, Maione D and Rinaudo D are listed as inventor on patents owned by the GSK group of companies. Uffe B. Skov Sørensen and Mogens Kilian received personal consultancy fees from Suzhou VACMICRO Biotech Co., Ltd. Non-financial interests: Markus Hufnagel has been invited by Novartis to an advisory board meeting on juvenile idiopathic arthritis. Pierrette Melin has provided one scientific consultation for GSK vaccines. All other authors have no financial or non-financial interests to disclose.
Ethics approval
The study has been approved by local ethics committees of each participating institution, and informed consent for study participation has been obtained from the legal caregivers. The study was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Footnotes
The list of “DEVANI Study Group” members were listed in Acknowledgements section.
Reinhard Berner and Pierrette Melin contributed equally to the publication.
Contributor Information
Pierrette Melin, Email: Pierrette.Melin@uliege.be.
for the DEVANI Study Group:
B. Petrunov, P. Krizova, K. Poulsen, L. Karstens, L. Baldassarri, M. Imperi, F. Rigat, A. Berardi, and G. Grandi
References
- 1.Seale AC, Bianchi-Jassir F, Russell NJ, Kohli-Lynch M, Tann CJ, Hall J, Madrid L, Blencowe H, Cousens S, Baker CJ, Bartlett L, Cutland C, Gravett MG, Heath PT, Ip M, Le Doare K, Madhi SA, Rubens CE, Saha SK, Schrag SJ, Sobanjo-Ter Meulen A, Vekemans J, Lawn JE. Estimates of the burden of group B streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis. 2017;65:S200–S219. doi: 10.1093/cid/cix664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker CJ, Barrett FF, Gordon RC, Yow MD. Suppurative meningitis due to streptococci of Lancefield group B: a study of 33 infants. J Pediatr. 1973;82:724–729. doi: 10.1016/S0022-3476(73)80606-7. [DOI] [PubMed] [Google Scholar]
- 3.Baker CJ. The spectrum of perinatal group B streptococcal disease. Vaccine. 2013;31(Suppl 4):D3–6. doi: 10.1016/j.vaccine.2013.02.030. [DOI] [PubMed] [Google Scholar]
- 4.Heath PT, Schuchat A. Perinatal group B streptococcal disease. Best Pract Res Clin Obstet Gynaecol. 2007;21:411–424. doi: 10.1016/j.bpobgyn.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Joubrel C, Tazi A, Six A, Dmytruk N, Touak G, Bidet P, Raymond J, Trieu Cuot P, Fouet A, Kernéis S, Poyart C. Group B streptococcus neonatal invasive infections, France 2007–2012. Clin Microbiol Infect. 2015;21:910–916. doi: 10.1016/j.cmi.2015.05.039. [DOI] [PubMed] [Google Scholar]
- 6.Tazi A, Plainvert C, Anselem O, Ballon M, Marcou V, Seco A, El Alaoui F, Joubrel C, El Helali N, Falloukh E, Frigo A, Raymond J, Trieu-Cuot P, Branger C, Le Monnier A, Azria E, Ancel P-Y, Jarreau PH, Mandelbrot L, Goffinet F, Poyart C. Risk factors for infant colonization by hypervirulent CC17 group B streptococcus: toward the understanding of late-onset disease. Clin Infect Dis. 2019;69:1740–1748. doi: 10.1093/cid/ciz033. [DOI] [PubMed] [Google Scholar]
- 7.Madrid L, Seale AC, Kohli-Lynch M, Edmond KM, Lawn JE, Heath PT, Madhi SA, Baker CJ, Bartlett L, Cutland C, Gravett MG, Ip M, Le Doare K, Rubens CE, Saha SK, Sobanjo-Ter Meulen A, Vekemans J, Schrag S, Infant GBS Disease Investigator Group Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65:S160–S172. doi: 10.1093/cid/cix656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagnew AF, Cunnington MC, Dube Q, Edwards MS, French N, Heyderman RS, Madhi SA, Slobod K, Clemens SAC. Variation in reported neonatal group B streptococcal disease incidence in developing countries. Clin Infect Dis. 2012;55:91–102. doi: 10.1093/cid/cis395. [DOI] [PubMed] [Google Scholar]
- 9.Bekker V, Bijlsma MW, van de Beek D, Kuijpers TW, van der Ende A. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect Dis. 2014;14:1083–1089. doi: 10.1016/S1473-3099(14)70919-3. [DOI] [PubMed] [Google Scholar]
- 10.Berardi A, Baroni L, BacchiReggiani ML, Ambretti S, Biasucci G, Bolognesi S, Capretti MG, Carretto E, Ciccia M, Fiorini V, Fortini C, Gargano G, Pedna MF, Rizzo V, Creti R, Ferrari F, GBS Prevention Working Group Emilia-Romagna The burden of early-onset sepsis in Emilia-Romagna (Italy): a 4-year, population-based study. J Matern-Fetal Neonatal Med. 2016;29:3126–3131. doi: 10.3109/14767058.2015.1114093. [DOI] [PubMed] [Google Scholar]
- 11.Lamagni TL, Keshishian C, Efstratiou A, Guy R, Henderson KL, Broughton K, Sheridan E. Emerging trends in the epidemiology of invasive group B streptococcal disease in England and Wales, 1991–2010. Clin Infect Dis. 2013;57:682–688. doi: 10.1093/cid/cit337. [DOI] [PubMed] [Google Scholar]
- 12.Di Renzo GC, Melin P, Berardi A, Blennow M, Carbonell-Estrany X, Donzelli GP, Hakansson S, Hod M, Hughes R, Kurtzer M, Poyart C, Shinwell E, Stray-Pedersen B, Wielgos M, El Helali N. Intrapartum GBS screening and antibiotic prophylaxis: a European consensus conference. J Matern Fetal Neonatal Med. 2015;28:766–782. doi: 10.3109/14767058.2014.934804. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi M, Vekemans J, Baker CJ, Ratner AJ, Le Doare K, Schrag SJ. Group B Streptococcus vaccine development: present status and future considerations, with emphasis on perspectives for low and middle income countries. F1000Research. 2016;5:2355. doi: 10.12688/f1000research.9363.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shabayek S, Spellerberg B. Group B streptococcal colonization, molecular characteristics, and epidemiology. Front Microbiol. 2018;9:437. doi: 10.3389/fmicb.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Remington JS, Wilson CB, Nizet V, Klein JO, Maldonado Y. Infectious diseases of the fetus and newborn E-book. Amsterdam: Elsevier Health Sciences; 2010. [Google Scholar]
- 16.Sørensen UBS, Klaas IC, Boes J, Farre M. The distribution of clones of Streptococcus agalactiae (group B streptococci) among herdspersons and dairy cows demonstrates lack of host specificity for some lineages. Vet Microbiol. 2019;235:71–79. doi: 10.1016/j.vetmic.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Plainvert C, Hays C, Touak G, Joubrel-Guyot C, Dmytruk N, Frigo A, Poyart C, Tazi A. Multidrug-resistant hypervirulent group B streptococcus in neonatal invasive infections, France, 2007–2019. Emerg Infect Dis. 2020;26:2721–2724. doi: 10.3201/eid2611.201669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones N, Bohnsack JF, Takahashi S, Oliver KA, Chan M-S, Kunst F, Glaser P, Rusniok C, Crook DWM, Harding RM, Bisharat N, Spratt BG. Multilocus sequence typing system for group B streptococcus. J Clin Microbiol. 2003;41:2530–2536. doi: 10.1128/JCM.41.6.2530-2536.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sørensen UBS, Poulsen K, Ghezzo C, Margarit I, Kilian M. Emergence and global dissemination of host-specific Streptococcus agalactiae clones. MBio. 2010;1:2. doi: 10.1128/mBio.00178-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGee L, Chochua S, Li Z, Mathis S, Rivers J, Metcalf B, Ryan A, Alden N, Farley MM, Harrison LH, Snippes Vagnone P, Lynfield R, Smelser C, Muse A, Thomas AR, Schrag S, Beall BW. Multistate, population-based distributions of candidate vaccine targets, clonal complexes, and resistance features of invasive group B streptococci within the United States, 2015–2017. Clin Infect Dis. 2021;72:1004–1013. doi: 10.1093/cid/ciaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes K, O’Halloran F, Cotter L. A review of antibiotic resistance in group B Streptococcus: the story so far. Crit Rev Microbiol. 2020;46:253–269. doi: 10.1080/1040841X.2020.1758626. [DOI] [PubMed] [Google Scholar]
- 22.Castor ML, Whitney CG, Como-Sabetti K, Facklam RR, Ferrieri P, Bartkus JM, Juni BA, Cieslak PR, Farley MM, Dumas NB. Antibiotic resistance patterns in invasive group B streptococcal isolates. Infect Dis Obstet Gynecol. 2008;2:2. doi: 10.1155/2008/727505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003;21:3468–3472. doi: 10.1016/S0264-410X(03)00353-0. [DOI] [PubMed] [Google Scholar]
- 24.Davies HG, Carreras-Abad C, Le Doare K, Heath PT. Group B streptococcus: trials and tribulations. Pediatr Infect Dis J. 2019;38:S72. doi: 10.1097/INF.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 25.Carreras-Abad C, Ramkhelawon L, Heath PT, Le Doare K. A vaccine against group B streptococcus: recent advances. Infect Drug Resist. 2020;13:1263–1272. doi: 10.2147/IDR.S203454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bianchi-Jassir F, Paul P, To K-N, Carreras-Abad C, Seale AC, Jauneikaite E, Madhi SA, Russell NJ, Hall J, Madrid L, Bassat Q, Kwatra G, Le Doare K, Lawn JE. Systematic review of Group B Streptococcal capsular types, sequence types and surface proteins as potential vaccine candidates. Vaccine. 2020;38:6682–6694. doi: 10.1016/j.vaccine.2020.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doare KL, Kampmann B, Vekemans J, Heath PT, Goldblatt D, Nahm MH, Baker C, Edwards MS, Kwatra G, Andrews N, Madhi SA, ter Meulen AS, Anderson AS, Corsaro B, Fischer P, Gorringe A. Serocorrelates of protection against infant group B streptococcus disease. Lancet Infect Dis. 2019;19:e162–e171. doi: 10.1016/S1473-3099(18)30659-5. [DOI] [PubMed] [Google Scholar]
- 28.Fabbrini M, Rigat F, Rinaudo CD, Passalaqua I, Khacheh S, Creti R, Baldassarri L, Carboni F, Anderloni G, Rosini R, Maione D, Grandi G, Telford JL, Margarit I. The protective value of maternal group B Streptococcus antibodies: quantitative and functional analysis of naturally acquired responses to capsular polysaccharides and pilus proteins in European maternal sera. Clin Infect Dis. 2016;63:746–753. doi: 10.1093/cid/ciw377. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez-Granger J, Alvargonzalez JC, Berardi A, Berner R, Kunze M, Hufnagel M, Melin P, Decheva A, Orefici G, Poyart C, Telford J, Efstratiou A, Killian M, Krizova P, Baldassarri L, Spellerberg B, Puertas A, Rosa-Fraile M. Prevention of group B streptococcal neonatal disease revisited. The DEVANI European project. Eur J Clin Microbiol Infect Dis. 2012;31:2097–2104. doi: 10.1007/s10096-012-1559-0. [DOI] [PubMed] [Google Scholar]
- 30.Afshar B, Broughton K, Creti R, Decheva A, Hufnagel M, Kriz P, Lambertsen L, Lovgren M, Melin P, Orefici G, Poyart C, Radtke A, Rodriguez-Granger J, Sørensen UBS, Telford J, Valinsky L, Zachariadou L, Members of the DEVANI Study Group. Efstratiou A. International external quality assurance for laboratory identification and typing of Streptococcus agalactiae (Group B streptococci) J Clin Microbiol. 2011;49:1475–1482. doi: 10.1128/JCM.02365-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao K, Poulsen K, Maione D, Rinaudo CD, Baldassarri L, Telford JL, Sorensen UBS, Members of the DEVANI Study Group. Kilian M. Capsular gene typing of streptococcus agalactiae compared to serotyping by latex agglutination. J Clin Microbiol. 2013;51:503–507. doi: 10.1128/JCM.02417-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imperi M, Pataracchia M, Alfarone G, Baldassarri L, Orefici G, Creti R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of Streptococcus agalactiae. J Microbiol Methods. 2010;80:212–214. doi: 10.1016/j.mimet.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 33.Poyart C, Tazi A, Réglier-Poupet H, Billoët A, Tavares N, Raymond J, Trieu-Cuot P. Multiplex PCR assay for rapid and accurate capsular typing of group B streptococci. J Clin Microbiol. 2007;45:1985–1988. doi: 10.1128/JCM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kong F, Lambertsen LM, Slotved H-C, Ko D, Wang H, Gilbert GL. Use of phenotypic and molecular serotype identification methods to characterize previously nonserotypeable group B streptococci. J Clin Microbiol. 2008;46:2745–2750. doi: 10.1128/JCM.00189-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berardi A, Cattelani C, Creti R, Berner R, Pietrangiolillo Z, Margarit I, Maione D, Ferrari F. Group B streptococcal infections in the newborn infant and the potential value of maternal vaccination. Expert Rev Anti Infect Ther. 2015;13:1387–1399. doi: 10.1586/14787210.2015.1079126. [DOI] [PubMed] [Google Scholar]
- 36.Schrag SJ, Verani JR. Intrapartum antibiotic prophylaxis for the prevention of perinatal group B streptococcal disease: experience in the United States and implications for a potential group B streptococcal vaccine. Vaccine. 2013;31(Suppl 4):D20–26. doi: 10.1016/j.vaccine.2012.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pulver LS, Hopfenbeck MM, Young PC, Stoddard GJ, Korgenski K, Daly J, Byington CL. Continued early onset group B streptococcal infections in the era of intrapartum prophylaxis. J Perinatol Off J Calif Perinat Assoc. 2009;29:20–25. doi: 10.1038/jp.2008.115. [DOI] [PubMed] [Google Scholar]
- 38.Stoll BJ, Hansen NI, Sánchez PJ, Faix RG, Poindexter BB, Van Meurs KP, Bizzarro MJ, Goldberg RN, Frantz ID, Hale EC, Shankaran S, Kennedy K, Carlo WA, Watterberg KL, Bell EF, Walsh MC, Schibler K, Laptook AR, Shane AL, Schrag SJ, Das A, Higgins RD, Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network Early onset neonatal sepsis: the burden of group B Streptococcal and E. coli disease continues. Pediatrics. 2011;127:817–826. doi: 10.1542/peds.2010-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benitz WE, Gould JB, Druzin ML. Risk factors for early-onset group B streptococcal sepsis: estimation of odds ratios by critical literature review. Pediatrics. 1999;103:e77–e77. doi: 10.1542/peds.103.6.e77. [DOI] [PubMed] [Google Scholar]
- 40.Verani JR, McGee L, Schrag SJ, Division of Bacterial Diseases, National Center for Immunization and Respiratory Diseases, Centers for Disease Control and Prevention Prevention of perinatal group B streptococcal disease–revised guidelines from CDC, 2010. MMWR Recomm Rep. 2010;59:1–36. [PubMed] [Google Scholar]
- 41.Romain A-S, Cohen R, Plainvert C, Joubrel C, Béchet S, Perret A, Tazi A, Poyart C, Levy C. Clinical and laboratory features of group B streptococcus meningitis in infants and newborns: study of 848 cases in France, 2001–2014. Clin Infect Dis. 2018;66:857–864. doi: 10.1093/cid/cix896. [DOI] [PubMed] [Google Scholar]
- 42.Ji W, Liu H, Madhi SA, Cunnington M, Zhang Z, Dangor Z, Zhou H, Mu X, Jin Z, Wang A, Qin X, Gao C, Zhu Y, Feng X, She S, Yang S, Liu J, Lei J, Jiang L, Liu Z, Li G, Li Q, Deng Q, Gao K, Fang Y. Clinical and molecular epidemiology of invasive group B streptococcus disease among infants, China. Emerg Infect Dis. 2019;25:2021–2030. doi: 10.3201/eid2511.181647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berardi A, Rossi C, Lugli L, Creti R, BacchiReggiani ML, Lanari M, Memo L, Pedna MF, Venturelli C, Perrone E, Ciccia M, Tridapalli E, Piepoli M, Contiero R, Ferrari F. Group B streptococcus late-onset disease: 2003–2010. Pediatrics. 2013;131:e361–368. doi: 10.1542/peds.2012-1231. [DOI] [PubMed] [Google Scholar]
- 44.Regan JA, Klebanoff MA, Nugent RP, Eschenbach DA, Blackwelder WC, Lou Y, Gibbs RS, Rettig PJ, Martin DH, Edelman R. Colonization with group B streptococci in pregnancy and adverse outcome. VIP Study Group. Am J Obstet Gynecol. 1996;174:1354–1360. doi: 10.1016/S0002-9378(96)70684-1. [DOI] [PubMed] [Google Scholar]
- 45.Fluegge K, Siedler A, Heinrich B, Schulte-Moenting J, Moennig M-J, Bartels DB, Dammann O, von Kries R, Berner R, German Pediatric Surveillance Unit Study Group Incidence and clinical presentation of invasive neonatal group B streptococcal infections in Germany. Pediatrics. 2006;117:e1139–1145. doi: 10.1542/peds.2005-2481. [DOI] [PubMed] [Google Scholar]
- 46.Libster R, Edwards KM, Levent F, Edwards MS, Rench MA, Castagnini LA, Cooper T, Sparks RC, Baker CJ, Shah PE. Long-term outcomes of group B streptococcal meningitis. Pediatrics. 2012;130:e8–e15. doi: 10.1542/peds.2011-3453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.